Effects of Sparassis crispa in Medical Therapeutics: A Systematic Review and Meta-Analysis of Randomized Controlled Trials
Abstract
1. Introduction
2. Results
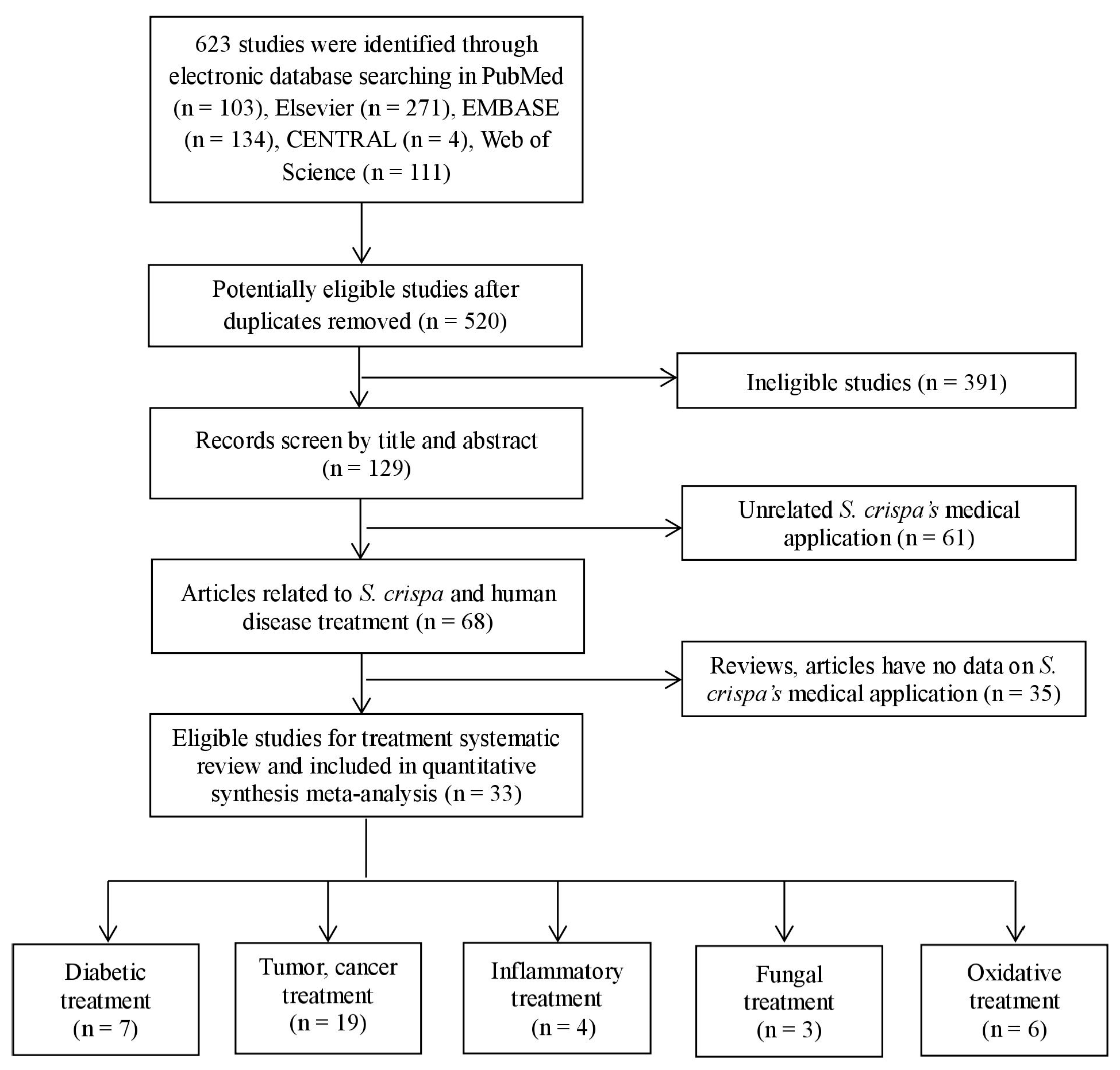
2.1. Characteristics of Included Studies
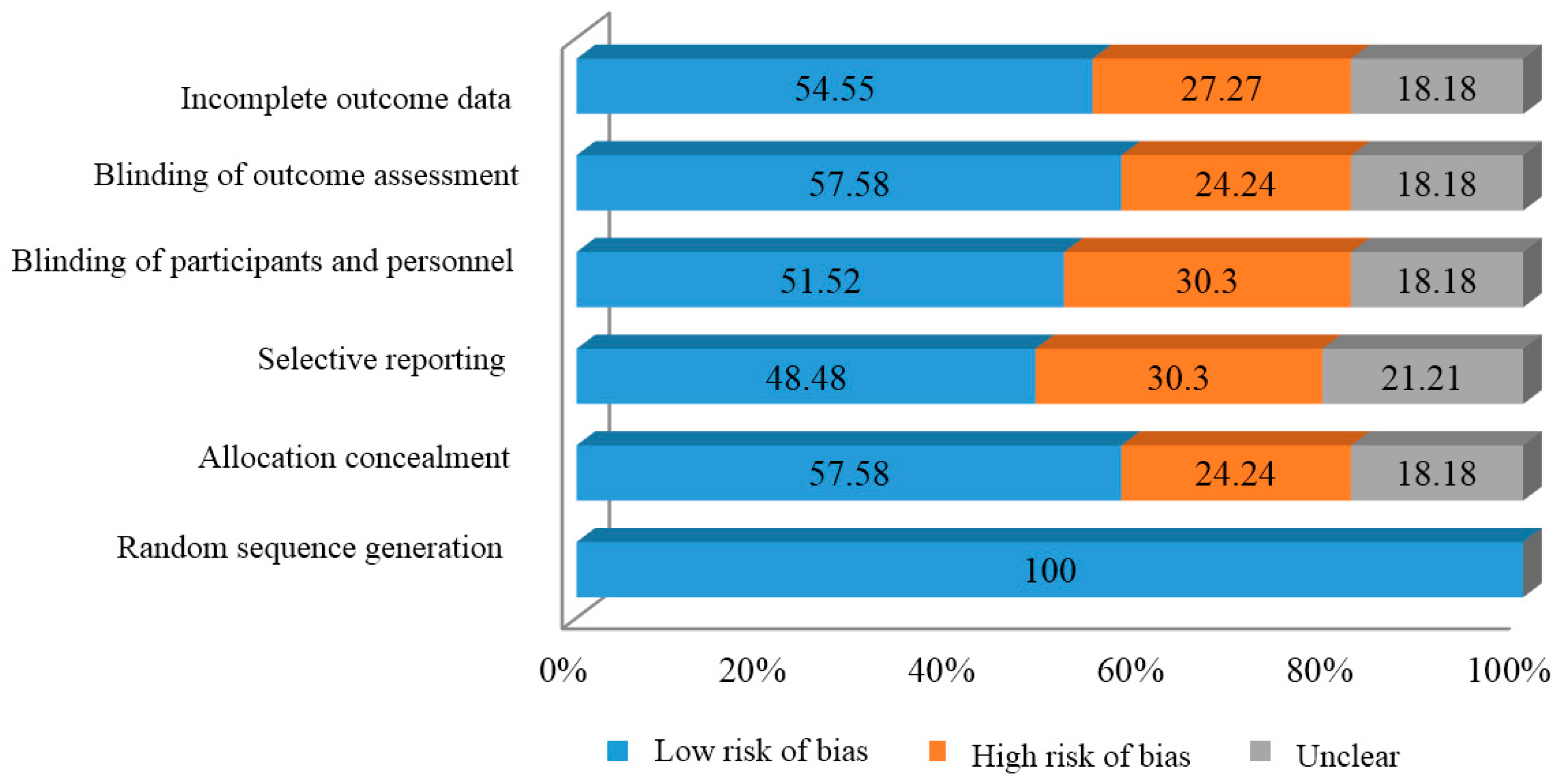
2.2. Risk of Bias
2.3. Diabetes Treatment
2.4. Cancer Treatment
2.5. Anti-Inflammatory Activity
2.6. Anti-Fungal Activity
2.7. Antioxidant Activity
2.8. Sensitivity Analysis
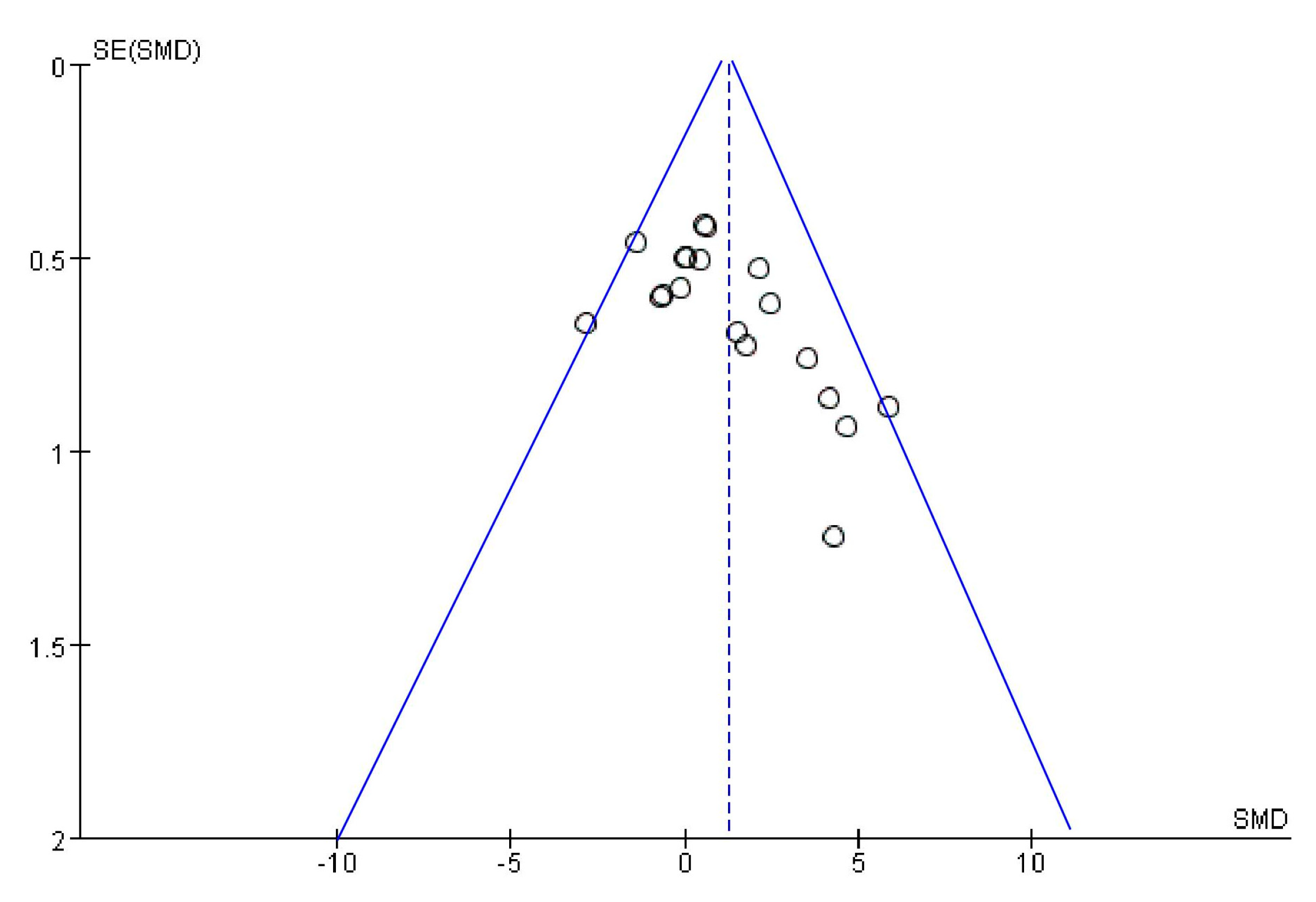
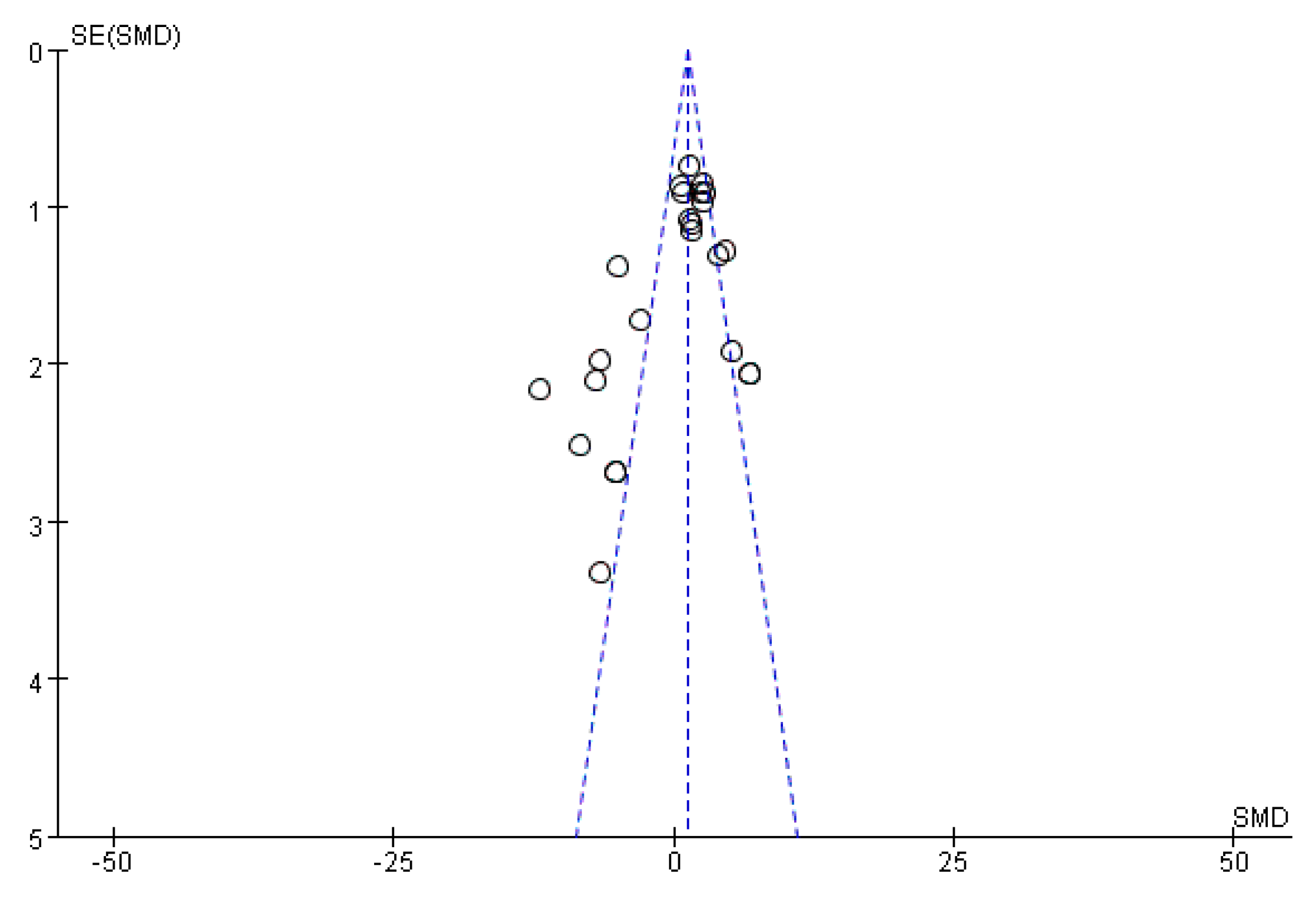
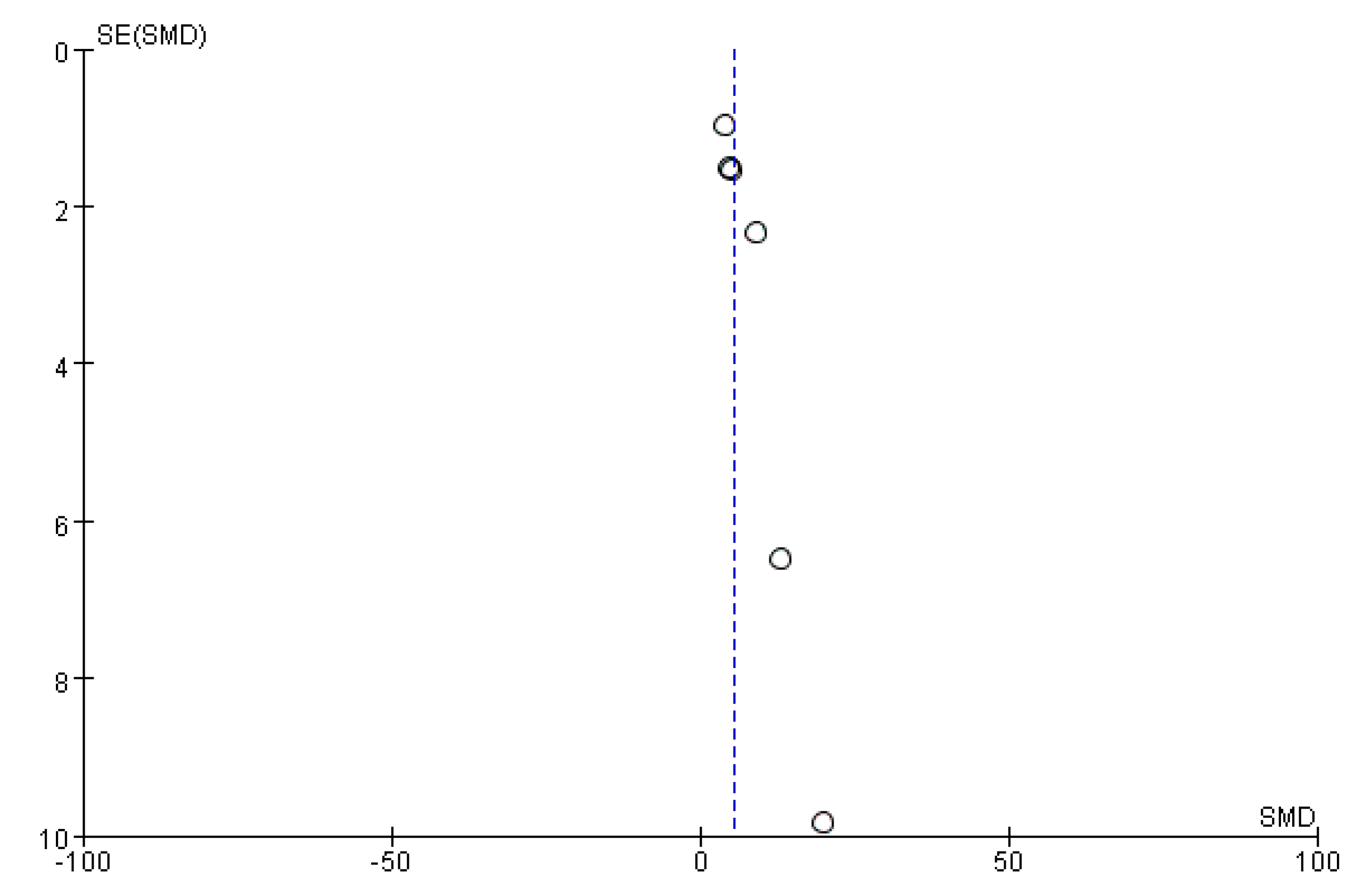
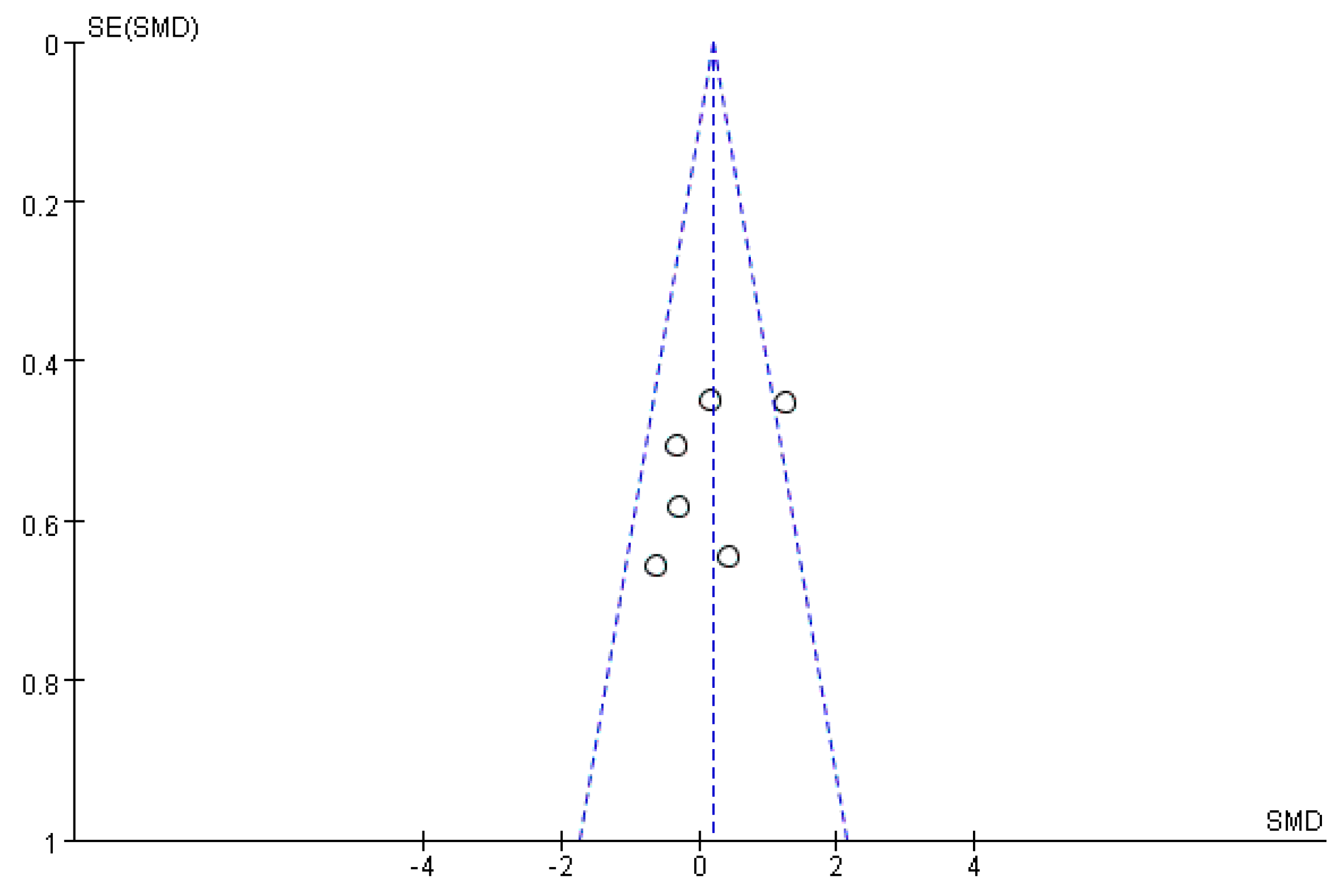
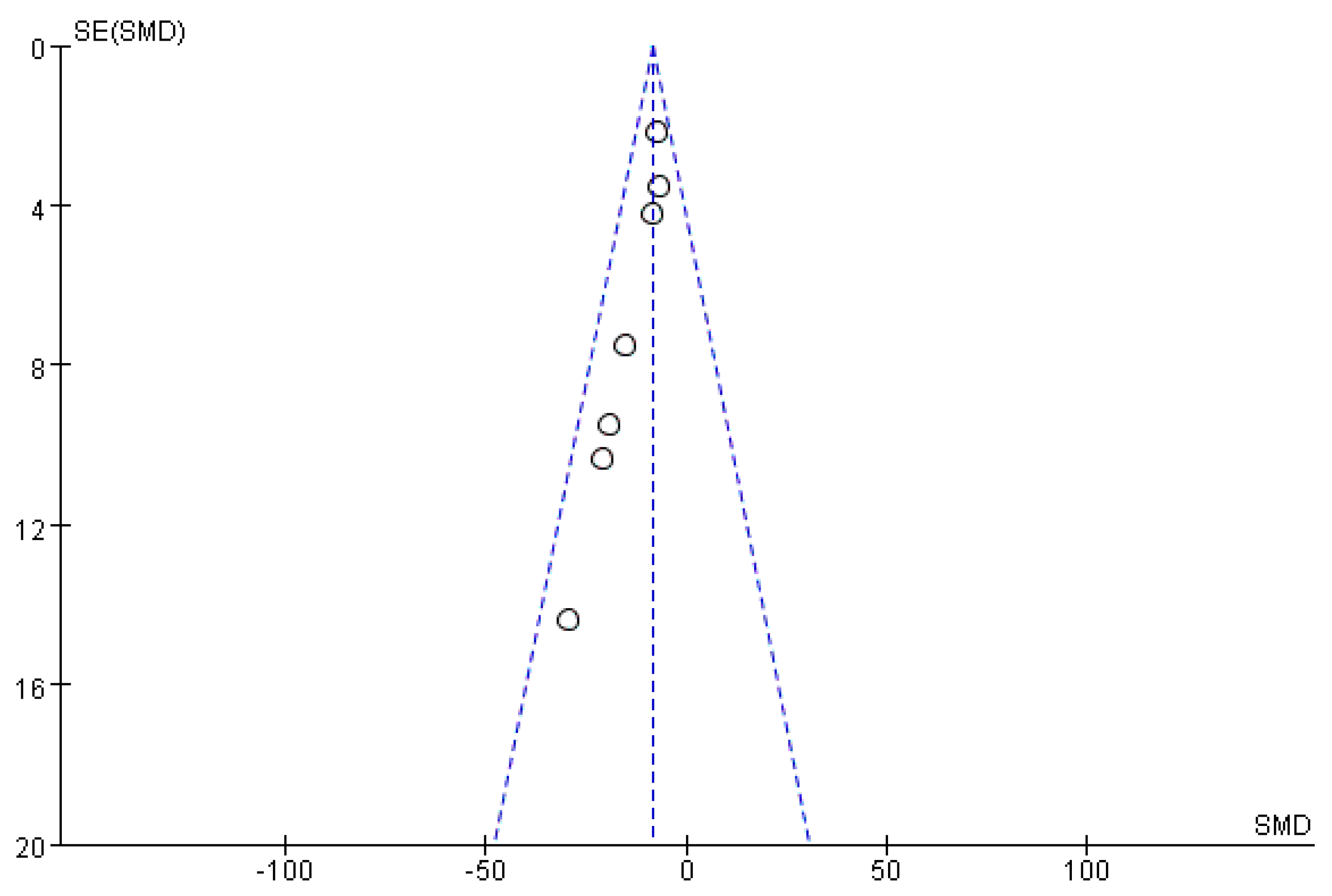
2.9. Bias Analysis
3. Discussion
4. Materials and Methods
4.1. Methods
4.2. Literature Search and Data Extraction
4.2.1. Database Research Strategy
4.2.2. Data Extraction
4.2.3. Exclusion Criteria
4.3. Meta-Analysis
5. Limitation of Study
6. Conclusions
Author Contributors
Acknowledgments
Conflicts of Interest
Abbreviations
| S. crispa | Sparassis crispa |
| SMD | Standardized mean difference |
References
- Elsayed, E.A.; El Enshasy, H.; Wadaan, M.A.; Aziz, R. Mushrooms: A potential natural source of anti-inflammatory compounds for medical applications. Mediat. Inflamm. 2014, 2014, 805841. [Google Scholar] [CrossRef] [PubMed]
- Molitoris, H.P. Mushroom in medicine. Folia Microbiol. 1994, 39, 91–98. [Google Scholar] [CrossRef]
- Kimura, T. Natural products and biological activity of the pharmacologically active Cauliflower mushroom Sparassis crispa. BioMed Res. Int. 2013, 2013, 156–167. [Google Scholar] [CrossRef] [PubMed]
- Sou, H.D.; Ryoo, R.; Ryu, S.R.; Ka, K.H.; Park, H.; Joo, S.H. Morphological and genetic characteristics of newly crossbred Cauliflower mushroom (Sparassis latifolia). J. Microbiol. 2013, 51, 552–557. [Google Scholar] [CrossRef] [PubMed]
- Kawagishi, H.; Hayashi, K.; Tokuyama, S.; Hashimoto, N.; Kimura, T.; Dombo, M. Novel bioactive compound from the Sparassis crispa mushroom. Biosci. Biotechnol. Biochem. 2007, 71, 1804–1806. [Google Scholar] [CrossRef] [PubMed]
- Kwon, A.H.; Qiu, Z.; Hashimoto, M.; Yamamoto, K.; Kimura, T. Effects of medicinal mushroom (Sparassis crispa) on wound healing in streptozotocin-induced diabetic rats. Am. J. Surg. 2009, 197, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, K.; Kokudo, N.; Hashimoto, T.; Yamamoto, K.; Inose, T.; Kimura, T. Novel phthalide compounds from Sparassis crispa (Hanabiratake), Hanabiratakelide A-C, exhibiting anti-cancer related activity. Biol. Pharm. Bull. 2010, 33, 1355–1359. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-Y.; Seguin, P.; Ahn, J.-K.; Kim, J.-J.; Chun, S.-C.; Kim, E.-H.; Seo, S.-H.; Kang, E.-Y.; Kim, S.-L.; Park, Y.-J.; et al. Phenolic compound concentration and antioxidant activities of edible and medicinal mushrooms from Korea. J. Agric. Food Chem. 2008, 56, 7265–7270. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-G.; Thi, N.N.; Kim, H.-G.; Lee, D.Y.; Lee, S.-E.; Kim, G.-S.; Baek, N.-I. Ergosterol peroxides from the fruit body of Sparassis crispa. Appl. Biol. Chem. 2016, 59, 313–316. [Google Scholar] [CrossRef]
