Th1-Biased Immunomodulation and In Vivo Antitumor Effect of a Novel Piperine Analogue
Abstract
1. Introduction
2. Results
2.1. Chemistry
2.2. Evaluation of Cytotoxicity
2.3. Assessment of Acute Preclinical Toxicity
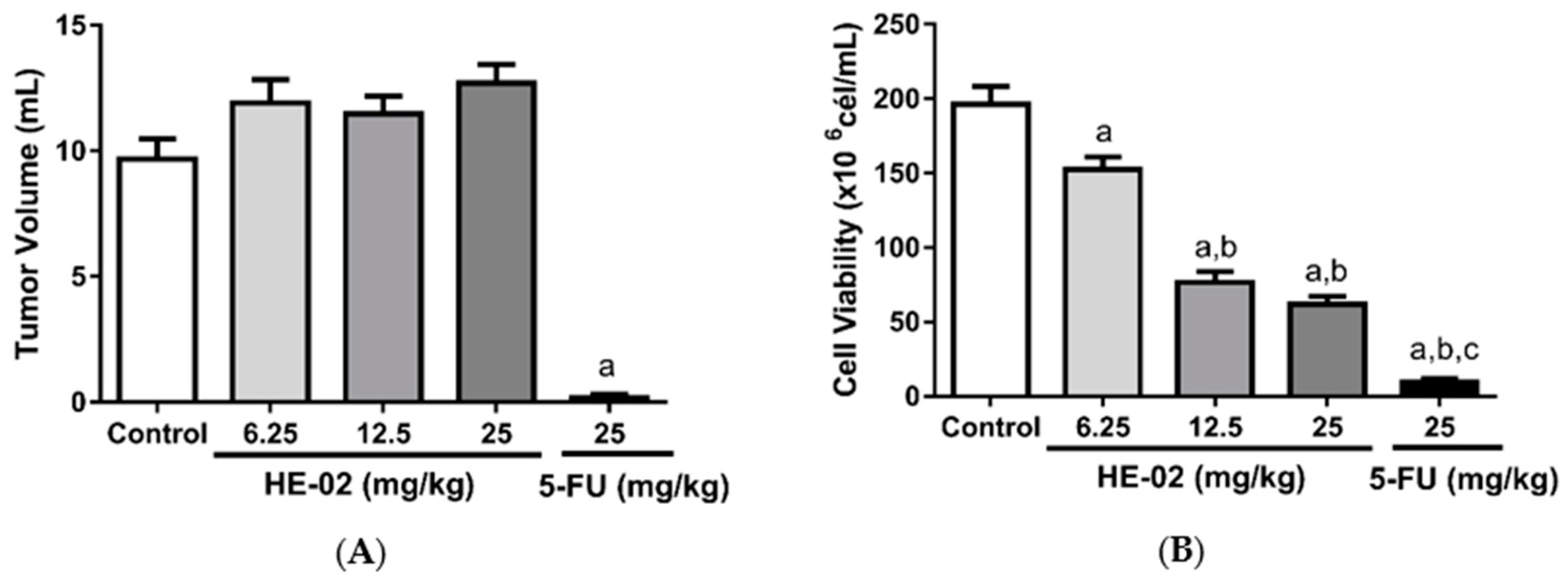
2.4. In Vivo Antitumor Activity
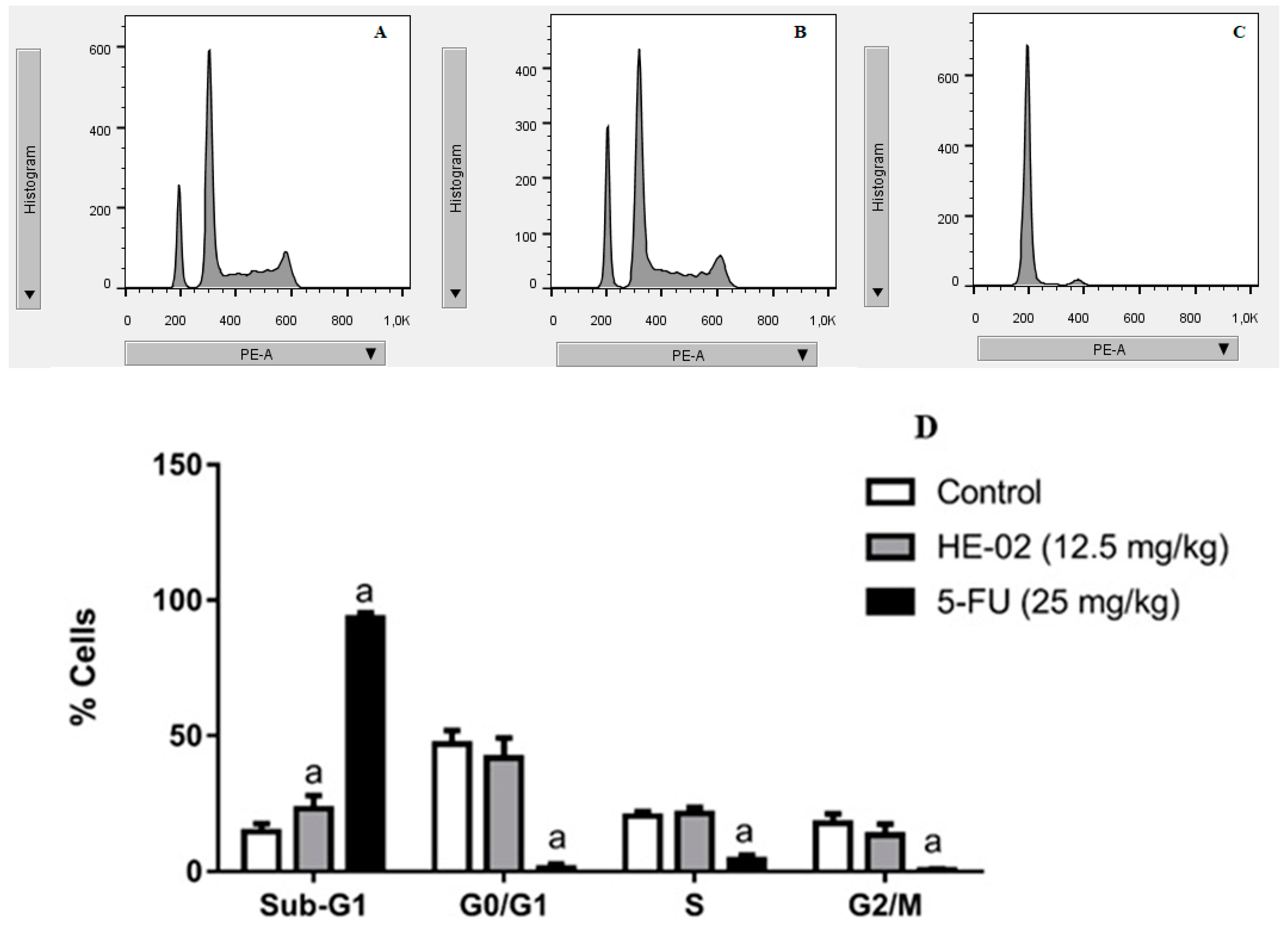
2.5. Cell Cycle Analysis
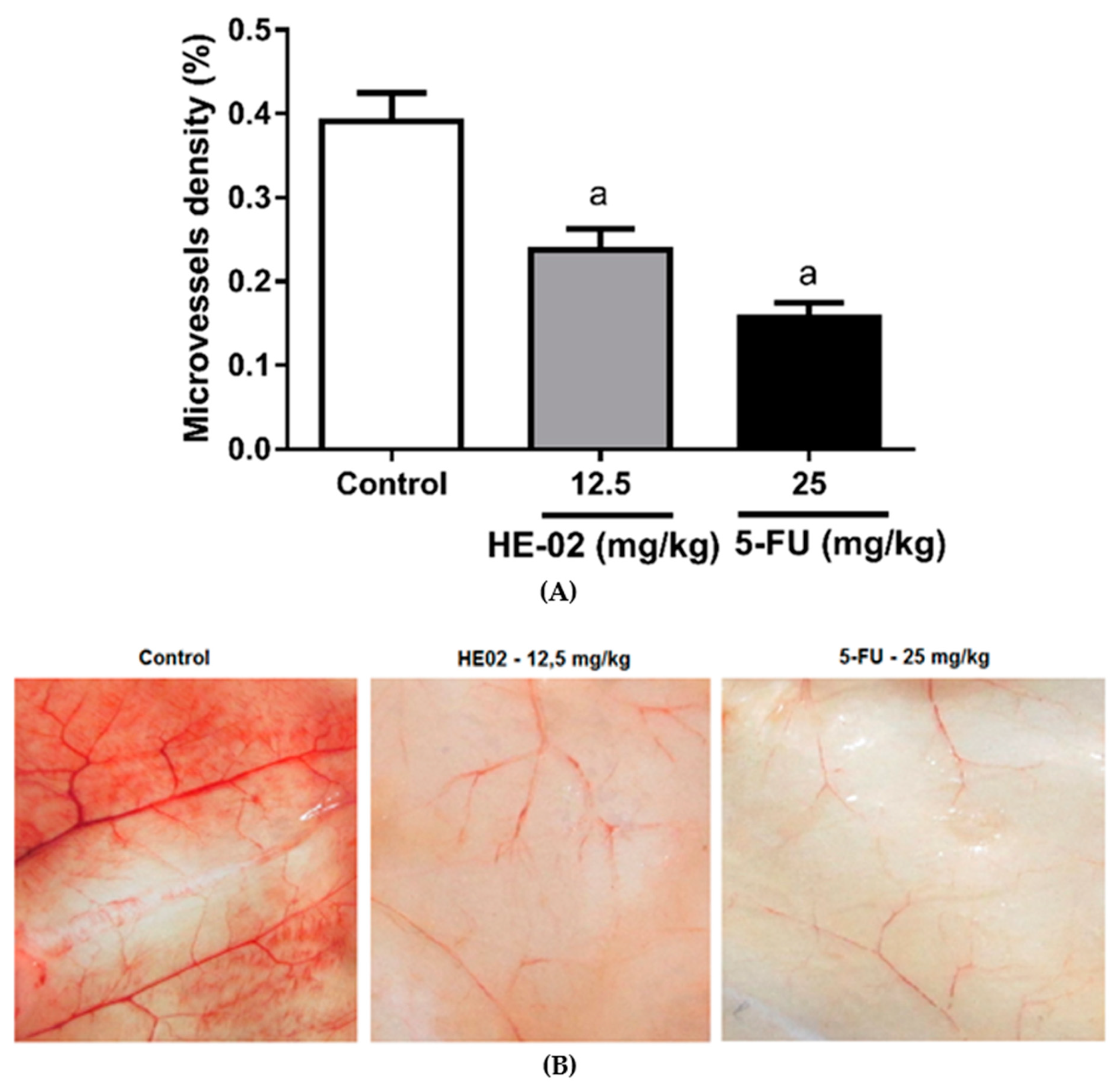
2.6. Evaluation of Antiangiogenic Effect
2.7. Quantification of Cytokines in the Peritoneal Lavage
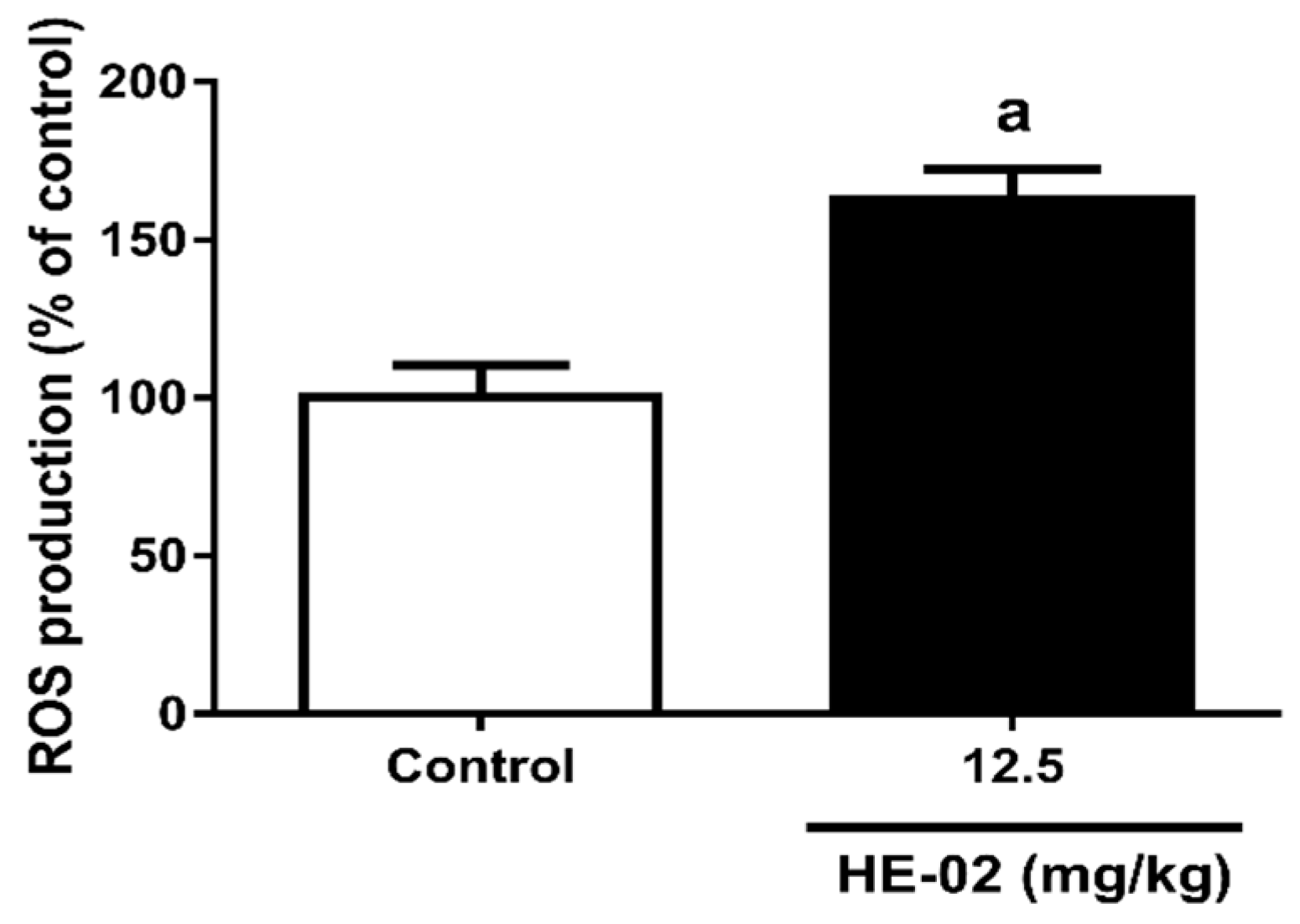
2.8. Quantification of Reactive Oxygen Species
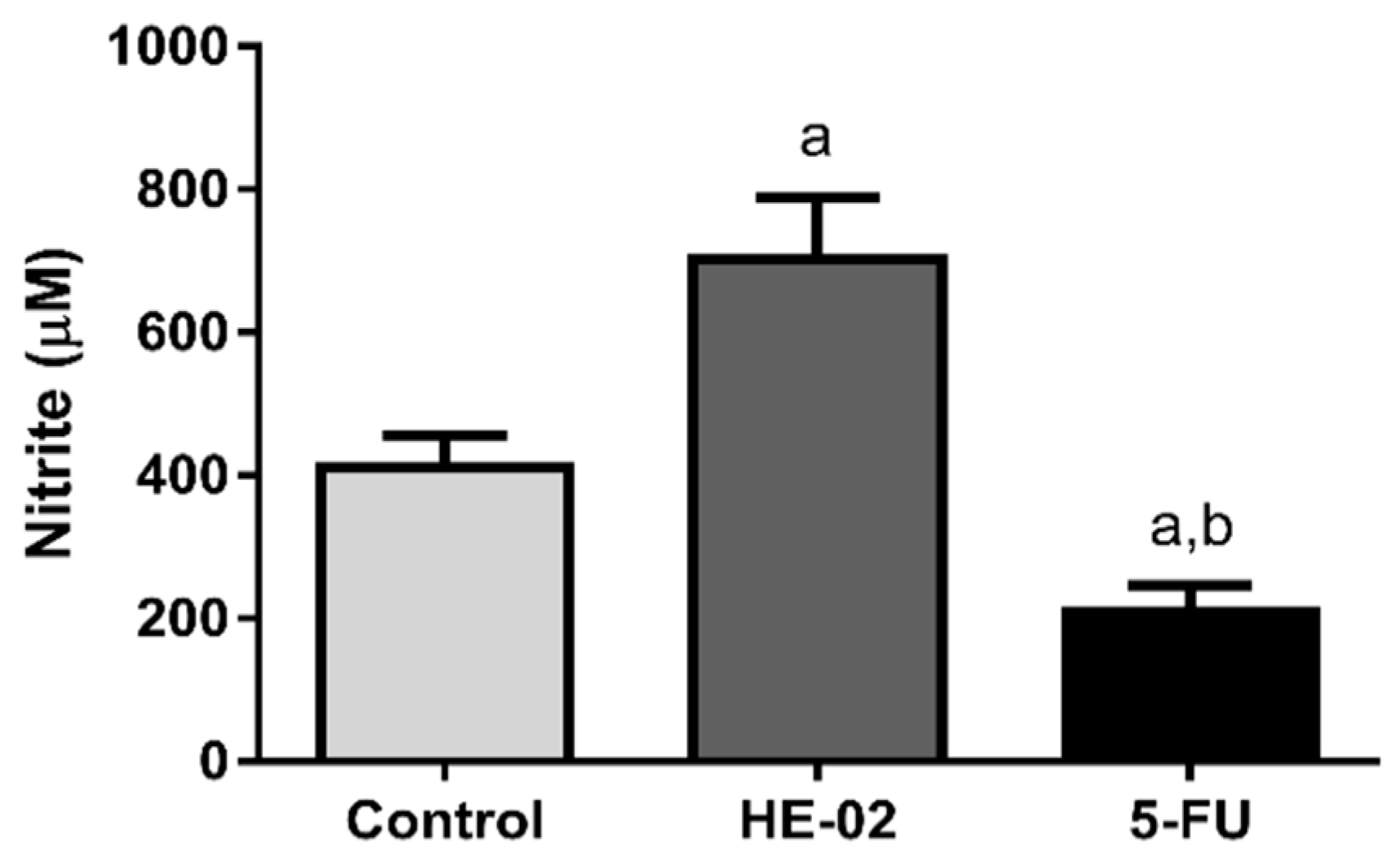
2.9. Quantification of Nitrite Levels
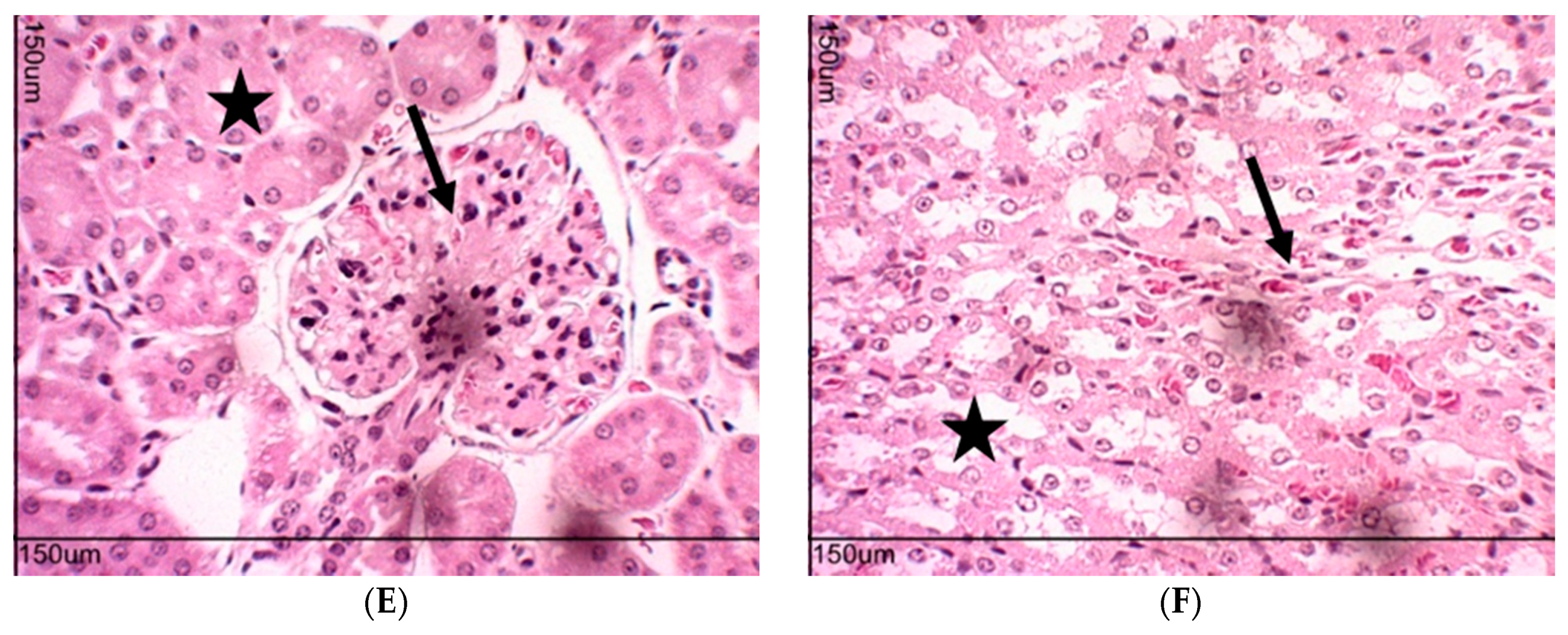
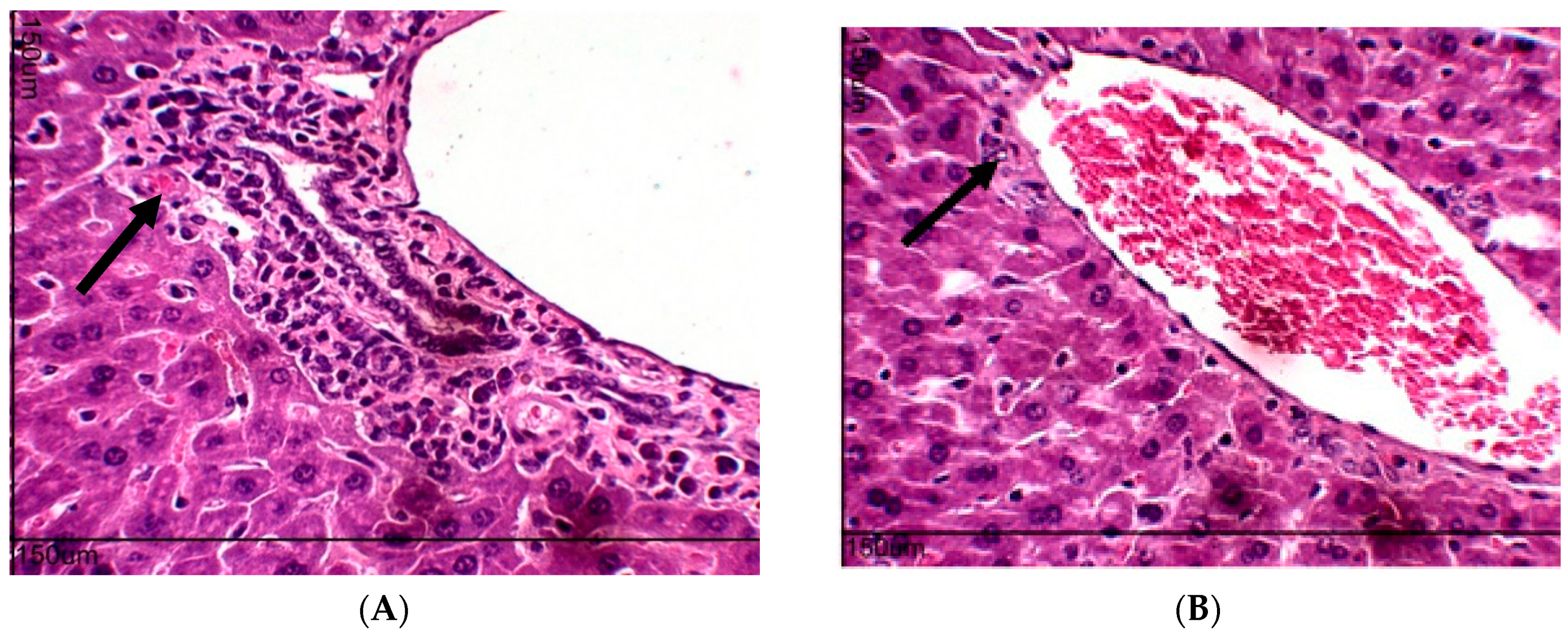
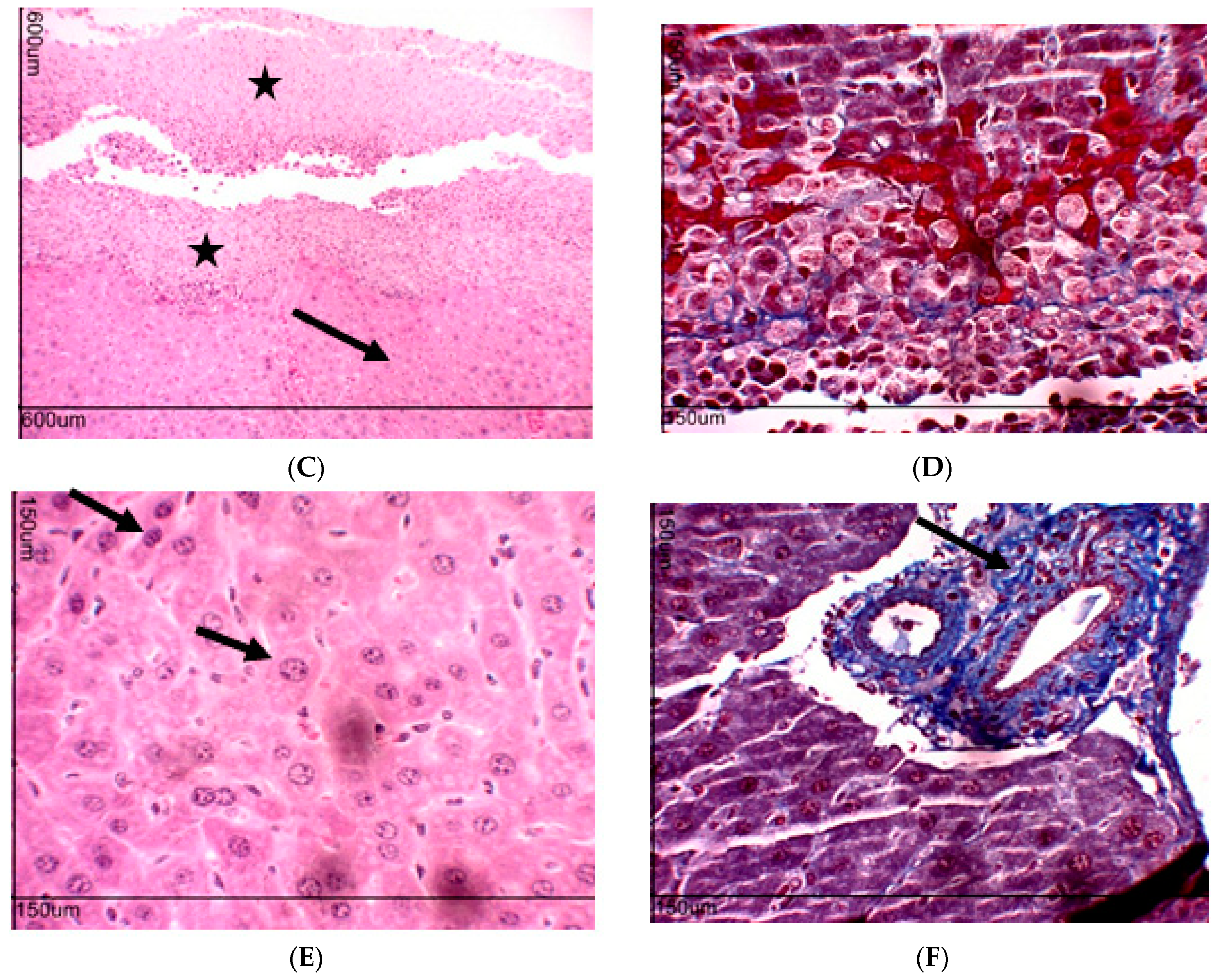
2.10. Toxicity Evaluation for Transplanted Mice
3. Discussion
4. Materials and Methods
4.1. Drugs and Reagents
4.2. Chemistry
4.2.1. Sinthesis of N-(p-Nitrophenyl)acetamide Piperinoate (HE-02)
Piperine Extraction (1)
Procedure for the Preparation of Piperinic Acid (2)
Procedure for the Preparation of 2-Chloro-N-(4-nitrophenyl)acetamide (5)
Procedure for Obtaining of N-(p-Nitrophenyl)acetamide Piperinoate (HE-02)
4.3. Animals
4.4. Toxicological Assays
4.4.1. Evaluation of Cytotoxicity in Erythrocytes
4.4.2. Evaluation of Cytotoxicity in RAW 264.7 Macrophage
4.4.3. Assessment of Acute Preclinical Toxicity
4.5. Evaluation of In Vivo Antitumor Activity in Ehrlich Ascitic Carcinoma Model
4.5.1. Cell Cycle Analysis
4.5.2. Evaluation of Antiangiogenic Effect
4.5.3. Quantification of Cytokines in the Peritoneal Lavage
4.5.4. Quantification of Reactive Oxygen Species
4.5.5. Quantification of Nitrite Levels
4.6. Toxicity Evaluation in Transplanted Animals with Ehrlich Tumor Cells
4.7. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| 5-FU | 5-Fluouracil |
| ALT | Alanine Aminotransferase |
| ANOVA | Analysis of Variance |
| ANS | Autonomic Nervous System |
| AST | Aspartate Aminotransferase |
| CCL2 | Chemokine (C-C motif) Ligand 2 |
| CEUA | Ethical Committee on the Use of Animals |
| CH50 | Hemolytic Complement |
| CNS | Central Nervous System |
| DCFH-DA | 2,7-Dichlorodihydrofluorescein Diacetate |
| DMF | Dimethylformamide |
| DMSO | Dimethylsufoxide |
| EAC | Ehrlich Ascitic Carcinoma |
| ELISA | Enzyme-Linked Immunosorbent Assay |
| FBS | Fetal Bovine Serum |
| HCl | Chloride Acid |
| HE-02 | Ethyl 2-oxo-2-(4-nitrophenylamine)-piperinoate |
| HFS | Hypotonic Fluorochemical Solution |
| HMQC | Heteronuclear Multiple-Quantum Correlation |
| IL-10 | Interleukin 10 |
