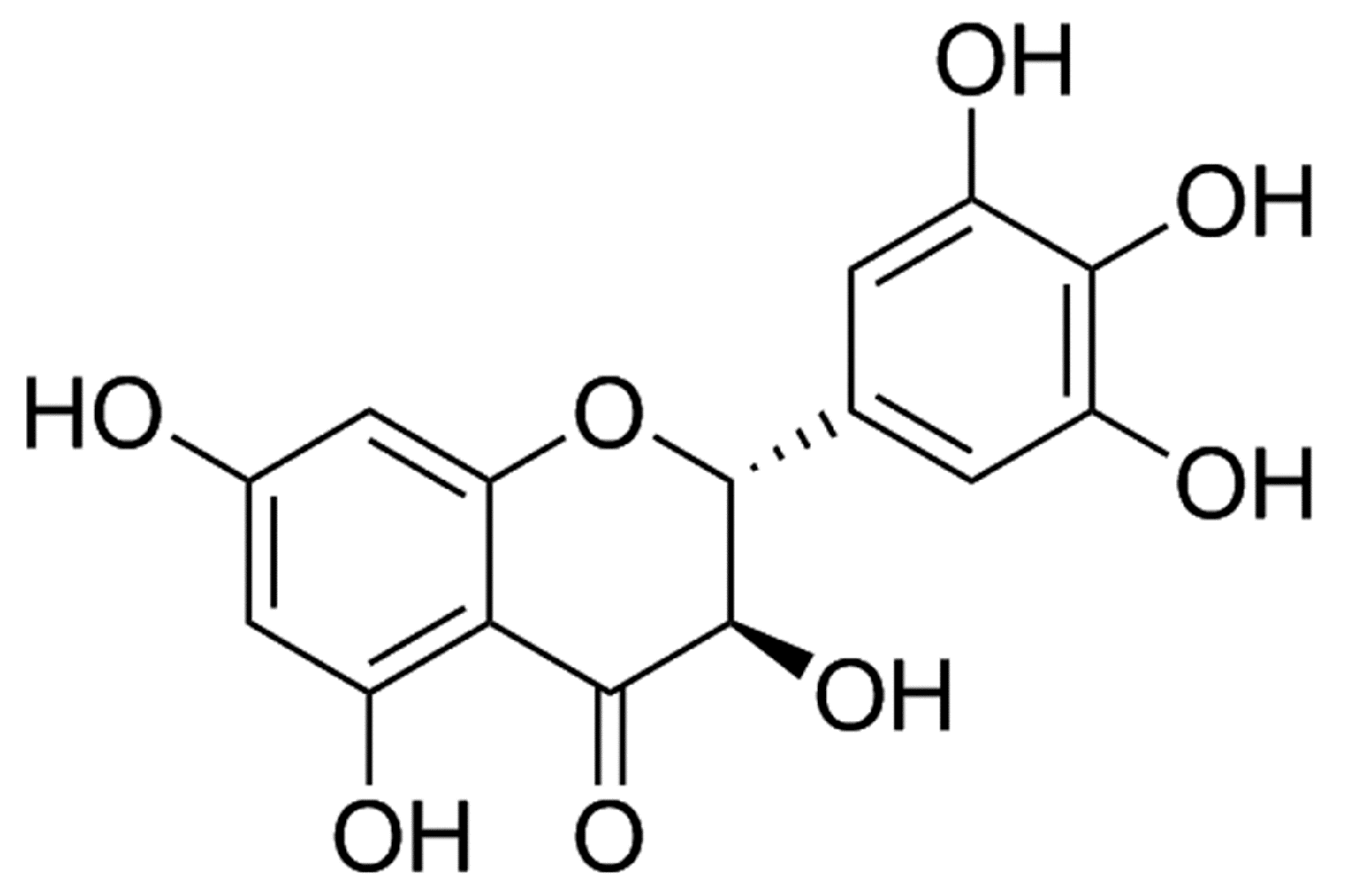
Dihydromyricetin Attenuates Myocardial Hypertrophy Induced by Transverse Aortic Constriction via Oxidative Stress Inhibition and SIRT3 Pathway Enhancement
Abstract
1. Introduction
2. Results
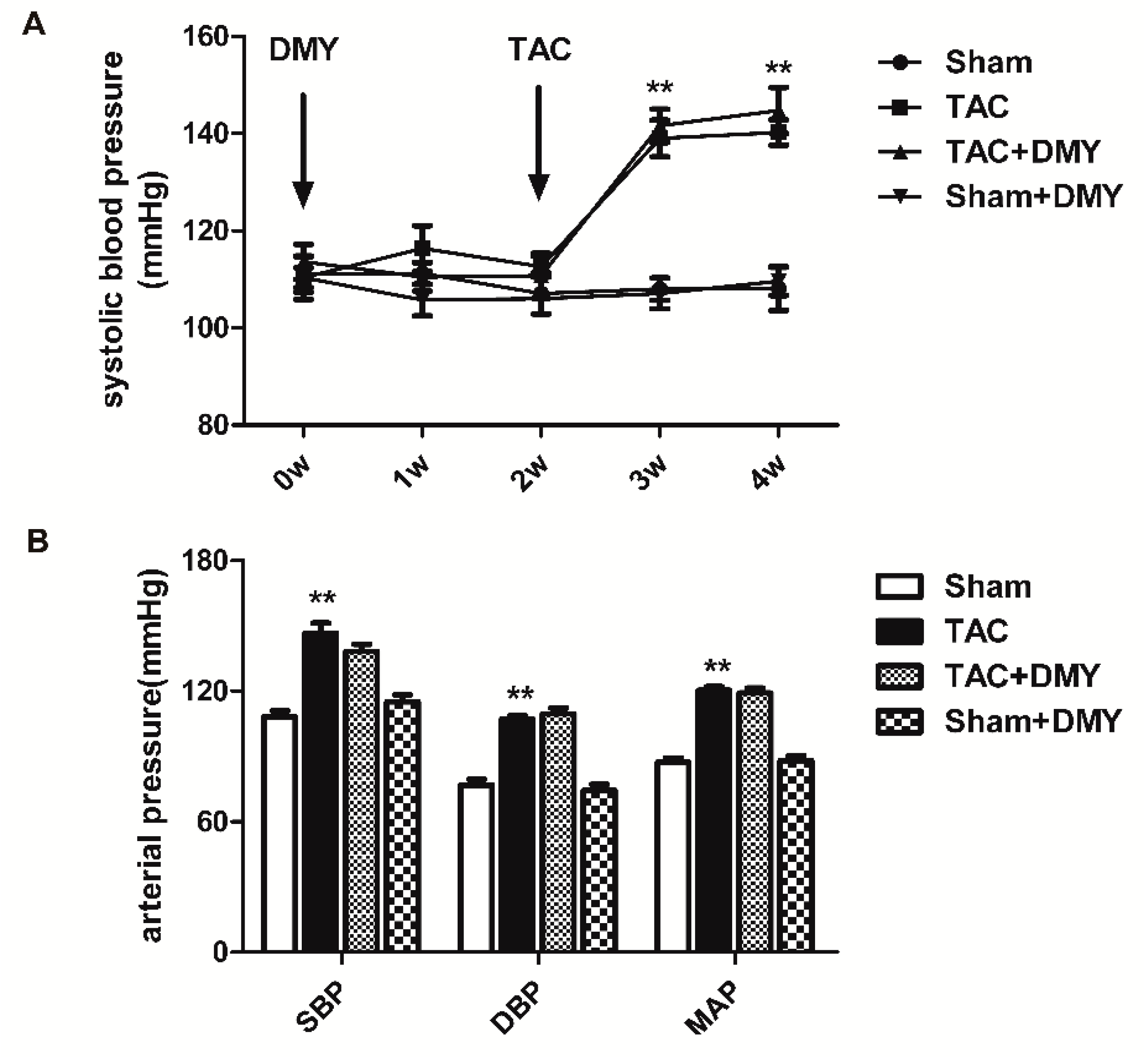
2.1. DMY Had No Significant Effects on Blood Pressure in Mice after TAC
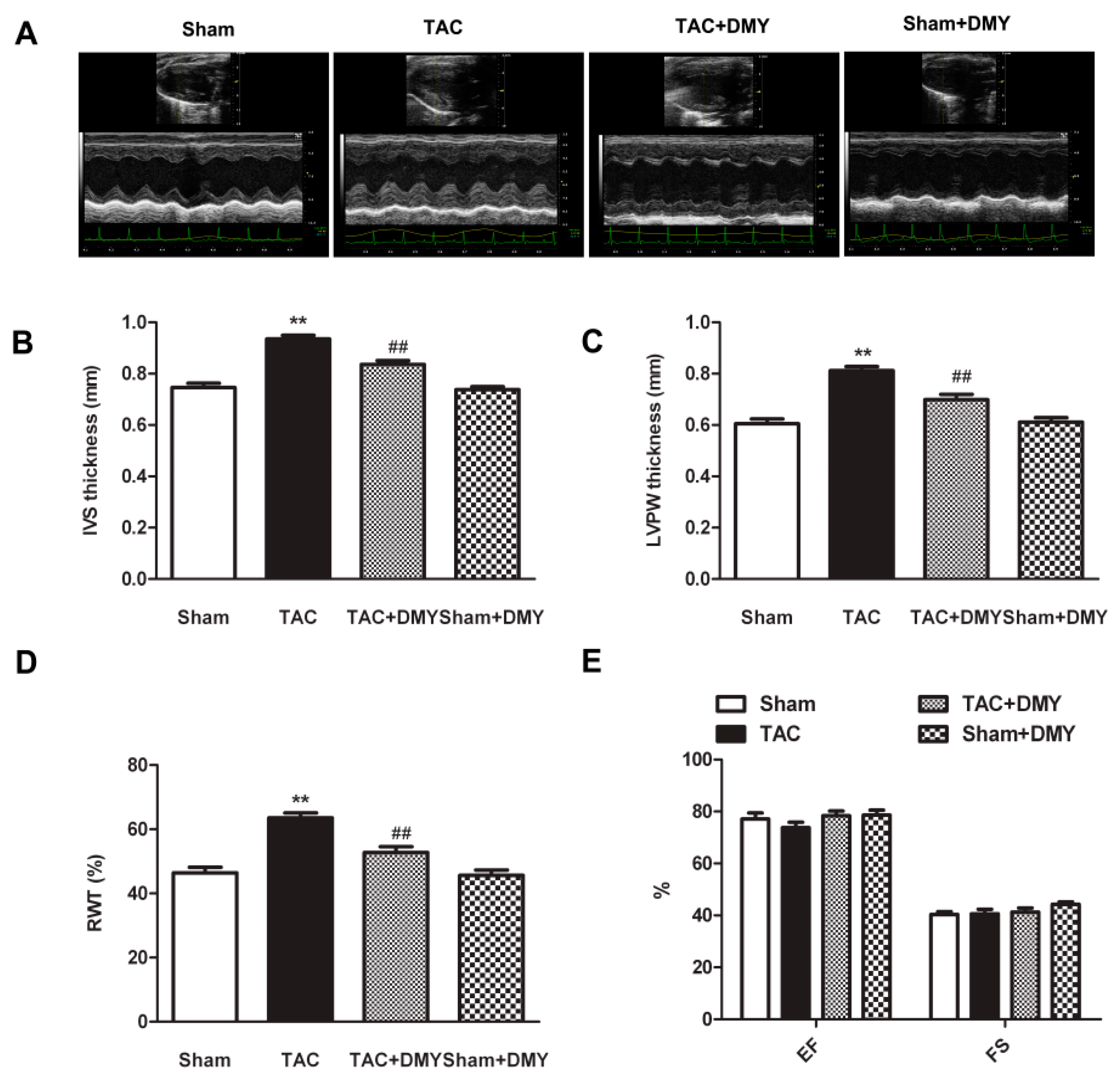
2.2. DMY Improved Myocardial Structure in Mice after TAC
2.3. DMY Reduced Cardiomyocyte Cross-Sectional Area and Cardiac Index in Mice after TAC
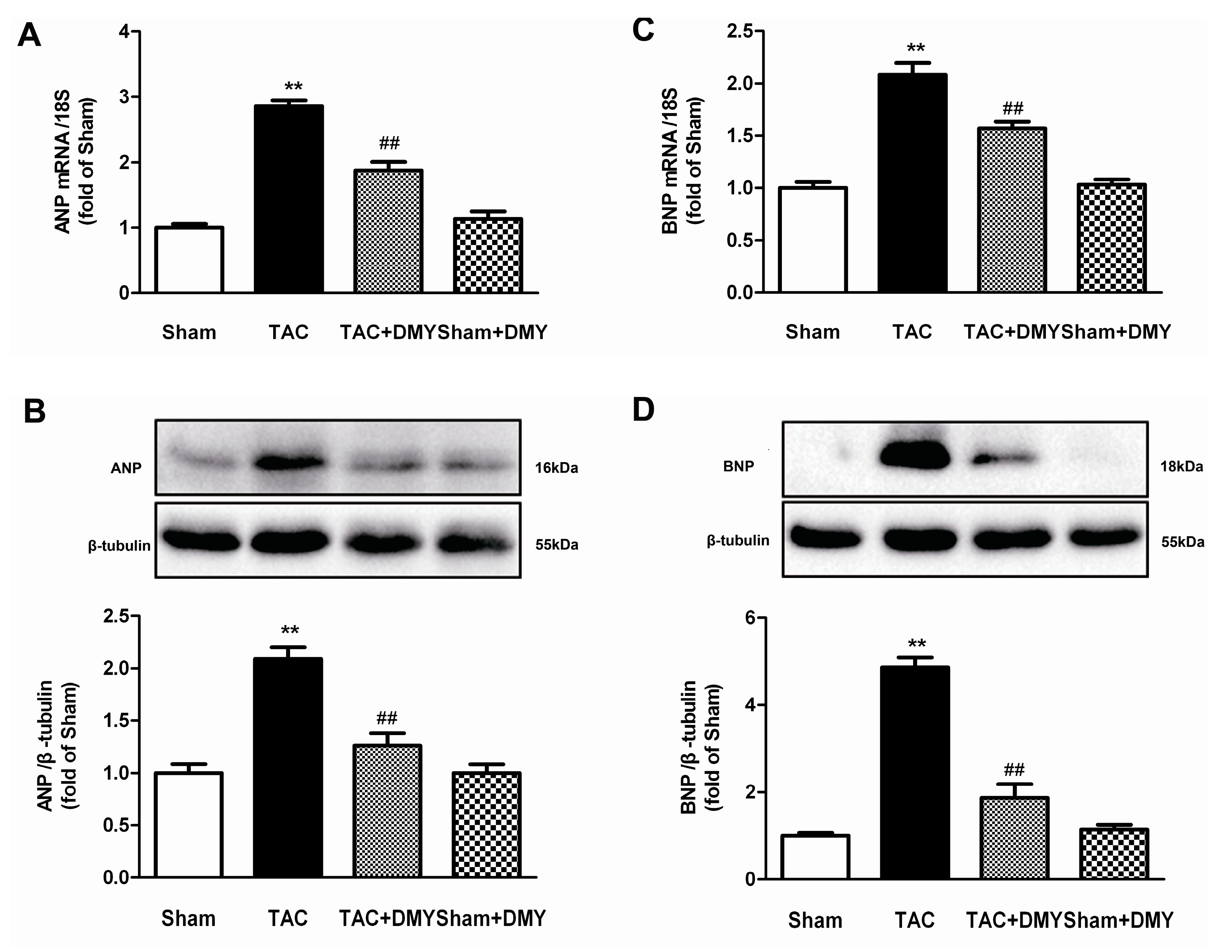
2.4. DMY Suppressed the Hypertrophic Genes Expression in the Myocardium of Mice after TAC
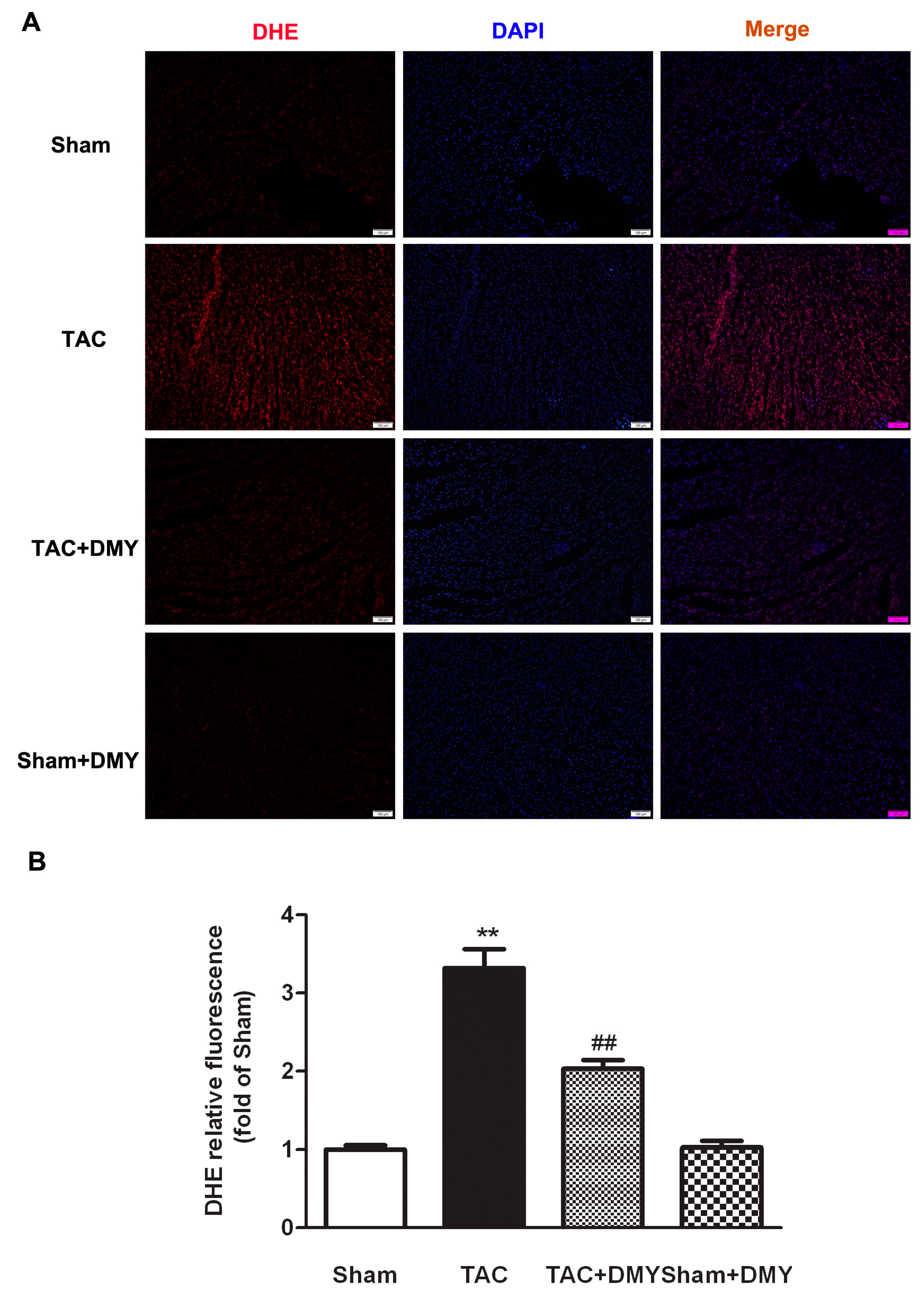
2.5. DMY Attenuated Oxidative Stress in the Myocardium of Mice after TAC
2.6. DMY Reduced Myocardial MDA Levels but Enhanced T-AOC and SOD Activity in Mice after TAC
2.7. DMY Increased SIRT3 Expression and Activity in the Myocardium of Mice after TAC
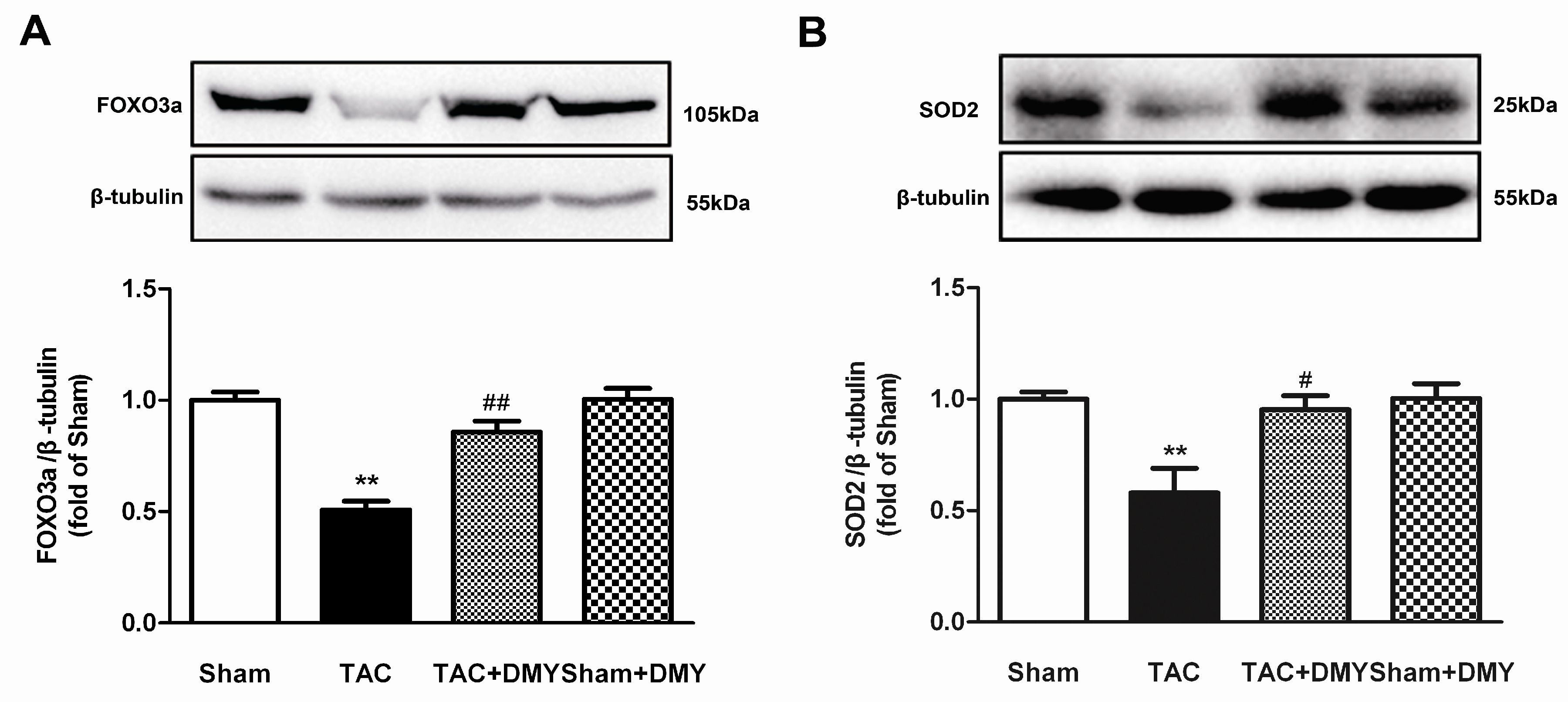
2.8. DMY Elevated FOXO3a and SOD2 Protein Expression in the Myocardium of Mice after TAC
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Transverse Aortic Constriction (TAC)
4.3. Blood Pressure Measurement
4.4. Echocardiography
4.5. Cardiac Index Determination
4.6. Wheat Germ Agglutinin (WGA) Staining
4.7. Histological Analysis
4.8. Oxidative Stress Evaluation
4.9. SIRT3 Activity
4.10. Quantitative Real-Time PCR
4.11. Western Blot
4.12. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ANP | Atrial natriuretic peptides |
| BNP | Brain natriuretic peptides |
| DBP | Diastolic blood pressure |
| DHE | Dihydroethidium |
| FOXO3a | Forkhead-box-protein 3a |
| HMI | Heart mass index |
| HW | Heart weight |
| IVS | Inter ventricular septum |
| LVMI | Left ventricular mass index |
| LVPW | Left ventricular posterior wall |
| MAP | Average mean artery pressure |
| RWT | Relative wall thickness |
| SBP | Systolic blood pressure |
| SOD | Superoxide dismutase |
| T-AOC | Total antioxidant capacity |
| WGA | Wheat germ agglutinin |
References
- Pagliaro, P.; Penna, C. Hypertension, hypertrophy, and reperfusion injury. J. Cardiovasc. Med. (Hagerstown) 2017, 18, 131–135. [Google Scholar] [CrossRef] [PubMed]
- Lazzeroni, D.; Rimoldi, O.; Camici, P.G. From left ventricular hypertrophy to dysfunction and failure. Circ. J. 2016, 80, 555–564. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.; Zhao, X.; Liu, S.; Ji, Y.; Yao, J.; Xu, H. Knockout of the prostaglandin E-2 receptor subtype 3 promotes eccentric cardiac hypertrophy and fibrosis in mice. J. Cardiovasc. Pharmacol. 2017, 22, 71–82. [Google Scholar]