- Yang, Y.H.; Kang, H.W.; Ro, H.S. Cloning and molecular characterization of β-1,3-glucan synthase from Sparassis crispa. Mycobiology 2014, 42, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Petrova, R.D.; Reznick, A.Z.; Wasser, S.P.; Denchev, C.M.; Nevo, E.; Mahajna, J. Fungal metabolites modulating NF-κB activity: An approach to cancer therapy and chemoprevention (review). Oncol. Rep. 2008, 19, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, K.; Kimura, T. Orally and topically administered Sparassis crispa (Hanabiratake) improved healing of skin wounds in mice with streptozotocin-induced diabetes. Biosci. Biotechnol. Biochem. 2014, 77, 1303–1305. [Google Scholar] [CrossRef] [PubMed]
- Ohno, N.; Miura, N.N.; Nakajima, M.; Yadomae, T. Antitumor 1,3-β-glucan from cultured fruit body of Sparassis crispa. Biol. Pharm. Bull. 2000, 23, 866–872. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, K.; Kimura, T. Dietary Sparassis crispa (Hanabiratake) ameliorates plasma levels of adiponectin and glucose in type 2 diabetic mice. J. Health Sci. 2010, 56, 541–546. [Google Scholar] [CrossRef]
- Harada, T.; Miura, N.; Adachi, Y.; Nakajima, M.; Yadomae, T.; Ohno, N. Effect of SCG, 1,3-β-d-glucan from Sparassis crispa on the hematopoietic response in cyclophosphamide induced leukopenic mice. Biol. Pharm. Bull. 2002, 25, 931–938. [Google Scholar] [CrossRef] [PubMed]
- Joshi, M.; Sagar, A. In vitro free radical scavenging activity of a wild edible mushroom, Sparassis crispa (Wulf.) Fr., from North Western Himalayas, India. J. Mycol. 2014, 2014, 748531. [Google Scholar] [CrossRef]
- Bang, S.; Chae, H.S.; Lee, C.; Choi, H.G.; Ryu, J.; Li, W.; Lee, H.; Jeong, G.S.; Chin, Y.W.; Shim, S.H. New aromatic compounds from the fruiting body of Sparassis crispa (Wulf.) and their inhibitory activities on proprotein convertase subtilisin/kexin type 9 mRNA expression. J. Agric. Food Chem. 2017, 65, 6152–6157. [Google Scholar] [CrossRef] [PubMed]
- Katarzyna, S.-Z.; Bozena, M.; Agnieszka, S. Antioxidant components of selected indigenous edible mushrooms of the obsolete order aphyllophorales. Rev. Iberoam. Micol. 2015, 32, 99–102. [Google Scholar] [CrossRef]
- Lee, E.J.; Kim, J.-E.; Park, M.-J.; Park, D.-C.; Lee, S.-P. Antimicrobial effect of the submerged culture of Sparassis crispa in soybean curd whey. Korean J. Food Preserv. 2013, 20, 111–120. [Google Scholar] [CrossRef]
- Ohno, N.; Harada, T.; Masuzawa, S.; Miura, N.N.; Adachi, Y.; Nakajima, M.; Yadomawe, T. Antitumor activity and hematopooietic response of a β-glucan extracted from the mushroom Sparassis crispa (Wulf) Fr. Int. J. Med. Mushrooms 2001, 3, 193–198. [Google Scholar] [CrossRef]
- Yamamoto, K.; Nishikawa, Y.; Kimura, T.; Dombo, M.; Matsuura, N.; Sugitachi, A. Antitumor activities of low molecular weight fraction derived from the cultured fruit body of Sparassis crispa in tumor-bearing mice. Nippon Shok. Kag. Kog. Kaishi 2007, 54, 419–423. [Google Scholar] [CrossRef][Green Version]
- Choi, W.-S.; Shin, P.-G.; Bok, Y.Y.; Jun, N.H.; Kim, G.-D. Anti-inflammatory effects of Sparassis crispa extracts. J. Mushroom 2013, 11, 46–51. [Google Scholar] [CrossRef]
- Choi, M.-H.; Han, H.-K.; Lee, Y.-J.; Jo, H.-G.; Shin, H.-J. In vitro anti-cancer activity of hydrophobic fractions of Sparassis latifolia extract using AGS, A529, and HepG2 cell lines. J. Mushroom 2014, 12, 304–310. [Google Scholar] [CrossRef]
- Kim, I.-K.; Yun, Y.C.; Shin, Y.C.; Yoo, J. Effect of Sparassis crispa extracts on immune cell activation and tumor growth inhibition. J. Life Sci. 2013, 23, 984–988. [Google Scholar] [CrossRef]
- Lee, J.-J.; Son, H.-Y.; Choi, Y.-M.; Cho, J.-H.; Min, J.-K.; Oh, H.-K. Physicochemical components and antioxidant activity of Sparassis crispa mixture fermented by lactic acid bacteria. Korean J. Food Preserv. 2016, 23, 361–368. [Google Scholar] [CrossRef]
