Effects of Iron Overload and Oxidative Damage on the Musculoskeletal System in the Space Environment: Data from Spaceflights and Ground-Based Simulation Models
Abstract
1. Introduction
2. The Effect of Spaceflight Environment and Its Analogs on Iron Metabolism
2.1. Evidence from Spaceflight
2.2. Evidence from Ground-Based Analog Models for Spaceflight
3. Iron Overload and Its Link to Spaceflight-Induced Oxidative Stress
3.1. The Relationship Between Iron and Oxidative Stress
3.2. Oxidative Stress in Spaceflight
3.3. Oxidative Stress in Ground-Based Models for Spaceflight
4. Iron and ROS Signaling in Spaceflight-Induced-Bone Loss and Muscle Atrophy
4.1. Iron and ROS Signaling in Spaceflight-Induced-Bone Loss
4.2. Iron and ROS Signaling in Spaceflight Induced-Muscle Atrophy
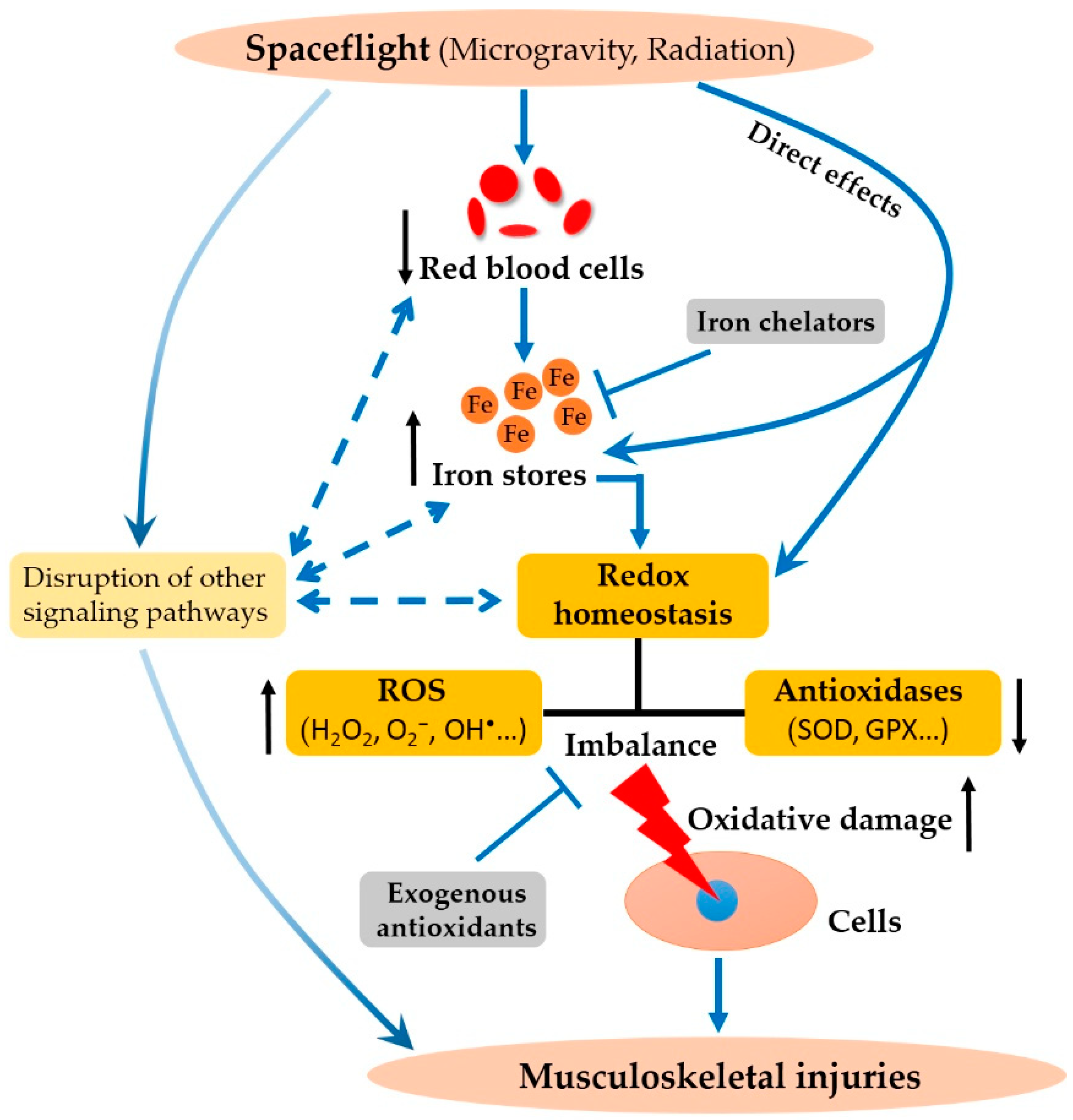
5. Implications for the Development of Spaceflight Countermeasures
6. Concluding Statements
Funding
Conflicts of Interest
References
- Crucian, B.E.; Chouker, A.; Simpson, R.J.; Mehta, S.; Marshall, G.; Smith, S.M.; Zwart, S.R.; Heer, M.; Ponomarev, S.; Whitmire, A.; et al. Immune system dysregulation during spaceflight: Potential countermeasures for deep space exploration missions. Front. Immunol. 2018, 9, 1437. [Google Scholar] [CrossRef] [PubMed]
- Hughson, R.L.; Helm, A.; Durante, M. Heart in space: Effect of the extraterrestrial environment on the cardiovascular system. Nat. Rev. Cardiol. 2018, 15, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Jandial, R.; Hoshide, R.; Waters, J.D.; Limoli, C.L. Space-brain: The negative effects of space exposure on the central nervous system. Surg. Neurol. Int. 2018, 9, 9. [Google Scholar] [PubMed]
- Vico, L.; Hargens, A. Skeletal changes during and after spaceflight. Nat. Rev. Rheumatol. 2018, 14, 229–245. [Google Scholar] [CrossRef] [PubMed]
- Radugina, E.A.; Almeida, E.A.C.; Blaber, E.; Poplinskaya, V.A.; Markitantova, Y.V.; Grigoryan, E.N. Exposure to microgravity for 30 days onboard Bion M1 caused muscle atrophy and impaired regeneration in murine femoral quadriceps. Life Sci. Space Res. 2018, 16, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Crielaard, B.J.; Lammers, T.; Rivella, S. Targeting iron metabolism in drug discovery and delivery. Nat. Rev. Drug Discov. 2017, 16, 400–423. [Google Scholar] [CrossRef] [PubMed]
- Dixon, S.J.; Stockwell, B.R. The role of iron and reactive oxygen species in cell death. Nat. Chem. Biol. 2014, 10, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Tsay, J.; Yang, Z.; Ross, F.P.; Cunningham-Rundles, S.; Lin, H.; Coleman, R.; Mayer-Kuckuk, P.; Doty, S.B.; Grady, R.W.; Giardina, P.J.; et al. Bone loss caused by iron overload in a murine model: Importance of oxidative stress. Blood 2010, 116, 2582–2589. [Google Scholar] [CrossRef] [PubMed]
- Tian, Q.; Wu, S.; Dai, Z.; Yang, J.; Zheng, J.; Zheng, Q.; Liu, Y. Iron overload induced death of osteoblasts in vitro: Involvement of the mitochondrial apoptotic pathway. PeerJ 2016, 4, e2611. [Google Scholar] [CrossRef] [PubMed]
- Balogh, E.; Tolnai, E.; Nagy, B., Jr.; Nagy, B.; Balla, G.; Balla, J.; Jeney, V. Iron overload inhibits osteogenic commitment and differentiation of mesenchymal stem cells via the induction of ferritin. Biochim. Biophys. Acta 2016, 1862, 1640–1649. [Google Scholar] [CrossRef] [PubMed]
- Reardon, T.F.; Allen, D.G. Iron injections in mice increase skeletal muscle iron content, induce oxidative stress and reduce exercise performance. Exp. Physiol. 2009, 94, 720–730. [Google Scholar] [CrossRef] [PubMed]
- Jeney, V. Clinical impact and cellular mechanisms of iron overload-associated bone loss. Front. Pharmacol. 2017, 8, 77. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G. Is iron accumulation a possible risk factor for sarcopenia? Biol. Trace Elem. Res. 2018, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Zwart, S.R.; Morgan, J.L.; Smith, S.M. Iron status and its relations with oxidative damage and bone loss during long-duration space flight on the international space station. Am. J. Clin. Nutr. 2013, 98, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.M. Red blood cell and iron metabolism during space flight. Nutrition 2002, 18, 864–866. [Google Scholar] [CrossRef]
- Zwart, S.R.; Mathews Oliver, S.A.; Fesperman, J.V.; Kala, G.; Krauhs, J.; Ericson, K.; Smith, S.M. Nutritional status assessment before, during, and after long-duration head-down bed rest. Aviat. Space Environ. Med. 2009, 80, 15–22. [Google Scholar] [CrossRef]
