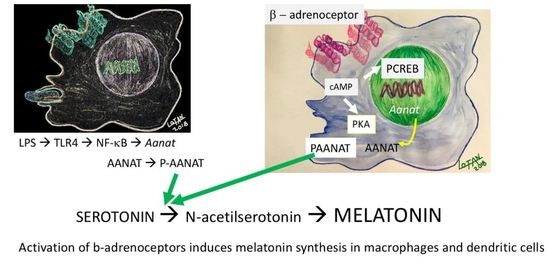
β-Adrenoceptors Trigger Melatonin Synthesis in Phagocytes
Abstract
:1. Introduction
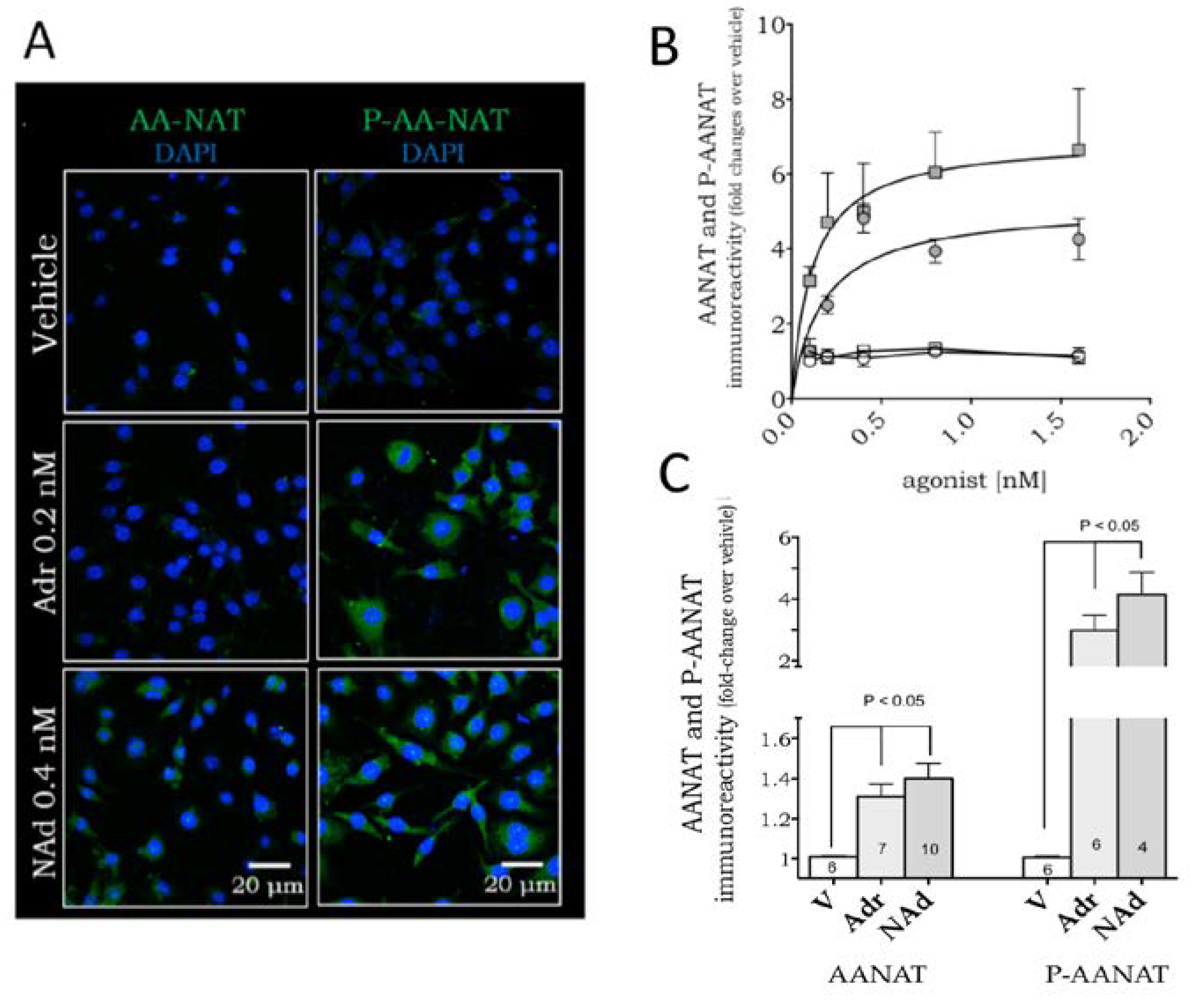
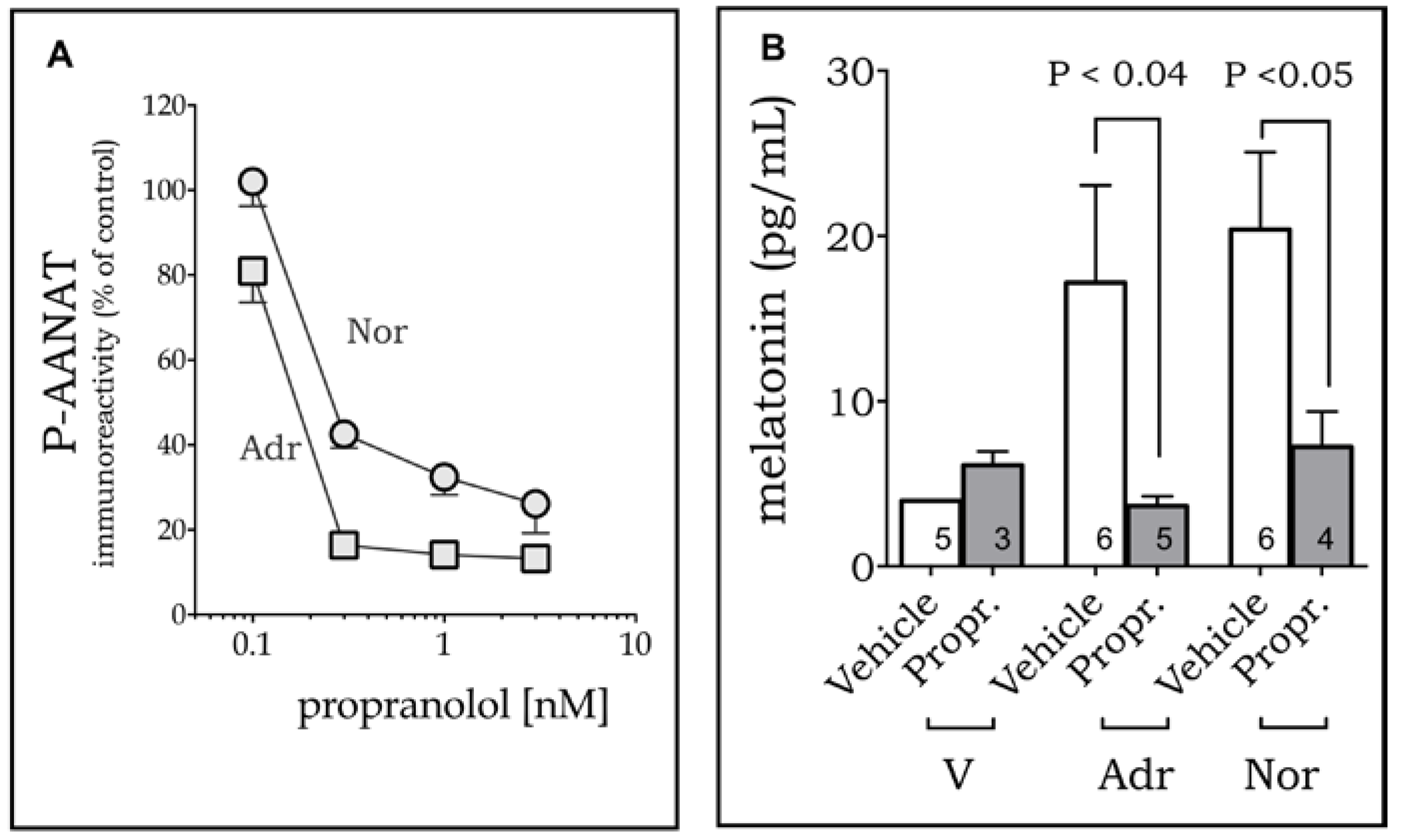
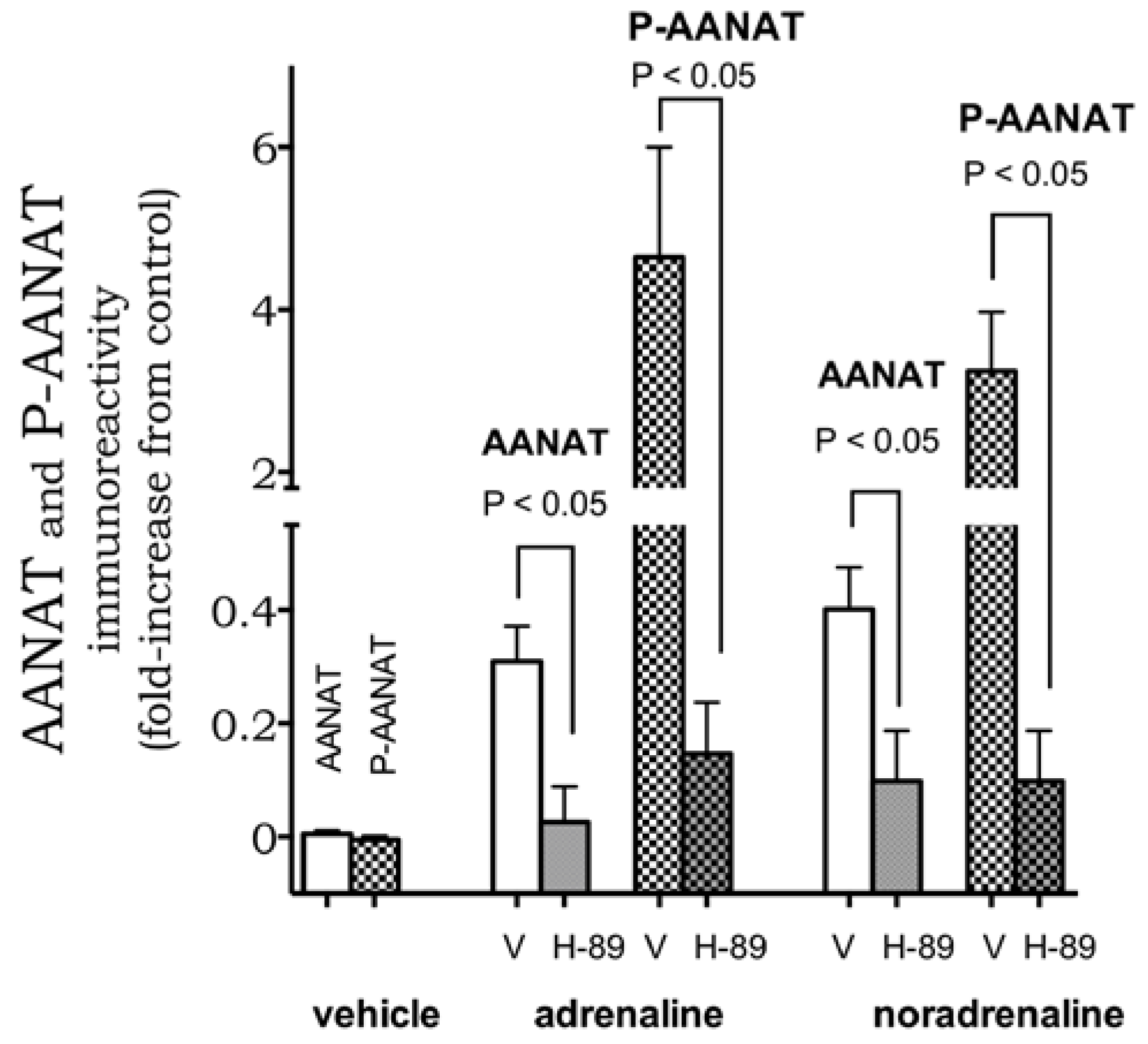
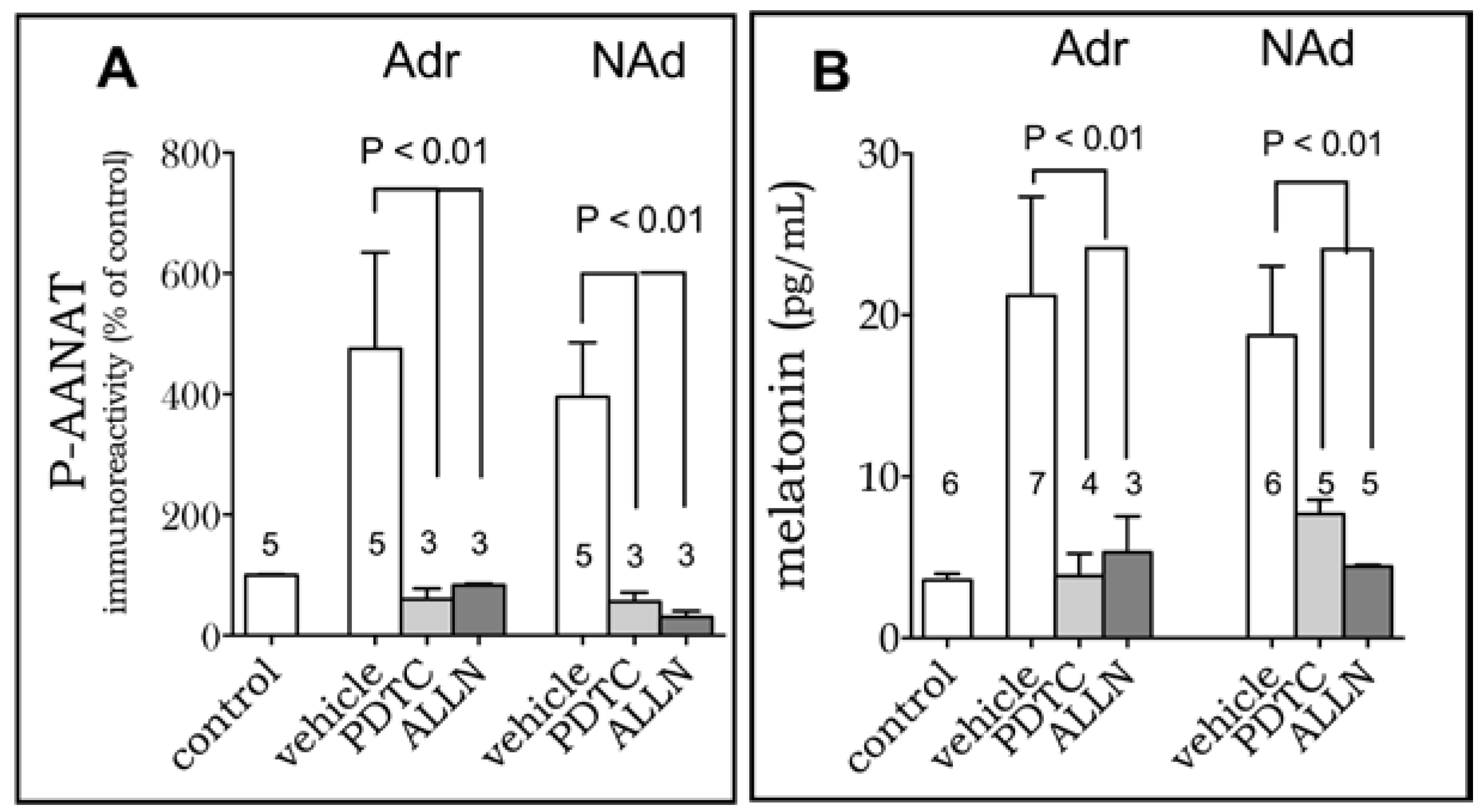
2. Results
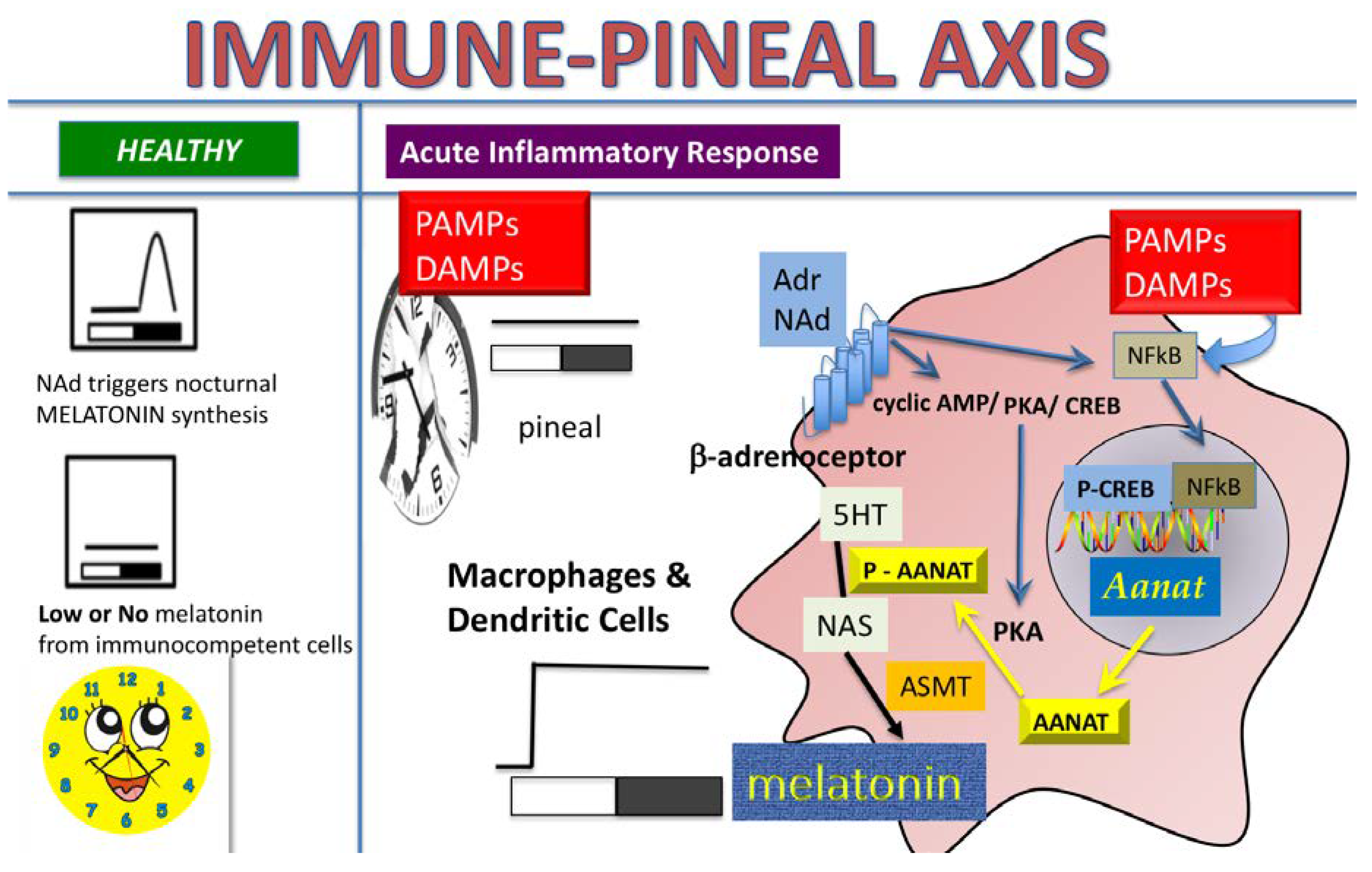
3. Discussion
4. Materials and Methods
4.1. Drugs and Reagents
4.2. Mice
4.3. Bone Marrow-Derived DC Generation and Culture
4.4. Cell Line RAW 264.7 Cultures
4.5. Immunofluorescence Detection of the Enzyme AANAT
4.6. Melatonin Dosage
4.7. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Carrillo-Vico, A.; Lardone, P.J.; Alvarez-Sánchez, N.; Rodríguez-Rodríguez, A.; Guerrero, J.M. Melatonin: Buffering the immune system. Int. J. Mol. Sci. 2013, 14, 8638–8683. [Google Scholar] [CrossRef] [PubMed]
- Lotufo, C.M.; Lopes, C.; Dubocovich, M.L.; Farsky, S.H.; Markus, R.P. Melatonin and N-acetylserotonin inhibit leukocyte rolling and adhesion to rat microcirculation. Eur. J. Pharmacol. 2001, 430, 351–357. [Google Scholar] [CrossRef]
- Markus, R.P.; Ferreira, Z.S.; Fernandes, P.A.; Cecon, E. The immune-pineal axis: A shuttle between endocrine and paracrine melatonin sources. Neuroimmunomodulation 2007, 14, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Markus, R.P.; Cecon, E.; Pires-Lapa, M.A. Immune-pineal axis: Nuclear factor κB (NF-κB) mediates the shift in the melatonin source from pinealocytes to immune competent cells. Int. J. Mol. Sci. 2013, 14, 10979–10997. [Google Scholar] [CrossRef] [PubMed]
- Muxel, S.M.; Pires-Lapa, M.A.; Monteiro, A.W.; Cecon, E.; Tamura, E.K.; Floeter-Winter, L.M.; Markus, R.P. NF-κB drives the synthesis of melatonin in RAW 264.7 macrophages by inducing the transcription of the arylalkylamine-N-acetyltransferase (AA-NAT) gene. PLoS ONE 2012, 7, e52010. [Google Scholar] [CrossRef] [PubMed]
