PpSARK Regulates Moss Senescence and Salt Tolerance through ABA Related Pathway
Abstract
1. Introduction
2. Results
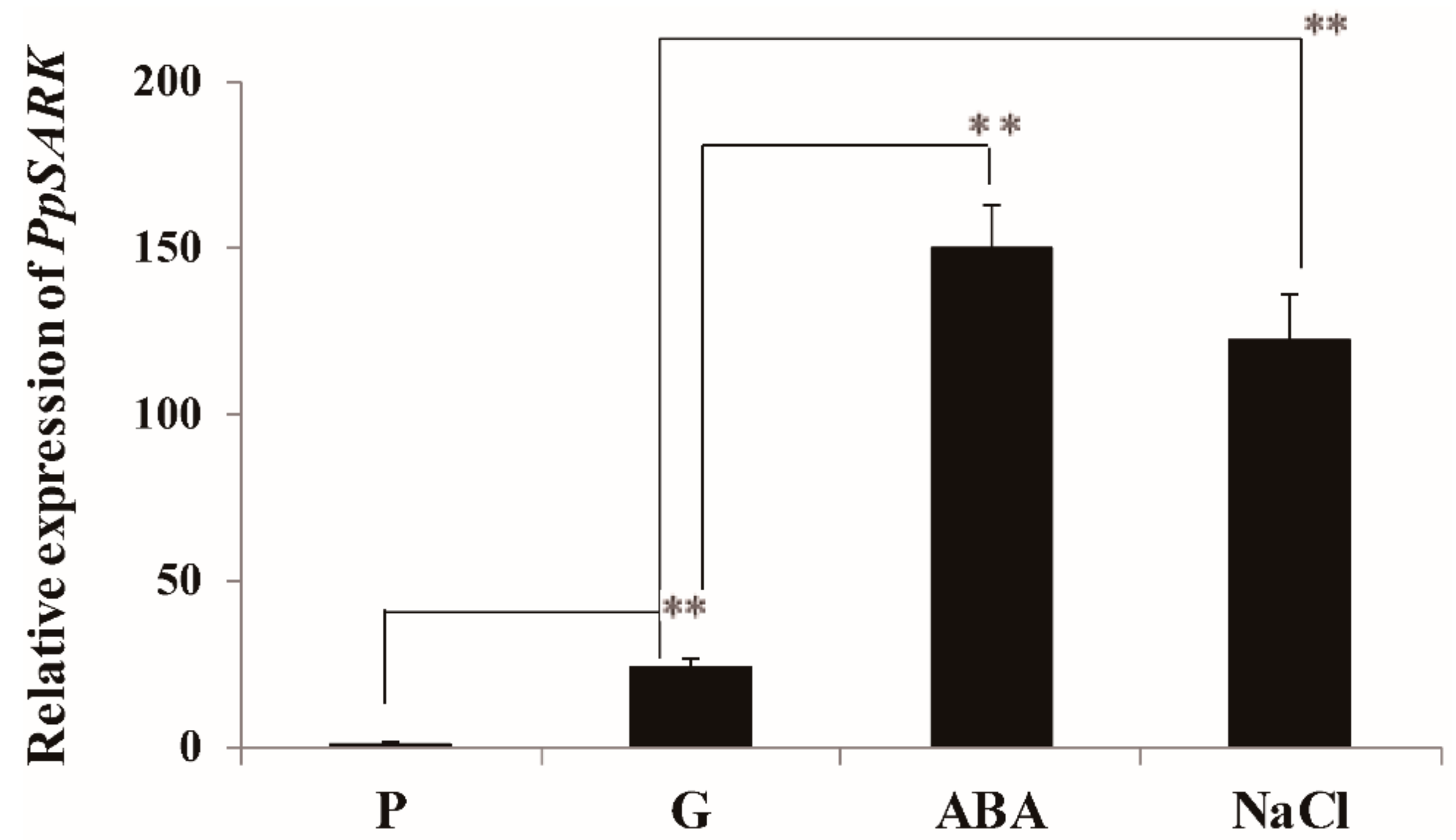
2.1. PpSARK Is a Development-Associated Gene Induced by ABA and NaCl
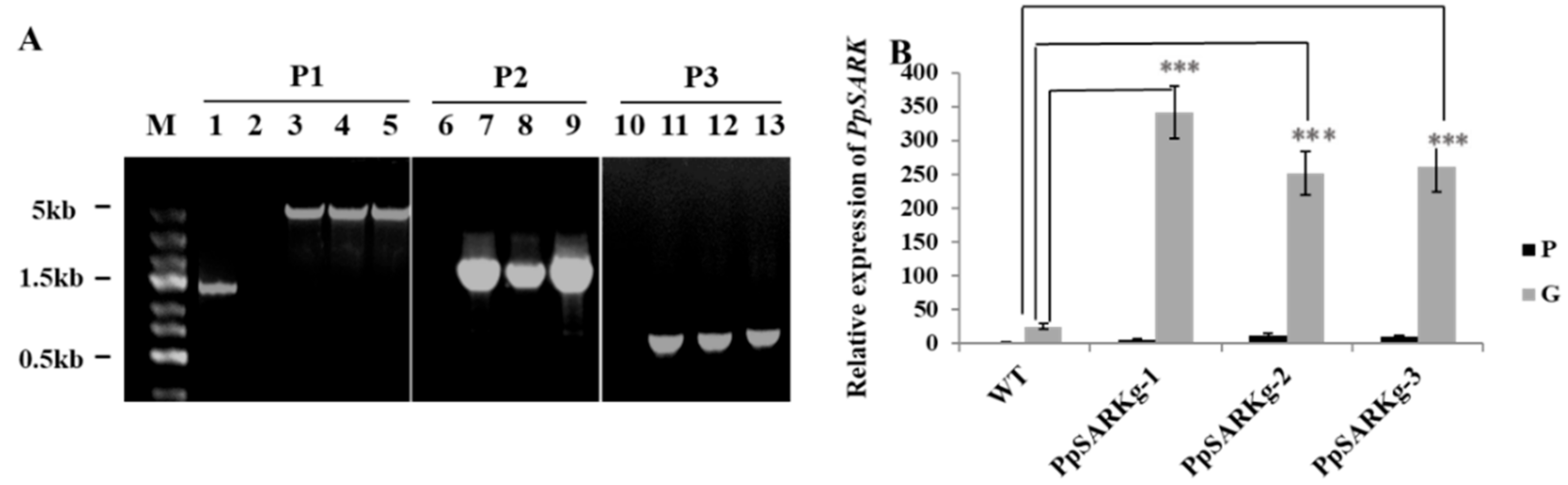
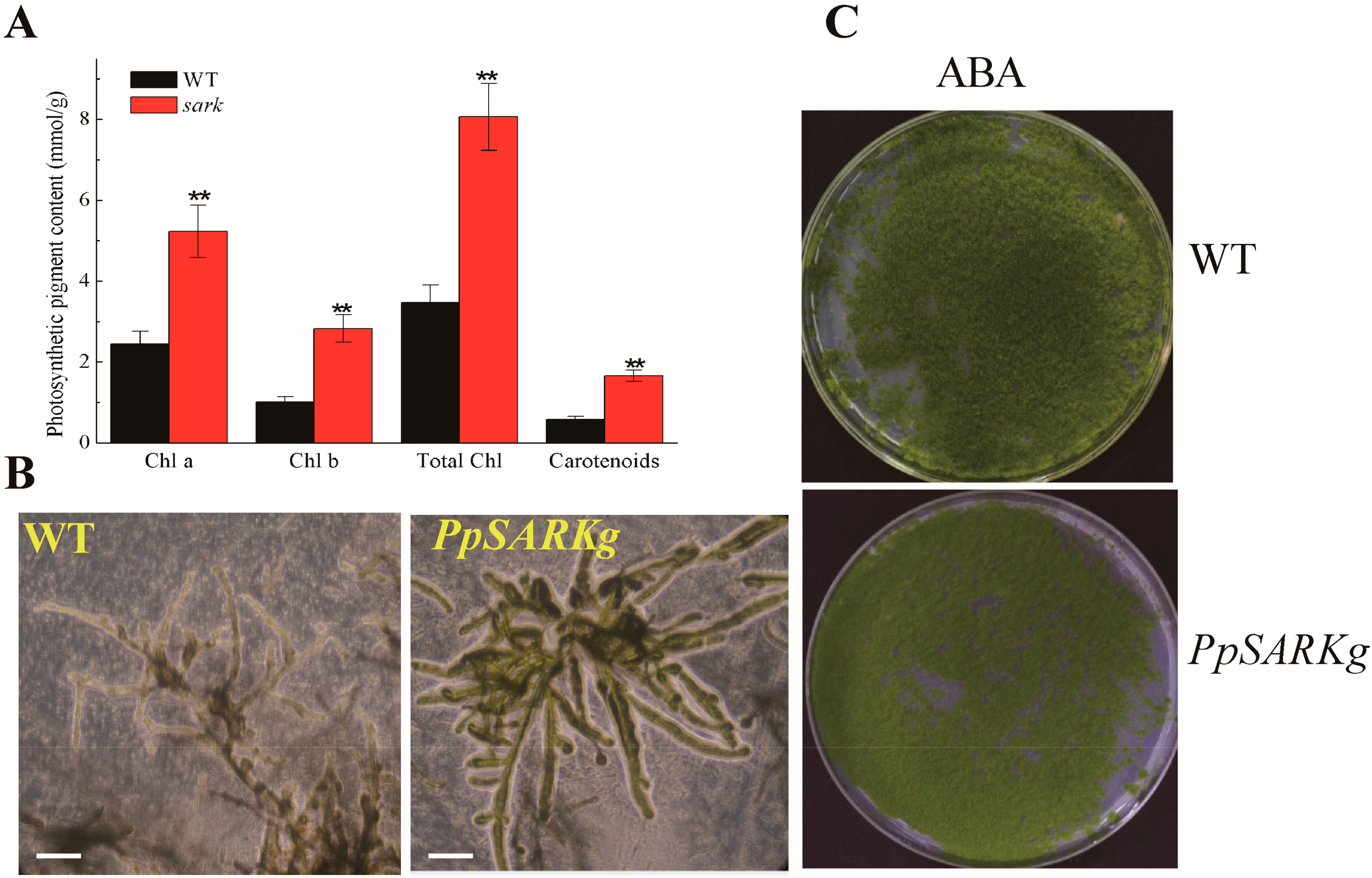
2.2. PpSARK Regulates Moss Senescence and Salt Tolerance
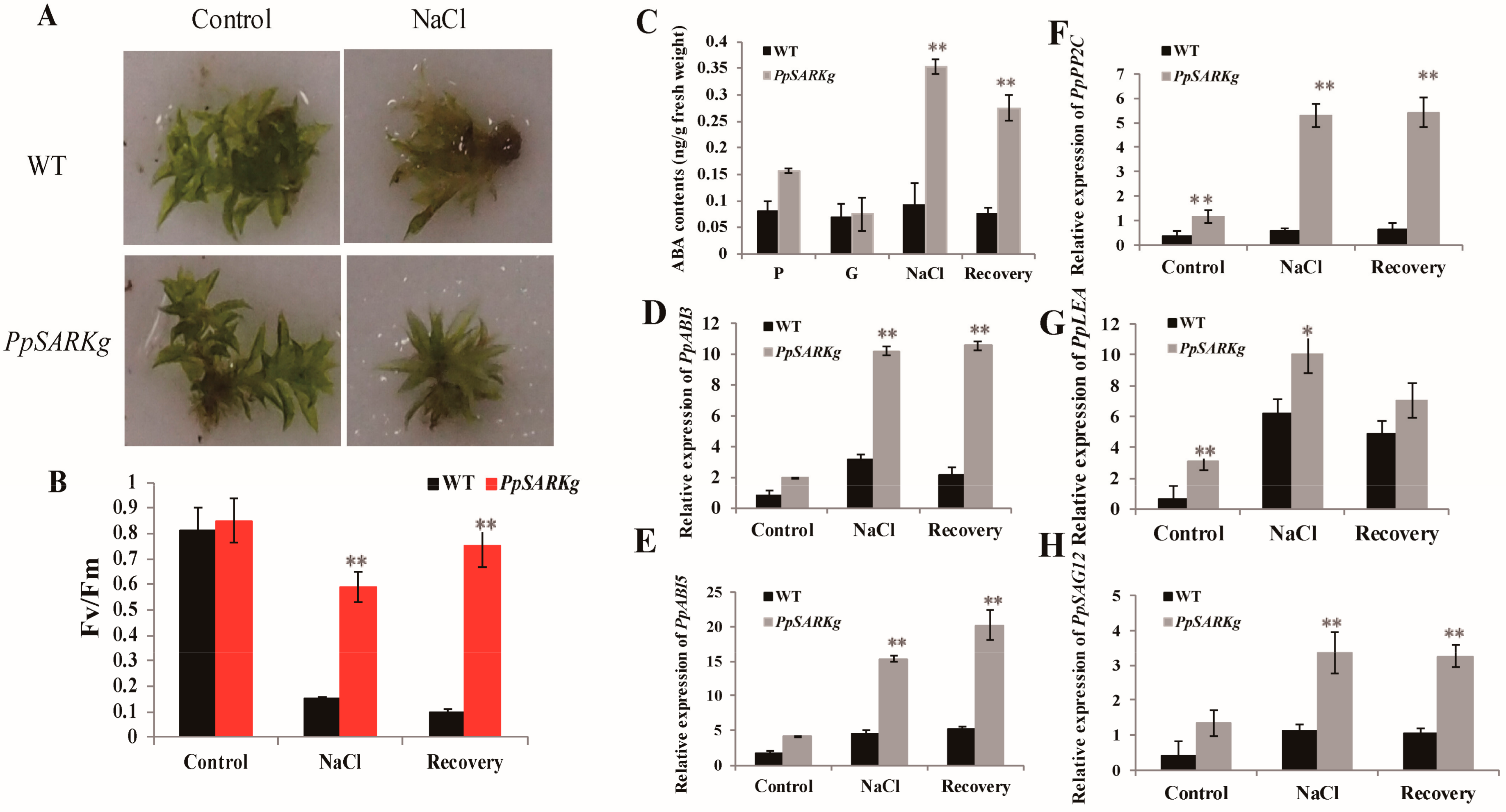
2.3. PpSARK Regulates Salt Tolerance and Senescence via an ABA-Related Pathway
3. Discussion
4. Materials and Methods
4.1. Moss Growth and Transformation
4.2. Plasmid Construction and Genotyping
4.3. Stress Treatments
4.4. Measurement of Chlorophyll Fluorescence (Fv/Fm) and Chlorophyll Content
4.5. Measurement of Endogenous ABA
4.6. RNA Isolation, cDNA Synthesis, and Quantitative RT-PCR
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflict of Interest
Accession Numbers
Abbreviations
| SARK | Senescence-associated receptor-like kinase |
References
- Wang, J.; Yuan, Z.; Zhang, Y. Alternative translation initiation from two in-frame start codons in DHX33 gene. Biochem. Biophys. Res. Commun. 2018, 502, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Chan, Z.; Gao, J.; Xing, L.; Cao, M.; Yu, C.; Hu, Y.; You, J.; Shi, H.; Zhu, Y.; et al. ABA receptor PYL9 promotes drought resistance and leaf senescence. Proc. Natl. Acad. Sci. USA 2016, 113, 1949–1954. [Google Scholar] [CrossRef] [PubMed]