- Park, S.-E.; Seo, S.-H.; Moon, Y.-S.; Lee, Y.-M.; Na, C.-S.; Son, H.-S. Antioxidant and immunological activities of Sparassis crispa fermented with Meyerozyma guilliermondii FM. J. Korean Soc. Food Sci. Nutr. 2016, 45, 1398–1405. [Google Scholar] [CrossRef]
- Lee, D.-S.; Kim, K.-H.; Yook, H.-S. Antioxidant activities of different parts of Sparassis crispa depending on extraction temperature. J. Korean Soc. Food Sci. Nutr. 2016, 45, 1617–1622. [Google Scholar] [CrossRef]
- Yamamoto, K.; Kimura, T.; Sugitachi, A.; Matsuurac, N. Anti-angiogenic and anti-metastatic effects of β-1,3-d-glucan purified from Hanabiratake, Sparassis crispa. Biol. Pharm. Bull. 2009, 32, 259–263. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.Y.; Kang, S.; Hua, C.S.; Ting, Z.; Park, S. Synbiotic effects of β-glucans from Cauliflower mushroom and Lactobacillus fermentum on metabolic changes and gut microbiome in estrogen-deficient rats. Genes Nutr. 2017, 12, 31. [Google Scholar] [CrossRef] [PubMed]
- Takashi, K. Antitumor Effects and Their Related Components of Sparassis crispa and Other Pharmacological Aspect of Sparassis crispa; Kyoto University: Kyoto, Japan, 2013; p. 96. [Google Scholar]
- Yoshitomi, H.; Iwaoka, E.; Kubo, M.; Shibata, M.; Gao, M. Beneficial effect of Sparassis crispa on stroke through activation of Akt/eNOS pathway in brain of SHRSP. J. Nat. Med. 2011, 65, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Kim, J.Y.; Ryu, H.S.; Park, H.G.; Kim, Y.O.; Kang, J.S.; Kim, H.M.; Hong, J.T.; Kim, Y.; Han, S.B. Induction of dendritic cell maturation by β-glucan isolated from Sparassis crispa. Int. Immunopharmacol. 2010, 10, 1284–1294. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Lee, Y.G.; Byeon, S.E.; Han, S.; Choi, S.S.; Kim, A.R.; Lee, J.; Lee, S.J.; Hong, S.; Cho, J.Y. Mitogen activated protein kinases are prime signalling enzymes in nitric oxide production induced by soluble β-glucan from Sparassis crispa. Arch. Pharm. Res. 2010, 33, 1753–1760. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Lee, H.J.; Kim, S. Purification and antithrombotic activity of wulfase, a fibrinolytic enzyme from the fruit bodies of the edible and medicinal mushroom Sparassis crispa Wulf. Ex. Fr. Appl. Biochem. Microbiol. 2016, 52, 608–614. [Google Scholar] [CrossRef]
- Harada, T.; Nagimura, N.; Adachi, Y.; Nakajima, M.; Ohno, T.Y.N. Antibody to soluble 1,3/1,6-β-d-glucan, SCG in sera of naive DBA/2 mice. Biol. Pharm. Bull. 2003, 26, 1225–1228. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Wang, D.; Zhang, J.; Du, M.; Cheng, Y.; Liu, Y.; Zhang, N.; Wang, D.; Wu, Y. Mitochondria related pathway is essential for polysaccharides purified from Sparassis crispa mediated neuro-protection against glutamate-induced toxicity in differentiated PC12 cells. Int. J. Mol. Sci. 2016, 17, 133. [Google Scholar] [CrossRef] [PubMed]
- Harada, T.; Miura, N.N.; Adachi, Y.; Nakajima, M.; Yadomae, T.; Ohno, N. IFN-γ induction by SCG, 1,3-β-d-glucan from Sparassis crispa, in DBA/2 mice in vitro. J. Interferon Cytokine Res. 2002, 22, 1227–1239. [Google Scholar] [CrossRef] [PubMed]
- Harada, T.; Miura, N.N.; Adachi, Y.; Nakajima, M.; Yadomae, T.; Ohno, N. Granulocyte-macrophage colony-stimulating factor (GM-CSF) regulates cytokine induction by 1,3-β-d-glucan SCG in DBA/2 mice in vitro. J. Interferon Cytokine Res. 2004, 24, 478–479. [Google Scholar] [CrossRef] [PubMed]