- Pires-Lapa, M.A.; Tamura, E.K.; Salustiano, E.M.; Markus, R.P. Melatonin synthesis in human colostrum mononuclear cells enhances dectin-1-mediated phagocytosis by mononuclear cells. J. Pineal Res. 2013, 55, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Pontes, G.N.; Cardoso, E.C.; Carneiro-Sampaio, M.M.; Markus, R.P. Injury switches melatonin production source from endocrine (pineal) to paracrine (phagocytes)—melatonin in human colostrum and colostrum phagocytes. J. Pineal Res. 2006, 41, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Pontes, G.N.; Cardoso, E.C.; Carneiro-Sampaio, M.M.; Markus, R.P. Pineal melatonin and the innate immune response: The TNF-alpha increase after cesarean section suppresses nocturnal melatonin production. J. Pineal Res. 2007, 43, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Tamura, E.K.; Fernandes, P.A.; Marçola, M.; da Silveira-Cruz-Machado, S.; Markus, R.P. Long-lasting priming of endothelial cells by plasma melatonin levels. PLoS ONE 2010, 5, e13958. [Google Scholar] [CrossRef] [PubMed]
- Goswami, S.; Haldar, C. UVB irradiation severely induces systemic tissue injury by augmenting oxidative load in a tropical rodent: Efficacy of melatonin as an antioxidant. J. Photochem. Photobiol. B 2014, 141, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Kaur, C.; Sivakumar, V.; Robinson, R.; Foulds, W.S.; Luu, C.D.; Ling, E.A. Neuroprotective effect of melatonin against hypoxia-induced retinal ganglion cell death in neonatal rats. J. Pineal Res. 2013, 54, 190–206. [Google Scholar] [CrossRef] [PubMed]
- Radogna, F.; Nuccitelli, S.; Mengoni, F.; Ghibelli, L. Neuroprotection by melatonin on astrocytoma cell death. Ann. N. Y. Acad. Sci. 2009, 1171, 509–513. [Google Scholar] [CrossRef] [PubMed]
- Shin, I.S.; Park, J.W.; Shin, N.R.; Jeon, C.M.; Kwon, O.K.; Kim, J.S.; Kim, J.C.; Oh, S.R.; Ahn, K.S. Melatonin reduces airway inflammation in ovalbumin-induced asthma. Immunobiology 2014, 219, 901–908. [Google Scholar] [CrossRef] [PubMed]
- Acuña-Castroviejo, D.; Escames, G.; Venegas, C.; Díaz-Casado, M.E.; Lima-Cabello, E.; López, L.C.; Rosales-Corral, S.; Tan, D.X.; Reiter, R.J. Extrapineal melatonin: Sources, regulation, and potential functions. Cell. Mol. Life Sci. 2014, 71, 2997–3025. [Google Scholar] [CrossRef] [PubMed]