- Dalla Libera, L.; Ravara, B.; Gobbo, V.; Tarricone, E.; Vitadello, M.; Biolo, G.; Vescovo, G.; Gorza, L. A transient antioxidant stress response accompanies the onset of disuse atrophy in human skeletal muscle. J. Appl. Physiol. 2009, 107, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Meng, X.; Dong, D.; Xue, Y.; Chen, X.; Wang, S.; Shen, Y.; Zhang, G.; Shang, P. Iron overload involved in the enhancement of unloading-induced bone loss by hypomagnetic field. Bone 2018, 114, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Morikawa, D.; Nojiri, H.; Saita, Y.; Kobayashi, K.; Watanabe, K.; Ozawa, Y.; Koike, M.; Asou, Y.; Takaku, T.; Kaneko, K.; et al. Cytoplasmic reactive oxygen species and sod1 regulate bone mass during mechanical unloading. J. Bone Miner. Res. 2013, 28, 2368–2380. [Google Scholar] [CrossRef] [PubMed]
- Hofer, T.; Marzetti, E.; Xu, J.; Seo, A.Y.; Gulec, S.; Knutson, M.D.; Leeuwenburgh, C.; Dupont-Versteegden, E.E. Increased iron content and rna oxidative damage in skeletal muscle with aging and disuse atrophy. Exp. Gerontol. 2008, 43, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Arosio, P.; Elia, L.; Poli, M. Ferritin, cellular iron storage and regulation. IUBMB Life 2017, 69, 414–422. [Google Scholar] [CrossRef] [PubMed]
- Daru, J.; Colman, K.; Stanworth, S.J.; De La Salle, B.; Wood, E.M.; Pasricha, S.R. Serum ferritin as an indicator of iron status: What do we need to know? Am. J. Clin Nutr. 2017, 106, 1634S–1639S. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.M.; Zwart, S.R.; Block, G.; Rice, B.L.; Davis-Street, J.E. The nutritional status of astronauts is altered after long-term space flight aboard the international space station. J. Nutr. 2005, 135, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Muckenthaler, M.U.; Rivella, S.; Hentze, M.W.; Galy, B. A red carpet for iron metabolism. Cell 2017, 168, 344–361. [Google Scholar] [CrossRef] [PubMed]
- Udden, M.M.; Driscoll, T.B.; Pickett, M.H.; Leachhuntoon, C.S.; Alfrey, C.P. Decreased production of red blood cells in human subjects exposed to microgravity. J. Lab. Clin. Med. 1995, 125, 442–449. [Google Scholar] [PubMed]
- Lane, H.W.; Alfrey, C.P.; Driscoll, T.B.; Smith, S.M.; Nyquist, L.E. Control of red blood cell mass during spaceflight. J. Gravit. Physiol. 1996, 3, 87–88. [Google Scholar] [PubMed]
- Xu, K.; Holubec, K.V.; Love, J.E.; Goodwin, T.J.; Sytkowski, A.J. Abnormal erythropoiesis in microgravity. Blood 2004, 104, 3701. [Google Scholar]
- De Santo, N.G.; Cirillo, M.; Kirsch, K.A.; Correale, G.; Drummer, C.; Frassl, W.; Perna, A.F.; Di Stazio, E.; Bellini, L.; Gunga, H.-C. Anemia and erythropoietin in space flights. Semin. Nephrol. 2005, 25, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, S.M.; Morukov, B.V.; Labetskaya, O.I.; Yarlikova, Y.V.; Levina, A.A.; Shishkanova, Z.G. Red blood of cosmonauts during missions aboard the international space station (iss). Hum. Physiol. 2010, 36, 877–881. [Google Scholar] [CrossRef]
- Fischer, C.L.; Johnson, P.C.; Berry, C.A. Red blood cell mass and plasma volume changes in manned space flight. JAMA 1967, 200, 579–583. [Google Scholar] [CrossRef] [PubMed]
- Leach, C.S.; Johnson, P.C. Influence of spaceflight on erythrokinetics in man. Science 1984, 225, 216–218. [Google Scholar] [CrossRef] [PubMed]
- Alfrey, C.P.; Rice, L.; Udden, M.M.; Driscoll, T.B. Neocytolysis: Physiological down-regulator of red-cell mass. Lancet 1997, 349, 1389–1390. [Google Scholar] [CrossRef]
- Davis, T.A.; Wiesmann, W.; Kidwell, W.; Cannon, T.; Kerns, L.; Serke, C.; Delaplaine, T.; Pranger, A.; Lee, K.P. Effect of spaceflight on human stem cell hematopoiesis: Suppression of erythropoiesis and myelopoiesis. J. Leukoc. Biol. 1996, 60, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Beguin, Y. Soluble transferrin receptor for the evaluation of erythropoiesis and iron status. Clin. Chim. Acta 2003, 329, 9–22. [Google Scholar] [CrossRef]
- Smith, S.M.; Zwart, S.R. Chapter 3 nutritional biochemistry of spaceflight. Adv. Clin. Chem. 2008, 46, 87–130. [Google Scholar] [PubMed]
- Mulavara, A.P.; Peters, B.T.; Miller, C.A.; Kofman, I.S.; Reschke, M.F.; Taylor, L.C.; Lawrence, E.L.; Wood, S.J.; Laurie, S.S.; Smc, L. Physiological and functional alterations after spaceflight and bed rest. Med. Sci. Sports Exerc. 2018, 50, 1961–1980. [Google Scholar] [CrossRef] [PubMed]