| IL-12 | Interleukin 12 |
| IL-1β | Interleukin 1 Beta |
| IL-4 | Interleukin 4 |
| INF-γ | Interferon Gamma |
| LD50 | Lethal Dose 50% |
| MCH | Mean Corpuscular Hemoglobin |
| MCHC | Mean Corpuscular Hemoglobin Concentration |
| MCV | Mean Corpuscular Volume |
| MDA-MB-231 | Human Breast Adenocarcinoma Cell Line |
| MDPI | Multidisciplinary Digital Publishing Institute |
| MTT | (3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide) |
| NMR | Nuclear Magnetic Resonance |
| NO | Oxide Nitric |
| OECD | Organization for Economic Co-operation and Development |
| PBS | Phosphate Buffered Saline |
| ROS | Reactive Oxygen Species |
| SK MEL 28 | Human Melanoma Cell Line |
| Th1 | T helper Cells Type 1 |
| Th2 | T helper Cells Type 2 |
| TNF-α | Tumor Necrosis Factor Alpha |
| UFPB | University Federal of Paraíba |
References
- Hanahan, D.; Weinberg, R.A. The hallmarks of cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef]
- Brindha, E.; Rajasekaran, R.; Aruna, P.; Koteeswaran, D.; Ganesan, S. High wavenumber Raman spectroscopy in the characterization of urinary metabolites of normal subjects, oral premalignant and malignant patients. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2017, 171, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Kasala, E.R.; Bodduluru, L.N.; Madana, R.M.; Athira, K.V.; Gogoi, R.; Barua, C.C. Chemopreventive and therapeutic potential of chrysin in cancer: Mechanistic perspectives. Toxicol. Lett. 2015, 233, 214–225. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Voronov, E.; Carmi, Y.; Apte, R.N. The role IL-1 in tumor-mediated angiogenesis. Front. Physiol. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Potente, M.; Gerhardt, H.; Carmeliet, P. Basic and therapeutic aspects of angiogenesis. Cell 2011, 146, 873–887. [Google Scholar] [CrossRef] [PubMed]
- Welti, J.; Loges, S.; Dimmeler, S.; Carmeliet, P. Recent molecular discoveries in angiogenesis and antiangiogenic therapies in cancer. J. Clin. Investig. 2013, 123, 3190–3200. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Coussens, L.M. Accessories to the crime: Functions of cells recruited to the tumor microenvironment. Cancer Cell 2012, 21, 309–322. [Google Scholar] [CrossRef] [PubMed]
- Bissell, M.J.; Hines, W.C. Why don’t we get more cancer? A proposed role of the microenvironment in restraining cancer progression. Nat. Med. 2011, 17, 320–329. [Google Scholar] [CrossRef] [PubMed]
- Elinav, E.; Nowarski, R.; Thaiss, C.A.; Hu, B.; Jin, C.; Flavell, R.A. Inflammation-induced cancer: Crosstalk between tumours, immune cells and microorganisms. Nat. Rev. Cancer 2013, 13, 759–771. [Google Scholar] [CrossRef] [PubMed]
