Are Colon and Rectal Cancer Two Different Tumor Entities? A Proposal to Abandon the Term Colorectal Cancer
Abstract
1. Introduction
2. Results
2.1. Anatomy and Topography
2.2. Epidemiology
2.3. Carcinogenic Risk
2.4. Formal Carcinogenesis
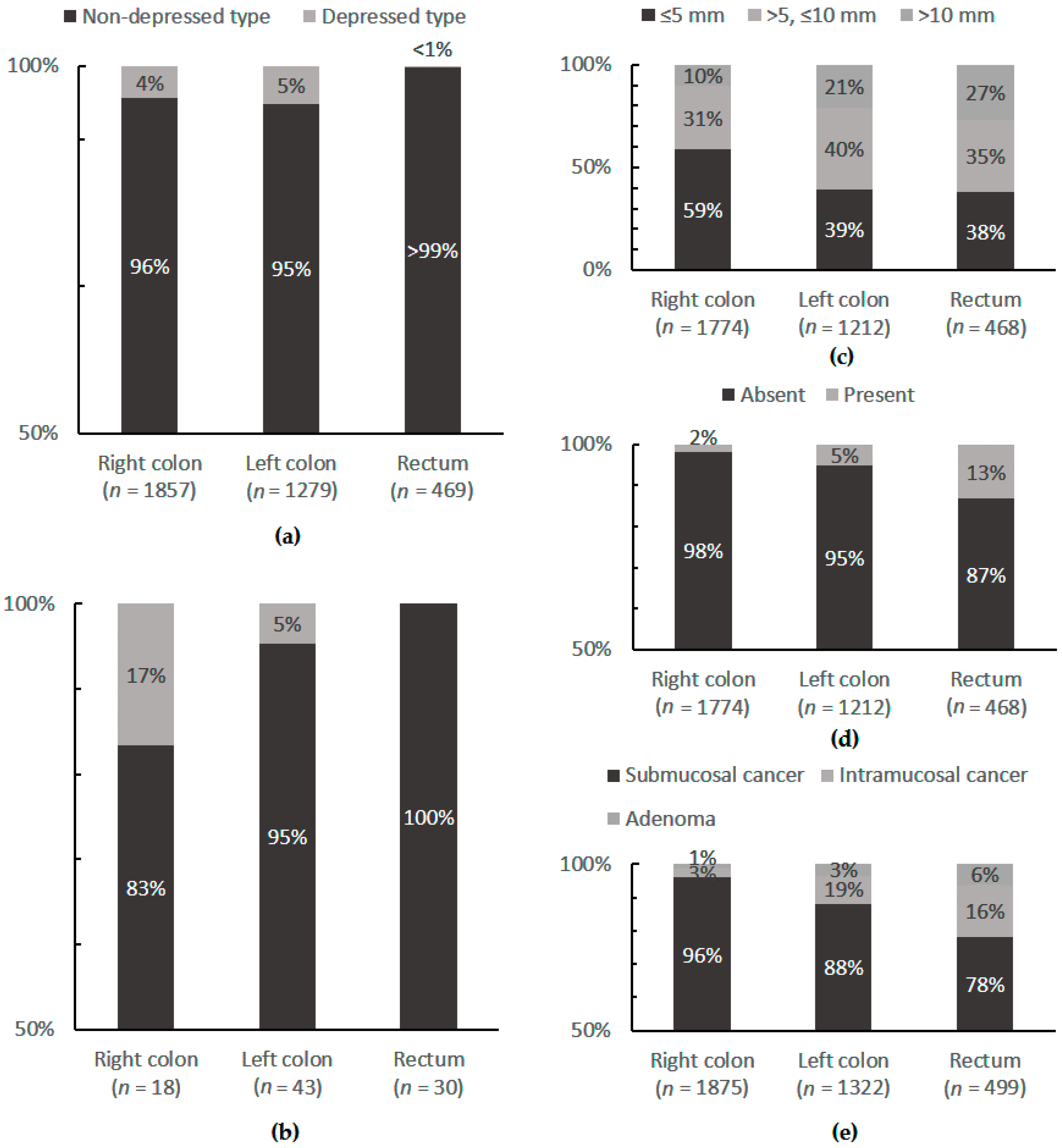
2.5. Macroscopy and Histopathology
2.6. Hereditary Syndromes and Molecular Carcinogenesis
2.7. Primary Preventive Measures
2.8. Clinical Symptoms and Examinations
2.9. Surgery
2.10. Biology
2.11 Multimodal Treatment
2.12. Prediction of Response to Multimodal Treatment
3. Discussion
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| CC | Colon cancer |
| CT | Chemotherapy |
| RC | Rectal cancer |
| CRC | Colorectal cancer |
| FOGT | Research Group Oncology of Gastrointestinal Tumors |
| LGIN/HGIN | Low grade or high grade intraepithelial neoplasia |
| FAP | Familial adenomatous polyposis |
| HNPCC | Hereditary non-polyposis colorectal cancer |
| APC | Adenomatous polyposis coli |
| MSI | Microsatellite instability |
| RCT | Radiochemotherapy |
References
- Link, K.-H.; Kornmann, M.; Staib, L.; Redenbacher, M.; Kron, M.; Beger, H.G.; Study Group Oncology of Gastrointestinal Tumors. Increase of survival benefit in advanced resectable colon cancer by extent of adjuvant treatment: Results of a randomized trial comparing modulation of 5-FU + levamisole with folinic acid or with interferon-alpha. Ann. Surg. 2005, 242, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Kornmann, M.; Staib, L.; Wiegel, T.; Kreuser, E.-D.; Kron, M.; Baumann, W.; Henne-Bruns, D.; Link, K.-H. Adjuvant chemoradiotherapy of advanced resectable rectal cancer: Results of a randomised trial comparing modulation of 5-fluorouracil with folinic acid or with interferon-α. Br. J. Cancer 2010, 103, 1163–1172. [Google Scholar] [CrossRef] [PubMed]
- Pox, C.; Aretz, S.; Bischoff, S.C.; Graeven, U.; Hass, M.; Heußner, P.; Hohenberger, W.; Holstege, A.; Hübner, J.; Kolligs, F.; et al. S3-Leitlinie Kolorektales Karzinom Version 1.0—Juni 2013 AWMF-Registernummer: 021/007OL. Z. Gastroenterol. 2013, 51, 753–854. [Google Scholar] [CrossRef] [PubMed]
- Heald, R.J.; Moran, B.J. Embryology and anatomy of the rectum. Semin. Surg. Oncol. 1998, 15, 66–71. [Google Scholar] [CrossRef]
- Köckerling, F.; Lippert, H.; Gastinger, I. Fortschritte in der Kolorektale Chirurgie; Med Science: Hannover, Germany, 2002; ISBN 978-3-9807862-0-1. [Google Scholar]
- Winkler, R.; Otto, P.; Schiedeck, T. Proktologie: Ein Leitfaden für die Praxis; Georg Thieme Verlag: Stuttgart, Germany, 2011; ISBN 9783131011626. [Google Scholar]
- Paulsen, F.; Waschke, J. Sobotta, Atlas der Anatomie des Menschen Band 1: Allgemeine Anatomie und Bewegungsapparat, 23rd ed.; Urban & Fischer Verlag/Elsevier GmbH: München, Germany, 2010; ISBN 978-3-437-44071-7. [Google Scholar]
- Willis, S.; Schumpelick, V. Onkologische Chirurgie. In Praxis der Viszeralchirurgie; Siewert, J.R., Ed.; Springer Medizin Verl: Heidelberg, Germany, 2010; pp. 713–732. ISBN 978-3-642-03807-5. [Google Scholar]
- Schumacher, G.H.; Aumüller, G. Topographische Anatomie des Menschen; Elsevier: München, Germany, 2004; pp. 256–296. ISBN 978-3-437-41367-4.8. [Google Scholar]
- Kornmann, M.; Link, K.-H.; Formentini, A. Differences in colon and rectal cancer chemosensitivity. Colorectal Cancer 2014, 3, 93–105. [Google Scholar] [CrossRef]
- Henne-Bruns, D.; Kremer, B.; Dürig, M. Chirurgie, 3rd ed.; Georg Thieme Verlag: Stuttgart, Germany, 2008; pp. 201–221. ISBN 978-3-13-125293-7. [Google Scholar]
- Schünke, M.; Schulte, E.; Schumacher, U. Prometheus—LernAtlas der Anatomie: Innere Organe, 4th ed.; Thieme: Stuttgart, Germany; New York, NY, USA, 2015; ISBN 978-3-13-139534-4. [Google Scholar]
- Ferlay, J.; Autier, P.; Boniol, M.; Heanue, M.; Colombet, M.; Boyle, P. Estimates of the cancer incidence and mortality in Europe in 2006. Ann. Oncol. 2007, 18, 581–592. [Google Scholar] [CrossRef] [PubMed]
- WHO Mortality Database 2009. Available online: https://www.health.govt.nz/system/files/documents/publications/mortality-and-demographic-data-2009.pdf (accessed on 12 February 2014).
