Impact of Fetuin-A (AHSG) on Tumor Progression and Type 2 Diabetes
Abstract
1. Introduction
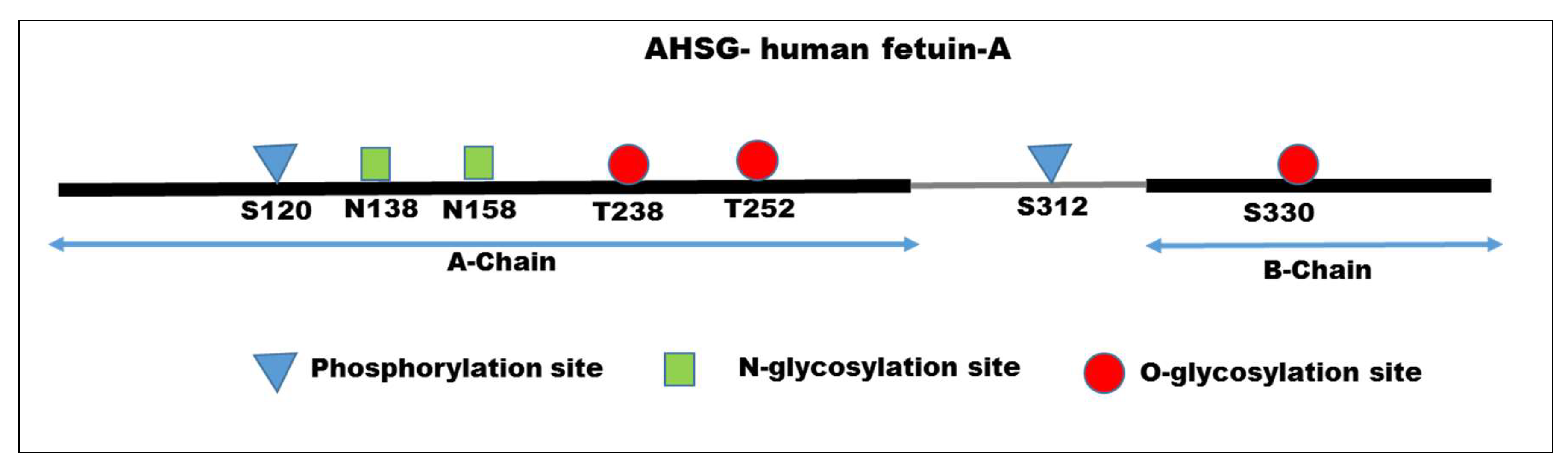
2. Structural Features of Fetuin-A
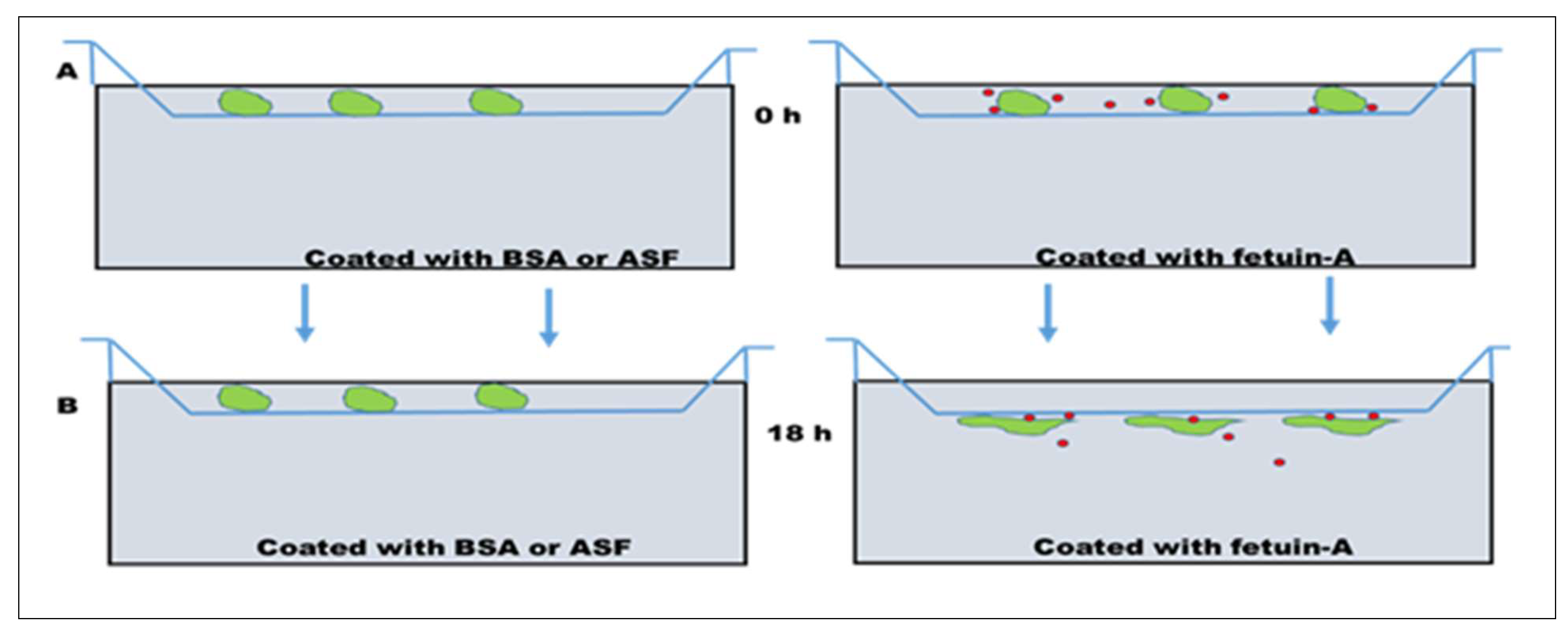
3. Role of Fetuin-A in Cell Attachment, Motility, and Invasion of Tumor Cells
4. Potential Role of Fetuin-A in a Bone Tumor Microenvironment
5. Role of Fetuin-A in Tumor Cell Growth, both in vitro and in vivo
6. Fetuin-A Autoantibodies and Cancer
7. Concentration of Fetuin-A in the Serum and Tumor Microenvironment during Tumor Progression
8. Fetuin-A and Toll-like Receptor 4
9. Fetuin-A and Tumor Progression—Take-home Lessons
10. Fetuin-A and Type 2 Diabetes Mellitus
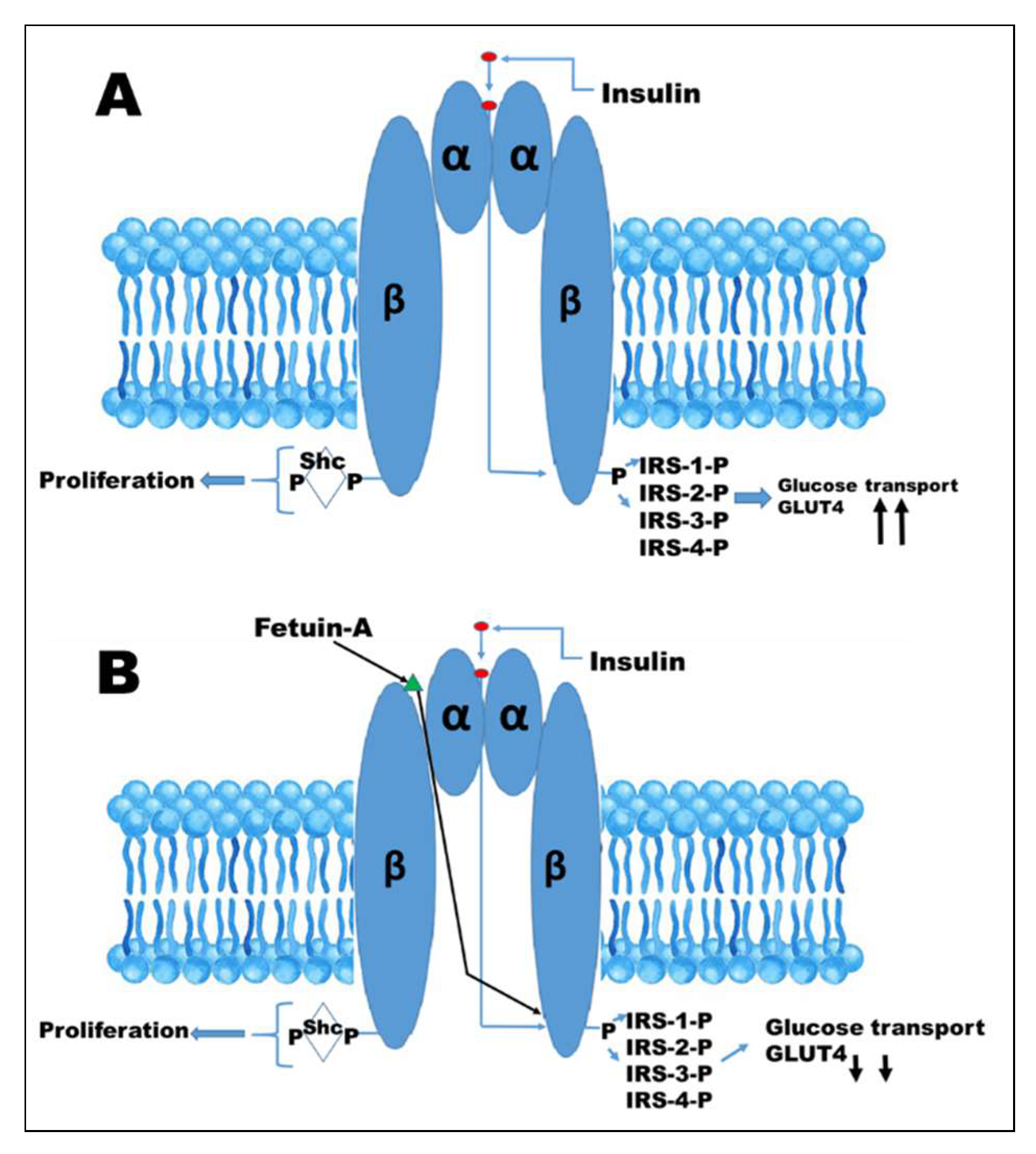
11. Action of Fetuin-A at the Insulin Receptor
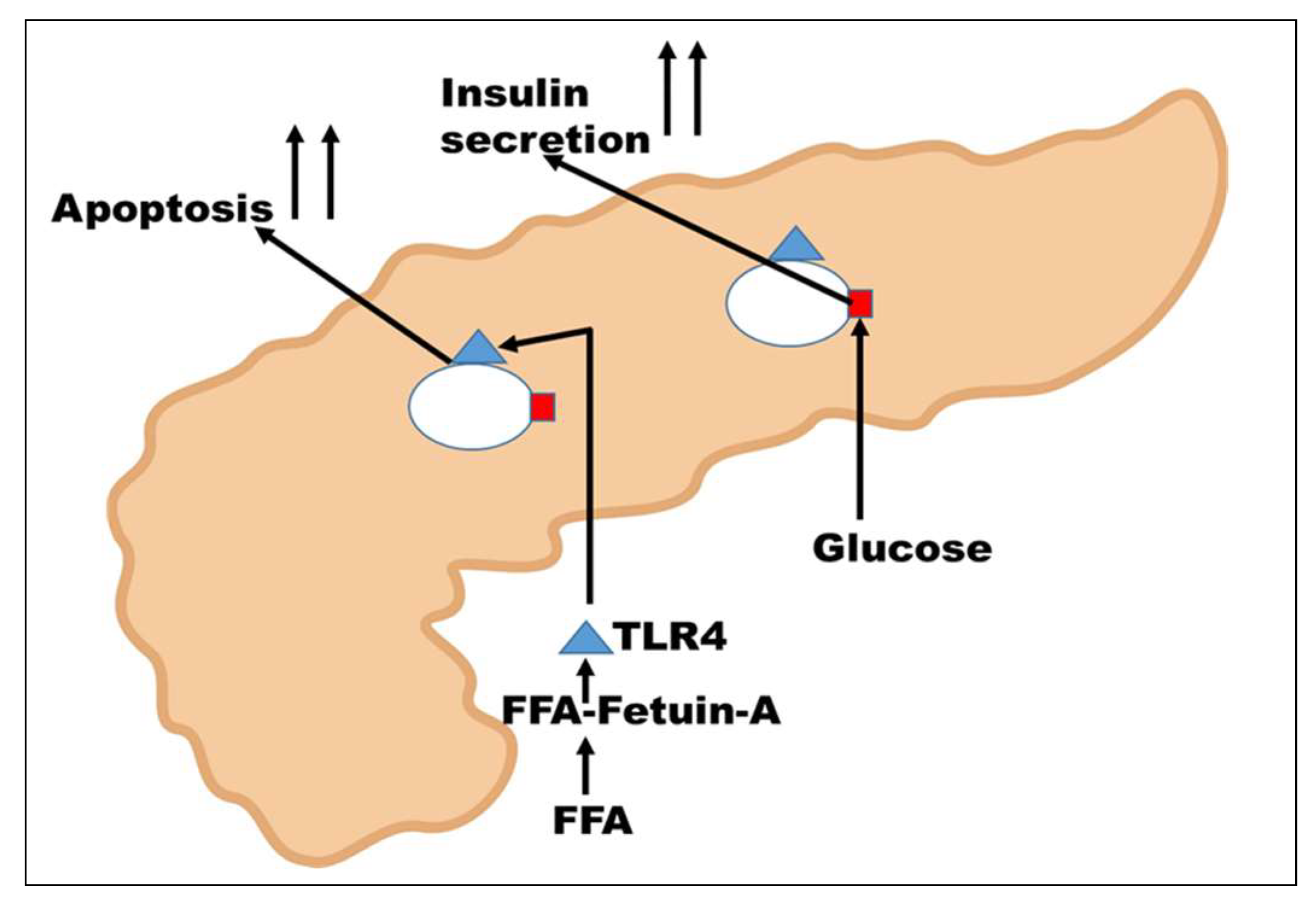
12. Action of Fetuin-A on β-islets Cells of the Pancreas
Author Contributions
Funding
Conflicts of Interest
References
- Brown, W.M.; Saunders, N.R.; Mollgard, K.; Dziegielewska, K.M. Fetuin—An old friend revisited. BioEssays. 1992, 14, 749–755. [Google Scholar] [CrossRef] [PubMed]
- Mori, K.; Emoto, M.; Inaba, M. Fetuin-A: A multifunctional protein. Recent Pat. Endocr. Metab. Immune Drug Discov. 2011, 5, 124–146. [Google Scholar] [CrossRef] [PubMed]
- Cintron, V.J.; Ko, M.S.; Chi, K.D.; Gross, J.P.; Srinivas, P.R.; Goustin, A.S.; Grunberger, G. Genetic mapping and functional studies of a natural inhibitor of the insulin receptor tyrosine kinase: The mouse ortholog of human α2-HS glycoprotein. Int. J. Exp. Diabetes Res. 2001, 1, 249–263. [Google Scholar] [CrossRef] [PubMed]
- Mathews, S.T.; Singh, G.P.; Ranalletta, M.; Cintron, V.J.; Qiang, X.; Goustin, A.S.; Jen, K.L.; Charron, M.J.; Jahnen-Dechent, W.; Grunberger, G. Improved insulin sensitivity and resistance to weight gain in mice null for the ahsg gene. Diabetes 2002, 51, 2450–2458. [Google Scholar] [CrossRef] [PubMed]
- Arnaud, P.; Kalabay, L. α2-HS glycoprotein: A protein in search of a function. Diabetes Metab. Res. Rev. 2002, 18, 311–314. [Google Scholar] [CrossRef] [PubMed]
- Miura, Y.; Iwazu, Y.; Shiizaki, K.; Akimoto, T.; Kotani, K.; Kurabayashi, M.; Kurosu, H.; Kuro, O.M. Identification and quantification of plasma calciprotein particles with distinct physical properties in patients with chronic kidney disease. Sci. Rep. 2018, 8, 1256. [Google Scholar] [CrossRef] [PubMed]
- Artunc, F.; Schleicher, E.; Weigert, C.; Fritsche, A.; Stefan, N.; Haring, H.U. The impact of insulin resistance on the kidney and vasculature. Nat. Rev. Nephrol. 2016, 12, 721–737. [Google Scholar] [CrossRef] [PubMed]
- Schafer, C.; Heiss, A.; Schwarz, A.; Westenfeld, R.; Ketteler, M.; Floege, J.; Muller-Esterl, W.; Schinke, T.; Jahnen-Dechent, W. The serum protein α2-Heremans-Schmid glycoprotein/fetuin-A is a systemically acting inhibitor of ectopic calcification. J. Clin. Invest. 2003, 112, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Fisher, H.W.; Puck, T.T.; Sato, G. Molecular growth requirements of single mammalian cells: The action of fetuin in promoting cell attachment to glass. Proc. Natl. Acad. Sci. USA 1958, 44, 4–10. [Google Scholar] [CrossRef]
- Pedersen, K.O. Fetuin, a new globulin isolated from serum. Nature 1944, 154, 575. [Google Scholar] [CrossRef]
- Nie, Z. Fetuin: Its enigmatic property of growth promotion. Am. J. Physiol. 1992, 263, C551–C562. [Google Scholar] [CrossRef] [PubMed]