- Harada, T.; Nagimura, N.; Adachi, Y.; Nakajima, M.; Ohno, T.Y.N. Mechanism of enhanced hematopoietic response by soluble β-glucan SCG in cyclophosphamide-treated mice. Microbiol. Immunol. 2006, 50, 687–690. [Google Scholar] [CrossRef] [PubMed]
- Nameda, S.; Harada, T.; Miura, N.N.; Adachi, Y.; Yadomae, T.; Nakajima, M.; Ohno, N. Enhanced cytokine synthesis of leukocytes by a β-glucan preparation, SCG, extracted from a medicinal mushroom, Sparassis crispa. Immunopharmacol. Immunotoxicol. 2003, 25, 321–335. [Google Scholar] [CrossRef] [PubMed]
- Yao, M.; Yamamoto, K.; Kimura, T.; Dombo, M. Effects of Hanabiratake (Sparassis crispa) on allergic rhinitis in ova-sensitized mice. Food Sci. Technol. Res. 2008, 14, 589–594. [Google Scholar] [CrossRef][Green Version]
- Kim, H.H.; Lee, S.; Singh, T.S.; Choi, J.K.; Shin, T.Y.; Kim, S.H. Sparassis crispa suppresses mast cell-mediated allergic inflammation: Role of calcium, mitogen-activated protein kinase and nuclear factor-kB. Int. J. Mol. Med. 2012, 30, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Woodward, S.; Sultan, H.Y.; Barrett, D.K.; Pearce, R.B. Two new antifungal metabolites produced and in decayed trees. J. Gen. Appl. Microbiol. 1992, 139, 153–159. [Google Scholar] [CrossRef]
- Puttaraju, N.G.; Venkateshaiah, S.U.; Dharmesh, S.M.; Urs, S.M.N.; Somasundaram, R. Antioxidant activity of indigenous edible mushrooms. J. Agric. Food Chem. 2006, 54, 9764–9772. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.R.; Hou, J.G.; Begum, S.; Xue, J.J.; Wang, Y.B.; Sung, C.K. Comparison of constituents, antioxidant potency, and acetylcholinesterase inhibition in Lentinus edodes, Sparassis crispa, and Mycoleptodonoides aitchisonii. Food Sci. Biotechnol. 2013, 22, 1747–1751. [Google Scholar] [CrossRef]
- Higgins, J.P.; Altman, D.G. The Cochrane Book Series; The Cochrane Collaboration: London, UK, 2008. [Google Scholar]
- Lull, C.; Wichers, H.J.; Savelkoul, H.F. Antiinflammatory and immunomodulating properties of fungal metabolites. Mediat. Inflamm. 2005, 2005, 63–80. [Google Scholar] [CrossRef] [PubMed]
- Novak, M.; Vetvicka, V. β-glucans, history, and the present: Immunomodulatory aspects and mechanisms of action. J. Immunotoxicol. 2008, 5, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Akramienė, D.; Kondrotas, A.; Didžiapetrienė, J.; Kėvelaitis, E. Effect of β-glucans on the immune system. Medicina 2007, 43, 597–606. [Google Scholar] [PubMed]
- Vannucci, L.; Krizan, J.; Sima, P.; Stakheev, D.; Caja, F.; Rajsiglova, L.; Horak, V.; Saieh, M. Immunostimulatory properties and antitumor activities of glucans (review). Int. J. Oncol. 2013, 43, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Chan, G.C.; Chan, W.K.; Sze, D.M. The effects of β-glucan on human immune and cancer cells. J. Hematol. Oncol. 2009, 2, 25. [Google Scholar] [CrossRef] [PubMed]
- Berdal, M. Wound Healing in Diabetes; Faculty of Health Sciences Metabolic and Renal Research Group: The Arctic University of Norway, Northern Norway, 2017; p. 73. [Google Scholar]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gotzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. PLoS Med. 2009, 6, e1000100. [Google Scholar] [CrossRef] [PubMed]
- Myung, S.-K. Systematic Review and Meta-Analysis; National Cancer Center: Gyeonggi-do, Korea, 2015; p. 125. [Google Scholar]
 ): SMD of individual studies; (◆): summary SMDs of the comparison.