- Riboli, E. Nutrition and cancer: Background and rationale of the European Prospective Investigation into Cancer and Nutrition (EPIC). Ann. Oncol. 1992, 3, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Ruf, G.; Hopt, U.; Otto, F. Kolorektales Karzinom, Empfehlungen zur Standardisierten Diagnostik, Therapie und Nachsorge; Tumorzentrum Freiburg am Universitätsklinikum: Freiburg, Germany, 2005. [Google Scholar]
- Schmidt-Decker, S.; Brannath, J.; Teichmann, W. The incidence and localization of colorectal adenomas and carcinomas in 4109 patients. Dtsch. Med. Wochenschr. 1990, 115, 1819–1824. [Google Scholar] [CrossRef] [PubMed]
- Mostafa, G.; Matthews, B.D.; Norton, H.J.; Kercher, K.W.; Sing, R.F.; Heniford, B.T. Influence of demographics on colorectal cancer. Am. Surg. 2004, 70, 259–264. [Google Scholar] [PubMed]
- Benedix, F.; Meyer, F.; Kube, R.; Gastinger, I.; Lippert, H. Right- and left-sided colonic cancer—different tumour entities. Zentralbl. Chir. 2010, 135, 312–317. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.; Ma, J.; Zou, Z.; Jemal, A. Cancer statistics, 2014. CA Cancer J. Clin. 2014, 64, 9–29. [Google Scholar] [CrossRef] [PubMed]
- American Cancer Society: Cancer Facts and Figures 2015; American Cancer Society: Atlanta, Ga, USA, 2015; Available online: http://www.cancer.org/acs/groups/content/@editorial/documents/document/acspc-044552.pdf (accessed on 7 January 2015).
- Werner, M. Allgemeine Onkogenese und Tumorpathologie. In Praxis der Viszeralchirurgie—Onkologische Chirurgie; Siewert, J.R., Ed.; Springer Medizin Verl: Heidelberg, Germany, 2010; pp. 4–10. ISBN 978-3-642-03807-5. [Google Scholar]
- Hermanek, P.; Mansmann, U.; Altendorf-Hofmann, A.; Hermanek, P.; Riedl, S.; Staimmer, D.; für dieStudiengruppe Kolorektales Karzinom (SGKRK). Vergleichende Beurteilung der onkologischen Ergebnisqualität beim colorectalen Carcinom Klinikvergleiche anhand von Surrogatendpunkten? Der Chirurg 1999, 70, 407–414. [Google Scholar] [CrossRef]
- Wittekind, C. Tumorklassifikationen. In Onkologische Chirurgie; Siewert, J.R., Ed.; Praxis der Viszeralchirurgie; Springer Medizin Verl: Heidelberg, Germany, 2010; pp. 20–28. ISBN 978-3-642-03807-5. [Google Scholar]
- Aaltonen, L.A.; Hamilton, S.R. Pathology and Genetics of Tumours of the Digestive System; World Health Organization, International Agency for Research on Cancer; World Health Organization Classification of Tumours; IARC Press: Lyon, France, 2000; ISBN 978-92-832-2410-5. [Google Scholar]
- Tannapfel, A. Präkanzerosen und molekulare Marker. In Praxis der Viszeralchirurgie—Onkologische Chirurgie; Siewert, J.R., Ed.; Springer Medizin Verl: Heidelberg, Germany, 2010; pp. 14–17. ISBN 978-3-642-03807-5. [Google Scholar]
- Hiddemann, W. Spezieller Teil: Solide Tumoren, Lymphome, Leukämien. In Die Onkologie, 2nd ed.; Bertram, C., Ed.; Springer Medizin: Heidelberg, Germany, 2010; p. 854. ISBN 978-3-540-79724-1. [Google Scholar]
- Konishi, K.; Fujii, T.; Boku, N.; Kato, S.; Koba, I.; Ohtsu, A.; Tajiri, H.; Ochiai, A.; Yoshida, S. Clinicopathological differences between colonic and rectal carcinomas: Are they based on the same mechanism of carcinogenesis? Gut 1999, 45, 818–821. [Google Scholar] [CrossRef] [PubMed]
- Lynch, H.T.; Smyrk, T.C. Classification of familial adenomatous polyposis: A diagnostic nightmare. Am. J. Hum. Genet. 1998, 62, 1288–1289. [Google Scholar] [CrossRef] [PubMed]
- Lynch, H.T.; Lynch, J.F.; Shaw, T.G.; Lubiński, J. HNPCC (Lynch Syndrome): Differential Diagnosis, Molecular Genetics and Management—A Review. Hered. Cancer Clin. Pract. 2003, 1, 7–18. [Google Scholar] [CrossRef]
- Nawa, T.; Kato, J.; Kawamoto, H.; Okada, H.; Yamamoto, H.; Kohno, H.; Endo, H.; Shiratori, Y. Differences between right- and left-sided colon cancer in patient characteristics, cancer morphology and histology. J. Gastroenterol. Hepatol. 2008, 23, 418–423. [Google Scholar] [CrossRef] [PubMed]
- Schmiegel, W.; Reinacher-Schick, A.; Arnold, D.; Graeven, U.; Heinemann, V.; Porschen, R.; Riemann, J.; Rödel, C.; Sauer, R.; Wieser, M.; et al. S3-Leitlinie “Kolorektales Karzinom”—Aktualisierung 2008. Z. Gastroenterol. 2008, 46, 799–840. [Google Scholar] [CrossRef] [PubMed]
- Lynch, H.T.; Smyrk, T.; McGinn, T.; Lanspa, S.; Cavalieri, J.; Lynch, J.; Slominski-Castor, S.; Cayouette, M.C.; Priluck, I.; Luce, M.C. Attenuated familial adenomatous polyposis (AFAP) a phenotypically and genotypically distinctive variant of FAP. Cancer 1995, 76, 2427–2433. [Google Scholar] [CrossRef]
- Jafarov, S. Unterschiede Zwischen Dick-und Enddarmkrebs in der Karzinogenese/Molekularbiologie, Biologischem Verlauf, Therapieverfahren, Funktionellen und Onkologischen Ergebnissen; Lehmanns Media: Berlin, Germany, 2018; ISBN 978-3-86541-957-6. [Google Scholar]
- Jass, J.R. Classification of colorectal cancer based on correlation of clinical, morphological and molecular features. Histopathology 2007, 50, 113–130. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.; Roepman, P.; Popovici, V.; Michaut, M.; Majewski, I.; Salazar, R.; Santos, C.; Rosenberg, R.; Nitsche, U.; Mesker, W.E.; et al. A robust genomic signature for the detection of colorectal cancer patients with microsatellite instability phenotype and high mutation frequency. J. Pathol. 2012, 228, 586–595. [Google Scholar] [CrossRef] [PubMed]
- Budinska, E.; Popovici, V.; Tejpar, S.; D’Ario, G.; Lapique, N.; Sikora, K.O.; Di Narzo, A.F.; Yan, P.; Hodgson, J.G.; Weinrich, S.; et al. Gene expression patterns unveil a new level of molecular heterogeneity in colorectal cancer. J. Pathol. 2013, 231, 63–76. [Google Scholar] [CrossRef] [PubMed]
- Nehls, O.; Okech, T.; Hsieh, C.-J.; Enzinger, T.; Sarbia, M.; Borchard, F.; Gruenagel, H.-H.; Gaco, V.; Hass, H.G.; Arkenau, H.T.; et al. Studies on p53, BAX and Bcl-2 protein expression and microsatellite instability in stage III (UICC) colon cancer treated by adjuvant chemotherapy: Major prognostic impact of proapoptotic BAX. Br. J. Cancer 2007, 96, 1409–1418. [Google Scholar] [CrossRef] [PubMed]
- Reimers, M.S.; Zeestraten, E.C.M.; Kuppen, P.J.K.; Liefers, G.J.; van de Velde, C.J.H. Biomarkers in precision therapy in colorectal cancer. Gastroenterol. Rep. 2013, 1, 166–183. [Google Scholar] [CrossRef] [PubMed]
- Peltomäki, P.; Vasen, HF. Mutations predisposing to hereditary nonpolyposis colorectal cancer: Database and results of a collaborative study. The International Collaborative Group on Hereditary Nonpolyposis Colorectal Cancer. Gastroenterology 1997, 113, 1146–1158. [Google Scholar] [CrossRef] [PubMed]
