Early Hyperbaric Oxygen Treatment Attenuates Burn-Induced Neuroinflammation by Inhibiting the Galectin-3-Dependent Toll-Like Receptor-4 Pathway in a Rat Model
Abstract
1. Introduction
2. Results
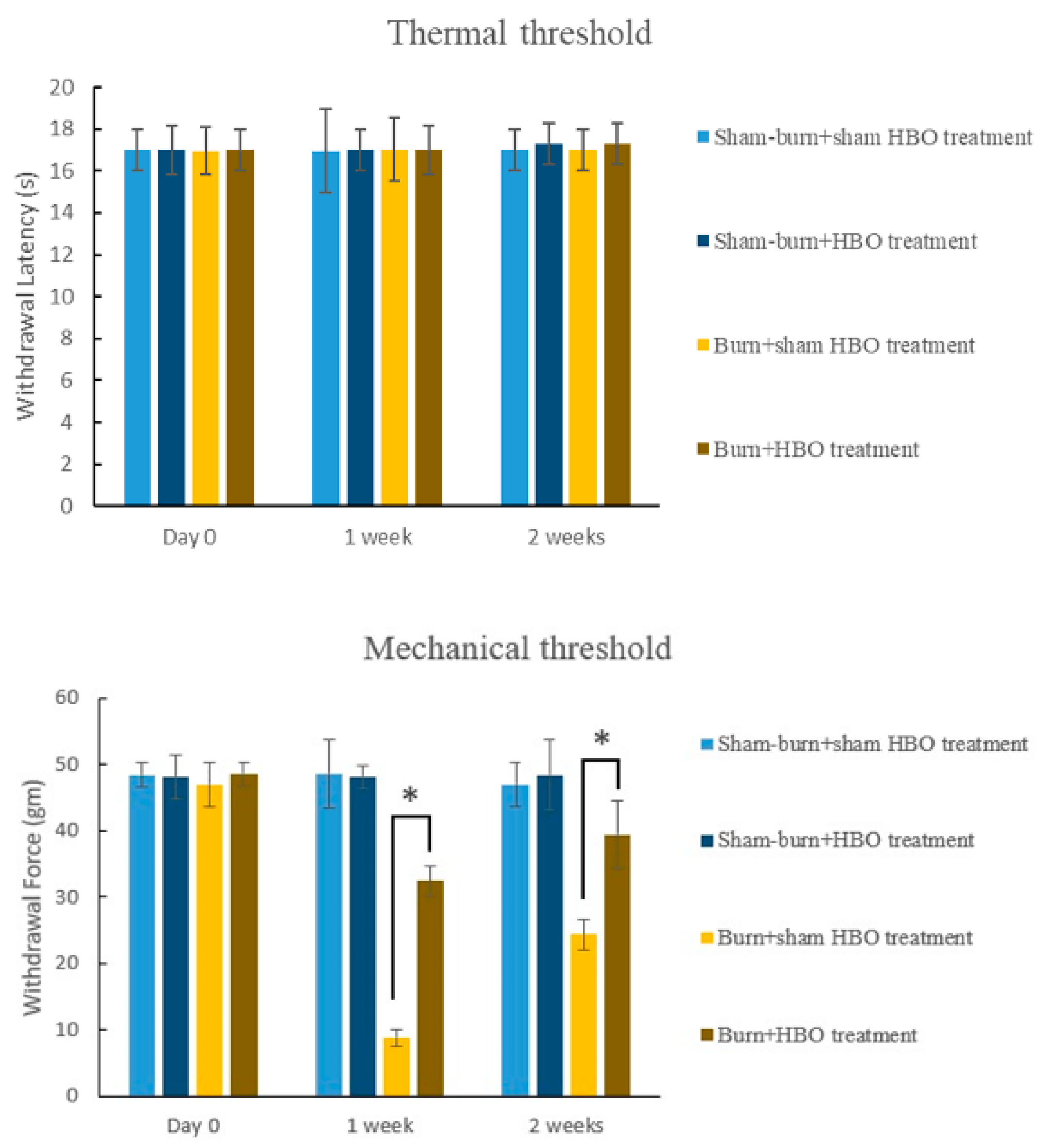
2.1. HBO Treatment Ameliorates Burn-Induced Mechanical Allodynia
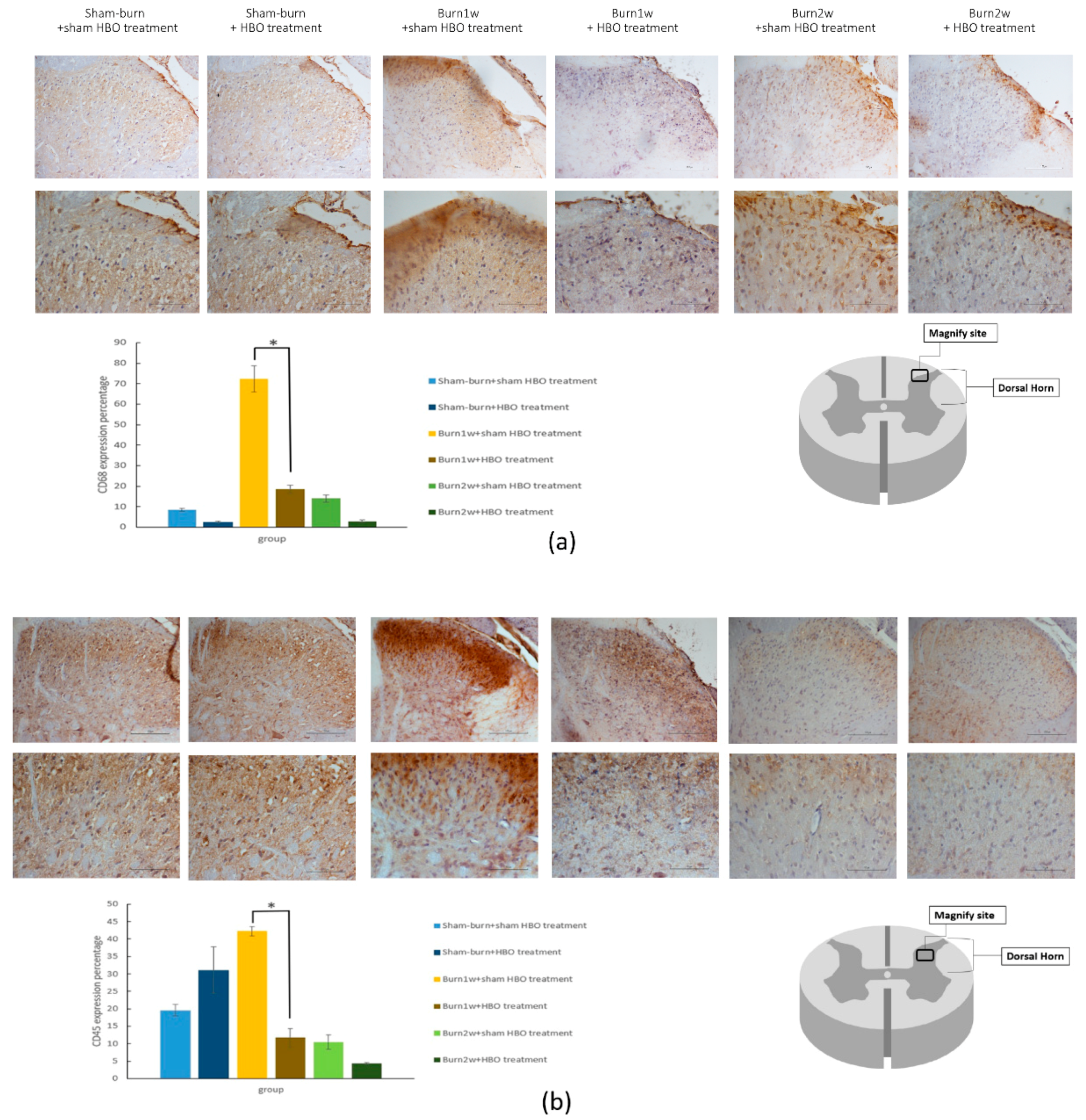
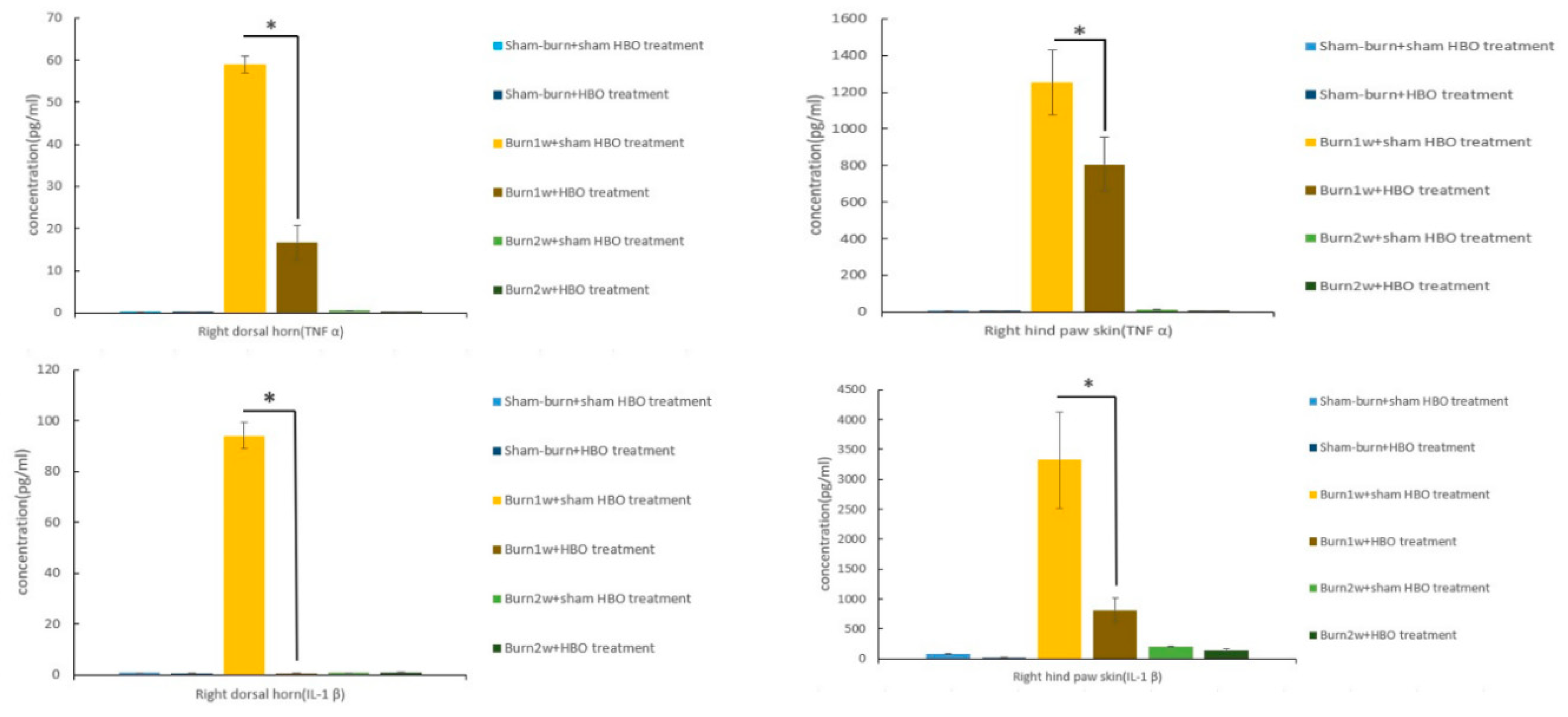
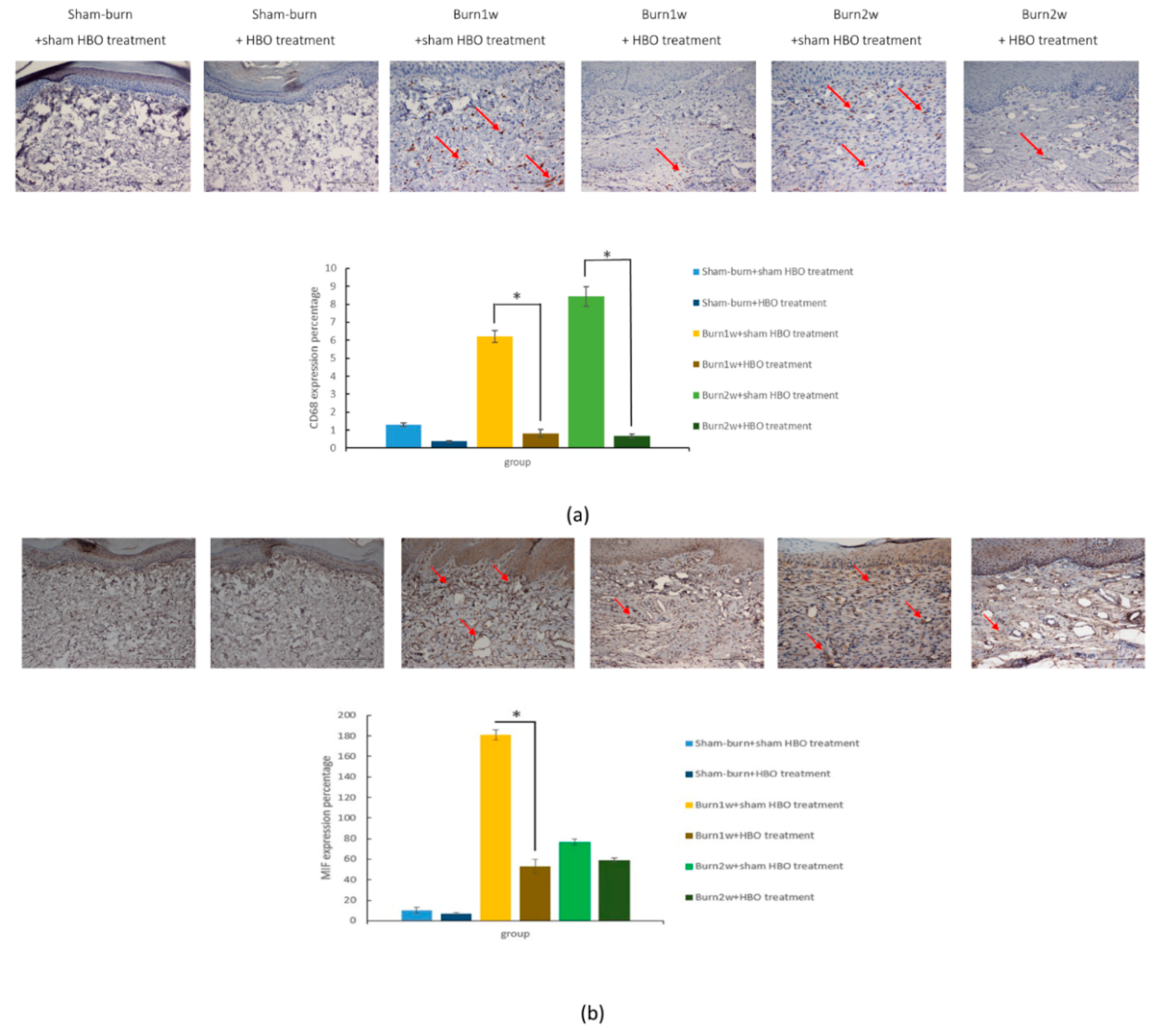
2.2. HBO Treatment Inhibits Microglial Cell Activation and Reduces Proinflammatory Cytokine Expression and Macrophage Recruitment
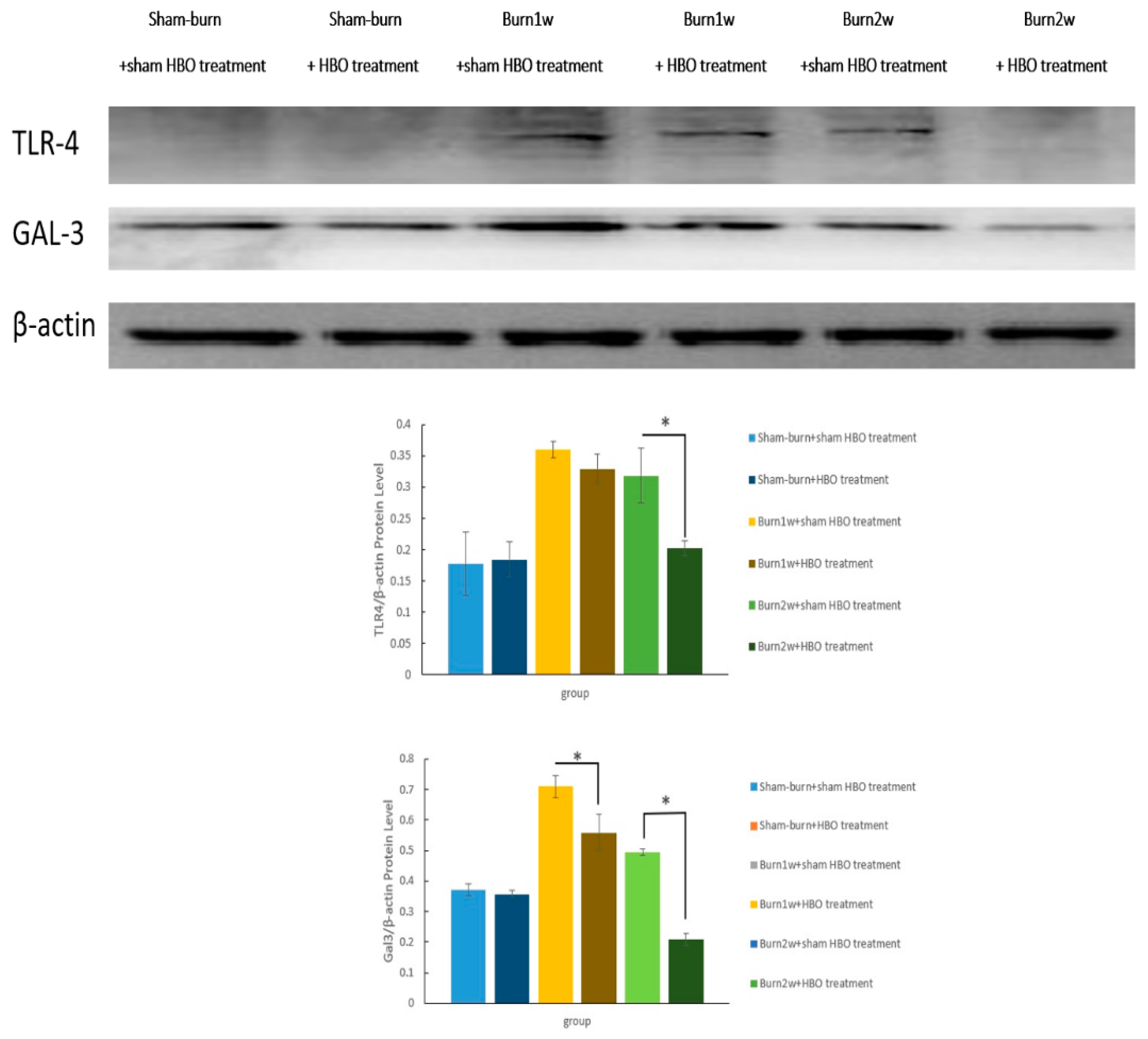
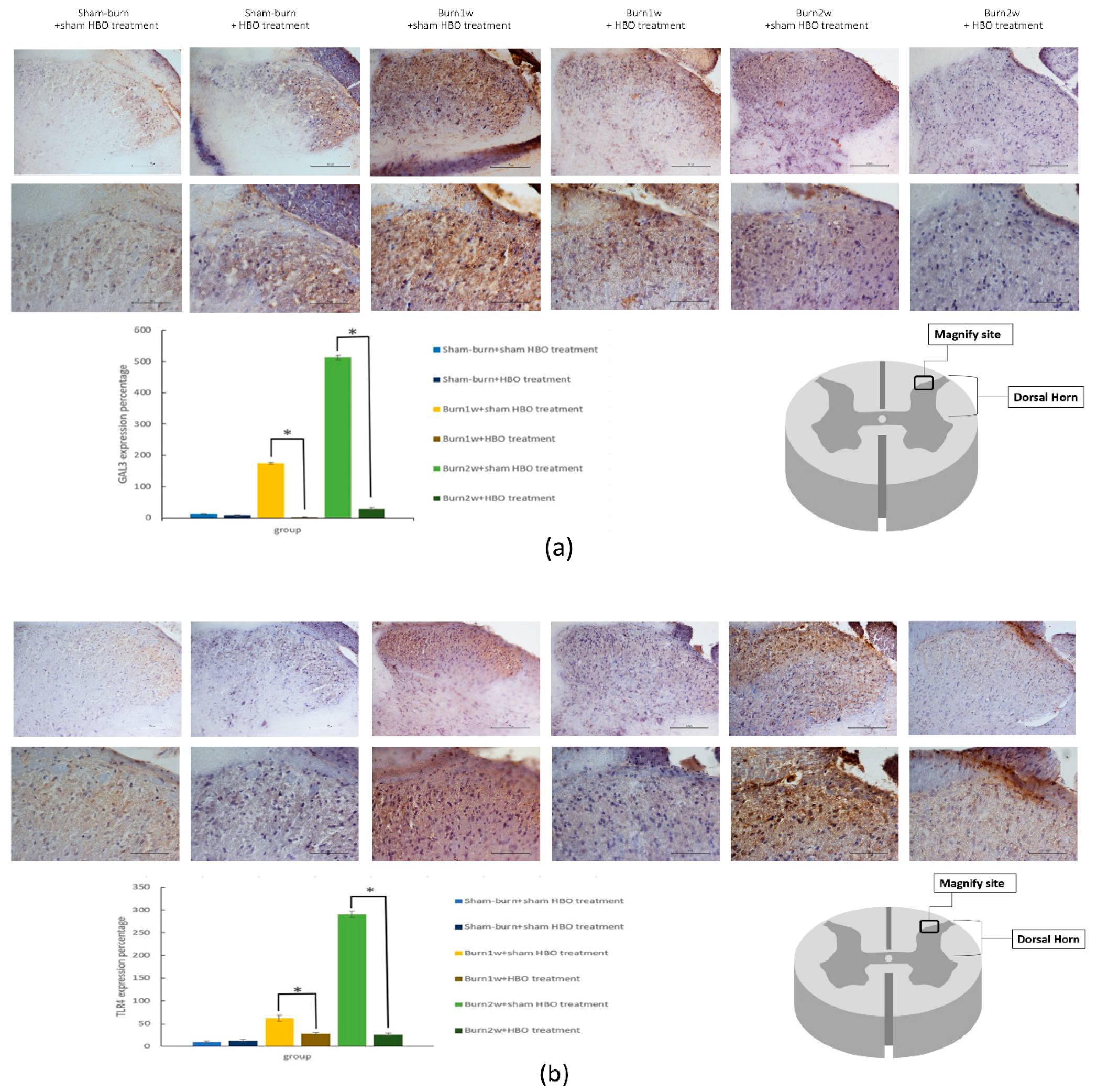
2.3. HBO Treatment Inhibits Gal-3 and TLR-4 Expression in the Dorsal Horns of the Spinal Cord and Gal-3 and TLR-4 Immunohistochemical Localization in the Hind Paw Skin
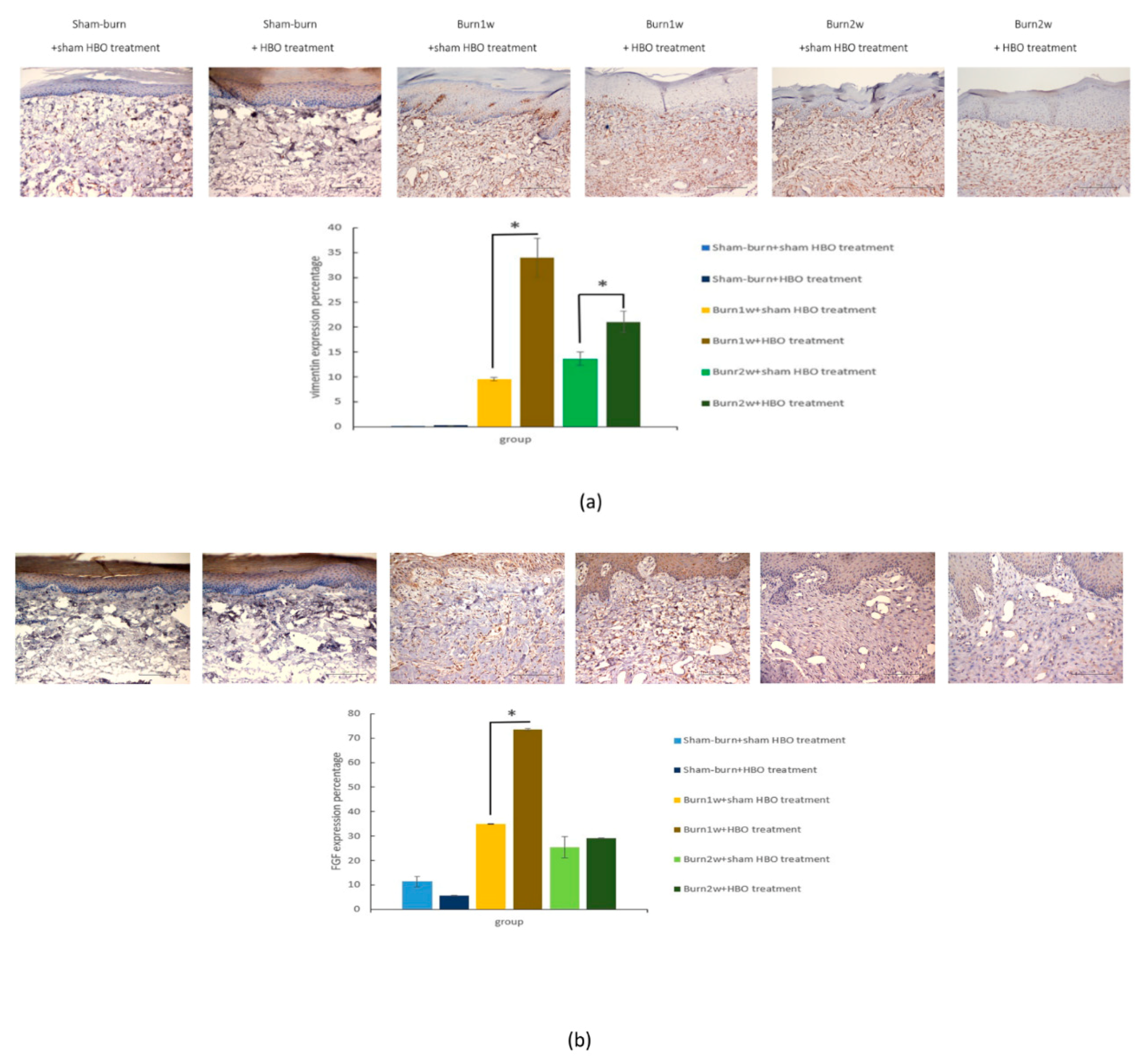
2.4. HBO Treatment Increases Growth Factor Expression in the Hind Paw Skin of Rats with Full-Thickness Burn Injury
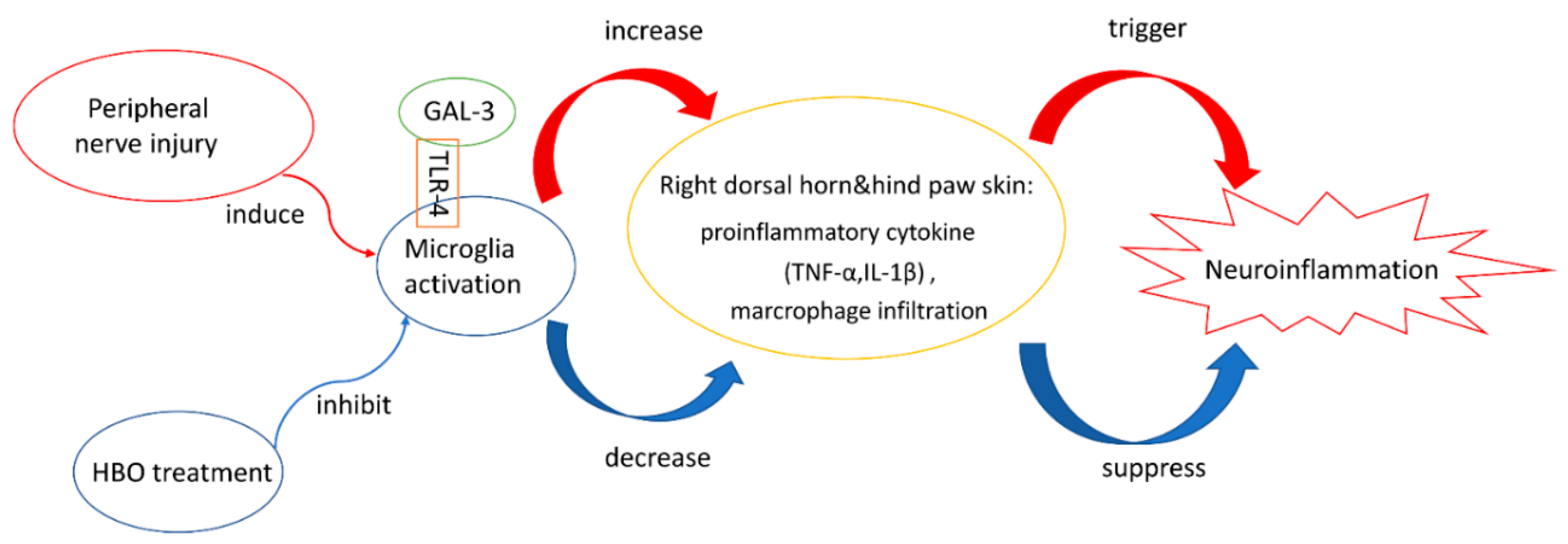
3. Discussion
4. Materials and Methods
4.1. Animal Preparation and Experimental Design
4.2. Full-Thickness Burn Injury Model
4.3. HBO Treatment
4.4. Behavior Test
4.5. Western Blot Assay
4.6. Immunohistochemical Assay
4.7. Enzyme-Linked Immunosorbent Assay
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nakagawa, Y.; Chiba, K. Diversity and plasticity of microglial cells in psychiatric and neurological disorders. Pharmacol. Ther. 2015, 154, 21–35. [Google Scholar] [CrossRef] [PubMed]
- Perry, V.H.; Nicoll, J.A.; Holmes, C. Microglia in neurodegenerative disease. Nat. Rev. Neurol. 2010, 6, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Perry, V.H.; Holmes, C. Microglial priming in neurodegenerative disease. Nat. Rev. Neurol. 2014, 10, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.J.; Tanasescu, R.; Gran, B. Innate immune regulation of autoimmunity in multiple sclerosis: Focus on the role of Toll-like receptor 2. J. Neuroimmunol. 2017, 304, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Moss, D.W.; Bates, T.E. Activation of murine microglial cell lines by lipopolysaccharide and interferon-gamma causes NO-mediated decreases in mitochondrial and cellular function. Eur. J. Neurosci. 2001, 13, 529–538. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Ma, J.; Liu, B.; Dai, C.; Xie, T.; Ma, X.; Li, M.; Dong, J.; Lan, Q.; Huang, Q. Hyperbaric oxygen therapy sensitizes nimustine treatment for glioma in mice. Cancer Med. 2016, 5, 3147–3155. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, E.C. Hyperbaric oxygenation in peripheral nerve repair and regeneration. Neurol. Res. 2007, 29, 184–198. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, S.; Luo, M.; Li, Y. Hyperbaric oxygen therapy improves local microenvironment after spinal cord injury. Neural Regen. Res. 2014, 9, 2182–2188. [Google Scholar] [CrossRef] [PubMed]
