ScFvs as Allosteric Inhibitors of VEGFR-2: Novel Tools to Harness VEGF Signaling
Abstract
:1. Introduction
2. Results
2.1. Selection, Production, and Purification of ScFv Antibodies
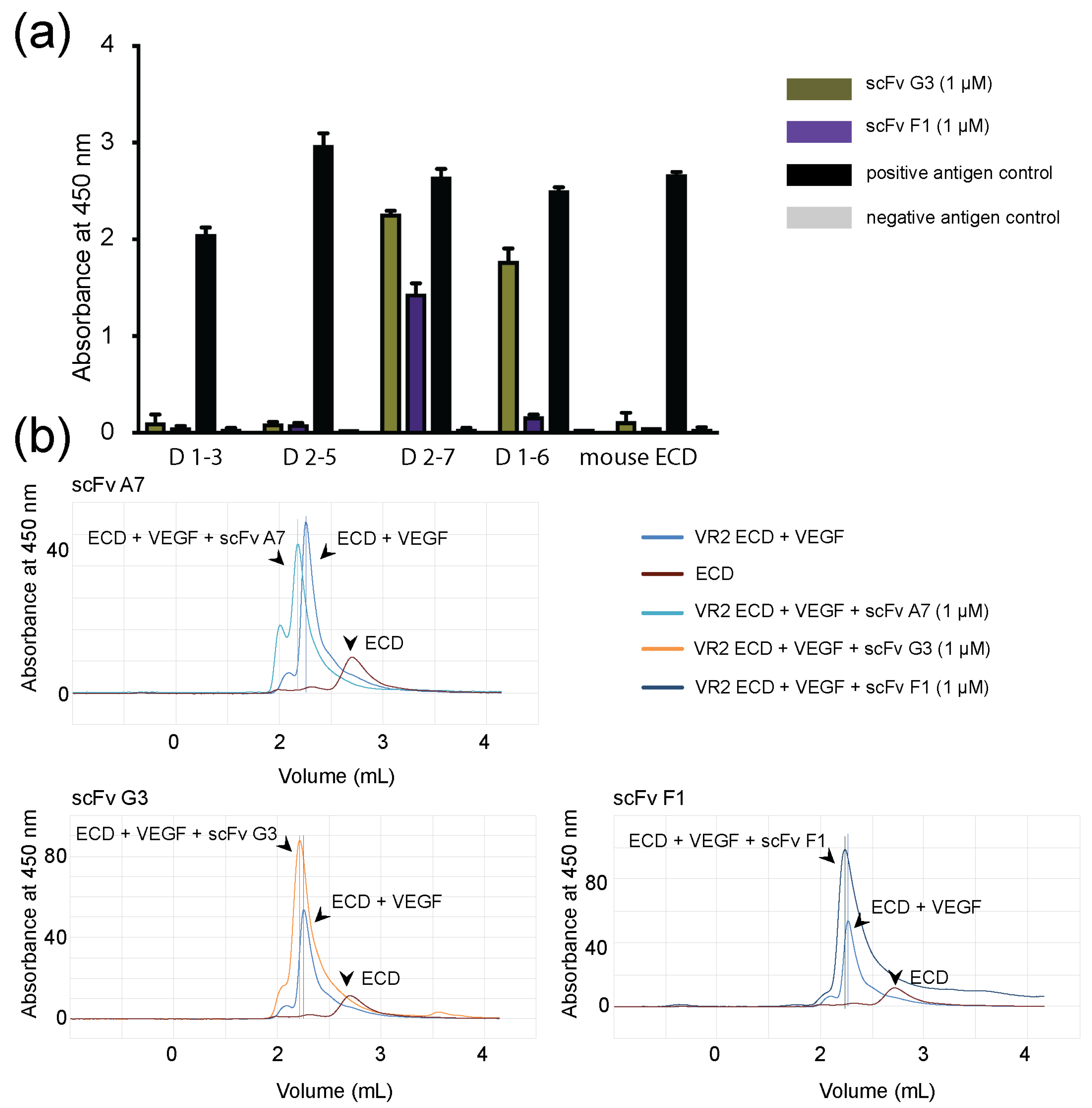
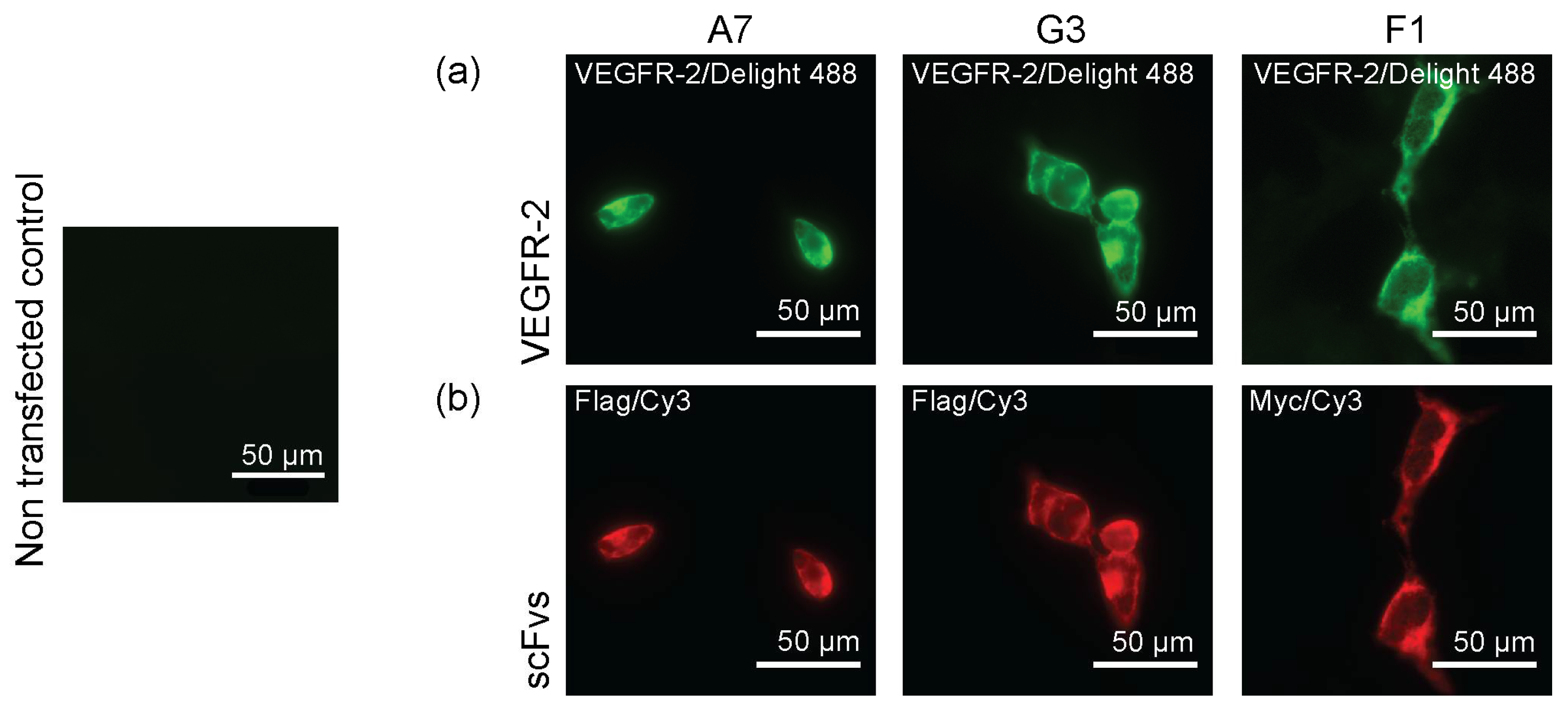
2.2. Binding of ScFvs to Recombinant and Endogenous VEGFR-2
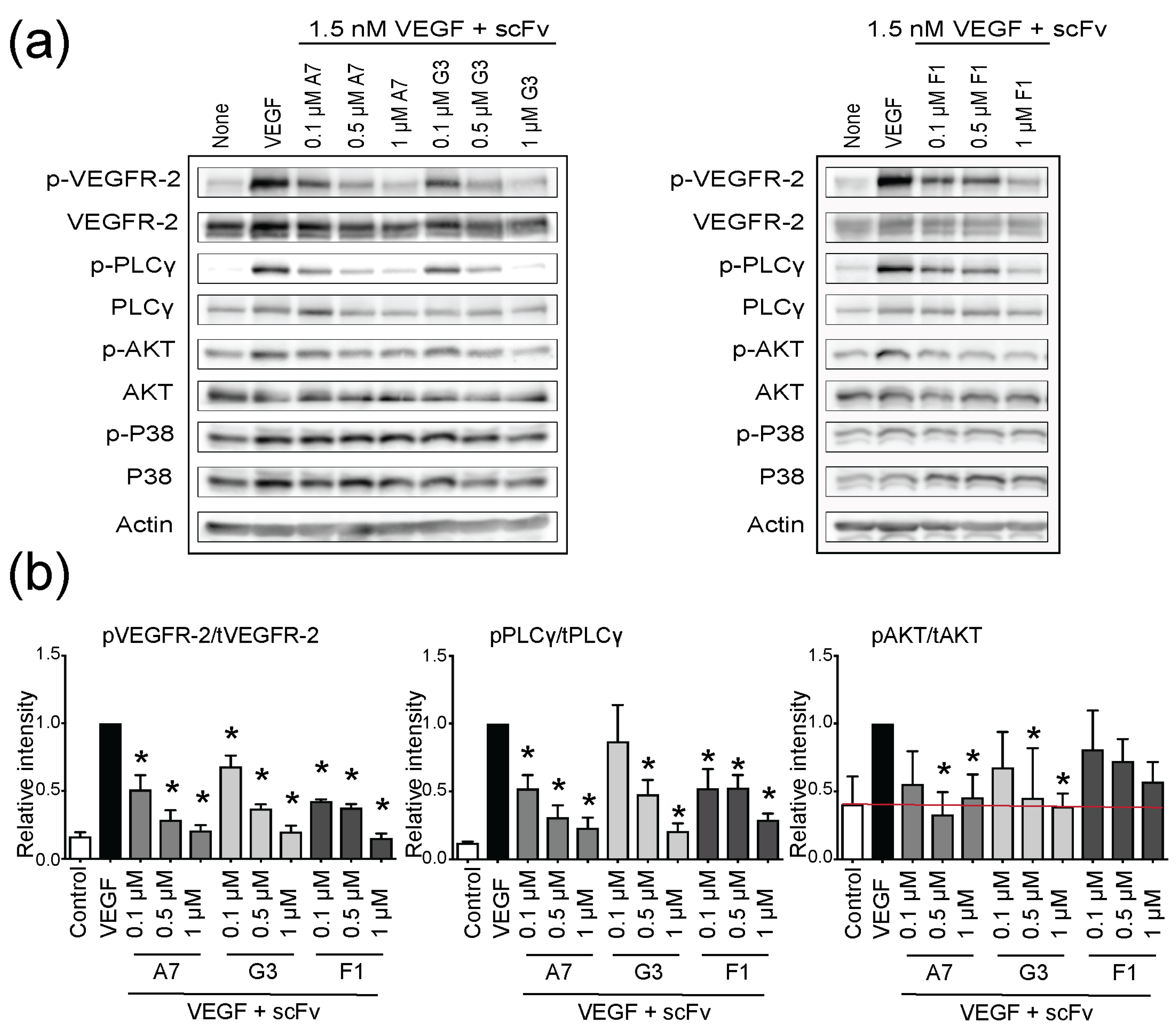
2.3. Functional Inhibition of VEGFR-2 Phosphorylation with ScFvs
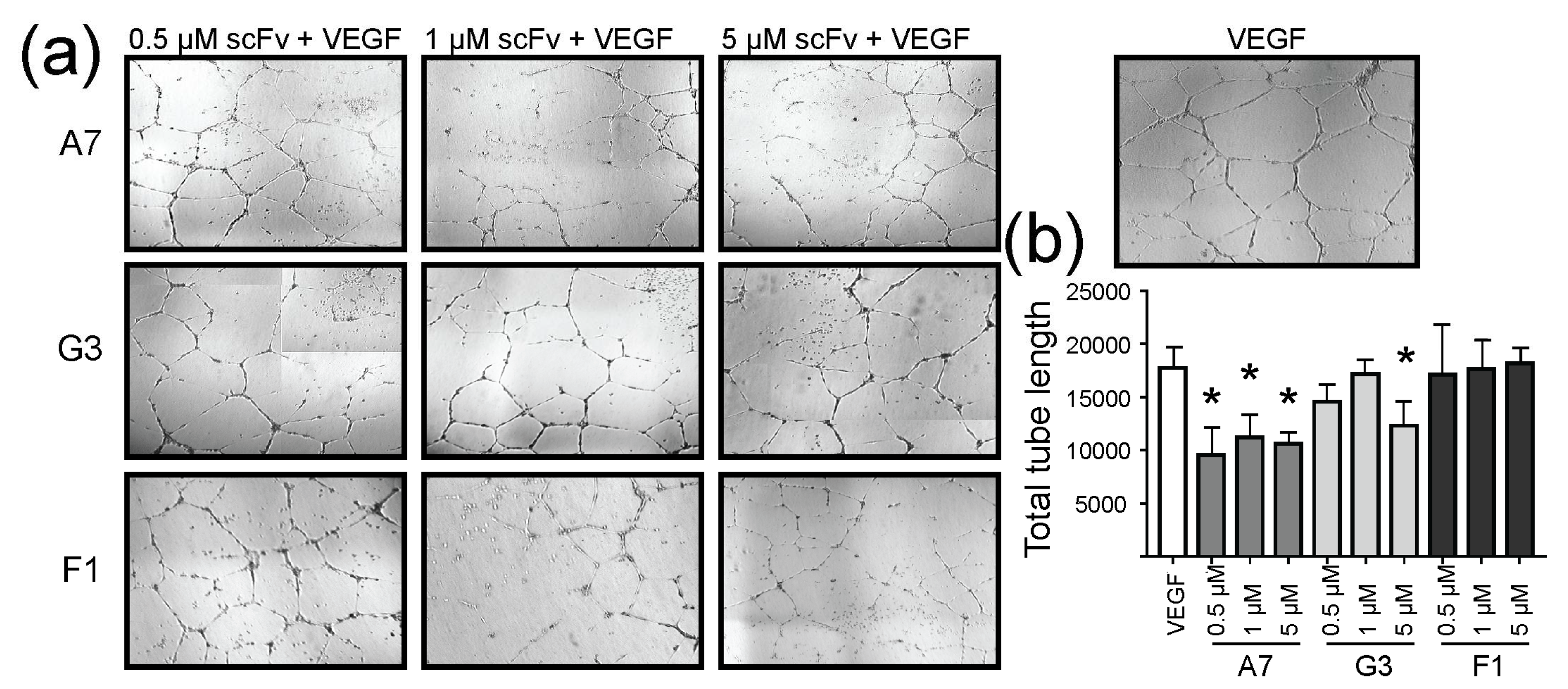
2.4. Effect of ScFvs on In Vitro Angiogenesis
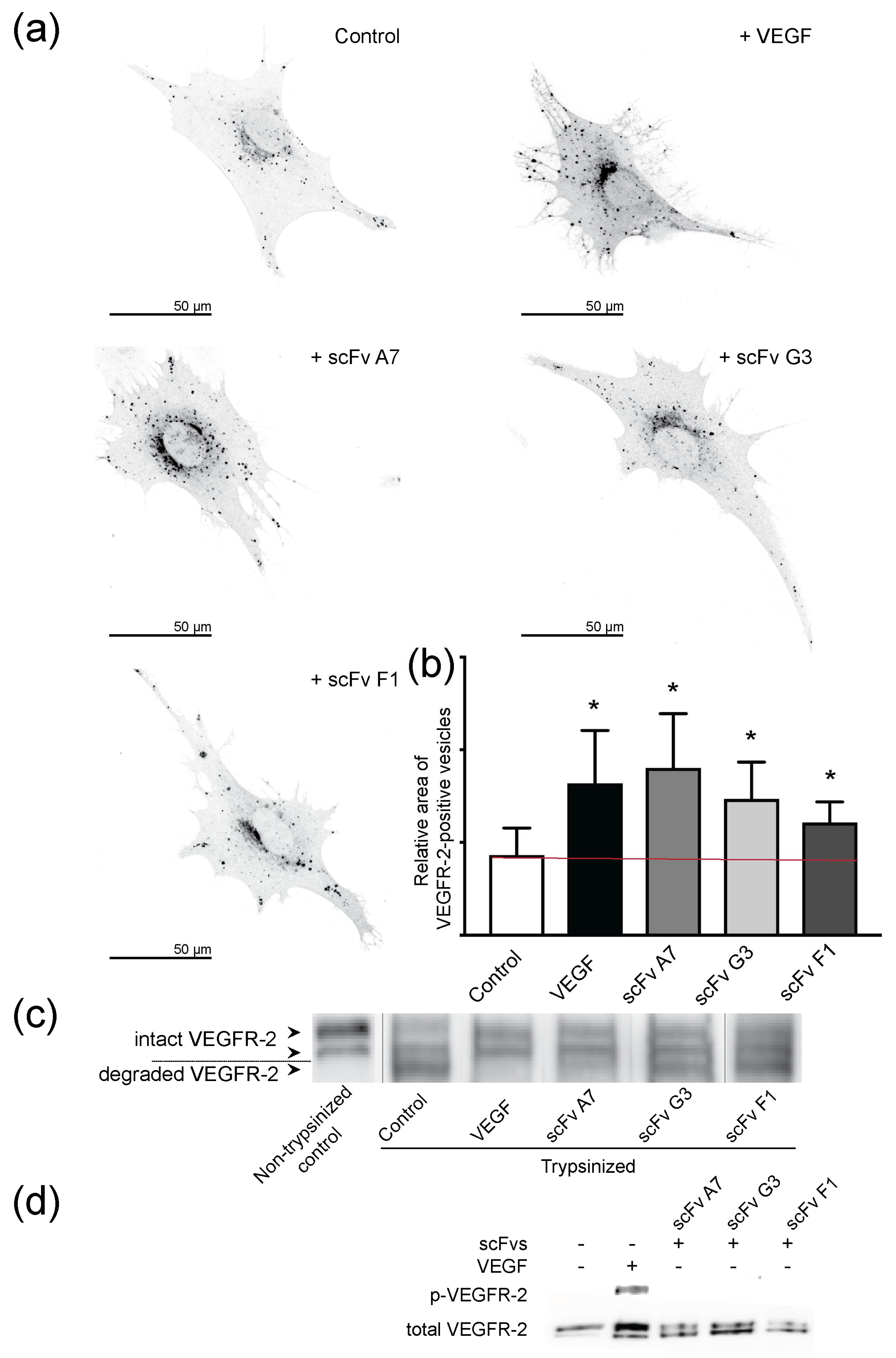
2.5. VEGF and ScFvs Promote Internalization of VEGFR-2
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Transient Transfection
4.3. VEGFR-2 Kinase Activity Assay
4.4. Immunofluorescence Microscopy
4.5. Phage Display and Selection of ScFv Antibodies
4.6. Subcloning of pCANTAB 5E ScFv A7
4.7. Expression and Purification of ScFvs
4.8. Enzyme-Linked Immunosorbent Assay (ELISA)
4.9. Size-Exclusion Chromatography (SEC)
4.10. Fluorescence Size-Exclusion Chromatography (FSEC)
4.11. Binding Affinity Determination by Isothermal Titration Calorimetry (ITC)
4.12. HUVEC Tube Formation Assay
4.13. HUVEC Migration Assay