- Khandelwal, A.; Cho, S.H.; Marella, H.; Sakata, Y.; Perroud, P.F.; Pan, A.; Quatrano, R.S. Role of ABA and ABI3 in desiccation tolerance. Science 2010, 327, 546. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; He, J.; Ji, S.; Wang, Q.; Pu, H.; Jiang, T.; Meng, L.; Yang, X.; Ji, J. Comparative studies of early liver dysfunction in senescence-accelerated mouse using mitochondrial proteomics approaches. Mol. Cell Proteomics 2008, 7, 1737–1747. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, Z.; Jiang, Z.; Zhao, Y.; Peng, J.; Jin, J.; Guo, H.; Luo, J. LSD: A leaf senescence database. Nucleic Acids Res. 2011, 39, D1103–D1107. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhao, Y.; Liu, X.; Jiang, Z.; Peng, J.; Jin, J.; Guo, H.; Luo, J. Construction of the Leaf Senescence Database and Functional Assessment of Senescence-Associated Genes. Methods Mol. Biol. 2017, 1533, 315–333. [Google Scholar] [PubMed]
- Han, X.; Wang, H.P.; Bo, J.J.; Hu, Y.R.; Chen, X.L.; Yu, D.Q. Arabidopsis WRKY8 Transcription Factor-Associated Genes VQ10 and VQ11 are Responsive to Multiple Abiotic Stresses. Plant Divers. 2015, 37, 760–766. [Google Scholar]
- Seok, H.Y.; Woo, D.H.; Nguyen, L.V.; Tran, H.T.; Tarte, V.N.; Mehdi, S.M.; Lee, S.Y.; Moon, Y.H. Arabidopsis AtNAP functions as a negative regulator via repression of AREB1 in salt stress response. Planta 2017, 245, 329–341. [Google Scholar] [CrossRef] [PubMed]
- Delatorre, C.A.; Cohen, Y.; Liu, L.; Peleg, Z.; Blumwald, E. The regulation of the SARK promoter activity by hormones and environmental signals. Plant Sci. 2012, 193–194, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Fu, R.; Zhang, M.; Zhao, Y.; He, X.; Ding, C.; Wang, S.; Feng, Y.; Song, X.; Li, P.; Wang, B. Identification of Salt Tolerance-related microRNAs and Their Targets in Maize (Zea mays L.) Using High-throughput Sequencing and Degradome Analysis. Front. Plant Sci. 2017, 8, 864. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Choi, J.; An, G.; Kim, S.R. Ectopic Expression of OsSta2 Enhances Salt Stress Tolerance in Rice. Front. Plant Sci. 2017, 8, 316. [Google Scholar]
- Liu, A.; Zhang, Y.; Chen, Q.; Zhang, C.; Xiong, Z.; He, Q.; Wang, G. Effects of salt stress on the growth and the photosynthesis in Alternanthera philoxeroides (Amaranthaceae). Acta Bot. Yunnanica 2007, 29, 85–90. [Google Scholar]
- Karan, R.; Subudhi, P.K. Overexpression of a nascent polypeptide associated complex gene (SaβNAC) of Spartina alterniflora improves tolerance to salinity and drought in transgenic Arabidopsis. Biochem. Biophys. Res. Commun. 2012, 4, 747–752. [Google Scholar] [CrossRef] [PubMed]
- Véry, A.A.; Nieves-Cordones, M.; Daly, M.; Khan, I.; Fizames, C.; Sentenac, H. Sentenac, Molecular biology of K+ transport across the plant cell membrane: What do we learn from comparison between plant species? J. Plant Physiol. 2014, 171, 748–769. [Google Scholar] [CrossRef] [PubMed]
- Xie, R.; Zhang, J.; Ma, Y.; Pan, X.; Dong, C.; Pang, S.; He, S.; Deng, L.; Yi, S.; Zheng, Y.; et al. Combined analysis of mRNA and miRNA identifies dehydration and salinity responsive key molecular players in citrus roots. Sci. Rep. 2017, 7, 42094. [Google Scholar] [CrossRef] [PubMed]