- Spiro, R.G. Studies on fetuin, a glycoprotein of fetal serum. I. Isolation, chemical composition, and physiocochemical properties. J. Biol. Chem. 1960, 235, 2860–2869. [Google Scholar] [PubMed]
- Yu, C.L.; Tsai, M.H. Fetal fetuin selectively induces apoptosis in cancer cell lines and shows anti-cancer activity in tumor animal models. Cancer Lett. 2001, 166, 173–184. [Google Scholar] [CrossRef]
- Tasheva, E.; An, K.; Conrad, G. Zinc affects cell proliferation and KSPG expression of corneal keratocytes. Invest. Ophth. Vis. Sci. 2004, 45, 3836. [Google Scholar]
- Norouzi, S.; Adulcikas, J.; Sohal, S.S.; Myers, S. Zinc transporters and insulin resistance: Therapeutic implications for type 2 diabetes and metabolic disease. J. Biomed. Sci. 2017, 24, 87. [Google Scholar] [CrossRef] [PubMed]
- Bozym, R.A.; Chimienti, F.; Giblin, L.J.; Gross, G.W.; Korichneva, I.; Li, Y.; Libert, S.; Maret, W.; Parviz, M.; Frederickson, C.J. Free zinc ions outside a narrow concentration range are toxic to a variety of cells in vitro. Exp. Biol. Med. 2010, 235, 741–750. [Google Scholar] [CrossRef] [PubMed]
- Sakwe, A.M.; Koumangoye, R.; Goodwin, S.J.; Ochieng, J. Fetuin-A (α2-HS glycoprotein) is a major serum adhesive protein that mediates growth signaling in breast tumor cells. J. Biol. Chem. 2010, 285, 41827–41835. [Google Scholar] [CrossRef] [PubMed]
- Goustin, A.S.; Abou-Samra, A.B. The “thrifty” gene encoding ahsg/fetuin-A meets the insulin receptor: Insights into the mechanism of insulin resistance. Cell. Signal. 2011, 23, 980–990. [Google Scholar] [CrossRef] [PubMed]
- Gerst, F.; Wagner, R.; Kaiser, G.; Panse, M.; Heni, M.; Machann, J.; Bongers, M.N.; Sartorius, T.; Sipos, B.; Fend, F.; et al. Metabolic crosstalk between fatty pancreas and fatty liver: Effects on local inflammation and insulin secretion. Diabetologia 2017, 60, 2240–2251. [Google Scholar] [CrossRef] [PubMed]
- Peter, A.; Kovarova, M.; Staiger, H.; Machann, J.; Schick, F.; Konigsrainer, A.; Konigsrainer, I.; Schleicher, E.; Fritsche, A.; Haring, H.U.; et al. The hepatokines fetuin-A and fetuin-B are upregulated in the state of hepatic steatosis and may differently impact on glucose homeostasis in humans. Am. J. Physiol. Endocrinol. Metab. 2018, 314, E266–E273. [Google Scholar] [CrossRef] [PubMed]
- Denecke, B.; Graber, S.; Schafer, C.; Heiss, A.; Woltje, M.; Jahnen-Dechent, W. Tissue distribution and activity testing suggest a similar but not identical function of fetuin-B and fetuin-A. Biochem. J. 2003, 376, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Araki, T.; Yoshioka, Y.; Schmid, K. The position of the disulfide bonds in human plasma α2-HS glycoprotein and the repeating double disulfide bonds in the domain structure. Biochim. Biophys. Acta 1989, 994, 195–199. [Google Scholar] [CrossRef]
- Schmid, K.; Burgi, W. Preparation and properties of the human plasma Ba-α2-glycoproteins. Biochim. Biophys. Acta 1961, 47, 440–453. [Google Scholar] [CrossRef]
- Yoshioka, Y.; Gejyo, F.; Marti, T.; Rickli, E.E.; Burgi, W.; Offner, G.D.; Troxler, R.F.; Schmid, K. The complete amino acid sequence of the A-chain of human plasma α2-HS glycoprotein. J. Biol. Chem. 1986, 261, 1665–1676. [Google Scholar] [PubMed]