- Geng, C.K.; Cao, H.H.; Ying, X.; Zhang, H.T.; Yu, H.L. The effects of hyperbaric oxygen on macrophage polarization after rat spinal cord injury. Brain Res. 2015, 1606, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Kang, N.; Hai, Y.; Yang, J.; Liang, F.; Gao, C.J. Hyperbaric oxygen intervention reduces secondary spinal cord injury in rats via regulation of HMGB1/TLR4/NF-kappaB signaling pathway. Int. J. Clin. Exp. Pathol. 2015, 8, 1141–1153. [Google Scholar] [PubMed]
- Liang, F.; Li, C.; Gao, C.; Li, Z.; Yang, J.; Liu, X.; Wang, Y. Effects of hyperbaric oxygen therapy on NACHT domain-leucine-rich-repeat- and pyrin domain-containing protein 3 inflammasome expression in rats following spinal cord injury. Mol. Med. Rep. 2015, 11, 4650–4656. [Google Scholar] [CrossRef] [PubMed]
- Wilson, H.D.; Wilson, J.R.; Fuchs, P.N. Hyperbaric oxygen treatment decreases inflammation and mechanical hypersensitivity in an animal model ofinflammatory pain. Brain Res. 2006, 1098, 126–128. [Google Scholar] [CrossRef] [PubMed]
- Wilson, H.D.; Toepfer, V.E.; Senapati, A.K.; Wilson, J.R.; Fuchs, P.N. Hyperbaric oxygen treatment iscomparable to acetylsalicylic acid treatment in an animal model of arthritis. J. Pain 2007, 8, 924–930. [Google Scholar] [CrossRef] [PubMed]
- Sümen, G.; Cimsit, M.; Eroglu, L. Hyperbaric oxygen treatment reduces carrageenan-induced acute inflammation in rats. Eur. J. Pharmacol. 2001, 431, 265–268. [Google Scholar] [CrossRef]
- Mychaskiw, G.; Pan, J.; Shah, S.; Zubkov, A.; Clower, B.; Badr, A.; Zhang, J.H. Effects of hyperbaric oxygen on skin blood flow and tissue morphology following sciatic nerve constriction. Pain Phys. 2005, 8, 157–161. [Google Scholar]
- Hui, J.; Zhang, Z.J.; Zhang, X.; Shen, Y.; Gao, Y.J. Repetitive hyperbaric oxygen treatment attenuates complete Freund’s adjuvant-induced pain and reduces glia-mediated neuroinflammation in the spinal cord. J. Pain 2013, 14, 747–758. [Google Scholar] [CrossRef] [PubMed]
- Burguillos, M.A.; Svensson, M.; Schulte, T.; Boza-Serrano, A.; Garcia-Quintanilla, A.; Kavanagh, E.; Santiago, M.; Viceconte, N.; Oliva-Martin, M.J.; Osman, A.M.; et al. Microglia-Secreted Galectin-3 Acts as a Toll-like Receptor 4 Ligand and Contributes to Microglial Activation. Cell Rep. 2015, 10, 1626–1638. [Google Scholar] [CrossRef] [PubMed]