4.14. Squassh Analysis of VEGFR-2 Internalization
4.15. Trypsin Digestion of Cell Surface-Exposed Receptors
4.16. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviations
| BSA | Bovine serum albumin |
| CDR | Complementarity determining region |
| DARPins | Designed ankyrin repeat proteins |
| DMEM | Dulbecco’s modified eagle’s medium |
| ECD | Extracellular domain |
| E. coli | Escherichia coli |
| EGFR | Epidermal growth factor receptor |
| ELISA | Enzyme linked immunosorbent assay |
| Fab | Fragment antigen binding |
| FSEC | Fluorescence size-exclusion chromatography |
| HEK293 | Human embryonic kidney cells 293 |
| HER2 | Human epidermal growth factor receptor 2 |
| HRP | Horseradish peroxidase |
| HUVECs | Human umbilical vein endothelial cells |
| IgG | Immunoglobulin G |
| IPTG | Isopropyl β-d-1-thiogalactopyranoside |
| ITC | Isothermal titration calorimetry |
| KDR | Kinase insert domain receptor |
| OD | Optical density |
| PAE-KDR | Porcine aortic endothelial cells overexpressing VEGFR-2 |
| PBS | Phosphate buffered saline |
| PCR | Polymerase chain reaction |
| PLCγ | Phospholipase C gamma |
| PVDF | Polyvinylidene fluoride |