- Haglund, A.C.; Ek, B.; Ek, P. Phosphorylation of human plasma α2-Heremans-Schmid glycoprotein (human fetuin) in vivo. Biochem. J. 2001, 357, 437–445. [Google Scholar] [PubMed]
- Lee, C.; Bongcam-Rudloff, E.; Sollner, C.; Jahnen-Dechent, W.; Claesson-Welsh, L. Type 3 cystatins; fetuins, kininogen and histidine-rich glycoprotein. Front. Biosci. 2009, 14, 2911–2922. [Google Scholar] [CrossRef]
- Han, G.; Ye, M.; Zhou, H.; Jiang, X.; Feng, S.; Jiang, X.; Tian, R.; Wan, D.; Zou, H.; Gu, J. Large-scale phosphoproteome analysis of human liver tissue by enrichment and fractionation of phosphopeptides with strong anion exchange chromatography. Proteomics 2008, 8, 1346–1361. [Google Scholar] [CrossRef] [PubMed]
- Oppermann, F.S.; Gnad, F.; Olsen, J.V.; Hornberger, R.; Greff, Z.; Keri, G.; Mann, M.; Daub, H. Large-scale proteomics analysis of the human kinome. Mol. Cell. Proteome 2009, 8, 1751–1764. [Google Scholar] [CrossRef] [PubMed]
- Magnuson, V.L.; McCombs, J.L.; Lee, C.C.; Yang, F.; Bowman, B.H.; McGill, J.R. Human α2-HS glycoprotein localized to 3q27→q29 by in situ hybridization. Cytogenet. Cell Genet. 1988, 47, 72–74. [Google Scholar] [CrossRef] [PubMed]
- Kundranda, M.N.; Ray, S.; Saria, M.; Friedman, D.; Matrisian, L.M.; Lukyanov, P.; Ochieng, J. Annexins expressed on the cell surface serve as receptors for adhesion to immobilized fetuin-A. Biochimica et Biophysica Biochim. Biophys. Acta 2004, 1693, 111–123. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.X.; O’Neill, K.D.; Chen, X.; Duan, D.; Wang, E.; Sturek, M.S.; Edwards, J.M.; Moe, S.M. Fetuin-A uptake in bovine vascular smooth muscle cells is calcium dependent and mediated by annexins. Am. J. Physiol. Renal. Physiol. 2007, 292, F599–F606. [Google Scholar] [CrossRef] [PubMed]
- Nangami, G.N.; Watson, K.; Parker-Johnson, K.; Okereke, K.O.; Sakwe, A.; Thompson, P.; Frimpong, N.; Ochieng, J. Fetuin-A (α2-HS glycoprotein) is a serum chemo-attractant that also promotes invasion of tumor cells through matrigel. Biochem. Biophys. Res. Commun. 2013, 438, 660–665. [Google Scholar] [CrossRef] [PubMed]
- Nangami, G.N.; Sakwe, A.M.; Izban, M.G.; Rana, T.; Lammers, P.E.; Thomas, P.; Chen, Z.; Ochieng, J. Fetuin-A (α2-HS glycoprotein) modulates growth, motility, invasion, and senescence in high-grade astrocytomas. Cancer Med. 2016, 5, 3532–3543. [Google Scholar] [CrossRef] [PubMed]
- Kundranda, M.N.; Henderson, M.; Carter, K.J.; Gorden, L.; Binhazim, A.; Ray, S.; Baptiste, T.; Shokrani, M.; Leite-Browning, M.L.; Jahnen-Dechent, W.; et al. The serum glycoprotein fetuin-A promotes lewis lung carcinoma tumorigenesis via adhesive-dependent and adhesive-independent mechanisms. Cancer Res. 2005, 65, 499–506. [Google Scholar] [PubMed]
- Koumangoye, R.B.; Sakwe, A.M.; Goodwin, J.S.; Patel, T.; Ochieng, J. Detachment of breast tumor cells induces rapid secretion of exosomes which subsequently mediate cellular adhesion and spreading. PLoS ONE 2011, 6, e24234. [Google Scholar] [CrossRef] [PubMed]
- Nangami, G.; Koumangoye, R.; Shawn Goodwin, J.; Sakwe, A.M.; Marshall, D.; Higginbotham, J.; Ochieng, J. Fetuin-A associates with histones intracellularly and shuttles them to exosomes to promote focal adhesion assembly resulting in rapid adhesion and spreading in breast carcinoma cells. Exp. Cell Res. 2014, 328, 388–400. [Google Scholar] [CrossRef] [PubMed]