): SMD of individual studies; (◆): summary SMDs of the comparison.
 ): SMD of individual studies; (◆): summary SMDs of the comparison.
): SMD of individual studies; (◆): summary SMDs of the comparison.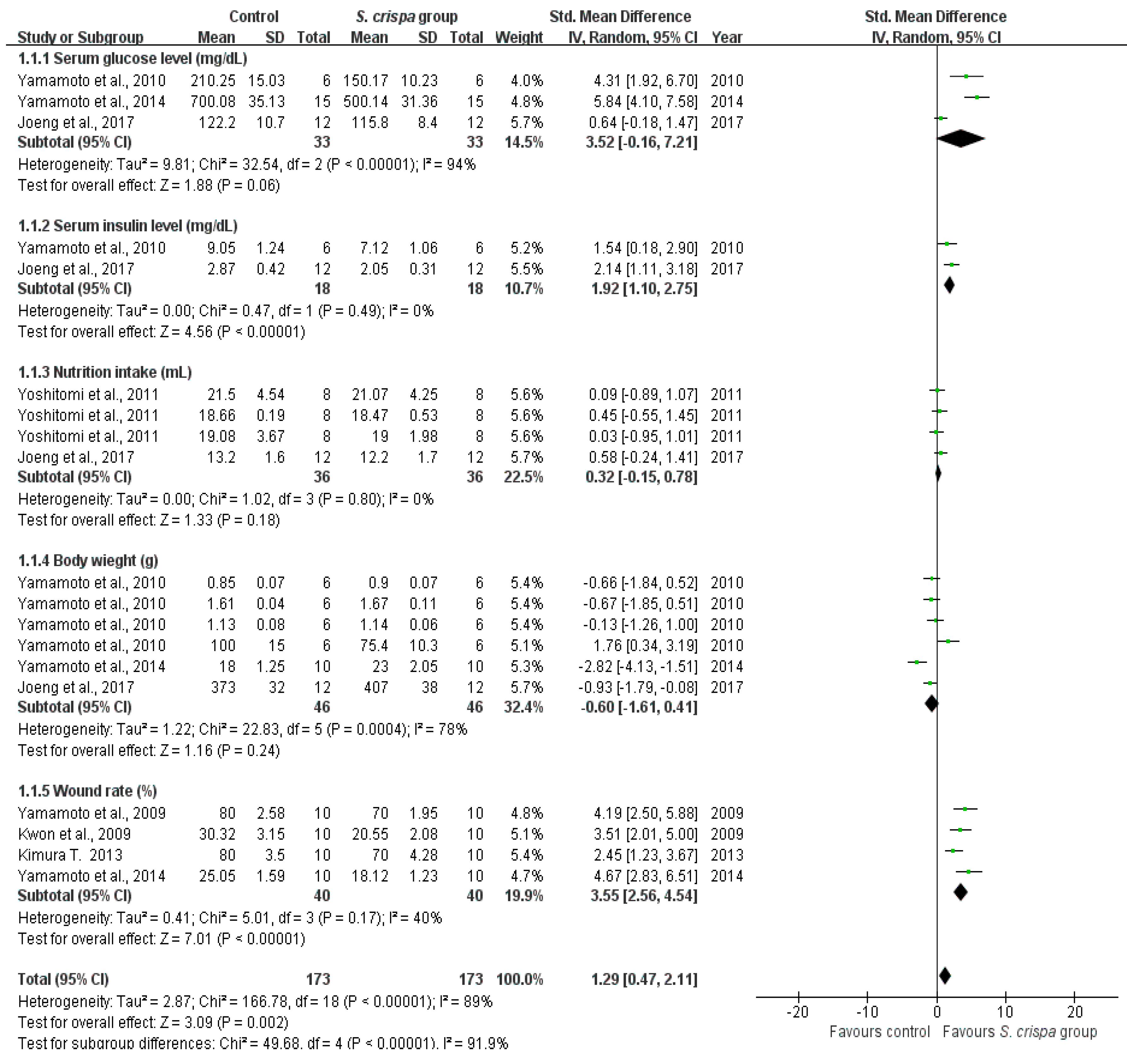
 ): SMD of individual studies; (◆): summary SMDs of the comparison.
): SMD of individual studies; (◆): summary SMDs of the comparison.
 ): SMD of individual studies; (◆): summary SMDs of the comparison.
): SMD of individual studies; (◆): summary SMDs of the comparison.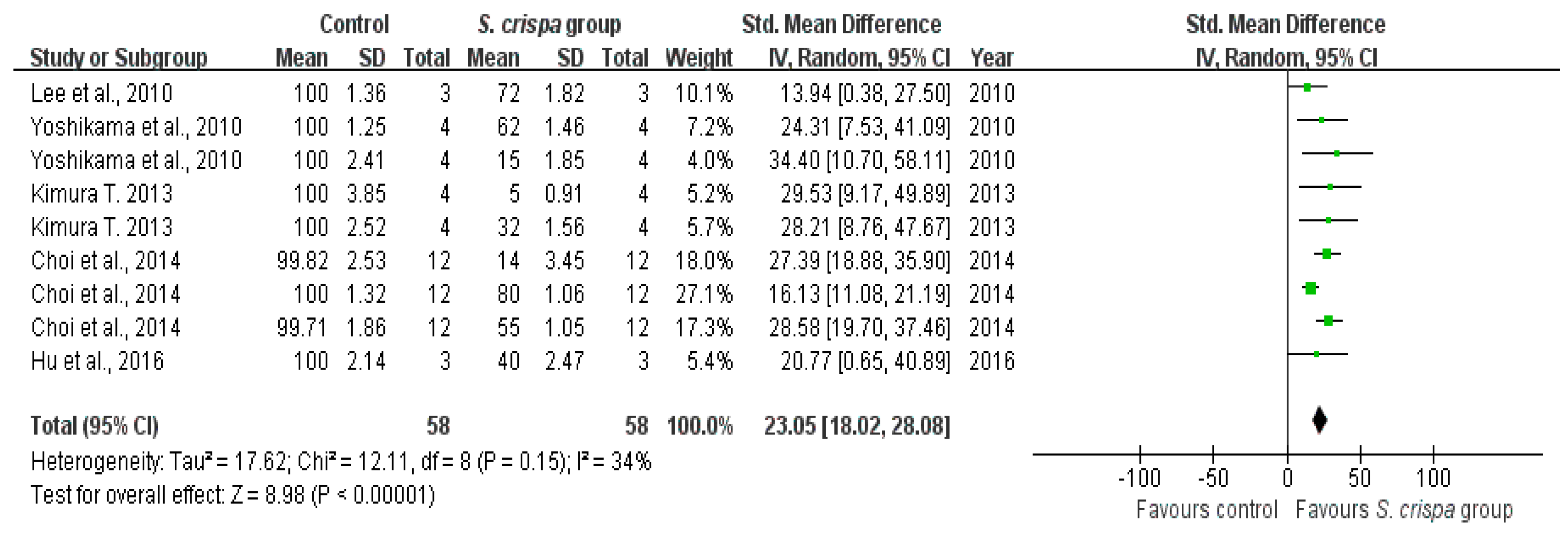
 ): SMD of individual studies; (◆): summary SMDs of the comparison.
): SMD of individual studies; (◆): summary SMDs of the comparison.
 ): SMD of individual studies; (◆): summary SMDs of the comparison.