| ScFv | Single chain variable fragment |
| SDS-PAGE | Sodium dodecylsulfate polyacrylamide gel electrophoresis |
| SEC | Size-exclusion chromatography |
| VEGF | Vascular endothelial growth factor |
| VEGFR | VEGF receptor |
| VH/VL | Antibody heavy/light chain variable region |
References
- Hurwitz, H.; Fehrenbacher, L.; Novotny, W.; Cartwright, T.; Hainsworth, J.; Heim, W.; Berlin, J.; Baron, A.; Griffing, S.; Holmgren, E.; et al. Bevacizumab plus Irinotecan, Fluorouracil, and Leucovorin for Metastatic Colorectal Cancer. N. Engl. J. Med. 2004, 350, 2335–2342. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, C.S.; Tomasek, J.; Yong, C.J.; Dumitru, F.; Passalacqua, R.; Goswami, C.; Safran, H.; Dos Santos, L.V.; Aprile, G.; Ferry, D.R.; et al. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): An international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet 2014, 383, 31–39. [Google Scholar] [CrossRef]
- Wilke, H.; Muro, K.; Van Cutsem, E.; Oh, S.-C.; Bodoky, G.; Shimada, Y.; Hironaka, S.; Sugimoto, N.; Lipatov, O.; Kim, T.-Y.; et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): A double-blind, randomised phase 3 trial. Lancet Oncol. 2014, 15, 1224–1235. [Google Scholar] [CrossRef]