- Carrillo-Vico, A.; Calvo, J.R.; Abreu, P.; Lardone, P.J.; García-Mauriño, S.; Reiter, R.J.; Guerrero, J.M. Evidence of melatonin synthesis by human lymphocytes and its physiological significance: Possible role as intracrine, autocrine, and/or paracrine substance. FASEB J. 2004, 18, 537–539. [Google Scholar] [CrossRef] [PubMed]
- Lardone, P.J.; Carrillo-Vico, A.; Naranjo, M.C.; De Felipe, B.; Vallejo, A.; Karasek, M.; Guerrero, J.M. Melatonin synthesized by Jurkat human leukemic T cell line is implicated in IL-2 production. J. Cell. Physiol. 2006, 206, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Conti, A.; Conconi, S.; Hertens, E.; Skwarlo-Sonta, K.; Markowska, M.; Maestroni, J.M. Evidence for melatonin synthesis in mouse and human bone marrow cells. J. Pineal Res. 2000, 28, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Finocchiaro, L.M.; Nahmod, V.E.; Launay, J.M. Melatonin biosynthesis and metabolism in peripheral blood mononuclear leucocytes. Biochem. J. 1991, 280, 727–731. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laranjeira-Silva, M.F.; Zampieri, R.A.; Muxel, S.M.; Floeter-Winter, L.M.; Markus, R.P. Melatonin attenuates Leishmania (L.) amazonensis infection by modulating arginine metabolism. J. Pineal Res. 2015, 59, 478–487. [Google Scholar] [CrossRef] [PubMed]
- Simonneaux, V.; Ribelayga, C. Generation of the melatonin endocrine message in mammals: A review of the complex regulation of melatonin synthesis by norepinephrine, peptides, and other pineal transmitters. Pharmacol. Rev. 2003, 55, 325–395. [Google Scholar] [CrossRef] [PubMed]
- Muxel, S.M.; Laranjeira-Silva, M.F.; Carvalho-Sousa, C.E.; Floeter-Winter, L.M.; Markus, R.P. The RelA/cRel nuclear factor-κB (NF-κB) dimer, crucial for inflammation resolution, mediates the transcription of the key enzyme in melatonin synthesis in RAW 264.7 macrophages. J. Pineal Res. 2016, 60, 394–404. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.K.; Mantovani, A. Macrophage plasticity and interaction with lymphocyte subsets: Cancer as a paradigm. Nat. Immunol. 2010, 11, 889–896. [Google Scholar] [CrossRef] [PubMed]
- Gastel, J.A.; Roseboom, P.H.; Rinaldi, P.A.; Weller, J.L.; Klein, D.C. Melatonin production: Proteasomal proteolysis in serotonin N-acetyltransferase regulation. Science 1998, 279, 1358–1360. [Google Scholar] [CrossRef] [PubMed]