- Liao, R.J.; Tong, L.J.; Huang, C.; Cao, W.W.; Wang, Y.Z.; Wang, J.; Chen, X.F.; Zhu, W.Z.; Zhang, W. Rescue of cardiac failing and remodelling by inhibition of protein phosphatase 1 gamma is associated with suppression of the alternative splicing factor-mediated splicing of Ca2+/calmodulin-dependent protein kinase delta. Clin. Exp. Pharmacol. Physiol. 2014, 41, 976–985. [Google Scholar] [CrossRef] [PubMed]
- Woo, A.Y.; Song, Y.; Xiao, R.P.; Zhu, W. Biased (2)-adrenoceptorsignalling in heart failure: pathophysiology and drug discovery. Br. J. Pharmacol. 2015, 172, 5444–5456. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Wu, D.; Sang, M.; Xu, Y.; Liu, Z.; Wu, Q. Stachydrine ameliorates isoproterenol-induced cardiac hypertrophy and fibrosis by suppressing inflammation and oxidative stress through inhibiting NF-kappa B and JAK/STAT signaling pathways in rats. Int. Immunopharmacol. 2017, 48, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Cervantes Gracia, K.; Llanas-Cornejo, D.; Husi, H. CVD and oxidative stress. J. Clin. Med. 2017, 6, E22. [Google Scholar] [CrossRef] [PubMed]
- Maulik, S.K.; Kumar, S. Oxidative stress and cardiac hypertrophy: a review. Toxicol. Mech. Methods 2012, 22, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Tsutsui, H.; Kinugawa, S.; Matsushima, S. Oxidative stress and heart failure. Am. J. Physiol. Heart Circ. Physiol. 2011, 301, H2181–H2190. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.Y.; Ding, Y.; Zhang, T.; Cai, Z.; Tao, J. Simultaneous determination of dihydromyricetin and resveratrol in ampelopsis sinica (Miq.) W.T. Wang by high-performance liquid chromatography coupled with a diode array detection method. J. Chromatogr. Sci. 2014, 52, 339–343. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhao, X.; Wan, J.; Ran, L.; Qin, Y.; Wang, X.; Gao, Y.; Shu, F.; Zhang, Y.; Liu, P.; et al. Dihydromyricetin improves glucose and lipid metabolism and exerts anti-inflammatory effects in nonalcoholic fatty liver disease: arandomized controlled trial. Pharmacol. Res. 2015, 99, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Hu, M.; Zhao, R.; Li, P.; Li, M. Dihydromyricetin suppresses the proliferation of hepatocellular carcinoma cells by inducing G2/M arrest through the Chk1/Chk2/Cdc25C pathway. Oncol. Rep. 2013, 30, 2467–2475. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Le, L.; Pan, H.; Hu, K.; Xu, L.; Xiao, P. Dihydromyricetin ameliorates the oxidative stress response induced by methylglyoxal via the AMPK/GLUT4 signal pathway in PC12 cells. Brain Res. Bull. 2014, 109, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Fu, L.; Wang, L.; Xu, L.; Yang, B. Electrophysiological study on the antiarrhythmic mechanism of ampelopsin in rats. Zhonghua Xin Xue Guan Bing Za Zhi 2014, 42, 675–679. (In Chinese) [Google Scholar] [PubMed]
- Liao, S.F.; Wang, H.T.; Yan, F.X.; Zheng, Y.X.; Zeng, Z.W.; Zheng, W.H. Protective effect and mechanisms of dihydromyricetin on PC12 cells induced by oxidative injury. Zhongyaocai 2014, 37, 1014–1020. (In Chinese) [Google Scholar] [PubMed]
- Wong, I.L.; Wang, B.C.; Yuan, J.; Duan, L.X.; Liu, Z.; Liu, T.; Li, X.M.; Hu, X.; Zhang, X.Y.; Jiang, T.; et al. Potent and nontoxic chemosensitizer of P-glycoprotein-mediated multidrug resistance in cancer: synthesis and evaluation of methylated epigallocatechin, gallocatechin, and dihydromyricetin derivatives. J. Med. Chem. 2015, 58, 4529–4549. [Google Scholar] [CrossRef] [PubMed]
- Song, Q.; Liu, L.; Yu, J.; Zhang, J.; Xu, M.; Sun, L.; Luo, H.; Feng, Z.; Meng, G. Dihydromyricetin attenuated Ang II induced cardiac fibroblasts proliferation related to inhibitory of oxidative stress. Eur. J. Pharmacol. 2017, 807, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Meng, G.; Yang, S.; Chen, Y.; Yao, W.; Zhu, H.; Zhang, W. Attenuating effects of dihydromyricetin on angiotensin II-induced rat cardiomyocyte hypertrophy related to antioxidative activity in a NO-dependent manner. Pharm. Biol. 2015, 53, 904–912. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Feng, H.; Li, S.; Meng, G.; Liu, S.; Tang, X.; Ma, Y.; Han, Y.; Xiao, Y.; Gu, Y.; et al. SIRT3 mediates the antioxidant effect of hydrogen sulfide in endothelial cells. Antioxid. Redox Signal. 2016, 24, 329–343. [Google Scholar] [CrossRef] [PubMed]
- Rangarajan, P.; Karthikeyan, A.; Lu, J.; Ling, E.A.; Dheen, S.T. Sirtuin 3 regulatesFoxo3a-mediated antioxidant pathway in microglia. Neuroscience 2015, 311, 398–414. [Google Scholar] [CrossRef] [PubMed]