- Li, L.C.; Li, J.; Gao, J. Functions of galectin-3 and its role in fibrotic diseases. J. Pharmacol. Exp. Ther. 2014, 351, 336–343. [Google Scholar] [CrossRef] [PubMed]
- Peacock, W.F.; Disomma, S. Emergency department use of galectin-3. Crit. Pathw. Cardiol. 2014, 13, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Ochieng, J.; Warfield, P.; Green-Jarvis, B.; Fentie, I. Galectin-3 regulates the adhesive interaction between breast carcinoma cells and elastin. J. Cell. Biochem. 1999, 75, 505–514. [Google Scholar] [CrossRef]
- Matarrese, P.; Fusco, O.; Tinari, N.; Natoli, C.; Liu, F.T.; Semeraro, M.L.; Malorni, W.; Iacobelli, S. Galectin-3 overexpression protects from apoptosis by improving cell adhesion properties. Int. J. Cancer 2000, 85, 545–554. [Google Scholar] [CrossRef]
- Lepur, A.; Carlsson, M.C.; Novak, R.; Dumic, J.; Nilsson, U.J.; Leffler, H. Galectin-3 endocytosis by carbohydrate independent and dependent pathways in different macrophage like cell types. Biochim. Biophys. Acta 2012, 1820, 804–818. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Guo, X.L. Molecular regulation of galectin-3 expression and therapeutic implication in cancer progression. Biomed. Pharmacother. 2016, 78, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Komai-Koma, M.; Gilchrist, D.S.; Hsu, D.K.; Liu, F.T.; Springall, T.; Xu, D. Galectin-3 is a negative regulator of lipopolysaccharide-mediated inflammation. J. Immunol. 2008, 181, 2781–2789. [Google Scholar] [CrossRef] [PubMed]
- Boza-Serrano, A.; Reyes, J.F.; Rey, N.L.; Leffler, H.; Bousset, L.; Nilsson, U.; Brundin, P.; Venero, J.L.; Burguillos, M.A.; Deierborg, T. The role of Galectin-3 in alpha-synuclein-induced microglial activation. Acta Neuropathol. Commun. 2014, 2, 156. [Google Scholar] [CrossRef] [PubMed]
- Jia, W.; Kidoya, H.; Yamakawa, D.; Naito, H.; Takakura, N. Galectin-3 accelerates M2 macrophage infiltration and angiogenesis in tumors. Am. J. Pathol. 2013, 182, 1821–1831. [Google Scholar] [CrossRef] [PubMed]
- Piper, S.E.; de Courcey, J.; Sherwood, R.A.; Amin-Youssef, G.F.; McDonagh, T.A. Serial galectin-3 for the monitoring of optimally treated stable chronic heart failure: A pilot study. Int. J. Cardiol. 2016, 207, 279–281. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, C.T.; Lood, C.; Ostergaard, O.; Iversen, L.V.; Voss, A.; Bengtsson, A.; Jacobsen, S.; Heegaard, N.H. Plasma levels of galectin-3-binding protein reflect type I interferon activity and are increased in patients with systemic lupus erythematosus. Lupus Sci. Med. 2014, 1, e000026. [Google Scholar] [CrossRef] [PubMed]