- Flierl, M.A.; Rittirsch, D.; Nadeau, B.A.; Chen, A.J.; Sarma, J.V.; Zetoune, F.S.; McGuire, S.R.; List, R.P.; Day, D.E.; Hoesel, L.M.; et al. Phagocyte-derived catecholamines enhance acute inflammatory injury. Nature 2007, 449, 721–725. [Google Scholar] [CrossRef] [PubMed]
- Flierl, M.A.; Rittirsch, D.; Nadeau, B.A.; Sarma, J.V.; Day, D.E.; Lentsch, A.B.; Huber-Lang, M.S.; Ward, P.A. Upregulation of phagocyte-derived catecholamines augments the acute inflammatory response. PLoS ONE 2009, 4, e4414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elenkov, I.J.; Wilder, R.L.; Chrousos, G.P.; Vizi, E.S. The sympathetic nerve—an integrative interface between two supersystems: The brain and the immune system. Pharmacol. Rev. 2000, 52, 595–638. [Google Scholar] [PubMed]
- Bosmann, M.; Grailer, J.J.; Zhu, K.; Matthay, M.A.; Sarma, J.V.; Zetoune, F.S.; Ward, P.A. Anti-inflammatory effects of β2 adrenergic receptor agonists in experimental acute lung injury. FASEB J. 2012, 26, 2137–2144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, S.T.; Su, H.; Zhang, Q.; Tang, H.Q.; Wang, C.J.; Zhou, Q.; Wei, W.; Zhu, H.Q.; Wang, Y. Melatonin Attenuates Aortic Endothelial Permeability and Arteriosclerosis in Streptozotocin-Induced Diabetic Rats: Possible Role of MLCK- and MLCP-Dependent MLC Phosphorylation. J. Cardiovasc. Pharmacol. Ther. 2016, 21, 82–92. [Google Scholar] [CrossRef] [PubMed]
- Assis-de-Brito, T.L.; Monte-Alto-Costa, A.; Romana-Souza, B. Propranolol impairs the closure of pressure ulcers in mice. Life Sci. 2014, 100, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Banchereau, J.; Steinman, R.M. Dendritic cells and the control of immunity. Nature 1998, 392, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Jockers, R.; Delagrange, P.; Dubocovich, M.L.; Markus, R.P.; Renault, N.; Tosini, G.; Cecon, E.; Zlotos, D.P. Update on melatonin receptors: IUPHAR Review 20. Br. J. Pharmacol. 