- Vogelsang, H.; Holinski-Feder, E.; Gross, M. Hereditäre Tumorerkrankungen des Gastrointestinaltrakts. In Tumorzentrum München: Manual Gastrointestinale Tumoren, 6th ed.; Tumorzentrum München, Zuckschwerdt: München, Germany, 2001; pp. 261–283. ISBN 978-3-88603-760-5. [Google Scholar]
- Kullmann, F.; Bocker, T.; Schölmerich, J.; Rüschoff, J. Microsatellite instability—A new aspects in genetics and molecular biology of hereditary nonpolyposis and sporadic colorectal tumors. Z. Gastroenterol. 1996, 34, 813–822. [Google Scholar] [PubMed]
- Popovici, V.; Budinska, E.; Tejpar, S.; Weinrich, S.; Estrella, H.; Hodgson, G.; Van Cutsem, E.; Xie, T.; Bosman, F.T.; Roth, A.D.; et al. Identification of a poor-prognosis BRAF-mutant-like population of patients with colon cancer. J. Clin. Oncol. 2012, 30, 1288–1295. [Google Scholar] [CrossRef] [PubMed]
- Rad, R.; Cadiñanos, J.; Rad, L.; Varela, I.; Strong, A.; Kriegl, L.; Constantino-Casas, F.; Eser, S.; Hieber, M.; Seidler, B.; et al. A genetic progression model of Braf(V600E)-induced intestinal tumorigenesis reveals targets for therapeutic intervention. Cancer Cell 2013, 24, 15–29. [Google Scholar] [CrossRef] [PubMed]
- Ogino, S.; Goel, A. Molecular classification and correlates in colorectal cancer. J. Mol. Diagn. 2008, 10, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Sanz-Pamplona, R.; Cordero, D.; Berenguer, A.; Lejbkowicz, F.; Rennert, H.; Salazar, R.; Biondo, S.; Sanjuan, X.; Pujana, M.A.; Rozek, L.; et al. Gene expression differences between colon and rectum tumors. Clin. Cancer Res. 2011, 17, 7303–7312. [Google Scholar] [CrossRef] [PubMed]
- Markowitz, S.D.; Bertagnolli, M.M. Molecular origins of cancer: Molecular basis of colorectal cancer. N. Engl. J. Med. 2009, 361, 2449–2460. [Google Scholar] [CrossRef] [PubMed]
- Rozek, L.S.; Lipkin, S.M.; Fearon, E.R.; Hanash, S.; Giordano, T.J.; Greenson, J.K.; Kuick, R.; Misek, D.E.; Taylor, J.M.G.; Douglas, J.A.; et al. CDX2 polymorphisms, RNA expression, and risk of colorectal cancer. Cancer Res. 2005, 65, 5488–5492. [Google Scholar] [CrossRef] [PubMed]
- Slattery, M.L.; Curtin, K.; Wolff, R.K.; Boucher, K.M.; Sweeney, C.; Edwards, S.; Caan, B.J.; Samowitz, W. A comparison of colon and rectal somatic DNA alterations. Dis. Colon Rectum 2009, 52, 1304–1311. [Google Scholar] [CrossRef] [PubMed]
- Iacopetta, B. Are there two sides to colorectal cancer? Int. J. Cancer 2002, 101, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Kobunai, T.; Toda, E.; Yamamoto, Y.; Kanazawa, T.; Kazama, Y.; Tanaka, J.; Tanaka, T.; Konishi, T.; Okayama, Y.; et al. Distal colorectal cancers with microsatellite instability (MSI) display distinct gene expression profiles that are different from proximal MSI cancers. Cancer Res. 2006, 66, 9804–9808. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.J.; Johnson, K.A.; Bryan, T.M.; Hill, D.E.; Markowitz, S.; Willson, J.K.; Paraskeva, C.; Petersen, G.M.; Hamilton, S.R.; Vogelstein, B. The APC gene product in normal and tumor cells. Proc. Natl. Acad. Sci. USA 1993, 90, 2846–2850. [Google Scholar] [CrossRef] [PubMed]
- Missiaglia, E.; Jacobs, B.; D’Ario, G.; Di Narzo, A.F.; Soneson, C.; Budinska, E.; Popovici, V.; Vecchione, L.; Gerster, S.; Yan, P.; et al. Distal and proximal colon cancers differ in terms of molecular, pathological, and clinical features. Ann. Oncol. 2014, 25, 1995–2001. [Google Scholar] [CrossRef] [PubMed]
- Klump, B.; Nehls, O.; Okech, T.; Hsieh, C.-J.; Gaco, V.; Gittinger, F.S.; Sarbia, M.; Borchard, F.; Greschniok, A.; Gruenagel, H.H.; et al. Molecular lesions in colorectal cancer: Impact on prognosis? Original data and review of the literature. Int. J. Colorectal Dis. 2004, 19, 23–42. [Google Scholar] [CrossRef] [PubMed]
- Aamodt, R.; Jonsdottir, K.; Andersen, S.N.; Bondi, J.; Bukholm, G.; Bukholm, I.R. Differences in protein expression and gene amplification of cyclins between colon and rectal adenocarcinomas. Gastroenterol. Res. Pract. 2009, 2009, 285830. [Google Scholar] [CrossRef] [PubMed]
- Chung, D.C. The genetic basis of colorectal cancer: Insights into critical pathways of tumorigenesis. Gastroenterology 2000, 119, 854–865. [Google Scholar] [CrossRef] [PubMed]
- Mann, B.; Gelos, M.; Siedow, A.; Hanski, M.L.; Gratchev, A.; Ilyas, M.; Bodmer, W.F.; Moyer, M.P.; Riecken, E.O.; Buhr, H.J.; et al. Target genes of beta-catenin-T cell-factor/lymphoid-enhancer-factor signaling in human colorectal carcinomas. Proc. Natl. Acad. Sci. USA 1999, 96, 1603–1608. [Google Scholar] [CrossRef] [PubMed]
- Harris, C.C.; Hollstein, M. Clinical implications of the p53 tumor-suppressor gene. N. Engl. J. Med. 1993, 329, 1318–1327. [Google Scholar] [CrossRef] [PubMed]
- Spears, C.P.; Gustavsson, B.G.; Berne, M.; Frösing, R.; Bernstein, L.; Hayes, A.A. Mechanisms of innate resistance to thymidylate synthase inhibition after 5-fluorouracil. Cancer Res. 1988, 48, 5894–5900. [Google Scholar] [PubMed]
- Lenz, H.J.; Danenberg, K.D.; Leichman, C.G.; Florentine, B.; Johnston, P.G.; Groshen, S.; Zhou, L.; Xiong, Y.P.; Danenberg, P.V.; Leichman, L.P. p53 and thymidylate synthase expression in untreated stage II colon cancer: Associations with recurrence, survival, and site. Clin. Cancer Res. 1998, 4, 1227–1234. [Google Scholar] [PubMed]
- Takenoue, T.; Nagawa, H.; Matsuda, K.; Fujii, S.; Nita, M.E.; Hatano, K.; Kitayama, J.; Tsuruo, T.; Muto, T. Relation between thymidylate synthase expression and survival in colon carcinoma, and determination of appropriate application of 5-fluorouracil by immunohistochemical method. Ann. Surg. Oncol. 2000, 7, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Kornmann, M.; Schwabe, W.; Sander, S.; Kron, M.; Sträter, J.; Polat, S.; Kettner, E.; Weiser, H.F.; Baumann, W.; Schramm, H.; et al. Thymidylate synthase and dihydropyrimidine dehydrogenase mRNA expression levels: Predictors for survival in colorectal cancer patients receiving adjuvant 5-fluorouracil. Clin. Cancer Res. 2003, 9, 4116–4124. [Google Scholar] [PubMed]
- Donada, M.; Bonin, S.; Nardon, E.; De Pellegrin, A.; Decorti, G.; Stanta, G. Thymidilate synthase expression predicts longer survival in patients with stage II colon cancer treated with 5-flurouracil independently of microsatellite instability. J. Cancer Res. Clin. Oncol. 2011, 137, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Edler, D.; Kressner, U.; Ragnhammar, P.; Johnston, P.G.; Magnusson, I.; Glimelius, B.; Påhlman, L.; Lindmark, G.; Blomgren, H. Immunohistochemically detected thymidylate synthase in colorectal cancer: An independent prognostic factor of survival. Clin. Cancer Res. 2000, 6, 488–492. [Google Scholar] [PubMed]