- Watson, K.; Koumangoye, R.; Thompson, P.; Sakwe, A.M.; Patel, T.; Pratap, S.; Ochieng, J. Fetuin-A triggers the secretion of a novel set of exosomes in detached tumor cells that mediate their adhesion and spreading. FEBS Lett. 2012, 586, 3458–3463. [Google Scholar] [CrossRef] [PubMed]
- Mbuyi, J.M.; Dequeker, J.; Bloemmen, F.; Stevens, E. Plasma proteins in human cortical bone: Enrichment of α2-HS glycoprotein, α1 acid-glycoprotein, and ige. Calcif. Tissue Int. 1982, 34, 229–231. [Google Scholar] [CrossRef] [PubMed]
- Esposito, M.; Guise, T.; Kang, Y. The biology of bone metastasis. Cold Spring Harb. Perspect. Biol. 2018, 8, a031252. [Google Scholar] [CrossRef] [PubMed]
- Le Pape, F.; Vargas, G.; Clézardin, P. The role of osteoclasts in breast cancer bone metastasis. J. Bone Oncol. 2016, 5, 93–95. [Google Scholar] [CrossRef] [PubMed]
- Roodman, G.D. Biology of osteoclast activation in cancer. J. Clin. Oncol. 2001, 19, 3562–3571. [Google Scholar] [CrossRef] [PubMed]
- Christoulas, D.; Terpos, E.; Dimopoulos, M.A. Pathogenesis and management of myeloma bone disease. Expert Rev. Hematol. 2009, 2, 385–398. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Baumann, M.J.; McCabe, L.R. Adsorption of serum fetuin to hydroxylapatite does not contribute to osteoblast phenotype modifications. J. Biomed. Mater. Res. A 2005, 73, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Malone, J.D.; Richards, M. α2-HS glycoprotein is chemotactic for mononuclear phagocytes. J. Cell Physiol. 1987, 132, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Mintz, P.J.; Rietz, A.C.; Cardo-Vila, M.; Ozawa, M.G.; Dondossola, E.; Do, K.A.; Kim, J.; Troncoso, P.; Logothetis, C.J.; Sidman, R.L.; et al. Discovery and horizontal follow-up of an autoantibody signature in human prostate cancer. Proc. Natl. Acad. Sci. USA. 2015, 112, 2515–2520. [Google Scholar] [CrossRef] [PubMed]
- Ray, S.; Lukyanov, P.; Ochieng, J. Members of the cystatin superfamily interact with MMP-9 and protect it from autolytic degradation without affecting its gelatinolytic activities. Biochim. Biophys. Acta 2003, 1652, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Stefan, N.; Hennige, A.M.; Staiger, H.; Machann, J.; Schick, F.; Kröber, S.M.; Machicao, F.; Fritsche, A.; Häring, H.-U. α2-Heremans-Schmid glycoprotein/fetuin-A is associated with insulin resistance and fat accumulation in the liver in humans. Diabetes Care 2006, 29, 853–857. [Google Scholar] [CrossRef] [PubMed]
- Ellem, K.A.; Kay, G.F. The nature of conditioning nutrients for human malignant melanoma cultures. J. Cell Sci. 1983, 62, 249–266. [Google Scholar] [PubMed]
- Riss, T.L.; Stewart, B.H.; Sirbasku, D.A. Rat pituitary tumor cells in serum-free culture. I. Selection of thyroid hormone-responsive and autonomous cells. In Vitro Cell. Dev. Biol. 1989, 25, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Swallow, C.J.; Partridge, E.A.; Macmillan, J.C.; Tajirian, T.; DiGuglielmo, G.M.; Hay, K.; Szweras, M.; Jahnen-Dechent, W.; Wrana, J.L.; Redston, M.; et al. α2-HS glycoprotein, an antagonist of transforming growth factor β in vivo, inhibits intestinal tumor progression. Cancer Res. 2004, 64, 6402–6409. [Google Scholar] [CrossRef] [PubMed]
- Guillory, B.; Sakwe, A.M.; Saria, M.; Thompson, P.; Adhiambo, C.; Koumangoye, R.; Ballard, B.; Binhazim, A.; Cone, C.; Jahanen-Dechent, W.; et al. Lack of fetuin-A (α2-HS glycoprotein) reduces mammary tumor incidence and prolongs tumor latency via the transforming growth factor-β signaling pathway in a mouse model of breast cancer. Am. J. Pathol. 2010, 177, 2635–2644. [Google Scholar] [CrossRef] [PubMed]