- Yue, Z.; Ma, Y.; You, J.; Li, Z.; Ding, Y.; He, P.; Lu, X.; Jiang, J.; Chen, S.; Liu, P. NMNAT3 is involved in the protective effect of SIRT3 in Ang II-induced cardiac hypertrophy. Exp. Cell Res. 2016, 347, 261–273. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chen, T.; Xiao, M.; Li, N.; Wang, S.; Su, H.; Guo, X.; Liu, H.; Yan, F.; Yang, Y.; et al. Mouse SIRT3 promotes autophagy in Ang II-induced myocardial hypertrophy through the deacetylation of FoxO1. Oncotarget 2016, 7, 86648–86659. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Zou, D.; Chen, K.; Zhou, Q.; Gao, Y.; Huang, Y.; Zhu, J.; Zhang, Q.; Mi, M. Dihydromyricetin improves hypobaric hypoxia-induced memory impairment via modulation of SIRT3 signaling. Mol. Neurobiol. 2016, 53, 7200–7212. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Yang, J.; Hu, O.; Huang, J.; Ran, L.; Chen, M.; Zhang, Y.; Zhou, X.; Zhu, J.; Zhang, Q.; et al. Dihydromyricetin ameliorates nonalcoholic fatty liver disease by improving mitochondrial respiratory capacity and redox homeostasis through modulation of SIRT3 signaling. Antioxid. Redox Signal. 2018. [Google Scholar] [CrossRef] [PubMed]
- Koentges, C.; Bode, C.; Bugger, H. SIRT3 in cardiac physiology and disease. Front. Cardiovasc. Med. 2016, 3, 38. [Google Scholar] [CrossRef] [PubMed]
- Tham, Y.K.; Bernardo, B.C.; Ooi, J.Y.; Weeks, K.L.; McMullen, J.R. Pathophysiology of cardiac hypertrophy and heart failure: signaling pathways and novel therapeutic targets. Arch. Toxicol. 2015, 89, 1401–1438. [Google Scholar] [CrossRef] [PubMed]
- Sundaresan, N.R.; Gupta, M.; Kim, G.; Rajamohan, S.B.; Isbatan, A.; Gupta, M.P. SIRT3 blocks the cardiac hypertrophic response by augmenting Foxo3a-dependent antioxidant defense mechanisms in mice. J. Clin. Investig. 2009, 119, 2758–2771. [Google Scholar] [CrossRef] [PubMed]
- Goh, V.J.; Le, T.T.; Bryant, J.; Wong, J.I.; Su, B.; Lee, C.H.; Pua, C.J.; Sim, C.P.Y.; Ang, B.; Aw, T.C.; Cook, S.A.; Chin, C.W.L. Novel index of maladaptive myocardial remodeling in hypertension. Circ. Cardiovasc. Imaging 2017, 10, e006840. [Google Scholar] [CrossRef] [PubMed]
- Froese, N.; Wang, H.; Zwadlo, C.; Wang, Y.; Grund, A.; Gigina, A.; Hofmann, M.; Kilian, K.; Scharf, G.; Korf-Klingebiel, M.; et al. Anti-androgenic therapy with finasteride improves cardiac function, attenuates remodeling and reverts pathologic gene-expression after myocardial infarction in mice. J. Mol. Cell. Cardiol. 2018, 122, 114–124. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Zeng, Y.; Xu, J.; Huang, X.L.; Zhang, W.; Xu, X.L. PPAR-gamma is involved in the protective effect of 2,3,4’,5-tetrahydroxystilbene-2-O-beta-d-glucoside against cardiac fibrosis in pressure-overloaded rats. Eur. J. Pharmacol. 