2016, 173, 2702–2725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ganguly, S.; Gastel, J.A.; Weller, J.L.; Schwartz, C.; Jaffe, H.; Namboodiri, M.A.; Coon, S.L.; Hickman, A.B.; Rollag, M.; Obsil, T.; et al. Role of a pineal cAMP-operated arylalkylamine N-acetyltransferase/14-3-3-binding switch in melatonin synthesis. Proc. Natl. Acad. Sci. USA 2001, 98, 8083–8088. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klein, D.C.; Ganguly, S.; Coon, S.; Weller, J.L.; Obsil, T.; Hickman, A.; Dyda, F. 14-3-3 Proteins and photoneuroendocrine transduction: Role in controlling the daily rhythm in melatonin. Biochem. Soc. Trans. 2002, 30, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, P.A.; Cecon, E.; Markus, R.P.; Ferreira, Z.S. Effect of TNF-alpha on the melatonin synthetic pathway in the rat pineal gland: Basis for a ‘feedback’ of the immune response on circadian timing. J. Pineal Res. 2006, 41, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Cecon, E.; Fernandes, P.A.; Pinato, L.; Ferreira, Z.S.; Markus, R.P. Daily variation of constitutively activated nuclear factor kappa B (NFKB) in rat pineal gland. Chronobiol. Int. 2010, 27, 52–67. [Google Scholar] [CrossRef] [PubMed]
- Da Silveira Cruz-Machado, S.; Tamura, E.K.; Pinato, L.; Fernandes, P.A.; Markus, R.P. Daily corticosterone rhythm modulates pineal function through NFκB-related gene transcriptional Program. Sci. Rep. 2017, 7, 2091. [Google Scholar] [CrossRef] [PubMed]
- Da Silveira Cruz-Machado, S.; Pinato, L.; Tamura, E.K.; Carvalho-Sousa, C.E.; Markus, R.P. Glia-pinealocyte network: The paracrine modulation of melatonin synthesis by tumor necrosis factor (TNF). PLoS ONE 2012, 7, e40142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Da Silveira Cruz-Machado, S.; Carvalho-Sousa, C.E.; Tamura, E.K.; Pinato, L.; Cecon, E.; Fernandes, P.A.; de Avellar, M.C.; Ferreira, Z.S.; Markus, R.P. TLR4 and CD14 receptors expressed in rat pineal gland trigger NFKB pathway. J. Pineal Res. 2010, 49, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Witherow, D.S.; Garrison, T.R.; Miller, W.E.; Lefkowitz, R.J. beta-Arrestin inhibits NF-kappaB activity by means of its interaction with the NF-kappaB inhibitor IkappaBalpha. Proc. Natl. Acad. Sci. USA 2004, 101, 8603–8607. [Google Scholar] [CrossRef] [PubMed]