- Morey-Holton, E.R.; Globus, R.K. Hindlimb unloading rodent model: Technical aspects. J. Appl. Physiol. 2002, 92, 1367–1377. [Google Scholar] [CrossRef] [PubMed]
- Dunn, C.D.R.; Lange, R.D.; Kimzey, S.L.; Johnson, P.C.; Leach, C.S. Serum erythropoietin titers during prolonged bedrest; relevance to the “anaemia” of space flight. Eur. J. Appl. Physiol. Occup. Physiol. 1984, 52, 178–182. [Google Scholar] [CrossRef] [PubMed]
- Trudel, G.; Uhthoff, H.K.; Laneuville, O. Hemolysis during and after 21 days of head-down-tilt bed rest. Physiol. Rep. 2017, 5, e13469. [Google Scholar] [CrossRef] [PubMed]
- Ryan, B.J.; Goodrich, J.A.; Schmidt, W.F.; Stothard, E.R.; Wright, K.P., Jr.; Byrnes, W.C. Haemoglobin mass alterations in healthy humans following four-day head-down tilt bed rest. Exp. Physiol. 2016, 101, 628–640. [Google Scholar] [CrossRef] [PubMed]
- Gunga, H.C.; Kirsch, K.; Baartz, F.; Maillet, A.; Gharib, C.; Nalishiti, W.; Rich, I.; Rocker, L. Erythropoietin under real and simulated microgravity conditions in humans. J. Appl. Physiol. 1996, 81, 761–773. [Google Scholar] [CrossRef] [PubMed]
- Morgan, J.L.; Zwart, S.R.; Heer, M.; Ploutz-Snyder, R.; Ericson, K.; Smith, S.M. Bone metabolism and nutritional status during 30-day head-down-tilt bed rest. J. Appl. Physiol. 2012, 113, 1519–1529. [Google Scholar] [CrossRef] [PubMed]
- Andrews, N.C. Disorders of iron metabolism. N. Engl. J. Med. 1999, 341, 1986–1995. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Sun, W.; Li, Y.; Ling, S.; Zhao, C.; Zhong, G.; Zhao, D.; Song, J.; Song, H.; Li, J.; et al. The regulation of iron metabolism by hepcidin contributes to unloading-induced bone loss. Bone 2016, 94, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Cavey, T.; Pierre, N.; Nay, K.; Allain, C.; Ropert, M.; Loreal, O.; Derbre, F. Simulated microgravity decreases circulating iron in rats: Role of inflammation-induced hepcidin upregulation. Exp. Physiol. 2017, 102, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Hohenberger, J.; Ray, K.; Meyer, K. The biology and chemistry of high-valent iron-oxo and iron-nitrido complexes. Nat. Commun. 2012, 3, 720. [Google Scholar] [CrossRef] [PubMed]
- Koppenol, W.H. The haber-weiss cycle--70 years later. Redox Rep. 2001, 6, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Zhao, B.; Zhang, L.; Wang, S.; Dong, D.; Lv, H.; Shang, P. Alterations in cellular iron metabolism provide more therapeutic opportunities for cancer. Int. J. Mol. Sci. 2018, 19, 1545. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, T.J.; Christofidou-Solomidou, M. Oxidative stress and space biology: An organ-based approach. Int. J. Mol. Sci. 2018, 19, 959. [Google Scholar] [CrossRef] [PubMed]
- Stein, T.; Leskiw, M. Oxidant damage during and after spaceflight. Am. J. Physiol. Endocrinol. Metab. 2000, 278, E37–E382. [Google Scholar] [CrossRef] [PubMed]
- Indo, H.P.; Majima, H.J.; Terada, M.; Suenaga, S.; Tomita, K.; Yamada, S.; Higashibata, A.; Ishioka, N.; Kanekura, T.; Nonaka, I. Changes in mitochondrial homeostasis and redox status in astronauts following long stays in space. Sci. Rep. 2016, 6, 39015. [Google Scholar] [CrossRef] [PubMed]
- De Luca, C.; Deeva, I.; Mariani, S.; Maiani, G.; Stancato, A.; Korkina, L. Monitoring antioxidant defenses and free radical production in space-flight, aviation and railway engine operators, for the prevention and treatment of oxidative stress, immunological impairment, and pre-mature cell aging. Toxicol. Ind. Health 2009, 25, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, A.M.; Corsetto, P.A.; Montorfano, G.; Milani, S.; Zava, S.; Tavella, S.; Cancedda, R.; Berra, B. Effects of long-term space flight on erythrocytes and oxidative stress of rodents. PLoS ONE 2012, 7, e32361. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Pecaut, M.; Stodieck, L.; Ferguson, V.; Bateman, T.; Bouxsein, M.; Gridley, D. Biological and metabolic response in sts-135 space-flown mouse skin. Free Radic. Res. 2014, 48, 890–897. [Google Scholar] [CrossRef] [PubMed]
- Blaber, E.A.; Pecaut, M.J.; Jonscher, K.R. Spaceflight activates autophagy programs and the proteasome in mouse liver. Int. J. Mol. Sci. 2017, 18, 2062. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.W.; Pecaut, M.J.; Stodieck, L.S.; Ferguson, V.L.; Bateman, T.A.; Bouxsein, M.; Jones, T.A.; Moldovan, M.; Cunningham, C.E.; Chieu, J.; et al. Spaceflight environment induces mitochondrial oxidative damage in ocular tissue. Radiat. Res. 2013, 180, 340–350. [Google Scholar] [CrossRef] [PubMed]