2016, 791, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Protsenko, Y.L.; Balakin, A.A.; Kuznetsov, D.A.; Kursanov, A.G.; Lisin, R.V.; Mukhlynina, E.A.; Lookin, O.N. Contractility of right ventricular myocardium in male and female rats during physiological and pathological hypertrophy. Bull. Exp. Biol. Med. 2017, 162, 303–305. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Lin, J.; Luo, J.; Han, D.; Fan, M.; Guo, T.; Tao, L.; Yuan, M.; Yi, F. Dihydromyricetin Protects against Diabetic Cardiomyopathy in Streptozotocin-Induced Diabetic Mice. Biomed. Res. Int. 2017, 2017, 3764370. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.Q.; Shao, L.; Wu, J.; Peng, N.; Jin, L.P.; Wei, G.Z.; Cheng, W.; Deng, C.J. Dihydromyricetin protects against lipopolysaccharide-induced cardiomyocyte injury through the toll-like receptor-4/nuclear factor-κB pathway. Mol. Med. Rep. 2017, 16, 8983–8988. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Ai, Q.; Feng, K.; Li, Y.; Liu, X. The cardioprotective effect of dihydromyricetin prevents ischemia-reperfusion-induced apoptosis in vivo and in vitro via the PI3K/Akt and HIF-1α signaling pathways. Apoptosis 2016, 21, 1366–1385. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.T.; Zeng, Y.; Tang, K.; Chen, X.; Zhang, W.; Xu, X.L. Dihydromyricetin ameliorates atherosclerosis in LDL receptor deficient mice. Atherosclerosis 2017, 262, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Luo, P.; Fu, Y.; Wang, J.; Dai, J.; Shao, J.; Yang, X.; Chang, L.; Weng, Q.; Yang, B.; et al. Dihydromyricetin prevents cardiotoxicity and enhances anticancer activity induced by adriamycin. Oncotarget 2015, 6, 3254–3267. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Peng, Y.; Tang, K.; Wang, Y.Q.; Zhao, Z.Y.; Wei, X.Y.; Xu, X.L. Dihydromyricetin ameliorates foam cell formation via LXRα-ABCA1/ABCG1-dependent cholesterol efflux in macrophages. Biomed. Pharmacother. 2018, 101, 542–552. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.L.; Zhu, Q.Y.; Zhao, C.; Wang, F.; Zhou, Z.Y.; Hu, Y.E.; Zhang, W. The Effect of 2,3,4’,5-Tetrahydroxystilbene-2-O-beta-d-Glucoside on pressure overload-induced cardiac remodeling in rats and its possible mechanism. Planta Med. 2014, 80, 130–138. [Google Scholar] [PubMed]