- Edler, D.; Hallström, M.; Johnston, P.G.; Magnusson, I.; Ragnhammar, P.; Blomgren, H. Thymidylate synthase expression: An independent prognostic factor for local recurrence, distant metastasis, disease-free and overall survival in rectal cancer. Clin. Cancer Res. 2000, 6, 1378–1384. [Google Scholar] [PubMed]
- Jakob, C.; Liersch, T.; Meyer, W.; Baretton, G.B.; Schwabe, W.; Häusler, P.; Kulle, B.; Becker, H.; Aust, D.E. Prognostic value of histologic tumor regression, thymidylate synthase, thymidine phosphorylase, and dihydropyrimidine dehydrogenase in rectal cancer UICC Stage II/III after neoadjuvant chemoradiotherapy. Am. J. Surg. Pathol. 2006, 30, 1169–1174. [Google Scholar] [CrossRef] [PubMed]
- Liersch, T.; Langer, C.; Ghadimi, B.M.; Kulle, B.; Aust, D.E.; Baretton, G.B.; Schwabe, W.; Häusler, P.; Becker, H.; Jakob, C. Lymph node status and TS gene expression are prognostic markers in stage II/III rectal cancer after neoadjuvant fluorouracil-based chemoradiotherapy. J. Clin. Oncol. 2006, 24, 4062–4068. [Google Scholar] [CrossRef] [PubMed]
- Conradi, L.-C.; Bleckmann, A.; Schirmer, M.; Sprenger, T.; Jo, P.; Homayounfar, K.; Wolff, H.A.; Rothe, H.; Middel, P.; Becker, H.; et al. Thymidylate synthase as a prognostic biomarker for locally advanced rectal cancer after multimodal treatment. Ann. Surg. Oncol. 2011, 18, 2442–2452. [Google Scholar] [CrossRef] [PubMed]
- Rijcken, F.E.M.; Hollema, H.; Kleibeuker, J.H. Proximal adenomas in hereditary non-polyposis colorectal cancer are prone to rapid malignant transformation. Gut 2002, 50, 382–386. [Google Scholar] [CrossRef] [PubMed]
- Petersen, G.M. Knowledge of the adenomatous polyposis coli gene and its clinical application. Ann. Med. 1994, 26, 205–208. [Google Scholar] [CrossRef] [PubMed]
- Kinzler, K.W.; Vogelstein, B. Lessons from hereditary colorectal cancer. Cell 1996, 87, 159–170. [Google Scholar] [CrossRef]
- Guinney, J.; Dienstmann, R.; Wang, X.; de Reyniès, A.; Schlicker, A.; Soneson, C.; Marisa, L.; Roepman, P.; Nyamundanda, G.; Angelino, P.; et al. The consensus molecular subtypes of colorectal cancer. Nat. Med. 2015, 21, 1350–1356. [Google Scholar] [CrossRef] [PubMed]
- Lal, N.; White, B.S.; Goussous, G.; Pickles, O.; Mason, M.J.; Beggs, A.D.; Taniere, P.; Willcox, B.E.; Guinney, J.; Middleton, G.W. KRAS Mutation and Consensus Molecular Subtypes 2 and 3 Are Independently Associated with Reduced Immune Infiltration and Reactivity in Colorectal Cancer. Clin. Cancer Res. 2018, 24, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Macfarlane, G.J.; Lowenfels, A.B. Physical activity and colon cancer. Eur. J. Cancer Prev. Off. J. Eur. Cancer Prev. Organ. ECP 1994, 3, 393–398. [Google Scholar] [CrossRef]
- Halle, M.; Schoenberg, M.H. Physical activity in the prevention and treatment of colorectal carcinoma. Dtsch. Arztebl. Int. 2009, 106, 722–727. [Google Scholar] [CrossRef] [PubMed]
- McTiernan, A. Physical activity after cancer: Physiologic outcomes. Cancer Investig. 2004, 22, 68–81. [Google Scholar] [CrossRef]
- Giovannucci, E.; Ascherio, A.; Rimm, E.B.; Colditz, G.A.; Stampfer, M.J.; Willett, W.C. Physical activity, obesity, and risk for colon cancer and adenoma in men. Ann. Intern. Med. 1995, 122, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Gerhardsson de Verdier, M.; Steineck, G.; Hagman, U.; Rieger, A.; Norell, S.E. Physical activity and colon cancer: A case-referent study in Stockholm. Int. J. Cancer 1990, 46, 985–989. [Google Scholar] [CrossRef] [PubMed]
- Chao, A.; Connell, C.J.; Jacobs, E.J.; McCullough, M.L.; Patel, A.V.; Calle, E.E.; Cokkinides, V.E.; Thun, M.J. Amount, type, and timing of recreational physical activity in relation to colon and rectal cancer in older adults: The Cancer Prevention Study II Nutrition Cohort. Cancer Epidemiol. Biomark. Prev. 2004, 13, 2187–2195. [Google Scholar]
- Larsson, S.C.; Wolk, A. Obesity and colon and rectal cancer risk: A meta-analysis of prospective studies. Am. J. Clin. Nutr. 2007, 86, 556–565. [Google Scholar] [CrossRef] [PubMed]
- Slattery, M.L.; Potter, J.; Caan, B.; Edwards, S.; Coates, A.; Ma, K.N.; Berry, T.D. Energy balance and colon cancer--beyond physical activity. Cancer Res. 1997, 57, 75–80. [Google Scholar] [PubMed]
- Friedenreich, C.; Norat, T.; Steindorf, K.; Boutron-Ruault, M.-C.; Pischon, T.; Mazuir, M.; Clavel-Chapelon, F.; Linseisen, J.; Boeing, H.; Bergman, M.; et al. Physical activity and risk of colon and rectal cancers: The European prospective investigation into cancer and nutrition. Cancer Epidemiol. Biomark. Prev. 2006, 15, 2398–2407. [Google Scholar] [CrossRef] [PubMed]
- Steinbach, G.; Lynch, P.M.; Phillips, R.K.; Wallace, M.H.; Hawk, E.; Gordon, G.B.; Wakabayashi, N.; Saunders, B.; Shen, Y.; Fujimura, T.; et al. The effect of celecoxib, a cyclooxygenase-2 inhibitor, in familial adenomatous polyposis. N. Engl. J. Med. 2000, 342, 1946–1952. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, T.; Iwama, T.; Yoshinaga, K.; Toyooka, M.; Taketo, M.M.; Sugihara, K. A randomized, double-blind, placebo-controlled trial of the effects of rofecoxib, a selective cyclooxygenase-2 inhibitor, on rectal polyps in familial adenomatous polyposis patients. Clin. Cancer Res. 2003, 9, 4756–4760. [Google Scholar] [PubMed]
- Almendingen, K.; Larsen, L.N.; Fausa, O.; Bratlie, J.; Høstmark, A.T.; Aabakken, L. Selective COX-2 inhibition affects fatty acids, but not COX mRNA expression in patients with FAP. Fam. Cancer 2010, 9, 571–580. [Google Scholar] [CrossRef] [PubMed]
- Rothwell, P.M.; Wilson, M.; Elwin, C.-E.; Norrving, B.; Algra, A.; Warlow, C.P.; Meade, T.W. Long-term effect of aspirin on colorectal cancer incidence and mortality: 20-year follow-up of five randomised trials. Lancet 2010, 376, 1741–1750. [Google Scholar] [CrossRef]
- Stelzner, S.; Koehler, C.; Stelzer, J.; Sims, A.; Witzigmann, H. Extended abdominoperineal excision vs. standard abdominoperineal excision in rectal cancer—A systematic overview. Int. J. Colorectal Dis. 2011, 26, 1227–1240. [Google Scholar] [CrossRef] [PubMed]
- Mori, T.; Takahashi, K.; Yasuno, M. Radical resection with autonomic nerve preservation and lymph node dissection techniques in lower rectal cancer surgery and its results: The impact of lateral lymph node dissection. Langenbecks Arch. Surg. 1998, 383, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Hohenberger, W.; Bittorf, B.; Papadopoulos, T.; Merkel, S. Survival after surgical treatment of cancer of the rectum. Langenbecks Arch. Surg. 2005, 390, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Link, K.H.; Hauser, H.; Mann, M.; Schlag, P.M. Kolonkarzinom. In Chirurgische Onkologie; Gnant, M., Schlag, P.M., Eds.; Springer-Verlag Wien: New York, NY, USA, 2008; pp. 315–329. ISBN 978-3-211-48612-2. [Google Scholar]