- Arnaud, P.; Miribel, L.; Emerson, D.L. α2-HS glycoprotein. Methods Enzymol. 1988, 163, 431–441. [Google Scholar] [PubMed]
- Thompson, P.D.; Sakwe, A.; Koumangoye, R.; Yarbrough, W.G.; Ochieng, J.; Marshall, D.R. α2-Heremans Schmid glycoprotein (AHSG) modulates signaling pathways in head and neck squamous cell carcinoma cell line sq20b. Exp. Cell Res. 2014, 321, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wu, W.; Chen, L.; Ma, X.; Zhao, Y.; Zhou, H.; Yang, R.; Hu, L. Expression and clinical significance of ahsg and complement C3 in pancreatic ductal adenocarcinoma. Zhonghua Yi Xue Za Zhi 2014, 94, 2175–2179. [Google Scholar] [PubMed]
- Chen, J.; Wu, W.; Chen, L.; Zhou, H.; Yang, R.; Hu, L.; Zhao, Y. Profiling the potential tumor markers of pancreatic ductal adenocarcinoma using 2D-DIGE and MALDI-TOF-MS: Up-regulation of complement C3 and α2-HS glycoprotein. Pancreatology 2013, 13, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Elmaci, I.; Altinoz, M.A. A metabolic inhibitory cocktail for grave cancers: Metformin, pioglitazone and lithium combination in treatment of pancreatic cancer and glioblastoma multiforme. Biochem. Genet. 2016, 54, 573–618. [Google Scholar] [CrossRef] [PubMed]
- Kamerkar, S.; LeBleu, V.S.; Sugimoto, H.; Yang, S.; Ruivo, C.F.; Melo, S.A.; Lee, J.J.; Kalluri, R. Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature 2017, 546, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Tkach, M.; Thery, C. Communication by extracellular vesicles: Where we are and where we need to go. Cell 2016, 164, 1226–1232. [Google Scholar] [CrossRef] [PubMed]
- Gyorffy, B.; Surowiak, P.; Budczies, J.; Lanczky, A. Online survival analysis software to assess the prognostic value of biomarkers using transcriptomic data in non-small-cell lung cancer. PLoS ONE 2013, 8, e82241. [Google Scholar] [CrossRef] [PubMed]
- Szasz, A.M.; Lanczky, A.; Nagy, A.; Forster, S.; Hark, K.; Green, J.E.; Boussioutas, A.; Busuttil, R.; Szabo, A.; Gyorffy, B. Cross-validation of survival associated biomarkers in gastric cancer using transcriptomic data of 1,065 patients. Oncotarget 2016, 7, 49322–49333. [Google Scholar] [CrossRef] [PubMed]
- Azuma, K.; Serada, S.; Takamatsu, S.; Terao, N.; Takeishi, S.; Kamada, Y.; Naka, T.; Miyoshi, E. Identification of sialylated glycoproteins in doxorubicin-treated hepatoma cells with glycoproteomic analyses. J. Proteome Res. 2014, 13, 4869–4877. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Grijalva, A.L.; Aguilar-Lemarroy, A.; Jave-Suarez, L.F.; Gutierrez-Ortega, A.; Godinez-Melgoza, P.A.; Herrera-Rodriguez, S.E.; Mariscal-Ramirez, I.; Martinez-Velazquez, M.; Gawinowicz, M.A.; Martinez-Silva, M.G.; et al. α2-HS glycoprotein, a tumor-associated antigen (TAA) detected in mexican patients with early-stage breast cancer. J. Proteome 2015, 112, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Yi, J.K.; Chang, J.W.; Han, W.; Lee, J.W.; Ko, E.; Kim, D.H.; Bae, J.Y.; Yu, J.; Lee, C.; Yu, M.H.; et al. Autoantibody to tumor antigen, α2-HS glycoprotein: A novel biomarker of breast cancer screening and diagnosis. Cancer Epidemiol. Biomarkers Prev. 2009, 18, 1357–1364. [Google Scholar] [CrossRef] [PubMed]