- Theriot, C.A.; Zanello, S.B. Molecular effects of spaceflight in the mouse eye after space shuttle mission sts-135. Gravit. Space Res. 2014, 2, 3–24. [Google Scholar]
- Connor, M.K.; Hood, D.A. Effect of microgravity on the expression of mitochondrial enzymes in rat cardiac and skeletal muscles. J. Appl. Physiol. 1998, 84, 593–598. [Google Scholar] [CrossRef] [PubMed]
- Baqai, F.P.; Gridley, D.S.; Slater, J.M.; Luo-Owen, X.; Stodieck, L.S.; Ferguson, V.; Chapes, S.K.; Pecaut, M.J. Effects of spaceflight on innate immune function and antioxidant gene expression. J. Appl. Physiol. 2009, 106, 1935–1942. [Google Scholar] [CrossRef] [PubMed]
- Hollander, J.; Gore, M.; Fiebig, R.; Mazzeo, R.; Ohishi, S.; Ohno, H.; Ji, L. Spaceflight downregulates antioxidant defense systems in rat liver. Free Radic. Biol. Med. 1998, 24, 385–390. [Google Scholar] [CrossRef]
- Debevec, T.; Pialoux, V.; Ehrström, S.; Ribon, A.; Eiken, O.; Mekjavic, I.B.; Millet, G.P. Femhab: The effects of bed rest and hypoxia on oxidative stress in healthy women. J. Appl. Physiol. 2016, 120, 930–938. [Google Scholar] [CrossRef] [PubMed]
- Rai, B.; Kaur, J.; Catalina, M.; Anand, S.; Jacobs, R.; Teughels, W. Effect of simulated microgravity on salivary and serum oxidants, antioxidants, and periodontal status. J. Periodontol. 2011, 82, 1478–1482. [Google Scholar] [CrossRef] [PubMed]
- Rai, B.; Kaur, J.; Catalina, M. Anti-oxidation actions of curcumin in two forms of bed rest: Oxidative stress serum and salivary markers. Asian Pac. J. Trop. Med. 2010, 3, 651–654. [Google Scholar] [CrossRef]
- Rai, B.; Kaur, J.; Jain, R. Salivary and serum 8-hydroxydeoxyguanosine level in simulated microgravity. Maced. J. Med. Sci. 2010, 3, 364–367. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Q.; Chen, H.; Liu, X.; Lv, K.; Wang, T.; Wang, Y.; Ji, G.; Cao, H.; Kan, G. Involvement of cholinergic dysfunction and oxidative damage in the effects of simulated weightlessness on learning and memory in rats. BioMed Res. Int. 2018, 2018, 2547532. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Javed, I.; Liu, Y.; Lu, S.; Peng, G.; Zhang, Y.; Qing, H.; Deng, Y. Effect of prolonged simulated microgravity on metabolic proteins in rat hippocampus: Steps toward safe space travel. J. Proteome Res. 2015, 15, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Ran, H.H.; Peng, L.; Xu, F.; Sun, J.F.; Zhang, L.N.; Fan, Y.Y.; Peng, L.; Cui, G. Mitochondrial regulation of nadph oxidase in hindlimb unweighting rat cerebral arteries. PLoS ONE 2014, 9, e95916. [Google Scholar] [CrossRef] [PubMed]
- Lawler, J.M.; Song, W.; Demaree, S.R. Hindlimb unloading increases oxidative stress and disrupt antioxidant capacity in skeletal muscle. Free Radic. Biol. Med. 2003, 35, 9–16. [Google Scholar] [CrossRef]
- Chowdhury, P.; Soulsby, M. Oxidant/anti-oxidant status in rats exposed to simulated weightlessness by hind-limb unloading and reloading. Open Clin. Chem. J. 2008, 1, 47–56. [Google Scholar] [CrossRef]
- Jayroe, J.; Soulsby, M.; Chowdhury, P. Attenuation of tissue oxidative stress by dietary restriction in rats on simulated microgravity. Ann. Clin. Lab. Sci. 2012, 42, 140–144. [Google Scholar] [PubMed]
- Chowdhury, P.; Soulsby, M.E.; Scott, J.L. Effects of aminoguanidine on tissue oxidative stress induced by hindlimb unloading in rats. Ann. Clin. Lab. Sci. 2009, 39, 64–70. [Google Scholar] [PubMed]
- Chowdhury, P.; Soulsby, M.; Kim, K. L-carnitine influence on oxidative stress induced by hind limb unloading in adult rats. Aviat. Space Environ. Med. 2007, 78, 554–556. [Google Scholar] [PubMed]
- Pietrofesa, R.A.; Turowski, J.B.; Arguiri, E.; Milovanova, T.N.; Solomides, C.C.; Thom, S.R.; Christofidou-Solomidou, M. Oxidative lung damage resulting from repeated exposure to radiation and hyperoxia associated with space exploration. J. Pulm. Respir Med. 2013, 3, 1000158. [Google Scholar] [PubMed]
- Christofidou-Solomidou, M.; Pietrofesa, R.A.; Arguiri, E.; Schweitzer, K.S.; Berdyshev, E.V.; McCarthy, M.; Corbitt, A.; Alwood, J.S.; Yu, Y.; Globus, R.K. Space radiation-associated lung injury in a murine model. Am. J. Physiol. Lung Cell. Mol. Physiol. 2015, 308, L416–L428. [Google Scholar] [CrossRef] [PubMed]