- Hou, X.; Tong, Q.; Wang, W.; Xiong, W.; Shi, C.; Fang, J. Dihydromyricetin protects endothelial cells from hydrogen peroxide-induced oxidative stress damage by regulating mitochondrial pathways. Life Sci. 2015, 130, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Chen, Z.; Zhang, C.; Xu, X.; Jin, J.; Zhan, W.; Han, T.; Wang, J. Dihydromyricetin ameliorates oleic acid-induced lipid accumulation in L02 and HepG2 cells by inhibiting lipogenesis and oxidative stress. Life Sci. 2016, 157, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, W.; Qiu, E. Protection of oxidative stress induced apoptosis in osteosarcoma cells by dihydromyricetin through down-regulation of caspase activation and up-regulation of BcL-2. Saudi J. Biol. Sci. 2017, 24, 837–842. [Google Scholar] [CrossRef] [PubMed]
- Elhassan, Y.S.; Philp, A.A.; Lavery, G.G. Targeting NAD+ in metabolic disease: new insight into an old molecule. J. Endocr. Soc. 2017, 1, 816–825. [Google Scholar] [CrossRef] [PubMed]
- Fan, D.; Yang, Z.; Liu, F.Y.; Jin, Y.G.; Zhang, N.; Ni, J.; Yuan, Y.; Liao, H.H.; Wu, Q.Q.; Xu, M.; et al. Sesamin Protects Against Cardiac Remodeling via SIRT3/ROS Pathway. Cell. Physiol. Biochem. 2017, 44, 2212–2227. [Google Scholar] [CrossRef] [PubMed]
- Hafner, A.V.; Dai, J.; Gomes, A.P.; Xiao, C.Y.; Palmeira, C.M.; Rosenzweig, A.; Sinclair, D.A. Regulation of the mPTP by SIRT3-mediated deacetylation of CypD at lysine 166 suppresses age-related cardiac hypertrophy. Aging 2010, 2, 914–923. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.P.; Oka, S.; Shao, D.; Hariharan, N.; Sadoshima, J. Nicotinamide phosphoribosyltransferase regulates cell survival through NAD+ synthesis in cardiac myocytes. Circ. Res. 2009, 105, 481–491. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Wang, Y.D.; Wang, X.Y.; Chen, H.; Cai, Z.J.; Xiang, M.X. SIRT3 in cardiovascular diseases: emerging roles and therapeutic implications. Int. J. Cardiol. 2016, 220, 700–705. [Google Scholar] [CrossRef] [PubMed]
- Osborne, B.; Bentley, N.L.; Montgomery, M.K.; Turner, N. The role of mitochondrial sirtuins in health and disease. Free Radic. Biol. Med. 2016, 100, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Pillai, V.B.; Bindu, S.; Sharp, W.; Fang, Y.H.; Kim, G.; Gupta, M.; Samant, S.; Gupta, M.P. SIRT3 protects mitochondrial DNA damage and blocks the development of doxorubicin-induced cardiomyopathy in mice. Am. J. Physiol. Heart Circ. Physiol. 2016, 310, H962–H972. [Google Scholar] [CrossRef] [PubMed]