- Raab, H.-R. R1 resection in rectal cancer. Chirurg 2017, 88, 771–776. [Google Scholar] [CrossRef] [PubMed]
- Köckerling, F.; Schug, C.; Hohenberger, W. Multiviszerale Resection beim Kolonkarzinom—Grenzen und Techniken. In Kolon- und Rektumchirurgie. Kurs der DGVC; Beger, H.G., Rühland, D., Siewert, J.R., Eds.; DCS: Singen, Germany, 1999; pp. 44–50. [Google Scholar]
- Kruschewski, M.; Pohlen, U.; Hotz, H.G.; Ritz, J.-P.; Kroesen, A.J.; Buhr, H.J. Results of multivisceral resection of primary colorectal cancer. Zentralbl. Chir. 2006, 131, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Lehnert, T.; Methner, M.; Pollok, A.; Schaible, A.; Hinz, U.; Herfarth, C. Multivisceral Resection for Locally Advanced Primary Colon and Rectal Cancer. Ann. Surg. 2002, 235, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Marusch, F.; Koch, A.; Schmidt, U.; Zippel, R.; Geissler, S.; Pross, M.; Roessner, A.; Köckerling, F.; Gastinger, I.; Lippert, H.; Studiengruppe “Kolon-/Rektumkarzinome (Primärtumor)”. Prospektive Multizenterstudien “Kolon-/Rektumkarzinome” als flächendeckende chirurgische Qualitätssicherung. Der Chirurg 2002, 73, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Scheele, J.; Lemke, J.; Meier, M.; Sander, S.; Henne-Bruns, D.; Kornmann, M. Quality of Life After Sphincter-Preserving Rectal Cancer Resection. Clin. Colorectal Cancer 2015, 14, e33–e40. [Google Scholar] [CrossRef] [PubMed]
- Köckerling, F.; Schug-Pass, C.; Scheidbach, H. Laparoskopische Resektion beim kolorektalen Karzinom – aktueller Stand und Perspektiven. Visc. Med. 2005, 21, 50–53. [Google Scholar] [CrossRef]
- Bonjer, H.J.; Hop, W.C.J.; Nelson, H.; Sargent, D.J.; Lacy, A.M.; Castells, A.; Guillou, P.J.; Thorpe, H.; Brown, J.; Delgado, S.; et al. Transatlantic Laparoscopically Assisted vs. Open Colectomy Trials Study Group Laparoscopically assisted vs open colectomy for colon cancer: A meta-analysis. Arch. Surg. 2007, 142, 298–303. [Google Scholar] [CrossRef] [PubMed]
- Colon Cancer Laparoscopic or Open Resection Study Group; Buunen, M.; Veldkamp, R.; Hop, W.C.J.; Kuhry, E.; Jeekel, J.; Haglind, E.; Påhlman, L.; Cuesta, M.A.; Msika, S.; et al. Survival after laparoscopic surgery versus open surgery for colon cancer: Long-term outcome of a randomised clinical trial. Lancet Oncol. 2009, 10, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Veldkamp, R.; Gholghesaei, M.; Bonjer, H.J.; Meijer, D.W.; Buunen, M.; Jeekel, J.; Anderberg, B.; Cuesta, M.A.; Cuschierl, A.; Fingerhut, A.; et al. European Association of Endoscopic Surgery (EAES) Laparoscopic resection of colon Cancer: Consensus of the European Association of Endoscopic Surgery (EAES). Surg. Endosc. 2004, 18, 1163–1185. [Google Scholar] [CrossRef] [PubMed]
- Schwenk, W.; Haase, O.; Neudecker, J.; Müller, J.M. Short term benefits for laparoscopic colorectal resection. Cochrane Database Syst. Rev. 2005, 20, CD003145. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Li, G.; Chen, P.; Yu, J. Laparoscopic versus open colorectal resection for cancer: A meta-analysis of results of randomized controlled trials on recurrence. Eur. J. Surg. Oncol. 2008, 34, 1217–1224. [Google Scholar] [CrossRef] [PubMed]
- Kuhry, E.; Schwenk, W.F.; Gaupset, R.; Romild, U.; Bonjer, H.J. Long-term results of laparoscopic colorectal cancer resection. Cochrane Database Syst. Rev. 2008, 16, CD003432. [Google Scholar] [CrossRef] [PubMed]
- Ptok, H.; Steinert, R.; Meyer, F.; Kröll, K.-P.; Scheele, C.; Köckerling, F.; Gastinger, I.; Lippert, H. Long-term oncological results after laparoscopic, converted and primary open procedures for rectal carcinoma. Results of a multicenter observational study. Chirurg 2006, 77, 709–717. [Google Scholar] [CrossRef] [PubMed]
- Breukink, S.; Pierie, J.; Wiggers, T. Laparoscopic versus open total mesorectal excision for rectal cancer. Cochrane Database Syst. Rev. 2006, 15, CD005200. [Google Scholar] [CrossRef]
- Müller-Stich, B.P.; Linke, G.R.; Wagner, M.; Steinemann, D.C. Laparoscopic versus open rectal cancer resection: Oncologically equal? Chirurg 2016, 87, 552–559. [Google Scholar] [CrossRef] [PubMed]
- Link, K.-H.; Kornmann, M.; Mann, M.; Bittner, R. Multimodale Therapie von Kolon- und Rektumkarzinomen: Qualitaetsparameter. Viszeralmedizin 2009, 25, 105–117. [Google Scholar] [CrossRef]
- Staib, L.; Link, K.H.; Blatz, A.; Beger, H.G. Surgery of colorectal cancer: Surgical morbidity and five- and ten-year results in 2400 patients--monoinstitutional experience. World J. Surg. 2002, 26, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Kornmann, M.; Staib, L.; Wiegel, T.; Kron, M.; Henne-Bruns, D.; Link, K.-H.; Formentini, A.; Study Group Oncology of Gastrointestinal Tumors (FOGT). Long-term results of 2 adjuvant trials reveal differences in chemosensitivity and the pattern of metastases between colon cancer and rectal cancer. Clin. Colorectal Cancer 2013, 12, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Link, K.H.; Staib, L.; Kreuser, E.D.; Beger, H.G. Adjuvant treatment of colon and rectal cancer: Impact of chemotherapy, radiotherapy, and immunotherapy on routine postsurgical patient management. Forschungsgruppe Onkologie Gastrointestinaler Tumoren (FOGT). Recent Results Cancer Res. 1996, 142, 311–352. [Google Scholar] [PubMed]
- Bethune, W.A. Carcinoma of the rectum: 508 patients with failure analysis and implication for adjuvant therapy. Can. Assoc. Radiol. J. 1987, 38, 209–214. [Google Scholar] [PubMed]
- Minsky, B.D.; Mies, C.; Rich, T.A.; Recht, A.; Chaffey, J.T. Potentially curative surgery of colon cancer: Patterns of failure and survival. J. Clin. Oncol. 1988, 6, 106–118. [Google Scholar] [CrossRef] [PubMed]
- Laurie, J.A.; Moertel, C.G.; Fleming, T.R.; Wieand, H.S.; Leigh, J.E.; Rubin, J.; McCormack, G.W.; Gerstner, J.B.; Krook, J.E.; Malliard, J. Surgical adjuvant therapy of large-bowel carcinoma: An evaluation of levamisole and the combination of levamisole and fluorouracil. The North Central Cancer Treatment Group and the Mayo Clinic. J. Clin. Oncol. 1989, 7, 1447–1456. [Google Scholar] [CrossRef] [PubMed]
- Metzger, U.; Laffer, U.; Egeli, R.; Arma, S.; Aeberhard, P.; Barras, J.P.; Martinoli, S.; Müller, W.; Castiglione, M.; Schroeder, R. (Status of portal perfusion in colorectal cancer. Swiss Study Group for Clinical Cancer Research). Chirurg 1994, 65, 509–513. [Google Scholar] [PubMed]