- Burkholder, B.; Huang, R.-Y.; Burgess, R.; Luo, S.; Jones, V.S.; Zhang, W.; Lv, Z.-Q.; Gao, C.-Y.; Wang, B.-L.; Zhang, Y.-M.; et al. Tumor-induced perturbations of cytokines and immune cell networks. Biochim. Biophys. Acta BBA-Rev. Cancer 2014, 1845, 182–201. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Zhang, J. Inflammasomes in inflammation-induced Cancer. Front. Immunol. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef] [PubMed]
- Sosa, V.; Moliné, T.; Somoza, R.; Paciucci, R.; Kondoh, H.; LLeonart, M.E. Oxidative stress and cancer: An overview. Ageing Res. Rev. 2013, 12, 376–390. [Google Scholar] [CrossRef] [PubMed]
- Liou, G.-Y.; Storz, P. Reactive oxygen species in cancer. Free Radic. Res. 2010, 44, 479–496. [Google Scholar] [CrossRef] [PubMed]
- Korde Choudhari, S.; Chaudhary, M.; Bagde, S.; Gadbail, A.R.; Joshi, V. Nitric oxide and cancer: A review. World J. Surg. Oncol. 2013, 11, 118. [Google Scholar] [CrossRef] [PubMed]
- Safarzadeh, E.; Shotorbani, S.S.; Baradaran, B. Herbal medicine as inducers of apoptosis in cancer treatment. Adv. Pharm. Bull. 2014, 4, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Gottesman, M.M.; Lavi, O.; Hall, M.D.; Gillet, J.-P. Toward a better understanding of the complexity of cancer drug resistance. Annu. Rev. Pharmacol. Toxicol. 2016, 56, 85–102. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Wu, Y.L.; Chen, G.; Feng, J.; Liu, X.-Q.; Wang, C.; Zhang, S.; Wang, J.; Zhou, S.; Ren, S.; et al. Final overall survival results from a randomised, phase III study of erlotinib versus chemotherapy as first-line treatment of EGFR mutation-positive advanced non-small-cell lung cancer (OPTIMAL, CTONG-0802). Ann. Oncol. 2015, 26, 1877–1883. [Google Scholar] [CrossRef] [PubMed]
- Rajopadhye, A.; Upadhye, A.; Mujumdar, A. HPTLC method for analysis of piperine in fruits of Piper species. J. Planar Chromatogr.-Mod. TLC 2011, 24, 57–59. [Google Scholar] [CrossRef]
- Fofaria, N.M.; Kim, S.-H.; Srivastava, S.K. Piperine Causes G1 Phase Cell Cycle Arrest and Apoptosis in Melanoma Cells through Checkpoint Kinase-1 Activation. PLoS ONE 2014, 9, e94298. [Google Scholar] [CrossRef] [PubMed]