- Tanno, M.; Kuno, A.; Horio, Y.; Miura, T. Emerging beneficial roles of sirtuins in heart failure. Basic Res. Cardiol. 2012, 107, 273. [Google Scholar] [CrossRef] [PubMed]
- Meng, G.; Liu, J.; Liu, S.; Song, Q.; Liu, L.; Xie, L.; Han, Y.; Ji, Y. Hydrogen sulfide pretreatment improves mitochondrial function in myocardial hypertrophy via a SIRT3-dependent manner. Br. J. Pharmacol. 2018, 175, 1126–1145. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, K.M.; Pennington, J.D.; Bisht, K.S.; Aykin-Burns, N.; Kim, H.S.; Mishra, M.; Sun, L.; Nquyen, P.; Ahn, B.H.; Leclerc, J.; et al. SIRT3 interacts with the daf-16 homolog FOXO3a in the mitochondria, as well as increases FOXO3a dependent gene expression. Int. J. Biol. Sci. 2008, 4, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Mitrovsky, G.; Vasanthi, H.R.; Das, D.K. Antiaging properties of a grape-derived antioxidant are regulated by mitochondrial balance of fusion and fission leading to mitophagy triggered by a signaling network of Sirt1-SIRT3-Foxo3-PINK1-PARKIN. Oxid. Med. Cell Longev. 2014, 2014, 345105. [Google Scholar] [CrossRef] [PubMed]
- Queiroz, E.A.; Puukila, S.; Eichler, R.; Sampaio, S.C.; Forsyth, H.L.; Lees, S.J.; Barbosa, A.M.; Dekker, R.F.; Fortes, Z.B.; Khaper, N. Metformin induces apoptosis and cell cycle arrest mediated by oxidative stress, AMPK and FOXO3a in MCF-7 breast cancer cells. PLoS ONE 2014, 9, e98207. [Google Scholar] [CrossRef] [PubMed]
- Tothova, Z.; Kollipara, R.; Huntly, B.J.; Lee, B.H.; Castrillon, D.H.; Cullen, D.E.; McDowell, E.P.; Lazo-Kallanian, S.; Williams, I.R.; Sears, C.; et al. FoxOs are critical mediators of hematopoietic stem cell resistance to physiologic oxidative stress. Cell 2007, 128, 325–339. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Zhao, J.; Fu, B.; Li, D.; Mao, T.; Peng, W.; Wu, H.; Zhang, Y. Melatonin-mediated upregulation of SIRT3 attenuates sodium fluoride-induced hepatotoxicity by activating the MT1-PI3K/AKT-PGC-1α signaling pathway. Free Radic. Biol. Med. 2017, 112, 616–630. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, J.; Lin, Y.; Lei, Q.; Guan, K.L.; Zhao, S.; Xiong, Y. Tumour suppressor SIRT3 deacetylates and activates manganese superoxide dismutase to scavenge ROS. EMBO Rep. 2011, 12, 534–541. [Google Scholar] [CrossRef] [PubMed]