- Kalabay, L.; Cseh, K.; Benedek, S.; Fekete, S.; Masszi, T.; Herjeczki, K.; Pozsonyi, T.; Jakab, L.; Jakab, L. Serum α2-HS glycoprotein concentration in patients with hematological malignancies. A follow-up study. Ann. Hematol. 1991, 63, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Kwak, J.Y.; Ma, T.Z.; Yoo, M.J.; Choi, B.H.; Kim, H.G.; Kim, S.R.; Yim, C.Y.; Kwak, Y.G. The comparative analysis of serum proteomes for the discovery of biomarkers for acute myeloid leukemia. Exp. Hematol. 2004, 32, 836–842. [Google Scholar] [CrossRef] [PubMed]
- Danda, R.; Ganapathy, K.; Sathe, G.; Madugundu, A.K.; Ramachandran, S.; Krishnan, U.M.; Khetan, V.; Rishi, P.; Keshava Prasad, T.S.; Pandey, A.; et al. Proteomic profiling of retinoblastoma by high resolution mass spectrometry. Clin. Proteome 2016, 13, 29. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, M.I.; Lee, C.C.; Mat Junit, S.; Ng, K.L.; Hashim, O.H. Tissue and serum samples of patients with papillary thyroid cancer with and without benign background demonstrate different altered expression of proteins. PeerJ. 2016, 4, e2450. [Google Scholar] [CrossRef] [PubMed]
- Schonemeier, B.; Metzger, J.; Klein, J.; Husi, H.; Bremer, B.; Armbrecht, N.; Dakna, M.; Schanstra, J.P.; Rosendahl, J.; Wiegand, J.; et al. Urinary peptide analysis differentiates pancreatic cancer from chronic pancreatitis. Pancreas 2016, 45, 1018–1026. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Fan, Y.X.; Yang, Y.; Liu, D.L.; Wu, K.; Wen, F.B.; Zhang, C.Y.; Zhu, D.Y.; Zhao, S. Identification of potential plasma biomarkers for esophageal squamous cell carcinoma by a proteomic method. Int. J. Clin. Exp. Pathol. 2015, 8, 1535–1544. [Google Scholar] [PubMed]
- Fan, N.J.; Kang, R.; Ge, X.Y.; Li, M.; Liu, Y.; Chen, H.M.; Gao, C.F. Identification α2-HS glycoprotein precursor and tubulin β-chain as serology diagnosis biomarker of colorectal cancer. Diagn. Pathol. 2014, 9, 53. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.J.; Wang, C.L.; Wang, C.I.; Chen, C.D.; Dan, Y.M.; Wu, C.C.; Wu, Y.C.; Lee, I.N.; Tsai, Y.H.; Chang, Y.S.; et al. Comprehensive proteome analysis of malignant pleural effusion for lung cancer biomarker discovery by using multidimensional protein identification technology. J. Proteome Res. 2011, 10, 4671–4682. [Google Scholar] [CrossRef] [PubMed]
- Valle, A.; Sastre-Serra, J.; Pol, C.; Miro, A.M.; Oliver, J.; Roca, P. Proteomic analysis of MCF-7 breast cancer cell line exposed to leptin. Anal. Cell. Pathol. 2011, 34, 147–157. [Google Scholar] [CrossRef]
- Rho, J.H.; Roehrl, M.H.; Wang, J.Y. Glycoproteomic analysis of human lung adenocarcinomas using glycoarrays and tandem mass spectrometry: Differential expression and glycosylation patterns of vimentin and fetuin a isoforms. Protein J. 2009, 28, 148–160. [Google Scholar] [CrossRef] [PubMed]
- Comunale, M.A.; Wang, M.; Hafner, J.; Krakover, J.; Rodemich, L.; Kopenhaver, B.; Long, R.E.; Junaidi, O.; Bisceglie, A.M.; Block, T.M.; et al. Identification and development of fucosylated glycoproteins as biomarkers of primary hepatocellular carcinoma. J. Proteome Res. 2009, 8, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Betesh, L.; Comunale, M.A.; Wang, M.; Liang, H.; Hafner, J.; Karabudak, A.; Giama, N.H.; Moser, C.D.; Miyoshi, E.; Roberts, L.R.; et al. Identification of fucosylated fetuin-A as a potential biomarker for cholangiocarcinoma. Proteome Clin. Appl. 2017, 11. [Google Scholar] [CrossRef] [PubMed]