): SMD of individual studies; (◆): summary SMDs of the comparison.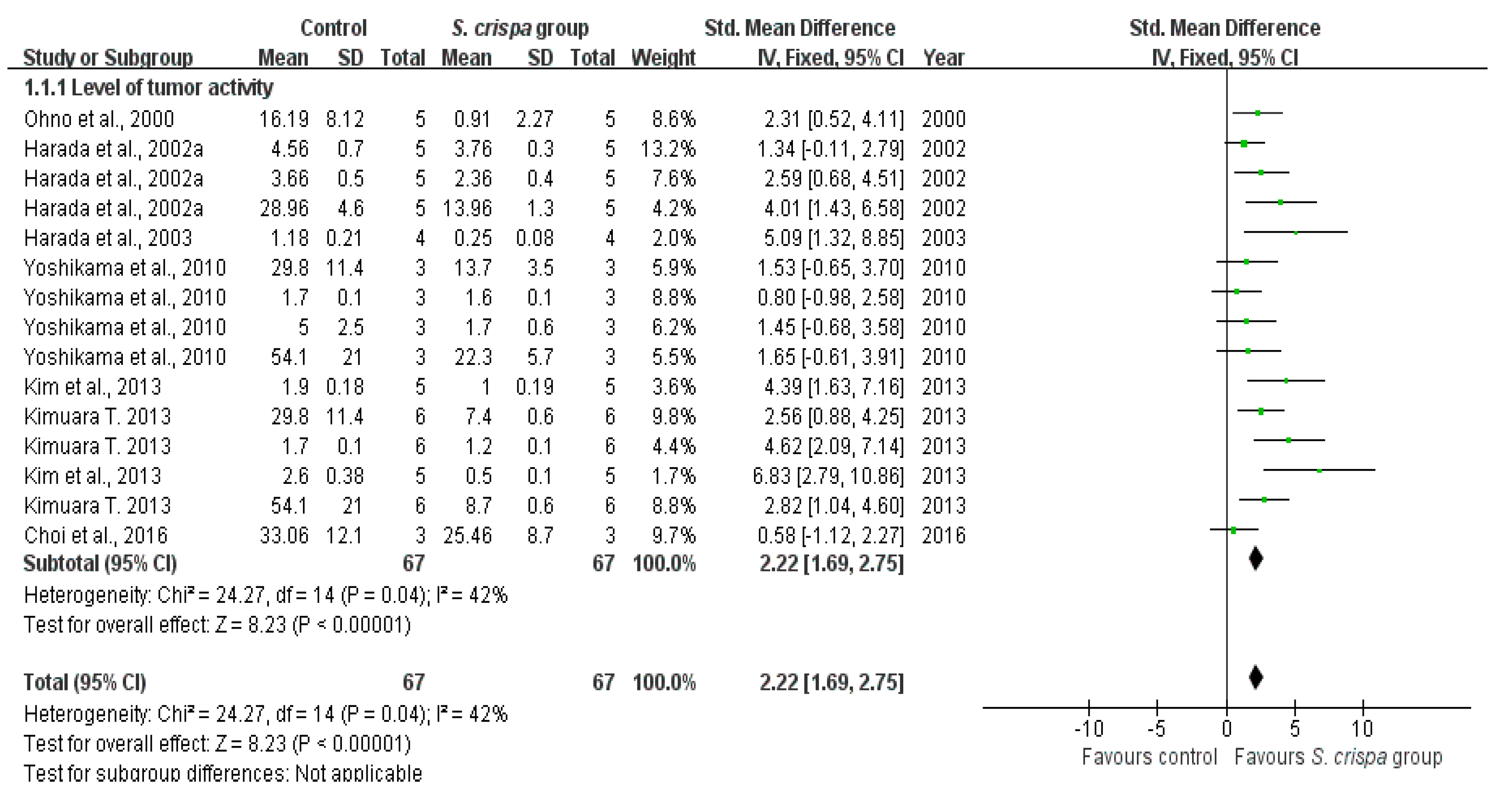
 ): SMD of individual studies; (◆): summary SMDs of the comparison.
): SMD of individual studies; (◆): summary SMDs of the comparison.
 ): SMD of individual studies; (◆): summary SMDs of the comparison.
): SMD of individual studies; (◆): summary SMDs of the comparison.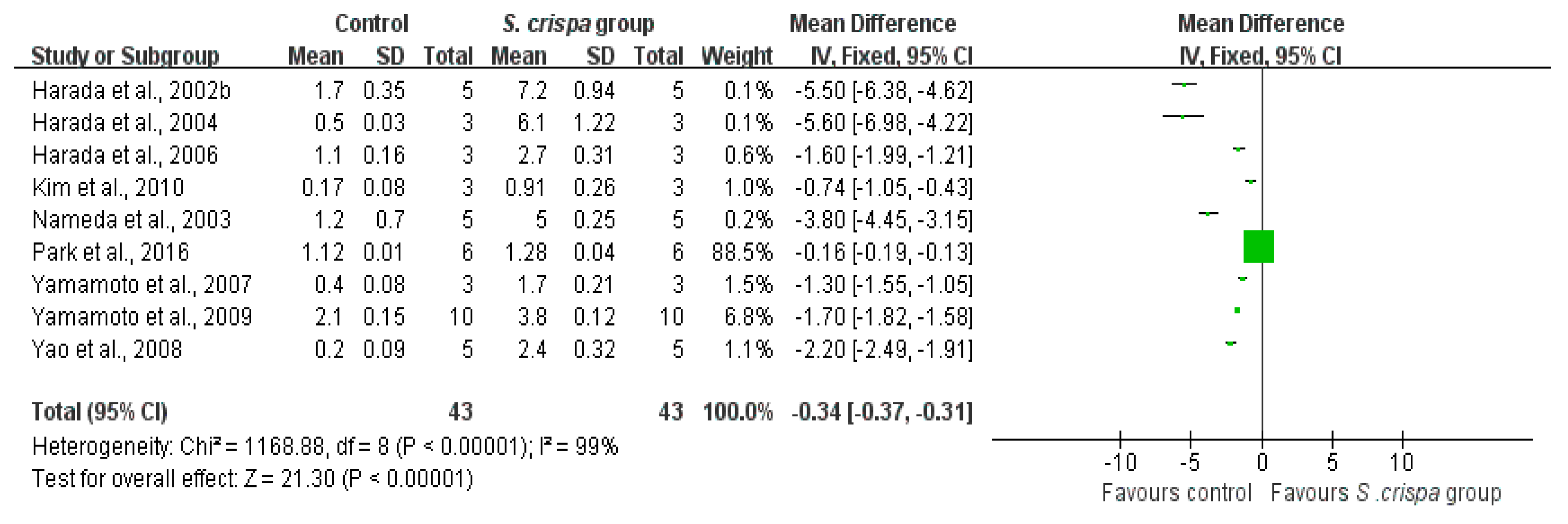
 ): SMD of individual studies; (◆): summary SMDs of the comparison.
): SMD of individual studies; (◆): summary SMDs of the comparison.
 ): SMD of individual studies; (◆): summary SMDs of the comparison.
): SMD of individual studies; (◆): summary SMDs of the comparison.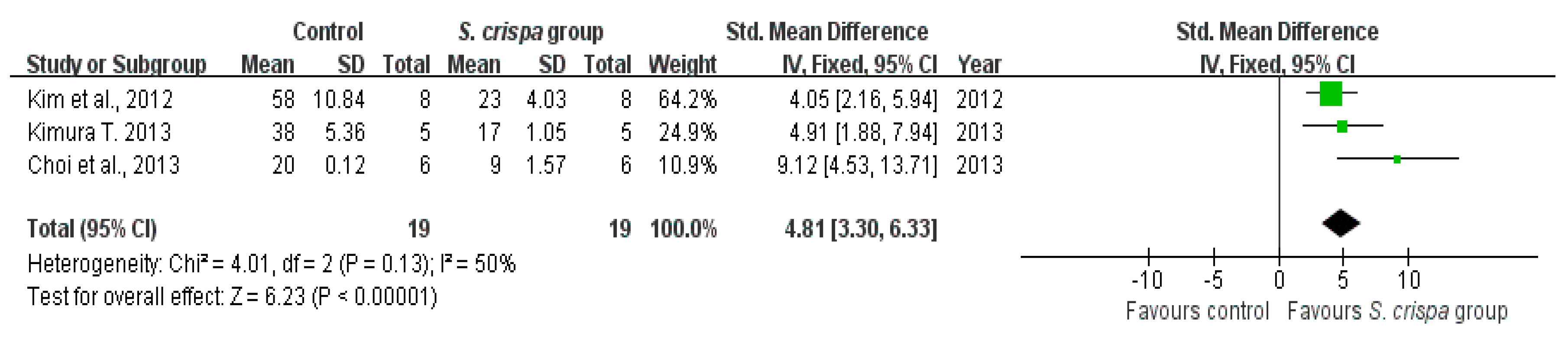
 ): SMD of individual studies; (◆): summary SMDs of the comparison.
): SMD of individual studies; (◆): summary SMDs of the comparison.
 ): SMD of individual studies; (◆): summary SMDs of the comparison.
): SMD of individual studies; (◆): summary SMDs of the comparison.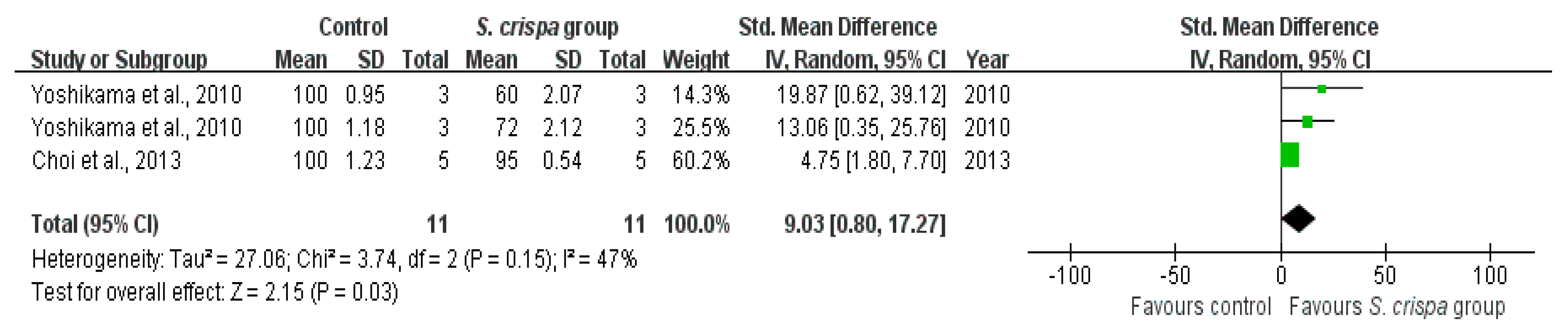
 ): SMD of individual studies; (◆): summary SMDs of the comparison.
): SMD of individual studies; (◆): summary SMDs of the comparison.
 ): SMD of individual studies; (◆): summary SMDs of the comparison.