- Mendel, D.B.; Laird, A.D.; Xin, X.; Louie, S.G.; Christensen, J.G.; Li, G.; Schreck, R.E.; Abrams, T.J.; Ngai, T.J.; Lee, L.B.; et al. In vivo antitumor activity of SU11248, a novel tyrosine kinase inhibitor targeting vascular endothelial growth factor and platelet-derived growth factor receptors: Determination of a pharmacokinetic/pharmacodynamic relationship. Clin. Cancer Res. 2003, 9, 327–337. [Google Scholar] [PubMed]
- Shinkai, A.; Ito, M.; Anazawa, H.; Yamaguchi, S.; Shitara, K.; Shibuya, M. Mapping of the Sites Involved in Ligand Association and Dissociation at the Extracellular Domain of the Kinase Insert Domain-containing Receptor for Vascular Endothelial Growth Factor. J. Biol. Chem. 1998, 273, 31283–31288. [Google Scholar] [CrossRef] [PubMed]
- Fuh, G.; Li, B.; Crowley, C.; Cunningham, B.; Wells, J.A. Requirements for binding and signaling of the kinase domain receptor for vascular endothelial growth factor. J. Biol. Chem. 1998, 273, 11197–11204. [Google Scholar] [CrossRef] [PubMed]
- Kisko, K.; Brozzo, M.S.; Missimer, J.; Schleier, T.; Menzel, A.; Leppänen, V.-M.; Alitalo, K.; Walzthoeni, T.; Aebersold, R.; Ballmer-Hofer, K. Structural analysis of vascular endothelial growth factor receptor-2/ligand complexes by small-angle X-ray solution scattering. FASEB J. 2011, 25, 2980–2986. [Google Scholar] [CrossRef] [PubMed]
- Ruch, C.; Skiniotis, G.; Steinmetz, M.O.; Walz, T.; Ballmer-Hofer, K. Structure of a VEGF–VEGF receptor complex determined by electron microscopy. Nat. Struct. Mol. Biol. 2007, 14, 249–250. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Xie, P.; Opatowsky, Y.; Schlessinger, J. Direct contacts between extracellular membrane-proximal domains are required for VEGF receptor activation and cell signaling. Proc. Natl. Acad. Sci. USA 2010, 107, 1906–1911. [Google Scholar] [CrossRef] [PubMed]
- Brozzo, M.S.; Leppanen, V.M.; Winkler, F.K.; Ballmer-Hofer, K. VEGFR-2/VEGF-A COMPLEX STRUCTURE. 3V2A 2012. [Google Scholar] [CrossRef]
- Hyde, C.A.C.; Giese, A.; Stuttfeld, E.; Abram Saliba, J.; Villemagne, D.; Schleier, T.; Binz, H.K.; Ballmer-Hofer, K. Targeting Extracellular Domains D4 and D7 of Vascular Endothelial Growth Factor Receptor 2 Reveals Allosteric Receptor Regulatory Sites. Mol. Cell. Biol. 2012, 32, 3802–3813. [Google Scholar] [CrossRef] [PubMed]