- Koca, S.S.; Akbas, F.; Ozgen, M.; Yolbas, S.; Ilhan, N.; Gundogdu, B.; Isik, A. Serum galectin-3 level in systemic sclerosis. Clin. Rheumatol. 2014, 33, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Han, Q.; Wang, X.; Ai, Z.; Zheng, Y. Galectin-3 Inhibition Is Associated with Neuropathic Pain Attenuation after Peripheral Nerve Injury. PLoS ONE 2016, 11, e0148792. [Google Scholar] [CrossRef] [PubMed]
- Arad, U.; Madar-Balakirski, N.; Angel-Korman, A.; Amir, S.; Tzadok, S.; Segal, O.; Menachem, A.; Gold, A.; Elkayam, O.; Caspi, D. Galectin-3 is a sensor-regulator of toll-like receptor pathways in synovial fibroblasts. Cytokine 2015, 73, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Hacker, H.; Vabulas, R.M.; Takeuchi, O.; Hoshino, K.; Akira, S.; Wagner, H. Immune cell activation by bacterial CpG-DNA through myeloid differentiation marker 88 and tumor necrosis factor receptor-associated factor (TRAF)6. J. Exp. Med. 2000, 192, 595–600. [Google Scholar] [CrossRef] [PubMed]
- Deborah, K.M. MAP kinase pathways. Cold Spring Harb. Perspect. Biol. 2012, 4, a011254. [Google Scholar]
- Sivori, S.; Falco, M.; Della, C.M.; Carlomagno, S.; Vitale, M.; Moretta, L.; Moretta, A. CpG and double-stranded RNA trigger human NK cells by Toll-like receptors: Induction of cytokine release and cytotoxicity against tumors and dendritic cells. Proc. Natl. Acad. Sci. USA 2004, 101, 10116–10121. [Google Scholar] [CrossRef] [PubMed]
- Ohashi, K.; Burkart, V.; Flohé, S.; Kolb, H. Cutting edge: Heat shock protein 60 is a putative endogenous ligand of the toll-like receptor-4 complex. J. Immunol. 2000, 164, 558–561. [Google Scholar] [CrossRef] [PubMed]
- Smiley, S.T.; King, J.A.; Hancock, W.W. Fibrinogen stimulates macrophage chemokine secretion through toll-like receptor 4. J. Immunol. 2001, 167, 2887–2894. [Google Scholar] [CrossRef] [PubMed]
- Kagan, J.C.; Medzhitov, R. Phosphoinositide-mediated adaptor recruitment controls toll-like receptor signaling. Cell. Mol. Neurobiol. 2006, 125, 943–955. [Google Scholar] [CrossRef] [PubMed]
- Johnson, G.B.; Brunn, G.J.; Kodaira, Y.; Platt, J.L. Receptor-mediated monitoring of tissue well-being via detection of soluble heparan sulfate by Toll-like receptor 4. J. Immunol. 2002, 168, 5233–5239. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Wang, C.; Li, W.H.; Liu, J.; He, H.H.; Long, J.H.; Yang, J.; Sui, X.; Wang, S.; You, Z.; Wang, Y.A. Madecassoside protects BV2 microglial cells from oxygen-glucose deprivation/reperfusion-induced injury via inhibition of the toll-like receptor 4 signaling pathway. Brain Res. 2018, 1679, 144–154. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.H.; Wu, S.H.; Chang, K.P.; Cheng, K.I.; Lee, S.S.; Kwan, A.L.; Yeh, J.L.; Tsai, H.P.; Lin, S.D.; Lai, C.S. Autologous fat grafting alleviates burn-induced neuropathic pain in rats. Plast. Reconstr. Surg. 2014, 133, 1396–1405. [Google Scholar] [CrossRef] [PubMed]
- Edwards, M.; Cooper, J.S. Hyperbaric, Thermal Burns; StatPearls: Treasure Island, FL, USA, 2017. [Google Scholar]
- O’Sullivan, S.T.; OConnor, T.P. Immunosuppression following thermal injury: The pathogenesis of immunodysfunction. Br. J. Plast. Surg. 1997, 50, 615–623. [Google Scholar] [CrossRef]
- Schwacha, M.G. Macrophages and post-burn immune dysfunction. Burns 2003, 29, 1–14. [Google Scholar] [CrossRef]
- Girardot, T.; Rimmele, T.; Venet, F.; Monneret, G. Apoptosis-induced lymphopenia in sepsis and other severe injuries. Apoptosis 2017, 22, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Huh, Y.; Ji, R.R.; Chen, G. Neuroinflammation, Bone Marrow Stem Cells, and Chronic Pain. Front. Immunol. 2017, 8, 1014. [Google Scholar] [CrossRef] [PubMed]
- Ji, R.R.; Xu, Z.Z.; Gao, Y.J. Emerging targets in neuroinflammation-driven chronic pain. Nat. Rev. Drug Discov. 2014, 13, 533–548. [Google Scholar] [CrossRef] [PubMed]
- Kettenmann, H.; Hanisch, U.K.; Noda, M.; Verkhratsky, A. Physiology of microglia. Physiol. Rev. 2011, 91, 461–553. [Google Scholar] [CrossRef] [PubMed]
- Rock, R.B.; Gekker, G.; Hu, S.; Sheng, W.S.; Cheeran, M.; Lokensgard, J.R.; Peterson, P.K. Role of microglia in central nervous system infections. Clin. Microbiol. Rev. 2004, 17, 942–964. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Andonegui, G.; Wong, C.H.; Kubes, P. Role of endothelial TLR4 for neutrophil recruitment into central nervous system microvessels in systemic inflammation. J. Immunol. 2009, 183, 5244–5250. [Google Scholar] [CrossRef] [PubMed]
- Rao, R.M.; Yang, L.; Garcia-Cardena, G.; Luscinskas, F.W. Endothelial-dependent mechanisms of leukocyte recruitment to the vascular wall. Circ. Res. 2007, 101, 234–247. [Google Scholar] [CrossRef] [PubMed]
- D’Mello, C.; Le, T.; Swain, M.G. Cerebral microglia recruit monocytes into the brain in response to tumor necrosis factoralpha signaling during peripheral organ inflammation. J. Neurosci. 2009, 29, 2089–2102. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.W.; Tan, A.; Saab, C.; Waxman, S. Unilateral focal burn injury is followed by long-lasting bilateral allodynia and neuronal hyperexcitability in spinal cord dorsal horn. J. Pain 2010, 11, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, G.C.; Ilarregui, J.M.; Rubel, C.J.; Toscano, M.A.; Gomez, S.A.; Beigier Bompadre, M.; Isturiz, M.A.; Rabinovich, G.A.; Palermo, M.S. Galectin-3 and soluble fibrinogen act in concert to modulate neutrophil activation and survival: Involvement of alternative MAPK pathways. Glycobiology 2005, 15, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.J.; Hao, W.R.; Chang, K.C.; Liu, J.C. The infiltrating macrophage-secreted galectin-3 plays an essential role in cardiac fibrosis and diastolic function in murine pressure-overload model. J. Hypertens. 2016, 34. [Google Scholar] [CrossRef]
- Nio-Kobayashi, J.; Boswell, L.; Amano, M.; Iwanaga, T.; Duncan, W.C. The loss of luteal progesterone production in women is associated with a galectin switch via α2,6-sialylation of glycoconjugates. J. Clin. Endocrinol. Metab. 2014, 99, 4616–4624. [Google Scholar] [CrossRef] [PubMed]
- Nishi, Y.; Sano, H.; Kawashima, T.; Okada, T.; Kuroda, T.; Kikkawa, K.; Kawashima, S.; Tanabe, M.; Goto, T.; Matsuzawa, Y.; et al. Role of galectin-3 in human pulmonary fibrosis. Allergol. Int. 2007, 56, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.J.; Yu, G.F.; Jie, Y.Q.; Fan, X.F.; Huang, Q.; Dai, W.M. Role of galectin-3 in plasma as a predictive biomarker of outcome after acute intracerebral hemorrhage. J. Neurol. Sci. 2016, 368, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Fritsch, K.; Mernberger, M.; Nist, A.; Stiewe, T.; Brehm, A.; Jacob, R. Galectin-3 interacts with components of the nuclear ribonucleoprotein complex. BMC Cancer 2016, 16, 502. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.G.; Kim, D.H.; Kim, S.J.; Cho, Y.; Jung, J.; Jang, W.; Chun, K.H. Galectin-3 supports stemness in ovarian cancer stem cells by activation of the Notch1 intracellular domain. Oncotarget 2016, 7, 68229–68241. [Google Scholar] [CrossRef] [PubMed]
- Frangogiannis, N.G. The inflammatory response in myocardial injury, repair, and remodelling. Nat. Rev. Cardiol. 2014, 11, 255. [Google Scholar] [CrossRef] [PubMed]
- Bergler, T.; Hoffmann, U.; Bergler, E.; Jung, B.; Banas, M.C.; Reinhold, S.W.; Kramer, B.K.; Banas, B. Toll-like receptor 4 in experimental kidney transplantation: Early mediator of endogenous danger signals. Nephron Exp. Nephrol. 2012, 121, e59–e70. [Google Scholar] [CrossRef] [PubMed]