- Hanin, M.; Ebel, C.; Ngom, M.; Laplaze, L.; Masmoudi, K. New Insights on Plant Salt Tolerance Mechanisms and Their Potential Use for Breeding. Front. Plant Sci. 2016, 7, 1787. [Google Scholar] [CrossRef] [PubMed]
- Hajouj, T.; Michelis, R.; Gepstein, S. Cloning and characterization of a receptor-like protein kinase gene associated with senescence. Plant Physiol. 2000, 124, 1305–1314. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Meng, T.; Li, P.; Yu, Y.; Cui, Y.; Wang, Y.; Gong, Q.; Wang, N.N. A soybean dual-specificity kinase, GmSARK, and its Arabidopsis homolog, AtSARK, regulate leaf senescence through synergistic actions of auxin and ethylene. Plant Physiol. 2011, 157, 2131–2153. [Google Scholar] [CrossRef] [PubMed]
- Xiao, D.; Cui, Y.; Xu, F.; Xu, X.; Gao, G.; Wang, Y.; Guo, Z.; Wang, D.; Wang, N.N. Senescence-Suppressed Protein Phosphatase Directly Interacts with the Cytoplasmic Domain of Senescence-Associated Receptor-Like Kinase and Negatively Regulates Leaf Senescence in Arabidopsis. Plant Physiol. 2015, 169, 1275–1291. [Google Scholar] [CrossRef] [PubMed]
- Peleg, Z.; Reguera, M.; Tumimbang, E.; Walia, H.; Blumwald, E. Cytokinin-mediated source/sink modifications improve drought tolerance and increase grain yield in rice under water-stress. Plant Biotechnol. J. 2011, 9, 747–758. [Google Scholar] [CrossRef] [PubMed]
- Rivero, R.M.; Kojima, M.; Gepstein, A.; Sakakibara, H.; Mittler, R.; Gepstein, S.; Blumwald, E. Delayed leaf senescence induces extreme drought tolerance in a flowering plant. Proc. Natl. Acad. Sci. USA 2007, 104, 19631–19636. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Ohtani, M.; Yamaguchi, M.; Toyooka, K.; Wakazaki, M.; Sato, M.; Kubo, M.; Nakano, Y.; Sano, R.; Hiwatashi, Y.; et al. Contribution of NAC transcription factors to plant adaptation to land. Science 2014, 343, 1505–1508. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, J.P.B.; Lang, D.; Zimmer, A.D.; Causier, B.; Reski, R.; Davies, B. The loss of SMG1 causes defects in quality control pathways in Physcomitrella patens. Nucleic Acids Res. 2018, 46, 5822–5836. [Google Scholar] [CrossRef] [PubMed]
- Rensing, S.A.; Lang, D.; Zimmer, A.D.; Terry, A.; Salamov, A.; Shapiro, H.; Nishiyama, T.; Perroud, P.F.; Lindquist, E.A.; Kamisugi, Y.; et al. The Physcomitrella genome reveals evolutionary insights into the conquest of land by plants. Science 2008, 319, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Qin, H.; Gu, Q.; Zhang, J.; Sun, L.; Kuppu, S.; Zhang, Y.; Burow, M.; Payton, P.; Blumwald, E.; Zhang, H. Regulated expression of an isopentenyltransferase gene (IPT) in peanut significantly improves drought tolerance and increases yield under field conditions. Plant Cell Physiol. 2011, 52, 1904–1914. [Google Scholar] [CrossRef] [PubMed]
- Thelander, M.; Landberg, K.; Sundberg, E. Auxin-mediated developmental control in the moss Physcomitrella patens. J. Exp. Bot. 2018, 69, 277–290. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, D.G. Gene targeting in Physcomitrella patens. Curr. Opin. Plant Biol. 2001, 4, 143–150. [Google Scholar] [CrossRef]