- Thieltges, K.M.; Avramovic, D.; Piscitelli, C.L.; Markovic-Mueller, S.; Binz, H.K.; Ballmer-Hofer, K. Characterization of a drug-targetable allosteric site regulating vascular endothelial growth factor signaling. Angiogenesis 2018, 38, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kendrew, J.; Eberlein, C.; Hedberg, B.; McDaid, K.; Smith, N.R.; Weir, H.M.; Wedge, S.R.; Blakey, D.C.; Foltz, I.; Zhou, J.; et al. An antibody targeted to VEGFR-2 Ig domains 4-7 inhibits VEGFR-2 activation and VEGFR-2-dependent angiogenesis without affecting ligand binding. Mol. Cancer Ther. 2011, 10, 770–783. [Google Scholar] [CrossRef] [PubMed]
- Marks, J.D.; Hoogenboom, H.R.; Bonnert, T.P.; McCafferty, J.; Griffiths, A.D.; Winter, G. By-passing immunization. Human antibodies from V-gene libraries displayed on phage. J. Mol. Biol. 1991, 222, 581–597. [Google Scholar] [CrossRef]
- Rosenblum, L.T.; Choyke, P.L.; Kobayashi, H. Quantitative and specific molecular imaging of cancer with labeled engineered monoclonal antibody fragments. Ther. Deliv. 2011, 2, 345–358. [Google Scholar] [CrossRef] [PubMed]