- Tepper, J.E.; O’Connell, M.J.; Petroni, G.R.; Hollis, D.; Cooke, E.; Benson, A.B.; Cummings, B.; Gunderson, L.L.; Macdonald, J.S.; Martenson, J.A. Adjuvant postoperative fluorouracil-modulated chemotherapy combined with pelvic radiation therapy for rectal cancer: Initial results of intergroup 0114. J. Clin. Oncol. 1997, 15, 2030–2039. [Google Scholar] [CrossRef] [PubMed]
- 1Mayer, B.; Sander, S.; Paschke, S.; Henne-Bruns, D.; Link, K.H.; Kornmann, M.; Study Group Oncology of Gastrointestinal Tumors (FOGT). Stratified Survival Analysis After Adjuvant Chemotherapy of Colon Cancer Reveals a Benefit for Older Patients. Anticancer Res. 2015, 35, 5587–5593. [Google Scholar]
- André, T.; Boni, C.; Navarro, M.; Tabernero, J.; Hickish, T.; Topham, C.; Bonetti, A.; Clingan, P.; Bridgewater, J.; Rivera, F.; de Gramont, A. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J. Clin. Oncol. 2009, 27, 3109–3116. [Google Scholar] [CrossRef] [PubMed]
- Kuebler, J.P.; Wieand, H.S.; O’Connell, M.J.; Smith, R.E.; Colangelo, L.H.; Yothers, G.; Petrelli, N.J.; Findlay, M.P.; Seay, T.E.; Atkins, J.N.; et al. Oxaliplatin combined with weekly bolus fluorouracil and leucovorin as surgical adjuvant chemotherapy for stage II and III colon cancer: Results from NSABP C-07. J. Clin. Oncol. 2007, 25, 2198–2204. [Google Scholar] [CrossRef] [PubMed]
- Rödel, C.; Graeven, U.; Fietkau, R.; Hohenberger, W.; Hothorn, T.; Arnold, D.; Hofheinz, R.-D.; Ghadimi, M.; Wolff, H.A.; Lang-Welzenbach, M.; et al. Oxaliplatin added to fluorouracil-based preoperative chemoradiotherapy and postoperative chemotherapy of locally advanced rectal cancer (the German CAO/ARO/AIO-04 study): Final results of the multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2015, 16, 979–989. [Google Scholar] [CrossRef]
- Roh, M.S.; Yothers, G.A.; O’Connell, M.J.; Beart, R.W.; Pitot, H.C.; Shields, A.F.; Parda, D.S.; Sharif, S.; Allegra, C.J.; Petrelli, N.J.; et al. The impact of capecitabine and oxaliplatin in the preoperative multimodality treatment in patients with carcinoma of the rectum: NSABP R-04. J. Clin. Oncol. 2011, 29, 3503. [Google Scholar] [CrossRef]
- Hong, Y.S.; Nam, B.-H.; Kim, K.-P.; Kim, J.E.; Park, S.J.; Park, Y.S.; Park, J.O.; Kim, S.Y.; Kim, T.-Y.; Kim, J.H.; et al. Oxaliplatin, fluorouracil, and leucovorin versus fluorouracil and leucovorin as adjuvant chemotherapy for locally advanced rectal cancer after preoperative chemoradiotherapy (ADORE): An open-label, multicentre, phase 2, randomised controlled trial. Lancet Oncol. 2014, 15, 1245–1253. [Google Scholar] [CrossRef]
- Schmoll, H.-J.; Haustermans, K.; Price, T.J.; Nordlinger, B.; Hofheinz, R.; Daisne, J.-F.; Janssens, J.; Brenner, B.; Schmidt, P.; Reinel, H.; et al. Preoperative chemoradiotherapy and postoperative chemotherapy with capecitabine and oxaliplatin versus capecitabine alone in locally advanced rectal cancer: Disease-free survival results at interim analysis. J. Clin. Oncol. 2014, 32, 3501. [Google Scholar] [CrossRef]
- Kapiteijn, E.; Marijnen, C.A.; Nagtegaal, I.D.; Putter, H.; Steup, W.H.; Wiggers, T.; Rutten, H.J.; Pahlman, L.; Glimelius, B.; van Krieken, J.H.; et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N. Engl. J. Med. 2001, 345, 638–646. [Google Scholar] [CrossRef] [PubMed]
- Erlandsson, J.; Holm, T.; Pettersson, D.; Berglund, Å.; Cedermark, B.; Radu, C.; Johansson, H.; Machado, M.; Hjern, F.; Hallböök, O.; Syk, I.; et al. Optimal fractionation of preoperative radiotherapy and timing to surgery for rectal cancer (Stockholm III): A multicentre, randomised, non-blinded, phase 3, non-inferiority trial. Lancet Oncol. 2017, 18, 336–346. [Google Scholar] [CrossRef]
- Langer, B.H.B.; Labianca, R.; Shepherd, L. Fluorouracil (FU) plus l-leucovorin (l-LV) versus observation after potentially curative resection of liver or lung metastases from colorectal cancer (CRC): Results of the ENG (EORTC/NCIC CTG/GIVIO) randomized trial. Proc. Am. Soc. Clin. Oncol. 2002, 21, 592. [Google Scholar]
- Nordlinger, B.; Sorbye, H.; Glimelius, B.; Poston, G.J.; Schlag, P.M.; Rougier, P.; Bechstein, W.O.; Primrose, J.N.; Walpole, E.T.; Finch-Jones, M.; et al. Perioperative chemotherapy with FOLFOX4 and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC Intergroup trial 40983): A randomised controlled trial. Lancet 2008, 371, 1007–1016. [Google Scholar] [CrossRef]
- Yamada, H.; Ichikawa, W.; Uetake, H.; Shirota, Y.; Nihei, Z.; Sugihara, K.; Hirayama, R. Thymidylate synthase gene expression in primary colorectal cancer and metastatic sites. Clin. Colorectal Cancer 2001, 1, 169–173; discussion 174. [Google Scholar] [CrossRef] [PubMed]
- Akagi, Y.; Kinugasa, T.; Mizobe, T.; Kawahara, A.; Kage, M.; Shirouzu, K. Expression of dihydropyrimidine dehydrogenase, orotate phosphoribosyl transferase and thymidylate synthase in patients with primary colorectal cancer, and associations with site of first metastasis. Anticancer Res. 2012, 32, 2277–2282. [Google Scholar] [PubMed]
- Link, K.H.; Sagban, T.A.; Mörschel, M.; Tischbirek, K.; Holtappels, M.; Apell, V.; Zayed, K.; Kornmann, M.; Staib, L. Colon cancer: Survival after curative surgery. Langenbecks Arch. Surg. 2005, 390, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Kornmann, M.; Hebart, H.; Danenberg, K.; Goeb, R.; Staib, L.; Kron, M.; Henne-Bruns, D.; Danenberg, P.; Link, K.-H. Response prediction in metastasised colorectal cancer using intratumoural thymidylate synthase: Results of a randomised multicentre trial. Eur. J. Cancer 2012, 48, 1443–1451. [Google Scholar] [CrossRef] [PubMed]
- Danenberg, P.V. Thymidylate synthetase—A target enzyme in cancer chemotherapy. Biochim. Biophys. Acta 1977, 473, 73–92. [Google Scholar] [CrossRef]
- Johnston, P.G.; Fisher, E.R.; Rockette, H.E.; Fisher, B.; Wolmark, N.; Drake, J.C.; Chabner, B.A.; Allegra, C.J. The role of thymidylate synthase expression in prognosis and outcome of adjuvant chemotherapy in patients with rectal cancer. J. Clin. Oncol. 1994, 12, 2640–2647. [Google Scholar] [CrossRef] [PubMed]
- Yamachika, T.; Nakanishi, H.; Inada, K.; Tsukamoto, T.; Kato, T.; Fukushima, M.; Inoue, M.; Tatematsu, M. A new prognostic factor for colorectal carcinoma, thymidylate synthase, and its therapeutic significance. Cancer 1998, 82, 70–77. [Google Scholar] [CrossRef]
- Edler, D.; Glimelius, B.; Hallström, M.; Jakobsen, A.; Johnston, P.G.; Magnusson, I.; Ragnhammar, P.; Blomgren, H. Thymidylate synthase expression in colorectal cancer: A prognostic and predictive marker of benefit from adjuvant fluorouracil-based chemotherapy. J. Clin. Oncol. 2002, 20, 1721–1728. [Google Scholar] [CrossRef] [PubMed]