- Pietrofesa, R.A.; Velalopoulou, A.; Lehman, S.L.; Arguiri, E.; Solomides, P.; Koch, C.J.; Mishra, O.P.; Koumenis, C.; Goodwin, T.J.; Christofidou-Solomidou, M. Novel double-hit model of radiation and hyperoxia-induced oxidative cell damage relevant to space travel. Int. J. Mol. Sci. 2016, 17, 953. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Boerma, M.; Rodriguez, D.; Campbell-Beachler, M.; Jones, T.; Stanbouly, S.; Sridharan, V.; Wroe, A.; Nelson, G. Acute effect of low-dose space radiation on mouse retina and retinal endothelial cells. Radiat. Res. 2018, 190, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Sasi, S.P.; Gee, H.; Lee, J.Y.; Yang, Y.; Mehrzad, R.; Onufrak, J.; Song, J.; Enderling, H.; Agarwal, A. Cardiovascular risks associated with low dose ionizing particle radiation. PLoS ONE 2014, 9, e110269. [Google Scholar] [CrossRef] [PubMed]
- Baluchamy, S.; Ravichandran, P.; Ramesh, V.; He, Z.; Zhang, Y.; Hall, J.C.; Jejelowo, O.; Gridley, D.S.; Wu, H.; Ramesh, G.T. Reactive oxygen species mediated tissue damage in high energy proton irradiated mouse brain. Mol. Cell. Biochem. 2012, 360, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Kondo, H.; Yumoto, K.; Alwood, J.S.; Mojarrab, R.; Wang, A.; Almeida, E.A.; Searby, N.D.; Limoli, C.L.; Globus, R.K. Oxidative stress and gamma radiation-induced cancellous bone loss with musculoskeletal disuse. J. Appl. Physiol. 2010, 108, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Baluchamy, S.; Ravichandran, P.; Periyakaruppan, A.; Ramesh, V.; Hall, J.C.; Zhang, Y.; Jejelowo, O.; Gridley, D.S.; Wu, H.; Ramesh, G.T. Induction of cell death through alteration of oxidants and antioxidants in lung epithelial cells exposed to high energy protons. J. Biol. Chem. 2010, 285, 24769–24774. [Google Scholar] [CrossRef] [PubMed]
- Limoli, C.L.; Giedzinski, E.; Baure, J.; Rola, R.; Fike, J.R. Altered growth and radiosensitivity in neural precursor cells subjected to oxidative stress. Int. J. Radiat. Biol. 2006, 82, 640–647. [Google Scholar] [CrossRef] [PubMed]
- Indo, H.P.; Tomiyoshi, T.; Suenaga, S.; Tomita, K.; Suzuki, H.; Masuda, D.; Terada, M.; Ishioka, N.; Gusev, O.; Cornette, R. Mnsod downregulation induced by extremely low 0.1 mgy single and fractionated x-rays and microgravity treatment in human neuroblastoma cell line, nb-1. J. Clin. Biochem. Nutr. 2015, 57, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Bai, B.; Zhang, Y. Bone abnormalities in young male rats with iron intervention and possible mechanisms. Chem. Biol. Interact. 2018, 279, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Hadjidakis, D.J.; Androulakis, I.I. Bone remodeling. Ann. N. Y. Acad. Sci. 2006, 1092, 385–396. [Google Scholar] [CrossRef] [PubMed]
- He, Y.F.; Ma, Y.; Gao, C.; Zhao, G.Y.; Zhang, L.L.; Li, G.F.; Pan, Y.Z.; Li, K.; Xu, Y.J. Iron overload inhibits osteoblast biological activity through oxidative stress. Biol. Trace Elem. Res. 2013, 152, 292–296. [Google Scholar] [CrossRef] [PubMed]
- Jia, P.; Xu, Y.J.; Zhang, Z.L.; Li, K.; Li, B.; Zhang, W.; Yang, H. Ferric ion could facilitate osteoclast differentiation and bone resorption through the production of reactive oxygen species. J. Orthop. Res. 2012, 30, 1843–1852. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.M.; Heer, M.A.; Shackelford, L.C.; Sibonga, J.D.; Ploutzsnyder, L.; Zwart, S.R. Benefits for bone from resistance exercise and nutrition in long-duration spaceflight: Evidence from biochemistry and densitometry. J. Bone Miner. Res. 2012, 27, 1896–1906. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.M.; Mccoy, T.; Gazda, D.; Morgan, J.L.; Heer, M.; Zwart, S.R. Space flight calcium: Implications for astronaut health, spacecraft operations, and earth. Nutrients 2012, 4, 2047–2068. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Li, L.; Jiang, Y.; Wang, C.; Geng, B.; Wang, Y.; Chen, J.; Liu, F.; Qiu, P.; Zhai, G.; et al. Space microgravity drives transdifferentiation of human bone marrow-derived mesenchymal stem cells from osteogenesis to adipogenesis. FASEB J. 2018, 32, 4444–4458. [Google Scholar] [CrossRef] [PubMed]
- Xin, M.; Yang, Y.; Zhang, D.; Wang, J.; Chen, S.; Zhou, D. Attenuation of hind-limb suspension-induced bone loss by curcumin is associated with reduced oxidative stress and increased vitamin d receptor expression. Osteoporos. Int. 2015, 26, 2665–2676. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Shuang, F.; Chen, D.; Zhou, R. Treatment of hydrogen molecule abates oxidative stress and alleviates bone loss induced by modeled microgravity in rats. Osteoporos. Int. 2013, 24, 969–978. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Qiu, X.; Xi, K.; Hu, W.; Pei, H.; Nie, J.; Wang, Z.; Ding, J.; Shang, P.; Li, B.; et al. Therapeutic ionizing radiation induced bone loss: A review of in vivo and in vitro findings. Connect. Tissue Res. 2018, 29, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Budagov, R.S.; Ashurov, A.A.; Zaĭchik, V.E. Blood trace elements as an indicator of the degree of severity in radiation lesions. Radiats. Biol. Radioecol. 1994, 34, 49–54. [Google Scholar] [PubMed]