- Yaffe, P.B.; Doucette, C.D.; Walsh, M.; Hoskin, D.W. Piperine impairs cell cycle progression and causes reactive oxygen species-dependent apoptosis in rectal cancer cells. Exp. Mol. Pathol. 2013, 94, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Lai, L.; Fu, Q.; Liu, Y.; Jiang, K.; Guo, Q.; Chen, Q.; Yan, B.; Wang, Q.; Shen, J. Piperine suppresses tumor growth and metastasis in vitro and in vivo in a 4T1 murine breast cancer model. Acta Pharmacol. Sin. 2012, 33, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Bezerra, D.P.; Castro, F.O.; Alves, A.P.N.N.; Pessoa, C.; Moraes, M.O.; Silveira, E.R.; Lima, M.A.S.; Elmiro, F.J.M.; Costa-Lotufo, L.V. In vivo growth-inhibition of Sarcoma 180 by piplartine and piperine, two alkaloid amides from Piper. Braz. J. Med. Biol. Res. 2006, 39, 801–807. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, P.D.; Guerra, F.S.; Sales, N.M.; Sardella, T.B.; Jancar, S.; Neves, J.S. Characterization of the inflammatory response during Ehrlich ascitic tumor development. J. Pharmacol. Toxicol. Methods 2015, 71, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Sadjadi, S.-A.; Annamaraju, P. Gemcitabine induced hemolytic uremic syndrome. Am. J. Case Rep. 2012, 13, 89–91. [Google Scholar] [CrossRef] [PubMed]
- Ying, X.; Yu, K.; Chen, X.; Chen, H.; Hong, J.; Cheng, S.; Peng, L. Piperine inhibits LPS induced expression of inflammatory mediators in RAW 264.7 cells. Cell. Immunol. 2013, 285, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Almeida, R.N.; Oliveira, T.M.L. Avaliação de drogas analgésicas de ação central. In Psicofarmacologia: Fundamentos Práticos, 1st ed.; Almeida, R.N., Ed.; Guarabara Koogan: Rio de Janeiro, Brazil, 2006; ISBN 8527711117. [Google Scholar]
- Amelo, W.; Nagpal, P.; Makonnen, E. Antiplasmodial activity of solvent fractions of methanolic root extract of Dodonaea angustifolia in Plasmodium berghei infected mice. BMC Complement. Altern. Med. 2014, 14. [Google Scholar] [CrossRef] [PubMed]
- Ozaslan, M.; Karagoz, I.; Kilic, I.; Guldur, M. Ehrlich ascites carcinoma. Afr. J. Biotechnol. 2011, 10, 2375–2378. [Google Scholar] [CrossRef]
- Ouyang, D.; Zeng, L.; Pan, H.; Xu, L.; Wang, Y.; Liu, K.; He, X. Piperine inhibits the proliferation of human prostate cancer cells via induction of cell cycle arrest and autophagy. Food Chem. Toxicol. 2013, 60, 424–430. [Google Scholar] [CrossRef] [PubMed]
- Kubota, Y. Tumor angiogenesis and anti-angiogenic therapy. Keio J. Med. 2012, 61, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Tecchio, C.; Cassatella, M.A. Neutrophil-derived cytokines involved in physiological and pathological angiogenesis. In Chemical Immunology and Allergy; Marone, G., Granata, F., Eds.; S. KARGER AG: Basel, Switzerland, 2013; Volume 99, pp. 123–137. ISBN 978-3-318-02480-7. [Google Scholar]