- Popat, S.; Matakidou, A.; Houlston, R.S. Thymidylate synthase expression and prognosis in colorectal cancer: A systematic review and meta-analysis. J. Clin. Oncol. 2004, 22, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Lochhead, P.; Kuchiba, A.; Imamura, Y.; Liao, X.; Yamauchi, M.; Nishihara, R.; Qian, Z.R.; Morikawa, T.; Shen, J.; Meyerhardt, J.A.; et al. Microsatellite instability and BRAF mutation testing in colorectal cancer prognostication. J. Natl. Cancer Inst. 2013, 105, 1151–1156. [Google Scholar] [CrossRef] [PubMed]
- Von Knebel Doeberitz, M.; Kloor, M. Towards a vaccine to prevent cancer in Lynch syndrome patients. Fam. Cancer 2013, 12, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Monson, K.M.; Bilchik, A.; Beutler, T.; Dan, A.G.; Schochet, E.; Wiese, D.; Kaushal, S.; Ganatra, B.; Desai, D. Comparative analysis of nodal upstaging between colon and rectal cancers by sentinel lymph node mapping: A prospective trial. Dis. Colon Rectum 2004, 47, 1767–1772. [Google Scholar] [CrossRef] [PubMed]
- Bilchik, A.J.; Hoon, D.S.B.; Saha, S.; Turner, R.R.; Wiese, D.; DiNome, M.; Koyanagi, K.; McCarter, M.; Shen, P.; Iddings, D.; et al. Prognostic impact of micrometastases in colon cancer: Interim results of a prospective multicenter trial. Ann. Surg. 2007, 246, 568–575; discussion 575–577. [Google Scholar] [CrossRef] [PubMed]
- Ritz, J.P.; Buhr, H.J. Kolonkarzinom. Praxis der Viszeralchirurgie—Onkologische Chirurgie; Siewert, J.R., Ed.; Springer Medizin Verl: Heidelberg, Germany, 2010; pp. 694–711. ISBN 978-3-642-03807-5. [Google Scholar]
- Weitz, J.; Koch, M.; Debus, J.; Höhler, T.; Galle, P.R.; Büchler, M.W. Colorectal cancer. Lancet Lond. Engl. 2005, 365, 153–165. [Google Scholar] [CrossRef]
- Senninger, N.; Colombo-Benkmann, M. Chirurgie von Kolon- und Rektum: Aktuelle Trends und Therapie; Philippka-Verlag: Münster, Germany, 2002; p. 9. [Google Scholar]
- Vogelstein, B.; Fearon, E.R.; Hamilton, S.R.; Kern, S.E.; Preisinger, A.C.; Leppert, M.; Nakamura, Y.; White, R.; Smits, A.M.; Bos, J.L. Genetic alterations during colorectal-tumor development. N. Engl. J. Med. 1988, 319, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Toribara, N.W.; Sleisenger, M.H. Screening for colorectal cancer. N. Engl. J. Med. 1995, 332, 861–867. [Google Scholar] [CrossRef] [PubMed]
- Vasen, H.F.; Watson, P.; Mecklin, J.P.; Lynch, H.T. New clinical criteria for hereditary nonpolyposis colorectal cancer (HNPCC, Lynch syndrome) proposed by the International Collaborative group on HNPCC. Gastroenterology 1999, 116, 1453–1456. [Google Scholar] [CrossRef]
- Vasen, H.F.A.; Möslein, G.; Alonso, A.; Bernstein, I.; Bertario, L.; Blanco, I.; Burn, J.; Capella, G.; Engel, C.; Frayling, I.; et al. Guidelines for the clinical management of Lynch syndrome (hereditary non-polyposis cancer). J. Med. Genet. 2007, 44, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Holch, J.W.; Ricard, I.; Stintzing, S.; Modest, D.P.; Heinemann, V. The relevance of primary tumour location in patients with metastatic colorectal cancer: A meta-analysis of first-line clinical trials. Eur. J. Cancer 2017, 70, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Siegmund-Schultze, N. Kolorektale Karzinome: Die Lage des Primarius zählt. Dtsch. Arztebl. 2017, 114, 20. [Google Scholar] [CrossRef]
- Holm, T.; Johansson, H.; Rutqvist, L.E.; Cedermark, B. Tumour location and the effects of preoperative radiotherapy in the treatment of rectal cancer. Br. J. Surg. 2001, 88, 839–843. [Google Scholar] [CrossRef] [PubMed]
- Link, K.H.; Kornmann, M.; Butzer, U.; Leder, G.; Sunelaitis, E.; Pillasch, J.; Salonga, D.; Danenberg, K.D.; Danenberg, P.V.; Beger, H.G. Thymidylate synthase quantitation and in vitro chemosensitivity testing predicts responses and survival of patients with isolated nonresectable liver tumors receiving hepatic arterial infusion chemotherapy. Cancer 2000, 89, 288–296. [Google Scholar] [CrossRef]
- Kornmann, M.; Link, K.H.; Lenz, H.-J.; Pillasch, J.; Metzger, R.; Butzer, U.; Leder, G.H.; Weindel, M.; Safi, F.; Danenberg, K.D.; et al. Thymidylate synthase is a predictor for response and resistance in hepatic artery infusion chemotherapy. Cancer Lett. 1997, 118, 29–35. [Google Scholar] [CrossRef]
- Leichman, C.G.; Lenz, H.J.; Leichman, L.; Danenberg, K.; Baranda, J.; Groshen, S.; Boswell, W.; Metzger, R.; Tan, M.; Danenberg, P.V. Quantitation of intratumoral thymidylate synthase expression predicts for disseminated colorectal cancer response and resistance to protracted-infusion fluorouracil and weekly leucovorin. J. Clin. Oncol. 1997, 15, 3223–3229. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | FAP | HNPCC |
|---|---|---|
| Prevalence rate | 1% of all colon and rectal cancers | 5% “CRC” |
| Phenotype | >100 polyps | Only a few polyps can be present |
| Genotype | APC gene mutations | Germline mutations of the DNA MMR genes |
| Age of onset | In most cases from 20 to 25 years | On average from year 44 onwards |
| Localization | Left colon, rectum; associated disease locations: bones, eyes, duodenum | Right colon; associated disease locations: endometrium and also (considerably rarer) stomach, ovaries, pancreas, ureter, renal pelvis, cystic ducts |
| Transformation to colon and/or rectum cancer | 100% to colon and/or rectum cancer | 50–70% to colon cancer |
| Mutation/Expression | Proximal CC | Distal CC and RC | Author (s) |
|---|---|---|---|
| Chromosome instability (CIN) | NO | YES | Ogino et al. 2008 [45]; Smith et al. 1993 [52] |
| Microsatellite instability (MSI) | YES | NO | Jass et al. 2007 [35] |
| EGFR and HER2 amplification | NO | YES | Missiaglia et al. 2014 [53] |
| CpG hypermethylation (CIMP) | YES | NO | Ogino et al. 2008 [45] |
| BRAF mutation (BRAF-like) | YES | NO | Popovici et al. 2012 [43] |
| KRAS | YES | NO | Slattery et al. 2009 [49] |
| p53 | NO | YES | Klump et al. 2004 [54] |
| HOX gene | YES | NO | Sanz-Pamplona et al. 2011 [46] |
| CDX2 gene | YES | NO | Rozek et al. 2005 [48] |
| Thymidylate synthase | YES | NO | Edler et al. 2000a [65]; Liersch et al. 2006 [66] |
| Cyclin D3 and c-Myc | YES | NO | Slattery et al. 2009 [49] |
| Cyclin D1, cyclin E and nuclear β-catenin | NO | YES | Slattery et al. 2009 [49] |
| Activation of MAPK pathways | YES | NO | Iacopetta 2002 [50] |
| Activation of Wnt pathways | NO | YES | Peltomaki + Vasen 1997 [40] |
| Mucosal lesions (non-depressed type) | YES | NO | Konishi et al. 1999 [28] |
| Submucosal lesions (non-depressed type) | NO | YES | Konishi et al. 1999 [28] |