- Wesley, U.V.; Vemuganti, R.; Ayvaci, E.R.; Dempsey, R.J. Galectin-3 enhances angiogenic and migratory potential of microglial cells via modulation of integrin linked kinase signaling. Brain Res. 2013, 1496, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Franco, R.; Fernandez-Suarez, D. Alternatively activated microglia and macrophages in the central nervous system. Prog. Neurobiol. 2015, 131, 65–86. [Google Scholar] [CrossRef] [PubMed]
- Boche, D.; Perry, V.H.; Nicoll, J.A. Activation patterns of microglia and their identification in the human brain. Neuropathol. Appl. Neurobiol. 2013, 39, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.Q.; Li, A.; Yang, X.; Xiao, X.; Hu, R.; Wang, T.W.; Dou, X.Y.; Yang, D.J.; Dong, Z. Paeoniflorin exerts neuroprotective effects by modulating the M1/M2 subset polarization of microglia/macrophages in the hippocampal CA1 region of vascular dementia rats via cannabinoid receptor 2. Chin. Med. 2018, 13, 14. [Google Scholar] [CrossRef] [PubMed]
- Jing, X.; Chen, S.S.; Jing, W.; Tan, Q.; Yu, M.X.; Tu, J.C. Diagnostic potential of differentially expressed Homer1, IL-1β and TNF-α in coronary artery disease. Int. J. Mol. Sci. 2014, 16, 535–546. [Google Scholar] [CrossRef] [PubMed]
- Gregory, J.L.; Morand, E.F.; McKeown, S.J.; Ralph, J.A.; Hall, P.; Yang, Y.H.; McColl, S.R.; Hickey, M.J. Macrophage migration inhibitory factor induces macrophage recruitment via CC chemokine ligand 2. J. Immunol. 2006, 177, 8072–8079. [Google Scholar] [CrossRef] [PubMed]
- Calandra, T.; Bernhagen, J.; Metz, C.N.; Spiegel, L.A.; Bacher, M.; Donnelly, T.; Cerami, A.; Bucala, R. MIF as a glucocorticoid-induced modulator of cytokine production. Nature 1995, 377, 68. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, H.; Quehenberger, O.; Kondratenko, N.; Green, S.; Steinberg, D. Minimally oxidized low-density lipoprotein increases expression of scavenger receptor A, CD36, and macrosialin in resident mouse peritoneal macrophages. Arterioscler. Thromb. Vasc. Biol. 1998, 18, 794–802. [Google Scholar] [CrossRef] [PubMed]
- Tsukamoto, K.; Kinoshita, M.; Kojima, K.; Mikuni, Y.; Kudo, M.; Mori, M.; Fujita, M.; Horie, E.; Shimaru, N.; Teramoto, T. Synergically increased expression of CD36, CLA-1 and CD68, but not of SR-A and LOX-1, with the progression to foam cells from macrophages. J. Atheroscler. Thromb. 2002, 9, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Ramprasad, M.P.; Terpstra, V.; Kondratenko, N.; Quehenberger, O.; Steinberg, D. Cell surface expression of mouse macrosialin and human CD68 and their role as macrophage receptors for oxidized low density lipoprotein. Proc. Natl. Acad. Sci. USA 1996, 93, 14833–14838. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Chen, A.; Wang, T.; Wang, M.; Ning, X.; He, M.; Hu, Y.; Yuan, L.; Li, S.; Wang, Q.; et al. Detecting cell-in-cell structures in human tumor samples by E-cadherin/CD68/CD45 triple staining. Oncotarget 2015, 6, 20278–20287. [Google Scholar] [CrossRef] [PubMed]