- Ribom, D.; Westman-Brinkmalm, A.; Smits, A.; Davidsson, P. Elevated levels of α2-Heremans-Schmid glycoprotein in CSF of patients with low-grade gliomas. Tumour Biol. 2003, 24, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Dowling, P.; O’Driscoll, L.; Meleady, P.; Henry, M.; Roy, S.; Ballot, J.; Moriarty, M.; Crown, J.; Clynes, M. 2-D difference gel electrophoresis of the lung squamous cell carcinoma versus normal sera demonstrates consistent alterations in the levels of ten specific proteins. Electrophoresis 2007, 28, 4302–4310. [Google Scholar] [CrossRef] [PubMed]
- Pal, D.; Dasgupta, S.; Kundu, R.; Maitra, S.; Das, G.; Mukhopadhyay, S.; Ray, S.; Majumdar, S.S.; Bhattacharya, S. Fetuin-A acts as an endogenous ligand of TLR4 to promote lipid-induced insulin resistance. Nat. Med. 2012, 18, 1279–1285. [Google Scholar] [CrossRef] [PubMed]
- Mukhuty, A.; Fouzder, C.; Mukherjee, S.; Malick, C.; Mukhopadhyay, S.; Bhattacharya, S.; Kundu, R. Palmitate induced fetuin-A secretion from pancreatic β-cells adversely affects its function and elicits inflammation. Biochem. Biophys. Res. Commun. 2017, 491, 1118–1124. [Google Scholar] [CrossRef] [PubMed]
- Sujana, C.; Huth, C.; Zierer, A.; Meesters, S.; Sudduth-Klinger, J.; Koenig, W.; Herder, C.; Peters, A.; Thorand, B. Association of fetuin-A with incident type 2 diabetes: Results from the monica/kora augsburg study and a systematic meta-analysis. Eur. J. Endocrinol. 2018, 178, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Guo, V.Y.; Cao, B.; Cai, C.; Cheng, K.K.; Cheung, B.M.Y. Fetuin-A levels and risk of type 2 diabetes mellitus: A systematic review and meta-analysis. Acta Diabetol. 2018, 55, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Trepanowski, J.F.; Mey, J.; Varady, K.A. Fetuin-A: A novel link between obesity and related complications. Int. J. Obes. 2015, 39, 734–741. [Google Scholar] [CrossRef] [PubMed]
- Mathews, S.T.; Rakhade, S.; Zhou, X.; Parker, G.C.; Coscina, D.V.; Grunberger, G. Fetuin-null mice are protected against obesity and insulin resistance associated with aging. Biochem. Biophys. Res. Commun. 2006, 350, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Srinivas, P.R.; Wagner, A.S.; Reddy, L.V.; Deutsch, D.D.; Leon, M.A.; Goustin, A.S.; Grunberger, G. Serum α2-HS glycoprotein is an inhibitor of the human insulin receptor at the tyrosine kinase level. Mol. Endocrinol. 1993, 7, 1445–1455. [Google Scholar] [PubMed]
- Chen, H.; Srinivas, P.R.; Cong, L.N.; Li, Y.; Grunberger, G.; Quon, M.J. α2-Heremans-Schmid glycoprotein inhibits insulin-stimulated Elk-1 phosphorylation, but not glucose transport, in rat adipose cells. Endocrinology 1998, 139, 4147–4154. [Google Scholar] [CrossRef] [PubMed]
- Boucher, J.; Kleinridders, A.; Kahn, C.R. Insulin receptor signaling in normal and insulin-resistant states. Cold Spring Harb. Perspect. Biol. 2014, 6. [Google Scholar] [CrossRef] [PubMed]
- Goustin, A.S.; Derar, N.; Abou-Samra, A.B. Ahsg-fetuin blocks the metabolic arm of insulin action through its interaction with the 95-kD β-subunit of the insulin receptor. Cell Signal. 2013, 25, 981–988. [Google Scholar] [CrossRef] [PubMed]