- Li, X.; Fang, P.; Mai, J.; Choi, E.T.; Wang, H.; Yang, X. Targeting mitochondrial reactive oxygen species as novel therapy for inflammatory diseases and cancers. J. Hematol. Oncol. 2013, 6, 19. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Ain, R. Nitric-oxide synthase trafficking inducer is a pleiotropic regulator of endothelial cell function and signaling. J. Biol. Chem. 2017, 292, 6600–6620. [Google Scholar] [CrossRef] [PubMed]
- Netea, M.G.; Simon, A.; van de Veerdonk, F.; Kullberg, B.-J.; Van der Meer, J.W.M.; Joosten, L.A.B. IL-1β Processing in host defense: Beyond the inflammasomes. PLoS Pathog. 2010, 6, e1000661. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Singhal, V.; Roshan, R.; Sharma, A.; Rembhotkar, G.W.; Ghosh, B. Piperine inhibits TNF-α induced adhesion of neutrophils to endothelial monolayer through suppression of NF-κB and IκB kinase activation. Eur. J. Pharmacol. 2007, 575, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Chuchawankul, S.; Khorana, N.; Poovorawan, Y. Piperine inhibits cytokine production by human peripheral blood mononuclear cells. Genet. Mol. Res. 2012, 11, 617–627. [Google Scholar] [CrossRef] [PubMed]
- Bae, G.-S.; Kim, J.-J.; Park, K.-C.; Koo, B.S.; Jo, I.-J.; Choi, S.B.; Lee, C.H.; Jung, W.-S.; Cho, J.-H.; Hong, S.-H.; et al. Piperine inhibits lipopolysaccharide-induced maturation of bone-marrow-derived dendritic cells through inhibition of ERK and JNK activation. Phytother. Res. 2012, 26, 1893–1897. [Google Scholar] [CrossRef] [PubMed]
- Tugues, S.; Burkhard, S.H.; Ohs, I.; Vrohlings, M.; Nussbaum, K.; Vom Berg, J.; Kulig, P.; Becher, B. New insights into IL-12-mediated tumor suppression. Cell Death Differ. 2015, 22, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Lasek, W.; Zagożdżon, R.; Jakobisiak, M. Interleukin 12: Still a promising candidate for tumor immunotherapy? Cancer Immunol. Immunother. 2014, 63, 419–435. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Terai, M.; Tamura, Y.; Alexeev, V.; Mastrangelo, M.J.; Selvan, S.R. Interleukin 10 in the tumor microenvironment: A target for anticancer immunotherapy. Immunol. Res. 2011, 51, 170–182. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, B.; Karuna, R.; Sreenivasa, R.S.; Haritha, K.; Sai, M.D.; Sasi, B.R.B.; Saralakumari, D. Effect of Commiphora mukul gum resin on hepatic marker enzymes, lipid peroxidation and antioxidants status in pancreas and heart of streptozotocin induced diabetic rats. Asian Pac. J. Trop. Biomed. 2012, 2, 895–900. [Google Scholar] [CrossRef]
- Ferreira, F.G.; Chin, E.W.K.; Santos, M.F.; Carvalho, D.L.M.; De Capua Junior, A. Índice de congestão portal e a ocorrência de trombose portal pós-dape. Rev. Assoc. Méd. Bras. 2005, 51, 233–236. [Google Scholar] [CrossRef] [PubMed]