- Kamisugi, Y.; Schlink, K.; Rensing, S.A.; Schween, G.; von Stackelberg, M.; Cuming, A.C.; Reski, R.; Cove, D.J. The mechanism of gene targeting in Physcomitrella patens: Homologous recombination, concatenation and multiple integration. Nucleic Acids Res. 2006, 34, 6205–6214. [Google Scholar] [CrossRef] [PubMed]
- Collonnier, C.; Epert, A.; Mara, K.; Maclot, F.; Guyon-Debast, A.; Charlot, F.; White, C.; Schaefer, D.G.; Nogué, F. CRISPR-Cas9-mediated efficient directed mutagenesis and RAD51-dependent and RAD51-independent gene targeting in the moss Physcomitrella patens. Plant Biotechnol. J. 2017, 15, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Collonnier, C.; Guyon-Debast, A.; Maclot, F.; Mara, K.; Charlot, F.; Nogué, F. Towards mastering CRISPR-induced gene knock-in in plants: Survey of key features and focus on the model Physcomitrella patens. Methods 2017, 121–122, 103–117. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.X.; Theg, S.M. A stromal heat shock protein 70 system functions in protein import into chloroplasts in the moss Physcomitrella patens. Plant Cell 2010, 22, 205–220. [Google Scholar] [CrossRef] [PubMed]
- Woo, N.S.; Badger, M.R.; Pogson, B.J. A rapid, non-invasive procedure for quantitative assessment of drought survival using chlorophyll fluorescence. Plant Methods 2008, 4, 27. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, R.; Ishimaru, T. An improved method for the determination of phytoplankton chlorophyll using N,N-dimethylformamide. J. Oceanogr. Soc. Jpn. 1990, 46, 190–194. [Google Scholar] [CrossRef]