): SMD of individual studies; (◆): summary SMDs of the comparison.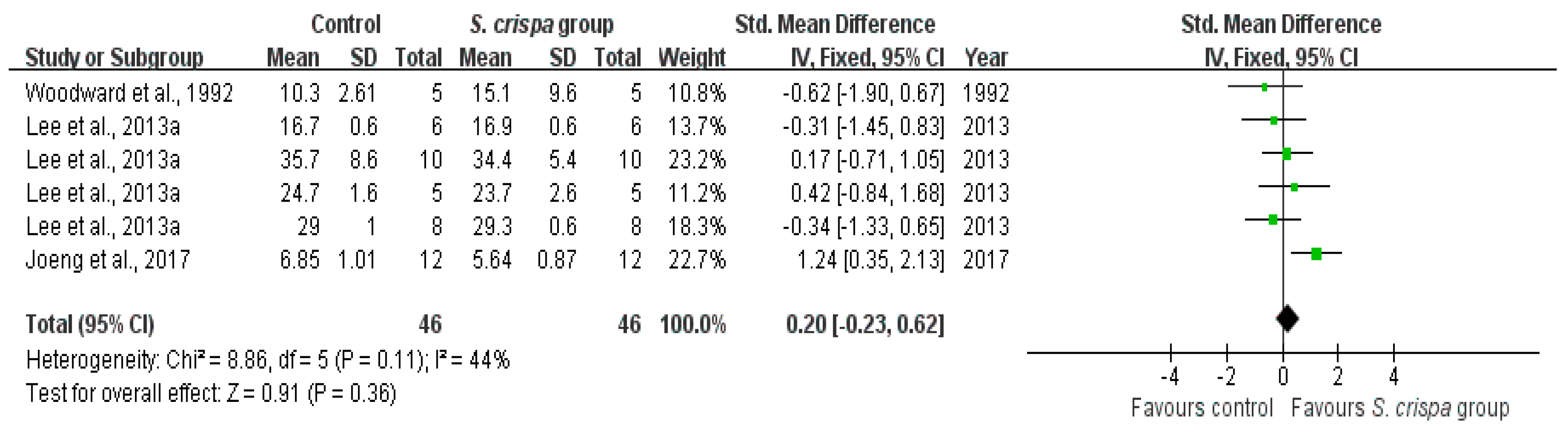
 ): SMD of individual studies; (◆): summary SMDs of the comparison.
): SMD of individual studies; (◆): summary SMDs of the comparison.
 ): SMD of individual studies; (◆): summary SMDs of the comparison.
): SMD of individual studies; (◆): summary SMDs of the comparison.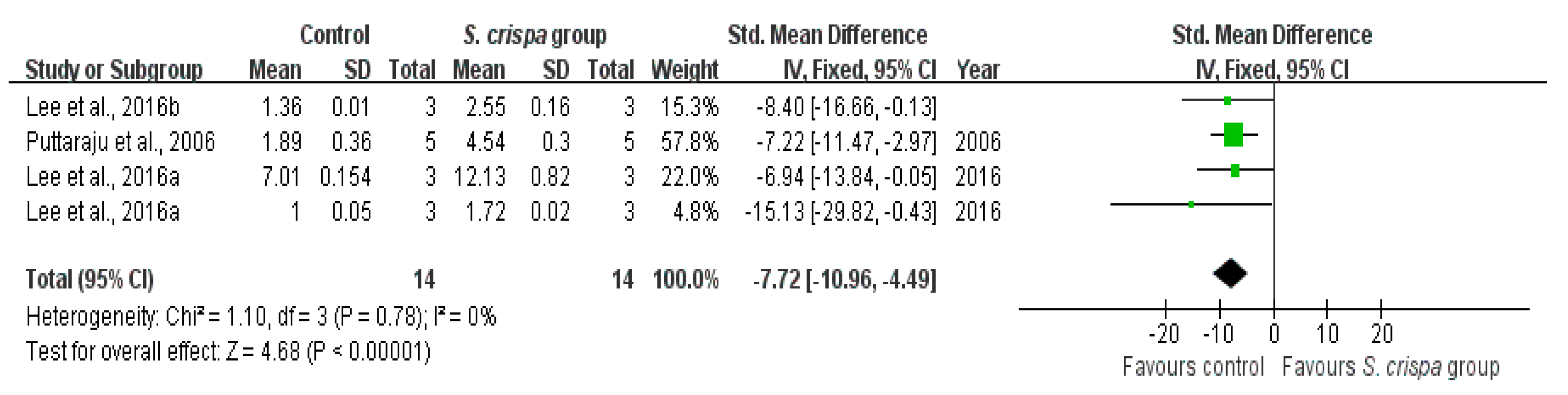
 ): SMD of individual studies; (◆): summary SMDs of the comparison.
): SMD of individual studies; (◆): summary SMDs of the comparison.
 ): SMD of individual studies; (◆): summary SMDs of the comparison.
): SMD of individual studies; (◆): summary SMDs of the comparison.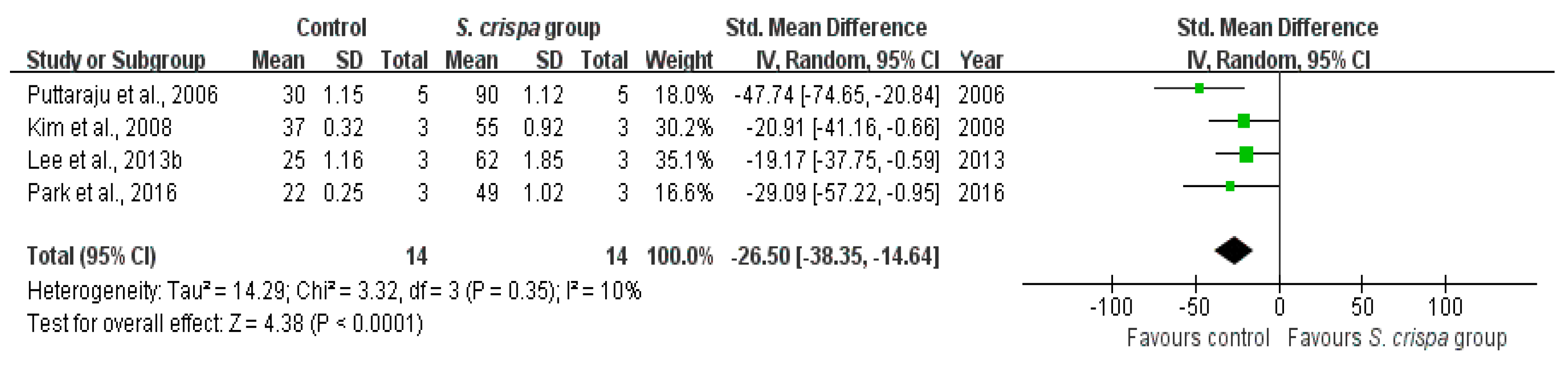
 ): S MD of individual studies; (◆): summary SMDs of the comparison.
): S MD of individual studies; (◆): summary SMDs of the comparison.
 ): S MD of individual studies; (◆): summary SMDs of the comparison.
): S MD of individual studies; (◆): summary SMDs of the comparison.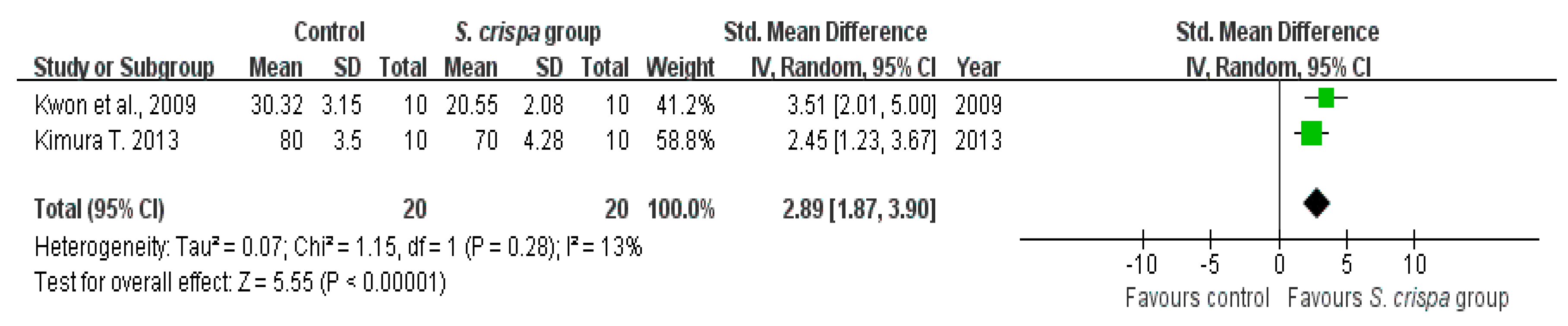
 ): SMD of individual studies; (◆): summary SMDs of the comparison.
): SMD of individual studies; (◆): summary SMDs of the comparison.
 ): SMD of individual studies; (◆): summary SMDs of the comparison.