- Pillai, V.B.; Samant, S.; Sundaresan, N.R.; Raghuraman, H.; Kim, G.; Bonner, M.Y.; Arbiser, J.L.; Walker, D.; Jones, D.P.; Gius, D.; et al. Honokiol blocks and reverses cardiac hypertrophy in mice by activating mitochondrial SIRT3. Nat. Commun. 2015, 6, 6656. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, L.; Wang, P.; Li, X.; Qiu, D.; Li, L.; Zhang, J.; Hou, X.; Han, L.; Ge, J.; et al. SIRT3-dependent deacetylation of SOD2 plays a protective role against oxidative stress in oocytes from diabetic mice. Cell Cycle 2017, 16, 1302–1308. [Google Scholar] [CrossRef] [PubMed]
- Valdecantos, M.P.; Pérez-Matute, P.; González-Muniesa, P.; Prieto-Hontoria, P.L.; Moreno-Aliaga, M.J.; Martínez, J.A. Lipoic acid improves mitochondrial function in nonalcoholic steatosis through the stimulation of sirtuin 1 and sirtuin 3. Obesity 2012, 20, 1974–1983. [Google Scholar] [CrossRef] [PubMed]
- De Almeida, A.C.; van Oort, R.J.; Wehrens, X.H. Transverse aortic constriction in mice. J. Vis. Exp. 2010, 38, 172. [Google Scholar]



© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Luo, H.-Q.; Sun, L.-L.; Xu, M.-T.; Yu, J.; Liu, L.-L.; Zhang, J.-Y.; Wang, Y.-Q.; Wang, H.-X.; Bao, X.-F.; et al. Dihydromyricetin Attenuates Myocardial Hypertrophy Induced by Transverse Aortic Constriction via Oxidative Stress Inhibition and SIRT3 Pathway Enhancement. Int. J. Mol. Sci. 2018, 19, 2592. https://doi.org/10.3390/ijms19092592
Chen Y, Luo H-Q, Sun L-L, Xu M-T, Yu J, Liu L-L, Zhang J-Y, Wang Y-Q, Wang H-X, Bao X-F, et al. Dihydromyricetin Attenuates Myocardial Hypertrophy Induced by Transverse Aortic Constriction via Oxidative Stress Inhibition and SIRT3 Pathway Enhancement. International Journal of Molecular Sciences. 2018; 19(9):2592. https://doi.org/10.3390/ijms19092592
Chicago/Turabian StyleChen, Yun, Hui-Qin Luo, Lin-Lin Sun, Meng-Ting Xu, Jin Yu, Lu-Lu Liu, Jing-Yao Zhang, Yu-Qin Wang, Hong-Xia Wang, Xiao-Feng Bao, and et al. 2018. "Dihydromyricetin Attenuates Myocardial Hypertrophy Induced by Transverse Aortic Constriction via Oxidative Stress Inhibition and SIRT3 Pathway Enhancement" International Journal of Molecular Sciences 19, no. 9: 2592. https://doi.org/10.3390/ijms19092592
APA StyleChen, Y., Luo, H.-Q., Sun, L.-L., Xu, M.-T., Yu, J., Liu, L.-L., Zhang, J.-Y., Wang, Y.-Q., Wang, H.-X., Bao, X.-F., & Meng, G.-L. (2018). Dihydromyricetin Attenuates Myocardial Hypertrophy Induced by Transverse Aortic Constriction via Oxidative Stress Inhibition and SIRT3 Pathway Enhancement. International Journal of Molecular Sciences, 19(9), 2592. https://doi.org/10.3390/ijms19092592