- Zhang, X.H.; Lou, Z.C.; Wang, A.L.; Hu, X.D.; Zhang, H.Q. Development of serum iron as a biological dosimeter in mice. Radiat. Res. 2013, 179, 684–689. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.H.; Zhang, X.H.; Hu, X.D.; Min, X.Y.; Zhou, Q.F.; Zhang, H.Q. Mechanisms of an increased level of serum iron in gamma-irradiated mice. Radiat. Environ. Biophys. 2016, 55, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Felice, P.A.; Ahsan, S.; Donneys, A.; Deshpande, S.S.; Nelson, N.S.; Buchman, S.R. Deferoxamine administration delivers translational optimization of distraction osteogenesis in the irradiated mandible. Plast. Reconstr. Surg. 2013, 132, 542e–548e. [Google Scholar] [CrossRef] [PubMed]
- Donneys, A.; Ahsan, S.; Perosky, J.E.; Deshpande, S.S.; Tchanque-Fossuo, C.N.; Levi, B.; Kozloff, K.M.; Buchman, S.R. Deferoxamine restores callus size, mineralization, and mechanical strength in fracture healing after radiotherapy. Plast. Reconstr. Surg. 2013, 131, 711e–719e. [Google Scholar] [CrossRef] [PubMed]
- Kook, S.H.; Kim, K.A.; Ji, H.; Lee, D.; Lee, J.C. Irradiation inhibits the maturation and mineralization of osteoblasts via the activation of nrf2/ho-1 pathway. Mol. Cell. Biochem. 2015, 410, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Kondo, H.; Limoli, C.; Searby, N.D.; Almeida, E.A.; Loftus, D.J.; Vercoutere, W.; Moreyholton, E.; Giedzinski, E.; Mojarrab, R.; Hilton, D. Shared oxidative pathways in response to gravity-dependent loading and gamma-irradiation of bone marrow-derived skeletal cell progenitors. Radiats. Biol. Radioecol. 2007, 47, 281–285. [Google Scholar] [PubMed]
- Alwood, J.S.; Tran, L.H.; Schreurs, A.S.; Shirazi-Fard, Y.; Kumar, A.; Hilton, D.; Tahimic, C.G.T.; Globus, R.K. Dose- and ion-dependent effects in the oxidative stress response to space-like radiation exposure in the skeletal system. Int. J. Mol. Sci. 2017, 18, 2117. [Google Scholar] [CrossRef] [PubMed]
- Fitts, R.H.; Trappe, S.W.; Costill, D.L.; Gallagher, P.M.; Creer, A.C.; Colloton, P.A.; Peters, J.R.; Romatowski, J.G.; Bain, J.L.; Riley, D.A. Prolonged space flight-induced alterations in the structure and function of human skeletal muscle fibres. J. Physiol. 2010, 588, 3567–3592. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Arfat, Y.; Wang, H.; Goswami, N. Muscle atrophy induced by mechanical unloading: Mechanisms and potential countermeasures. Front. Physiol. 2018, 9, 235. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, K.M.; Haddad, F.; Pandorf, C.E.; Roy, R.R.; Edgerton, V.R. Alterations in muscle mass and contractile phenotype in response to unloading models: Role of transcriptional/pretranslational mechanisms. Front. Physiol. 2013, 4, 284. [Google Scholar] [CrossRef] [PubMed]
- Altun, M.; Edstrom, E.; Spooner, E.; Flores-Moralez, A.; Bergman, E.; Tollet-Egnell, P.; Norstedt, G.; Kessler, B.M.; Ulfhake, B. Iron load and redox stress in skeletal muscle of aged rats. Muscle Nerve 2007, 36, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, Y.; Imao, M.; Satoh, A.; Watanabe, H.; Hamano, H.; Horinouchi, Y.; Izawa-Ishizawa, Y.; Kihira, Y.; Miyamoto, L.; Ishizawa, K.; et al. Iron-induced skeletal muscle atrophy involves an akt-forkhead box o3-e3 ubiquitin ligase-dependent pathway. J. Trace Elem. Med. Biol. 2016, 35, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Kasztura, M.; Dzięgała, M.; Kobak, K.; Bania, J.; Mazur, G.; Banasiak, W.; Ponikowski, P.; Jankowska, E.A. Both iron excess and iron depletion impair viability of rat h9c2 cardiomyocytes and l6g8c5 myocytes. Kardiol. Pol. 2017, 75, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, C.; Inaba, M.; Ishimura, E.; Yamakawa, T.; Shoji, S.; Okuno, S. Association of increased serum ferritin with impaired muscle strength/quality in hemodialysis patients. J. Ren. Nutr. 2016, 26, 253–257. [Google Scholar] [CrossRef] [PubMed]
- Agostini, F.; Dalla Libera, L.; Rittweger, J.; Mazzucco, S.; Jurdana, M.; Mekjavic, I.B.; Pisot, R.; Gorza, L.; Narici, M.; Biolo, G. Effects of inactivity on human muscle glutathione synthesis by a double-tracer and single-biopsy approach. J. Physiol. 2010, 588, 5089–5104. [Google Scholar] [CrossRef] [PubMed]