): SMD of individual studies; (◆): summary SMDs of the comparison.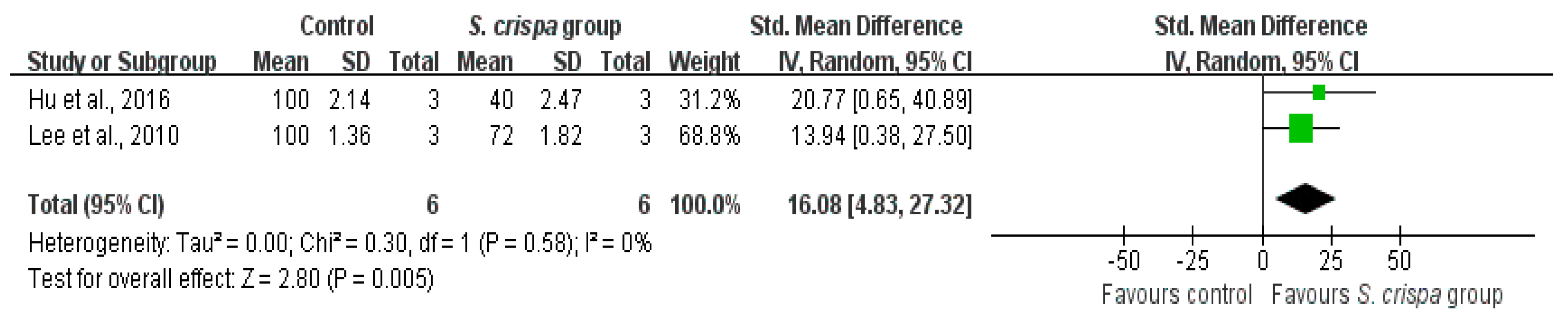
| Reference | Characteristic of Object | S. crispa Extract Compound | Medical Therapeutic | n | Dosage | Location |
|---|---|---|---|---|---|---|
| [6] Kwon et al., 2009 | Mice | β-glucan | Anti-diabetic | 10 | 100 µg/mL β-glucan | Korea |
| [32] Kim et al., 2010 | Dendritic cell | β-glucan | Anti-tumor | 3 | 100 µg/mL β-glucan | Korea |
| [31] Yoshitomi et al., 2011 | Mice | β-glucan | Anti-diabetic | 8 | 100 µg/mL β-glucan | Japan |
| [33] Lee et al., 2010 | RAW 264.7 cell | β-glucan | Anti-tumor | 3 | 250 µg/mL β-glucan | Korea |
| [34] Choi et al., 2016 | Human fibrinogen | Wulfase | Anti-tumor | 3 | 200 µg/mL Wulfase | Korea |
| [15] Harada et al., 2002a | CD 41 and CD 81 cell | β-glucan | Anti-tumor | 3 | 200–250 µg/mL β-glucan | Japan |
| [35] Harada et al., 2003 | Mice | β-glucan | Anti-tumor | 4 | 25 µg/mL β-glucan | Japan |
| [28] Yamamoto et al., 2009 | C57BL/6J cell/Mice cell line | β-glucan | Anti-diabetic Anti-tumor | 10 | 160 µg/mL β-glucan | Japan |
| [7] Yoshikama et al., 2010 | RAW 264.7 cell | Phthalide compounds | Anti-tumor | 3–4 | 100 µg/mL phthalide compound | Japan |
| [21] Yamamoto et al., 2007 | Sarcoma180 cell | S. crispa extract | Anti-tumor | 3 | 35 µg/mL β-glucan | Japan |
| [14] Yamamoto et al., 2010 | Mice | S. crispa extract | Anti-diabetic | 6–8 | 100 µg/mL β-glucan | Japan |
| [29] Jeong et al., 2017 | Mice | β-glucan | Anti-diabetic Anti-fungal | 12 | 100 µg/mL β-glucan | Korea |
| [22] Choi et al., 2013 | RAW 264.7 cell | β-glucan | Anti-inflammatory | 3 | 200 µg/mL β-glucan | Korea |
| [23] Choi et al., 2014 | A529 cell HepG2 cell AGS cell | β-glucan | Anti-tumor | 12 | 250 µg/mL β-glucan | Korea |
| [30] Kimura T. 2013 | Sarcoma 180 cell Mice Colon cancer cell F3444N/Rat | β-glucan | Anti-diabetic Anti-tumor Anti-inflammatory | 3–5 | 100 µg/mL β-glucan | Japan |
| [42] Kim et al., 2012 | Mast cell (HMC-1) | S. crispa extract | Anti-inflammatory | 3 | 200 µg/mL S. crispa extract | Korea |
| [36] Hu et al., 2016 | PC12 cell | β-glucan | Anti-tumor | 6 | 250 µg/mL β-glucan | China |
| [44] Puttaraju et al., 2006 | Mice | S. crispa extract | Antioxidant | 3 | 30 µg/mL β-glucan | India |
| [8] Kim et al., 2008 | Mice or cell | S. crispa extract | Antioxidant | 3 | 100 µg/mL S. crispa extract | Korea |
| [43] Woodward et al., 1992 | Botrytis cinerea | Antibiotic compounds | Anti-fungal | 10 | 100 µg/mL antibiotic compound | United Kingdom |
| [13] Ohno et al., 2000 | Mice | S. crispa extract | Anti-tumor | 10 | 250 µg/mL S. crispa extract | Japan |
| [12] Yamamoto et al., 2014 | Mice | β-glucan | Anti-diabetic | 10–18 | 250 µg/mL β-glucan | Japan |
| [19] Lee et al., 2013a | Soybean | S. crispa extract | Anti-fungal | 3 | 125 µg/mL S. crispa extract | Korea |
| [24] Kim et al., 2013 | Raw 264.7 cell | β-glucan | Anti-tumor | 5 | 100 µg/mL β-glucan | Korea |
| [37] Harada et al., 2002b | Mice | β-glucan | Anti-tumor | 5 | 100 µg/mL β-glucan | Japan |
| [38] Harada et al., 2004 | Mice | β-glucan | Anti-tumor | 3 | 100 µg/mL β-glucan | Japan |
| [39] Harada et al., 2006 | Mice | β-glucan | Anti-tumor | 3 | 100 µg/mL β-glucan | Japan |
| [40] Nameda et al., 2003 | Mice | β-glucan | Anti-tumor | 3 | 50 µg/mL β-glucan | Japan |
| [41] Yao et al., 2008 | Mice | β-glucan | Anti-tumor | 10 | 120 µg/mL β-glucan | China |
| [25] Lee et al., 2016a | Soybean | β-glucan | Antioxidant | 3 | 200µg/mL β-glucan | Korea |
| [26] Park et al., 2016 | Mice | S. crispa extract | Antioxidant | 6 | 200 µg/mL S. crispa extract | Korea |
| [27] Lee et al., 2016b | Cell | S. crispa extract | Antioxidant | 3 | 50 µg/mL β-glucan | Korea |
| [45] Lee et al., 2013b | Mice | Phenolic compounds | Antioxidant | 3 | 200 µg/mL phenolic compounds | Korea |
| Study | Random Sequence Generation | Allocation Concealment | Selective Reporting | Blinding of Participants | Blinding of Outcome Assessment | Incomplete Outcome Data |
|---|---|---|---|---|---|---|
| [6] Kwon et al., 2009 | ||||||
| [32] Kim et al., 2010 | ||||||
| [31] Yoshitomi et al., 2011 | ||||||
| [33] Lee et al., 2010 | ||||||
| [34] Choi et al., 2016 | ||||||
| [15] Harada et al., 2002a | ||||||
| [35] Harada et al., 2003 | ||||||
| [28] Yamamoto et al., 2009 | ||||||
| [7] Yoshikama et al., 2010 | ||||||
| [21] Yamamoto et al., 2007 | ||||||
| [14] Yamamoto et al., 2010 | ||||||
| [29] Jeong et al., 2017 | ||||||
| [22] Choi et al., 2013 | ||||||
| [23] Choi et al., 2014 | ||||||
| [30] Kimura T. 2013 | ||||||
| [42] Kim et al., 2012 | ||||||
| [36] Hu et al., 2016 | ||||||
| [44] Puttaraju et al., 2006 | ||||||
| [8] Kim et al., 2008 | ||||||
| [43] Woodward et al., 1992 | ||||||
| [13] Ohno et al., 2000 | ||||||
| [12] Yamamoto et al., 2014 | ||||||
| [19] Lee et al., 2013a | ||||||
| [24] Kim et al., 2013 | ||||||
| [37] Harada et al., 2002b | ||||||
| [38] Harada et al., 2004 | ||||||
| [39] Harada et al., 2006 | ||||||
| [40] Nameda et al., 2003 | ||||||
| [41] Yao et al., 2008 | ||||||
| [25] Lee et al., 2016a | ||||||
| [26] Park et al., 2016 | ||||||
| [27] Lee et al., 2016b | ||||||
| [45] Lee et al., 2013b | ||||||
| Risk of bias rating | Low risk of bias | High risk of bias | Unclear |
| Medical Application | No. of Studies | Healing Effect | n | SMD (95% CI) | I2 | Total Effect |
|---|---|---|---|---|---|---|
| Diabetes treatment | 7 | Serum glucose level (mg/dL) | 33 | 3.52 (−0.16, 7.21) | 94% | SMD = 1.29 95% CI (0.47, 2.11) I2 = 91.9% |
| Serum insulin level (mg/dL) | 18 | 1.92 (1.10, 2.75) | 0% | |||
| Nutrition intake (mL) | 36 | 0.32 (−0.15, 0.78) | 0% | |||
| Body weight (g) | 46 | −0.60 (−1.61, 0.41) | 78% | |||
| Wound closure rate (%) | 40 | 3.55 (2.56, 4.54) | 40% | |||
| Cancer treatment | 19 | Tumor activity | 67 | 2.22 (1.69, 2.75) | 42% | |
| Cancer cell survival (%) | 58 | 23.05 (18.02, 28.08) | 34% | |||
| IFN- γ production (ng/mL) | 43 | −0.34 (−0.37, −0.31) | 99% | |||
| Anti-inflammatory activity | 4 | NO production (mg) | 19 | 4.81 (3.30, 6.33) | 50% | |
| Inflammatory cell survival (%) | 11 | 9.03 (0.80, 17.27) | 47% | |||
| Anti-fungal activity | 3 | Anti-fungal activity | 46 | 0.20 (−0.23, 0.62) | 44% | |
| Antioxidant activity | 6 | Anti-oxidant activity | 14 | −7.72 (−10.96, −4.49) | 0% | |
| DPPH (%) | 14 | −26.50 (−38.35, −14.64) | 10% |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thi Nhu Ngoc, L.; Oh, Y.-K.; Lee, Y.-J.; Lee, Y.-C. Effects of Sparassis crispa in Medical Therapeutics: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Int. J. Mol. Sci. 2018, 19, 1487. https://doi.org/10.3390/ijms19051487
Thi Nhu Ngoc L, Oh Y-K, Lee Y-J, Lee Y-C. Effects of Sparassis crispa in Medical Therapeutics: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. International Journal of Molecular Sciences. 2018; 19(5):1487. https://doi.org/10.3390/ijms19051487
Chicago/Turabian StyleThi Nhu Ngoc, Le, You-Kwan Oh, Young-Jong Lee, and Young-Chul Lee. 2018. "Effects of Sparassis crispa in Medical Therapeutics: A Systematic Review and Meta-Analysis of Randomized Controlled Trials" International Journal of Molecular Sciences 19, no. 5: 1487. https://doi.org/10.3390/ijms19051487
APA StyleThi Nhu Ngoc, L., Oh, Y.-K., Lee, Y.-J., & Lee, Y.-C. (2018). Effects of Sparassis crispa in Medical Therapeutics: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. International Journal of Molecular Sciences, 19(5), 1487. https://doi.org/10.3390/ijms19051487