- Baliga, R.; Zhang, Z.; Baliga, M.; Ueda, N.; Shah, S.V. In vitro and in vivo evidence suggesting a role for iron in cisplatin-induced nephrotoxicity. Kidney Int. 1998, 53, 394–401. [Google Scholar] [CrossRef] [PubMed]
- Fukasawa, H.; Furuya, R.; Yasuda, H.; Yamamoto, T.; Hishida, A.; Kitagawa, M. Anti-cancer agent-induced nephrotoxicity. Anticancer Agents Med. Chem. 2014, 14, 921–927. [Google Scholar] [CrossRef] [PubMed]
- Sodré, F.L.; Costa, J.C.B.; Lima, J.C.C. Avaliação da função e da lesão renal: Um desafio laboratorial. J. Bras. Patol. E Med. Lab. 2007, 43. [Google Scholar] [CrossRef]
- Gowda, S.; Desai, P.B.; Kulkarni, S.S.; Hull, V.V.; Math, A.A.K.; Vernekar, S.N. Markers of renal function tests. North Am. J. Med. Sci. 2010, 2, 170–173. [Google Scholar]
- Dogra, R.K.; Khanna, S.; Shanker, R. Immunotoxicological effects of piperine in mice. Toxicology 2004, 196, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Meghwal, M.; Goswami, T.K. Piper nigrum and piperine: An update. Phytother. Res. 2013, 27, 1121–1130. [Google Scholar] [CrossRef] [PubMed]
- Ikan, R. Nitrogenous compounds. In Natural Products. A Laboratory Guide, 2nd ed.; Ikan, R., Ed.; Academic Press: New York, NY, USA, 1991; pp. 233–238. ISBN 9780080512426. [Google Scholar]
- Peixoto, I.N.; Souza, H.D.S.; Lira, B.F.; Silva, D.F.; Lima, E.O.; Barbosa-Filho, J.M.; Athayde-Filho, P.F. Synthesis and antifungal activity against Candida strains of mesoionic system derived from 1,3-thyazolium-5-thiolate. J. Braz. Chem. Soc. 2016, 27, 1807–1813. [Google Scholar] [CrossRef]
- Kang, C.; Munawir, A.; Cha, M.; Sohn, E.-T.; Lee, H.; Kim, J.-S.; Yoon, W.D.; Lim, D.; Kim, E. Cytotoxicity and hemolytic activity of jellyfish Nemopilema nomurai (Scyphozoa: Rhizostomeae) venom. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2009, 150, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Organisation for Economic Co-operation and Development (OECD). Guideline for Testing of Chemicals n. 423: Acute Oral Toxicity; OECD: Paris, France, 2001; pp. 1–14. [Google Scholar]
- Dolai, N.; Karmakar, I.; Suresh Kumar, R.B.; Kar, B.; Bala, A.; Haldar, P.K. Evaluation of antitumor activity and in vivo antioxidant status of Anthocephalus cadamba on Ehrlich ascites carcinoma treated mice. J. Ethnopharmacol. 2012, 142, 865–870. [Google Scholar] [CrossRef] [PubMed]
- Pozarowski, P.; Darzynkiewicz, Z. Analysis of cell cycle by flow cytometry. In Checkpoint Controls and Cancer; Humana Press: Totowa, NJ, USA, 2004; pp. 301–312. ISBN 978-1-59259-811-3. [Google Scholar]