- Loniewski, K.; Shi, Y.; Pestka, J.; Parameswaran, N. Toll-like receptors differentially regulate GPCR kinases and arrestins in primary macrophages. Mol. Immunol. 2008, 45, 2312–2322. [Google Scholar] [CrossRef] [PubMed]
- Kizaki, T.; Izawa, T.; Sakurai, T.; Haga, S.; Taniguchi, N.; Tajiri, H.; Watanabe, K.; Day, N.K.; Toba, K.; Ohno, H. Beta2-adrenergic receptor regulates Toll-like receptor-4-induced nuclear factor-kappaB activation through beta-arrestin 2. Immunology 2008, 124, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Kizaki, T.; Shirato, K.; Sakurai, T.; Ogasawara, J.E.; Oh-ishi, S.; Matsuoka, T.; Izawa, T.; Imaizumi, K.; Haga, S.; Ohno, H. Beta2-adrenergic receptor regulate Toll-like receptor 4-induced late-phase NF-kappaB activation. Mol. Immunol. 2009, 46, 1195–1203. [Google Scholar] [CrossRef] [PubMed]
- Watari, K.; Nakaya, M.; Nishida, M.; Kim, K.M.; Kurose, H. β-arrestin2 in infiltrated macrophages inhibits excessive inflammation after myocardial infarction. PLoS ONE 2013, 8, e68351. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Yao, W.; Li, S.; Xi, J. Norepinephrine induces the expression of interleukin-6 via β-adrenoreceptor-NAD(P)H oxidase system-NF-κB dependent signal pathway in U937 macrophages. Biochem. Biophys. Res. Commun. 2015, 460, 1029–1034. [Google Scholar] [CrossRef] [PubMed]
- Pinato, L.; da Silveira Cruz-Machado, S.; Franco, D.G.; Campos, L.M.; Cecon, E.; Fernandes, P.A.; Bittencourt, J.C.; Markus, R.P. Selective protection of the cerebellum against intracerebroventricular LPS is mediated by local melatonin synthesis. Brain Struct. Funct. 2015, 220, 827–840. [Google Scholar] [CrossRef] [PubMed]
- Kinker, G.S.; Oba-Shinjo, S.M.; Carvalho-Sousa, C.E.; Muxel, S.M.; Marie, S.K.; Markus, R.P.; Fernandes, P.A. Melatonergic system-based two-gene index is prognostic in human gliomas. J. Pineal Res. 2016, 60, 84–94. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira Tatsch-Dias, M.; Levandovski, R.M.; Custódio de Souza, I.C.; Gregianin Rocha, M.; Magno Fernandes, P.A.; Torres, I.L.; Hidalgo, M.P.; Markus, R.P.; Caumo, W. The concept of the immune-pineal axis tested in patients undergoing an abdominal hysterectomy. Neuroimmunomodulation 