| Mucosal and submucosal lesions (depressed type) | YES | NO | Konishi et al. 1999 [28] |
| Prevention Measure | Decreased Incidence | |
|---|---|---|
| Colon Cancer | Rectal Cancer | |
| Physical activity | YES (Halle and Schoenberg 2009 [75]; Slattery et al. 1997 [81]; Macfarlane and Lowenfels 1994 [74]; Chao et al. 2004 [79]; Larsson et al. 2007 [80]; Gerhardsson de Verdier et al. 1990 [78]) | NO (Halle and Schoenberg 2009 [75]; Larsson et al. 2007 [80]; Chao et al. 2004 [79]; Gerhardsson de Verdier et al. 1990 [78]) |
| Low BMI | YES (Slattery et al. 1997 [81]; Friedenreich et al. 2006 [82]; Larsson et al. 2007 [80]) | NO (Friedenreich et al. 2006 [82]; Larsson et al. 2007 [80]) |
| Reduced energy uptake | YES (Slattery et al. 1997 [81]) | NO (Friedenreich et al. 2006 [82]) |
| COX-2 inhibitors | NO in case of HNPCC (there are no sufficient data) | YES in case of FAP (Steinbach et al. 2000 [83]; Higuchi et al. 2003 [84]; Almendingen et al. 2010 [85]) |
| Aspirin | YES (Rothwell et al. 2010 [86]) | NO (Rothwell et al. 2010 [86]) |
| Header | Laparoscopic Resections | Converted Resections | Open Resections | p |
|---|---|---|---|---|
| FU rate [n] | 150/192 | 27/33 | 4611/5782 | – |
| Follow-up [months], mean (SD) | 34 (12.3) | 37 (12.7) | 33 (13.4) | 0.208 |
| Local recurrences [n] ([%]) | 3 (2.0) | 3 (11.1) | 314 (6.8) | – |
| Metastasis [n] ([%]) | 18 (12.0) | 4 (14.8) | 553 (11.9) | – |
| Disease-free survival time (DFS) [months], mean (95% CI) | 58.3 (55.3–61.3) | 55.3 (47.2–63.4) | 59.6 (58.8–60.5) | 0.585 |
| 5-year survival rate [%] | 83.8% (0.036) | 73.4% (0.096) | 74.5 (0.018) | |
| UICC stage-adapted | 0.797 | |||
| Tumor Type | Colon Cancer | Rectum Cancer | ||
|---|---|---|---|---|
| N | 7-Year Survival Rates (95% CI) | N | 7-Year Survival Rates (95% CI) | |
| Treatment | ||||
| 5-FU | 282 | 54.1% (46.5–61.0) | 282 | 50.6% (43.0–57.7) |
| 5-FU + FA | 295 | 66.8% (59.4–73.1) | 291 | 56.3% (49.4–62.7) |
| 5-FU + IFN-α | 278 | 56.7% (49.3–63.4) | 223 | 54.8% (46.7–62.2) |
| Treatment—UICC stage II | ||||
| 5-FU | 21 | 61.9% (38.1–78.8) | 93 | 57.5% (43.4–69.3) |
| 5-FU + FA | 23 | 78.3% (55.4–90.3) | 97 | 75.9% (62.8–85.0) |
| 5-FU + IFN-α | 24 | 69.6% (46.6–84.2) | 81 | 69.3% (55.0–79.9) |
| Treatment—UICC stage III | ||||
| 5-FU | 261 | 52.8% (44.6–60.4) | 189 | 47.7% (38.8–56.2) |
| 5-FU + FA | 272 | 65.6% (57.7–72.3) | 194 | 46.6%(38.6–54.2) |
| 5-FU + IFN-α | 254 | 55.3% (47.4–62.4) | 142 | 46.1% (36.0–55.5) |
| UICC substages | ||||
| II (pT3–4pN0) * | 68 * | 70.1% (57.5–79.5) | 271 * | 67.2% (59.4–73.9) |
| IIIa (pT1–2pN1) | 72 | 79.5% (62.4–89.4) | 71 | 61.5% (47.9–72.4) |
| IIIb (pT3–4pN1) | 424 | 62.2% (55.7–68.0) | 227 | 52.7% (44.7–60.0) |
| IIIc (pT1–4pN2) | 291 | 46.4% (39.4–53.2) | 227 | 35.7% (26.9–44.5) |
| Tumor grade | ||||
| 1–2 | 601 | 60.4% (55.2–65.2) | 605 | 56.8% (51.8–61.5) |
| 3 | 215 | 57.2% (48.5–64.9) | 158 | 43.1% (34.3–51.6) |
| Type of resection | ||||
| Colon (all) | 855 | 59.2% (54.9–63.2) | – | – |
| AR | – | – | 359 | 57.0% (50.5–62.9) |
| APRE | – | – | 188 | 45.3% (36.9–53.4) |
| Unknown | – | – | 249 | 57.6% (50.5–64.0) |
| Tumor Location | UICC Stage | Local Recurrence Rate (Only) | Combined Local Metastases Including Distant Metastases |
|---|---|---|---|
| Colon | I | 7–8% a | 7–8% a |
| II | 0–4% a | 14–43% a | |
| III | 0–7% a | 22–67% a | |
| Rectum | I | 6–17% b | 12–18% b |
| II | 13–24% b | 32% b | |
| III | 3–50% b | 37–64% b |
| Tumor Type | Colon Cancer | Rectum Cancer | ||||||
|---|---|---|---|---|---|---|---|---|
| Variability | FOGT-1, Treatment | N | FOGT-2, Treatment | N | ||||
| A | B | C | A | B | C | |||
| Basic Treatment | 5-FU | 5-FU | 5-FU | 5-FU | 5-FU | 5-FU | ||
| Additional Treatment | – | +FA | +IFNα | – | +FA | +IFNα | ||
| Number of Patients | 282 | 295 | 278 | 855 | 282 | 291 | 223 | 796 |
| N | 128 | 112 | 112 | 352 | 129 | 123 | 97 | 349 |
| Total recurrence rate | 45.5% | 38.0% | 40.3% | 41.2% | 45.7% | 42.3% | 43.5% | 43.8% |
| Local recurrence (only) | 10 | 14 | 13 | 37 | 21 | 16 | 18 | 55 |
| 3.5% | 4.7% | 4.7% | 4.2% | 7.4% | 5.5% | 8.1% | 6.9% | |
| Local recurrence with distant metastases | 16 | 10 | 9 | 35 | 18 | 15 | 12 | 45 |
| 5.7% | 3.4% | 3.2% | 4.1% | 6.4% | 5.2% | 5.4% | 5.7% | |
| Distant metastasis rate (only) | 99 | 82 | 88 | 269 | 88 | 88 | 63 | 239 |
| 35.1% | 27.8% | 31.7% | 31.5% | 31.2% | 30.2% | 28.3% | 30.0% | |
| Recurrence n.s. for the location | 3 | 6 | 2 | 11 | 2 | 4 | 4 | 10 |
| 1.1% | 2.0% | 0.7% | 1.3% | 0.7% | 1.4% | 1.8% | 1.3% | |
| Distant metastases a | ||||||||
| Liver | 66 | 52 | 60 | 178 | 60 | 54 | 42 | 156 |
| 23.4% | 17.7% | 21.6% | 20.8% | 21.2% | 18.5% | 18.8% | 19.5% | |
| Lung | 29 | 17 | 16 | 62 | 41 | 35 | 25 | 101 |
| 10.2% | 5.7% | 5.7% | 7.25% | 14.5% | 12.0% | 11.2% | 12.6% | |
| Peritoneum | 28 | 21 | 27 | 76 | 16 | 8 | 8 | 32 |
| 10% | 7.1% | 9.7% | 8.9% | 5.6% | 2.7% | 3.5% | 4.0% | |
| Bone | 0 | 3 | 3 | 6 | 2 | 11 | 4 | 17 |
| 1.0% | 1.0% | 0.7% | 0.7% | 0.4% | 1.8% | 2.1% | ||
| Other locations | 29 | 24 | 26 | 79 | 23 | 22 | 17 | 62 |
| 10.2% | 8.1% | 9.3% | 9.2% | 8.1% | 7.6% | 7.6% | 7.8% | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paschke, S.; Jafarov, S.; Staib, L.; Kreuser, E.-D.; Maulbecker-Armstrong, C.; Roitman, M.; Holm, T.; Harris, C.C.; Link, K.-H.; Kornmann, M. Are Colon and Rectal Cancer Two Different Tumor Entities? A Proposal to Abandon the Term Colorectal Cancer. Int. J. Mol. Sci. 2018, 19, 2577. https://doi.org/10.3390/ijms19092577
Paschke S, Jafarov S, Staib L, Kreuser E-D, Maulbecker-Armstrong C, Roitman M, Holm T, Harris CC, Link K-H, Kornmann M. Are Colon and Rectal Cancer Two Different Tumor Entities? A Proposal to Abandon the Term Colorectal Cancer. International Journal of Molecular Sciences. 2018; 19(9):2577. https://doi.org/10.3390/ijms19092577
Chicago/Turabian StylePaschke, Stephan, Sakhavat Jafarov, Ludger Staib, Ernst-Dietrich Kreuser, Catharina Maulbecker-Armstrong, Marc Roitman, Torbjörn Holm, Curtis C. Harris, Karl-Heinrich Link, and Marko Kornmann. 2018. "Are Colon and Rectal Cancer Two Different Tumor Entities? A Proposal to Abandon the Term Colorectal Cancer" International Journal of Molecular Sciences 19, no. 9: 2577. https://doi.org/10.3390/ijms19092577
APA StylePaschke, S., Jafarov, S., Staib, L., Kreuser, E.-D., Maulbecker-Armstrong, C., Roitman, M., Holm, T., Harris, C. C., Link, K.-H., & Kornmann, M. (2018). Are Colon and Rectal Cancer Two Different Tumor Entities? A Proposal to Abandon the Term Colorectal Cancer. International Journal of Molecular Sciences, 19(9), 2577. https://doi.org/10.3390/ijms19092577