- Kahle, A.C.; Cooper, J.S. Hyperbaric, Physiological and Pharmacological Effects Gases; StatPearls: Treasure Island, FL, USA, 2017. [Google Scholar]
- Schulze, J.; Kaiser, O.; Paasche, G.; Lamm, H.; Pich, A.; Hoffmann, A.; Lenarz, T.; Warnecke, A. Effect of hyperbaric oxygen on BDNF-release and neuroprotection: Investigations with human mesenchymal stem cells and genetically modified NIH3T3 fibroblasts as putative cell therapeutics. PLoS ONE 2017, 12, e0178182. [Google Scholar] [CrossRef] [PubMed]
- Asano, T.; Kaneko, E.; Shinozaki, S.; Imai, Y.; Shibayama, M.; Chiba, T.; Ai, M.; Kawakami, A.; Asaoka, H.; Nakayama, T.; et al. Hyperbaric oxygen induces basic fibroblast growth factor and hepatocyte growth factor expression, and enhances blood perfusion and muscle regeneration in mouse ischemic hind limbs. Circ. J. 2007, 71, 405–411. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Z.-S.; Lo, J.-J.; Wu, S.-H.; Wang, C.-Z.; Chen, R.-F.; Lee, S.-S.; Chai, C.-Y.; Huang, S.-H. Early Hyperbaric Oxygen Treatment Attenuates Burn-Induced Neuroinflammation by Inhibiting the Galectin-3-Dependent Toll-Like Receptor-4 Pathway in a Rat Model. Int. J. Mol. Sci. 2018, 19, 2195. https://doi.org/10.3390/ijms19082195
Wu Z-S, Lo J-J, Wu S-H, Wang C-Z, Chen R-F, Lee S-S, Chai C-Y, Huang S-H. Early Hyperbaric Oxygen Treatment Attenuates Burn-Induced Neuroinflammation by Inhibiting the Galectin-3-Dependent Toll-Like Receptor-4 Pathway in a Rat Model. International Journal of Molecular Sciences. 2018; 19(8):2195. https://doi.org/10.3390/ijms19082195
Chicago/Turabian StyleWu, Zong-Sheng, Jing-Jou Lo, Sheng-Hua Wu, Chau-Zen Wang, Rong-Fu Chen, Su-Shin Lee, Chee-Yin Chai, and Shu-Hung Huang. 2018. "Early Hyperbaric Oxygen Treatment Attenuates Burn-Induced Neuroinflammation by Inhibiting the Galectin-3-Dependent Toll-Like Receptor-4 Pathway in a Rat Model" International Journal of Molecular Sciences 19, no. 8: 2195. https://doi.org/10.3390/ijms19082195
APA StyleWu, Z.-S., Lo, J.-J., Wu, S.-H., Wang, C.-Z., Chen, R.-F., Lee, S.-S., Chai, C.-Y., & Huang, S.-H. (2018). Early Hyperbaric Oxygen Treatment Attenuates Burn-Induced Neuroinflammation by Inhibiting the Galectin-3-Dependent Toll-Like Receptor-4 Pathway in a Rat Model. International Journal of Molecular Sciences, 19(8), 2195. https://doi.org/10.3390/ijms19082195