- Roder, P.V.; Wu, B.; Liu, Y.; Han, W. Pancreatic regulation of glucose homeostasis. Exp. Mol. Med. 2016, 48, e219. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Yang, L.; Yan, S.; Zheng, H.; Liang, L.; Cai, X.; Liao, M. Fetuin a promotes lipotoxicity in β cells through the TLR4 signaling pathway and the role of pioglitazone in anti-lipotoxicity. Mol. Cell. Endocrinol. 2015, 412, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Draznin, B. Molecular mechanisms of insulin resistance: Serine phosphorylation of insulin receptor substrate-1 and increased expression of p85α: The two sides of a coin. Diabetes 2006, 55, 2392–2397. [Google Scholar] [CrossRef] [PubMed]
- Jialal, I.; Devaraj, S.; Bettaieb, A.; Haj, F.; Adams-Huet, B. Increased adipose tissue secretion of fetuin-A, lipopolysaccharide-binding protein and high-mobility group box protein 1 in metabolic syndrome. Atherosclerosis 2015, 241, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, S.; Mondal, S.A.; Kumar, M.; Dutta, D. Proinflammatory and antiinflammatory attributes of fetuin-A: A novel hepatokine modulating cardiovascular and glycemic outcomes in metabolic syndrome. Endocr. Pract. 2014, 20, 1345–1351. [Google Scholar] [PubMed]
| Changes in the Levels of Fetuin-A (Protein or mRNA) and Post-Translational Status of the Glycoprotein During Tumor Progression | Reference |
|---|---|
| Increased fetuin expression in tumor tissue (Protein) | [66,67,68,69] |
| Increased fetuin expression in tumor tissue (Protein and mRNA) | [33,53,54] |
| Increased fetuin-A expression in serum | [70] |
| Increased in malignant pleural effusion of lung cancer | [71] |
| Increased uptake of fetuin-A by tumor cells | [72] |
| Increased high mannose glycan structures of fetuin-A in lung adenocarcinoma but not in control normal lung | [73] |
| Increased fucosylation of fetuin-A in hepatocellular carcinoma and cholangiosarcoma | [74,75] |
| Increased levels of fetuin-A in CSF of low grade glioma patients | [76] |
| Reduced levels of fetuin-A in sera of patients with hematological malignancies. | [64,65] |
| Reduced levels of fetuin-A in the microenvironment during the progression of GI tumors | [50] |
| Reduced levels of fetuin-A in lung squamous cell carcinoma | [77] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ochieng, J.; Nangami, G.; Sakwe, A.; Moye, C.; Alvarez, J.; Whalen, D.; Thomas, P.; Lammers, P. Impact of Fetuin-A (AHSG) on Tumor Progression and Type 2 Diabetes. Int. J. Mol. Sci. 2018, 19, 2211. https://doi.org/10.3390/ijms19082211
Ochieng J, Nangami G, Sakwe A, Moye C, Alvarez J, Whalen D, Thomas P, Lammers P. Impact of Fetuin-A (AHSG) on Tumor Progression and Type 2 Diabetes. International Journal of Molecular Sciences. 2018; 19(8):2211. https://doi.org/10.3390/ijms19082211
Chicago/Turabian StyleOchieng, Josiah, Gladys Nangami, Amos Sakwe, Cierra Moye, Joel Alvarez, Diva Whalen, Portia Thomas, and Philip Lammers. 2018. "Impact of Fetuin-A (AHSG) on Tumor Progression and Type 2 Diabetes" International Journal of Molecular Sciences 19, no. 8: 2211. https://doi.org/10.3390/ijms19082211
APA StyleOchieng, J., Nangami, G., Sakwe, A., Moye, C., Alvarez, J., Whalen, D., Thomas, P., & Lammers, P. (2018). Impact of Fetuin-A (AHSG) on Tumor Progression and Type 2 Diabetes. International Journal of Molecular Sciences, 19(8), 2211. https://doi.org/10.3390/ijms19082211