- Motterlini, R. Heme oxygenase-1: A key step in counteracting cellular dysfunction. Cell. Mol. Biol. 2005, 51, 343–346. [Google Scholar] [PubMed]
- Reich, K.A.; Chen, Y.W.; Thompson, P.D.; Hoffman, E.P.; Clarkson, P.M. Forty-eight hours of unloading and 24 h of reloading lead to changes in global gene expression patterns related to ubiquitination and oxidative stress in humans. J. Appl. Physiol. 2010, 109, 1404–1415. [Google Scholar] [CrossRef] [PubMed]
- Reggiani, C. Not all disuse protocols are equal: New insight into the signalling pathways to muscle atrophy. J. Physiol. 2015, 593, 5227–5228. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Hwang, J.C.; Lees, H.A.; Wohlgemuth, S.E.; Knutson, M.D.; Judge, A.R.; Dupont-Versteegden, E.E.; Marzetti, E.; Leeuwenburgh, C. Long-term perturbation of muscle iron homeostasis following hindlimb suspension in old rats is associated with high levels of oxidative stress and impaired recovery from atrophy. Exp. Gerontol. 2012, 47, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Nuoc, T.N.; Kim, S.; Ahn, S.H.; Lee, J.S.; Park, B.J.; Lee, T.H. The analysis of antioxidant expression during muscle atrophy induced by hindlimb suspension in mice. J. Physiol. Sci. 2017, 67, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Hord, J.M.; Lawler, J.M. ROS and nNOS in the regulation of disuse-induced skeletal muscle atrophy. In The Plasticity of Skeletal Muscle; Sakuma, K., Ed.; Springer: Singapore, 2017; pp. 231–250. [Google Scholar]
- Talbert, E.E.; Smuder, A.J.; Min, K.; Kwon, O.S.; Szeto, H.H.; Powers, S.K. Immobilization-induced activation of key proteolytic systems in skeletal muscles is prevented by a mitochondria-targeted antioxidant. J. Appl. Physiol. 2013, 115, 529–538. [Google Scholar] [CrossRef] [PubMed]
- Lawler, J.M.; Kunst, M.; Hord, J.M.; Lee, Y.; Joshi, K.; Botchlett, R.E.; Ramirez, A.; Martinez, D.A. Euk-134 ameliorates nnosμ translocation and skeletal muscle fiber atrophy during short-term mechanical unloading. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2014, 306, R470–R482. [Google Scholar] [CrossRef] [PubMed]
- Ikemoto, M.; Nikawa, T.; Kano, M.; Hirasaka, K.; Kitano, T.; Watanabe, C.; Tanaka, R.; Yamamoto, T.; Kamada, M.; Kishi, K. Cysteine supplementation prevents unweighting-induced ubiquitination in association with redox regulation in rat skeletal muscle. Biol. Chem. 2002, 383, 715–721. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Wen, X.; Liu, W.; Hu, H.; Ye, B.; Zhou, Y. Comparison of the effects of deferasirox, deferoxamine, and combination of deferasirox and deferoxamine on an aplastic anemia mouse model complicated with iron overload. Drug Des. Dev. Ther. 2018, 12, 1081–1091. [Google Scholar] [CrossRef] [PubMed]
- Farberg, A.S.; Jing, X.L.; Monson, L.A.; Donneys, A.; Tchanquefossuo, C.N.; Deshpande, S.S.; Buchman, S.R. Deferoxamine reverses radiation induced hypovascularity during bone regeneration and repair in the murine mandible. Bone 2012, 50, 1184. [Google Scholar] [CrossRef] [PubMed]
- Kondo, H.; Miura, M.; Kodama, J.; Ahmed, S.M.; Itokawa, Y. Role of iron in oxidative stress in skeletal muscle atrophied by immobilization. Pflügers Arch. 1992, 421, 295–297. [Google Scholar] [CrossRef] [PubMed]
- Matuszczak, Y.; Arbogast, S.; Reid, M.B. Allopurinol mitigates muscle contractile dysfunction caused by hindlimb unloading in mice. Aviat. Space Environ. Med. 2004, 75, 581–588. [Google Scholar] [PubMed]
- Mansour, H.H.; Hafez, H.F.; Fahmy, N.M.; Hanafi, N. Protective effect of n-acetylcysteine against radiation induced DNA damage and hepatic toxicity in rats. Biochem. Pharmacol. 2008, 75, 773–780. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.J.; Lucas, E.A.; Turner, R.T.; Evans, G.L.; Lerner, M.R.; Brackett, D.J.; Stoecker, B.J.; Arjmandi, B.H. Vitamin e provides protection for bone in mature hindlimb unloaded male rats. Calcif. Tissue Int. 2005, 76, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Servais, S.; Letexier, D.; Favier, R.; Duchamp, C.; Desplanches, D. Prevention of unloading-induced atrophy by vitamin e supplementation: Links between oxidative stress and soleus muscle proteolysis? Free Radic. Biol. Med. 2007, 42, 627–635. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Su, Y.; Wang, D.; Chen, Y.; Wu, T.; Li, G.; Sun, X.; Cui, L. Tanshinol attenuates the deleterious effects of oxidative stress on osteoblastic differentiation via wnt/foxo3a signaling. Oxid. Med. Cell. Longev. 2013, 2013, 351895. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Diao, Y.; Kuang, W.; Li, Q.; Tian, Y.; Gao, J.; Dai, L.; Cao, L.; Wang, W.; Wei, L. Salvianolic acid b alleviate the osteoblast activity decreasing under simulated microgravity by Keap1/Nrf2/ARE signaling pathway. J. Funct. Foods 2018, 46, 288–294. [Google Scholar] [CrossRef]