- Cai, B.D.; Ye, E.C.; Yuan, B.F.; Feng, Y.Q. Sequential solvent induced phase transition extraction for profiling of endogenous phytohormones in plants by liquid chromatography-mass spectrometry. J. Chromatogr. B 2015, 1004, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.M.; Radhakrishnan, R.; Kang, S.M.; Kim, J.H.; Lee, I.Y.; Moon, B.K.; Yoon, B.W.; Lee, I.J. Phytotoxic mechanisms of bur cucumber seed extracts on lettuce with special reference to analysis of chloroplast proteins, phytohormones, and nutritional elements. Ecotoxicol. Environ. Saf. 2015, 122, 230–237. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, P.; Yang, H.; Liu, G.; Ma, W.; Li, C.; Huo, H.; He, J.; Liu, L. PpSARK Regulates Moss Senescence and Salt Tolerance through ABA Related Pathway. Int. J. Mol. Sci. 2018, 19, 2609. https://doi.org/10.3390/ijms19092609
Li P, Yang H, Liu G, Ma W, Li C, Huo H, He J, Liu L. PpSARK Regulates Moss Senescence and Salt Tolerance through ABA Related Pathway. International Journal of Molecular Sciences. 2018; 19(9):2609. https://doi.org/10.3390/ijms19092609
Chicago/Turabian StyleLi, Ping, Hong Yang, Gaojing Liu, Wenzhang Ma, Chuanhong Li, Heqiang Huo, Jianfang He, and Li Liu. 2018. "PpSARK Regulates Moss Senescence and Salt Tolerance through ABA Related Pathway" International Journal of Molecular Sciences 19, no. 9: 2609. https://doi.org/10.3390/ijms19092609
APA StyleLi, P., Yang, H., Liu, G., Ma, W., Li, C., Huo, H., He, J., & Liu, L. (2018). PpSARK Regulates Moss Senescence and Salt Tolerance through ABA Related Pathway. International Journal of Molecular Sciences, 19(9), 2609. https://doi.org/10.3390/ijms19092609