2013, 20, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Suofu, Y.; Li, W.; Jean-Alphonse, F.G.; Jia, J.; Khattar, N.K.; Li, J.; Baranov, S.V.; Leronni, D.; Mihalik, A.C.; He, Y.; et al. Dual role of mitochondria in producing melatonin and driving GPCR signaling to block cytochrome c release. Proc. Natl. Acad. Sci. USA 2017, 114, E7997–E8006. [Google Scholar] [CrossRef] [PubMed]
- Takenaka, M.C.; Guereschi, M.G.; Basso, A.S. Neuroimmune interactions: Dendritic cell modulation by the sympathetic nervous system. Semin. Immunopathol. 2017, 39, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Nijhuis, L.E.; Olivier, B.J.; Dhawan, S.; Hilbers, F.W.; Boon, L.; Wolkers, M.C.; Samsom, J.N.; de Jonge, W.J. Adrenergic β2 receptor activation stimulates anti-inflammatory properties of dendritic cells in vitro. PLoS ONE 2014, 9, e85086. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takenaka, M.C.; Araujo, L.P.; Maricato, J.T.; Nascimento, V.M.; Guereschi, M.G.; Rezende, R.M.; Quintana, F.J.; Basso, A.S. Norepinephrine Controls Effector T Cell Differentiation through β2-Adrenergic Receptor-Mediated Inhibition of NF-κB and AP-1 in Dendritic Cells. J. Immunol. 2016, 196, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Guereschi, M.G.; Araujo, L.P.; Maricato, J.T.; Takenaka, M.C.; Nascimento, V.M.; Vivanco, B.C.; Reis, V.O.; Keller, A.C.; Brum, P.C.; Basso, A.S. Beta2-adrenergic receptor signaling in CD4+ Foxp3+ regulatory T cells enhances their suppressive function in a PKA-dependent manner. Eur. J. Immunol. 2013, 43, 1001–1012. [Google Scholar] [CrossRef] [PubMed]
- Nakai, A.; Hayano, Y.; Furuta, F.; Noda, M.; Suzuki, K. Control of lymphocyte egress from lymph nodes through β2-adrenergic receptors. J. Exp. Med. 2014, 211, 2583–2598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wirth, T.; Westendorf, A.M.; Bloemker, D.; Wildmann, J.; Engler, H.; Mollerus, S.; Wadwa, M.; Schäfer, M.K.; Schedlowski, M.; del Rey, A. The sympathetic nervous system modulates CD4(+)Foxp3(+) regulatory T cells via noradrenaline-dependent apoptosis in a murine model of lymphoproliferative disease. Brain Behav. Immun. 2014, 38, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Scheiermann, C.; Kunisaki, Y.; Lucas, D.; Chow, A.; Jang, J.E.; Zhang, D.; Hashimoto, D.; Merad, M.; Frenette, P.S. Adrenergic nerves govern circadian leukocyte recruitment to tissues. Immunity 2012, 37, 290–301. [Google Scholar] [CrossRef] [PubMed]