- Qu, L.; Chen, H.; Liu, X.; Bi, L.; Xiong, J.; Mao, Z.; Li, Y. Protective effects of flavonoids against oxidative stress induced by simulated microgravity in sh-sy5y cells. Neurochem. Res. 2010, 35, 1445–1454. [Google Scholar] [CrossRef] [PubMed]
- Mukai, R.; Terao, J. Role of dietary flavonoids in oxidative stress and prevention of muscle atrophy. J. Phys. Fitness Sports Med. 2013, 2, 385–392. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, Y.; Wang, Z.; Xu, Z.; Zhang, Q.; Yin, M. Effects of dietary resveratrol on excess-iron-induced bone loss via antioxidative character. J. Nutr. Biochem. 2015, 26, 1174–1182. [Google Scholar] [CrossRef] [PubMed]
- Habold, C.; Momken, I.; Ouadi, A.; Bekaert, V.; Brasse, D. Effect of prior treatment with resveratrol on density and structure of rat long bones under tail-suspension. J. Bone Miner. Metab. 2011, 29, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Momken, I.; Stevens, L.; Bergouignan, A.; Desplanches, D.; Rudwill, F.; Chery, I.; Zahariev, A.; Zahn, S.; Stein, T.P.; Sebedio, J.L. Resveratrol prevents the wasting disorders of mechanical unloading by acting as a physical exercise mimetic in the rat. FASEB J. 2011, 25, 3646–3660. [Google Scholar] [CrossRef] [PubMed]
- Arbogast, S.; Smith, J.; Matuszczak, Y.; Hardin, B.J.; Moylan, J.S.; Smith, J.D.; Ware, J.; Kennedy, A.R.; Reid, M.B. Bowman-birk inhibitor concentrate prevents atrophy, weakness, and oxidative stress in soleus muscle of hindlimb-unloaded mice. J. Appl. Physiol. 2007, 102, 956–964. [Google Scholar] [CrossRef] [PubMed]
- Vinson, J.A.; Zubik, L.; Bose, P.; Samman, N.; Proch, J. Dried fruits: Excellent in vitro and in vivo antioxidants. J. Am. Coll. Nutr. 2005, 24, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Schreurs, A.S.; Shirazi-Fard, Y.; Shahnazari, M.; Alwood, J.S.; Truong, T.A.; Tahimic, C.G.T.; Limoli, C.L.; Turner, N.D.; Halloran, B.; Globus, R.K. Dried plum diet protects from bone loss caused by ionizing radiation. Sci. Rep. 2016, 6, 21343. [Google Scholar] [CrossRef] [PubMed]
- Ohsawa, I.; Ishikawa, M.; Takahashi, K.; Watanabe, M.; Nishimaki, K.; Yamagata, K.; Katsura, K.I.; Katayama, Y.; Asoh, S.; Ohta, S. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat. Med. 2007, 13, 688. [Google Scholar] [CrossRef] [PubMed]
- Schoenfeld, M.P.; Ansari, R.R.; Zakrajsek, J.F.; Billiar, T.R.; Toyoda, Y.; Wink, D.A.; Nakao, A. Hydrogen therapy may reduce the risks related to radiation-induced oxidative stress in space flight. Med. Hypotheses 2011, 76, 117–118. [Google Scholar] [CrossRef] [PubMed]
- Morrison, S.J.; Scadden, D.T. The bone marrow niche for haematopoietic stem cells. Nature 2014, 505, 327–334. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.; Zhang, G.; Dong, D.; Shang, P. Effects of Iron Overload and Oxidative Damage on the Musculoskeletal System in the Space Environment: Data from Spaceflights and Ground-Based Simulation Models. Int. J. Mol. Sci. 2018, 19, 2608. https://doi.org/10.3390/ijms19092608
Yang J, Zhang G, Dong D, Shang P. Effects of Iron Overload and Oxidative Damage on the Musculoskeletal System in the Space Environment: Data from Spaceflights and Ground-Based Simulation Models. International Journal of Molecular Sciences. 2018; 19(9):2608. https://doi.org/10.3390/ijms19092608
Chicago/Turabian StyleYang, Jiancheng, Gejing Zhang, Dandan Dong, and Peng Shang. 2018. "Effects of Iron Overload and Oxidative Damage on the Musculoskeletal System in the Space Environment: Data from Spaceflights and Ground-Based Simulation Models" International Journal of Molecular Sciences 19, no. 9: 2608. https://doi.org/10.3390/ijms19092608
APA StyleYang, J., Zhang, G., Dong, D., & Shang, P. (2018). Effects of Iron Overload and Oxidative Damage on the Musculoskeletal System in the Space Environment: Data from Spaceflights and Ground-Based Simulation Models. International Journal of Molecular Sciences, 19(9), 2608. https://doi.org/10.3390/ijms19092608