- Agrawal, S.S.; Saraswati, S.; Mathur, R.; Pandey, M. Cytotoxic and antitumor effects of brucine on Ehrlich ascites tumor and human cancer cell line. Life Sci. 2011, 89, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Hasui, M.; Hirabayashi, Y.; Kobayashi, Y. Simultaneous measurement by flow cytometry of phagocytosis and hydrogen peroxide production of neutrophils in whole blood. J. Immunol. Methods 1989, 117, 53–58. [Google Scholar] [CrossRef]
- Green, L.C.; Wagner, D.A.; Glogowski, J.; Skipper, P.L.; Wishnok, J.S.; Tannenbaum, S.R. Analysis of nitrate, nitrite, and [15N] nitrate in biological fluids. Anal. Biochem. 1982, 126, 131–138. [Google Scholar] [CrossRef]
- Mangueira, V.M.; Batista, T.M.; Brito, M.T.; Sousa, T.K.G.; Cruz, R.M.D.; Abrantes, R.A.; Veras, R.C.; Medeiros, I.A.; Medeiros, K.K.P.; Pereira, A.L.C.; et al. A new acridine derivative induces cell cycle arrest and antiangiogenic effect on Ehrlich ascites carcinoma model. Biomed. Pharmacother. 2017, 90, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Rolim, T.L.; Meireles, D.R.P.; Batista, T.M.; Sousa, T.K.G.; Mangueira, V.M.; Abrantes, R.A.; Pita, J.C.L.R.; Xavier, A.L.; Costa, V.C.O.; Batista, L.M.; et al. Erratum to: Toxicity and antitumor potential of Mesosphaerum sidifolium (Lamiaceae) oil and fenchone, its major component. BMC Complement. Altern. Med. 2017, 17. [Google Scholar] [CrossRef] [PubMed]
- Junqueira, L.C.; Carneiro, J. Histologia Básica, 12th ed.; Guarabara Koogan: Rio de Janeiro, Brazil, 2013; ISBN 978-85-277-2311-4. [Google Scholar]



| Group | D/T 1 | Effects | |
|---|---|---|---|
| Control | 0/3 | None | |
| 2000 mg/kg | Experiment 1 | 0/3 | Diarrhea |
| Experiment 2 | 2/3 | None | |
| 300 mg/kg | Experiment 3 | 0/3 | None |
| Groups | Dose (mg/kg) | Water Intake (mL) | Food Intake (g) | Starting Weight (g) | Final Weight (g) |
|---|---|---|---|---|---|
| Control | 38.82 ± 6.25 | 31.12 ± 1.19 | 31.50 ± 0.45 | 30.71 ± 0.72 | |
| HE-02 | 12.5 | 32.27 ± 6.84 a | 18.15 ± 2.84 a | 30.88 ± 0.82 | 23.22 ± 0.40 a |
| 5-FU | 25 | 38.36 ± 3.93 | 35.93 ± 6.69 a | 32.00 ± 0.62 | 26.69 ± 0.98 a |
| Group | Dose (mg/kg) | AST (U/L) | ALT (U/L) | Urea (mg/dL) | Creatinine |
|---|---|---|---|---|---|
| Control | 181.80 ± 25.28 | 25.33 ± 3.84 | 36.83 ± 3.46 | 0.24 ± 0.1 | |
| HE-02 | 12.5 | 247.80 ± 45.19 | 21.75 ± 2.32 | 55.00 ± 5.30 a | 0.31 ± 0.03 |
| 5-FU | 25 | 155.20 ± 20.78 | 22.33 ± 4.55 | 37.80 ± 1.71 | 0.26 ± 0.01 |
| Group | Control | HE-02 (12.5 mg/kg) | 5-FU (25 mg/kg) |
|---|---|---|---|