- Gabanyi, I.; Muller, P.A.; Feighery, L.; Oliveira, T.Y.; Costa-Pinto, F.A.; Mucida, D. Neuro-immune Interactions Drive Tissue Programming in Intestinal Macrophages. Cell 2016, 164, 378–391. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, P.A.; Tamura, E.K.; D’Argenio-Garcia, L.; Muxel, S.M.; da Silveira Cruz-Machado, S.; Marçola, M.; Carvalho-Sousa, C.E.; Cecon, E.; Ferreira, Z.S.; Markus, R.P. Dual Effect of Catecholamines and Corticosterone Crosstalk on Pineal Gland Melatonin Synthesis. Neuroendocrinology 2017, 104, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Koga, M.M.; Bizzarro, B.; Sa-Nunes, A.; Rios, F.J.O.; Jancar, S. Activation of PAF-receptor induces regulatory dendritic cells through PGE2 and IL-10. Prostaglandins Leukot. Essent. Fat. Acids 2013, 89, 319–326. [Google Scholar] [CrossRef] [PubMed]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pires-Lapa, M.A.; Carvalho-Sousa, C.E.; Cecon, E.; Fernandes, P.A.; Markus, R.P. β-Adrenoceptors Trigger Melatonin Synthesis in Phagocytes. Int. J. Mol. Sci. 2018, 19, 2182. https://doi.org/10.3390/ijms19082182
Pires-Lapa MA, Carvalho-Sousa CE, Cecon E, Fernandes PA, Markus RP. β-Adrenoceptors Trigger Melatonin Synthesis in Phagocytes. International Journal of Molecular Sciences. 2018; 19(8):2182. https://doi.org/10.3390/ijms19082182
Chicago/Turabian StylePires-Lapa, Marco A., Claudia E. Carvalho-Sousa, Erika Cecon, Pedro A. Fernandes, and Regina P. Markus. 2018. "β-Adrenoceptors Trigger Melatonin Synthesis in Phagocytes" International Journal of Molecular Sciences 19, no. 8: 2182. https://doi.org/10.3390/ijms19082182
APA StylePires-Lapa, M. A., Carvalho-Sousa, C. E., Cecon, E., Fernandes, P. A., & Markus, R. P. (2018). β-Adrenoceptors Trigger Melatonin Synthesis in Phagocytes. International Journal of Molecular Sciences, 19(8), 2182. https://doi.org/10.3390/ijms19082182