| Erythrocytes (106/mm3) | 7.91 ± 0.18 | 7.31 ± 0.29 | 7.33 ± 0.22 |
| Hemoglobin (g/dL) | 11.59 ± 0.24 | 11.28 ± 0.31 | 10.88 ± 0.31 |
| Hematocrit | 38.71 ± 1.09 | 37.17 ± 1.84 | 34.47 ± 1.39 |
| MCV (fm3) | 50.19 ± 1.10 | 50.66 ± 1.42 | 47.17 ± 1.15 |
| MCH (pg) | 14.99 ± 0.40 | 15.34 ± 0.22 | 15.02 ± 0.49 |
| MCHC (g/dL) | 30.11 ± 0.91 | 30.63 ± 0.94 | 31.43 ± 1.29 |
| Total Leukocytes (103/mm3) | 10.88 ± 0.73 | 24.70 ± 2.65 a | 4.62 ± 0.36 a,b |
| Lymphocytes (%) | 77.00 ± 3.44 | 31.20 ± 2.13 a | 92.33 ± 0.67 a,b |
| Segmented (%) | 19.80 ± 0.49 | 67.40 ± 1.48 a | 5.83 ± 0.48 a,b |
| Monocytes (%) | 1.00 ± 0.24 | 1.40 ± 0.24 | 1.20 ± 0.37 |
| Eosinophils (%) | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| Group | Dose (mg/kg) | Heart Index (mg/g) | Liver Index (mg/g) | Kidney Index (mg/g) | Thymus Index (mg/g) | Spleen Index (mg/g) |
|---|---|---|---|---|---|---|
| Control | 3.48 ± 0.10 | 67.23 ± 1.84 | 10.58 ± 0.24 | 3.23 ± 0.16 | 5.38 ± 0.38 | |
| HE-02 | 12.5 | 3.13 ± 0.12 | 71.80 ± 1.36 | 9.75 ± 0.24 | 2.02 ± 0.22 a | 3.22 ± 0.23 a |
| 5-FU | 25 | 4.05 ± 0.08 a | 58.96 ± 1.41 a | 10.56 ± 0.44 | 2.08 ± 0.12 a | 5.43 ± 0.18 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, J.; Brito, M.; Ferreira, R.; Moura, A.P.; Sousa, T.; Batista, T.; Mangueira, V.; Leite, F.; Cruz, R.; Vieira, G.; et al. Th1-Biased Immunomodulation and In Vivo Antitumor Effect of a Novel Piperine Analogue. Int. J. Mol. Sci. 2018, 19, 2594. https://doi.org/10.3390/ijms19092594
Santos J, Brito M, Ferreira R, Moura AP, Sousa T, Batista T, Mangueira V, Leite F, Cruz R, Vieira G, et al. Th1-Biased Immunomodulation and In Vivo Antitumor Effect of a Novel Piperine Analogue. International Journal of Molecular Sciences. 2018; 19(9):2594. https://doi.org/10.3390/ijms19092594
Chicago/Turabian StyleSantos, Jephesson, Monalisa Brito, Rafael Ferreira, Ana Paula Moura, Tatyanna Sousa, Tatianne Batista, Vivianne Mangueira, Fagner Leite, Ryldene Cruz, Giciane Vieira, and et al. 2018. "Th1-Biased Immunomodulation and In Vivo Antitumor Effect of a Novel Piperine Analogue" International Journal of Molecular Sciences 19, no. 9: 2594. https://doi.org/10.3390/ijms19092594
APA StyleSantos, J., Brito, M., Ferreira, R., Moura, A. P., Sousa, T., Batista, T., Mangueira, V., Leite, F., Cruz, R., Vieira, G., Lira, B., Athayde-Filho, P., Souza, H., Costa, N., Veras, R., Barbosa-Filho, J. M., Magalhães, H., & Sobral, M. (2018). Th1-Biased Immunomodulation and In Vivo Antitumor Effect of a Novel Piperine Analogue. International Journal of Molecular Sciences, 19(9), 2594. https://doi.org/10.3390/ijms19092594