- Yokota, T.; Milenic, D.E.; Whitlow, M.; Schlom, J. Rapid tumor penetration of a single-chain Fv and comparison with other immunoglobulin forms. Cancer Res. 1992, 52, 3402–3408. [Google Scholar] [PubMed]
- Thurber, G.M.; Schmidt, M.M.; Wittrup, K.D. Antibody tumor penetration: Transport opposed by systemic and antigen-mediated clearance. Adv. Drug Deliv. Rev. 2008, 60, 1421–1434. [Google Scholar] [CrossRef] [PubMed]
- Monnier, P.; Vigouroux, R.; Tassew, N. In Vivo Applications of Single Chain Fv (Variable Domain) (scFv) Fragments. Antibodies 2013, 2, 193–208. [Google Scholar] [CrossRef]
- Kim, H.-Y.; Wang, X.; Wahlberg, B.; Edwards, W.B. Discovery of hapten-specific scFv from a phage display library and applications for HER2-positive tumor imaging. Bioconjug. Chem. 2014, 25, 1311–1322. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Stuckert, P.; Bosch, I.; Marks, J.D.; Marasco, W.A. Single-chain antibody-mediated gene delivery into ErbB2-positive human breast cancer cells. Cancer Gene Ther. 2001, 8, 555–565. [Google Scholar] [CrossRef] [PubMed]
- Safdari, Y.; Ahmadzadeh, V. Use of Single-Chain Antibody Derivatives for Targeted Drug Delivery. Mol. Med. 2016, 22, 258–270. [Google Scholar] [CrossRef] [PubMed]
- Silacci, M.; Brack, S.; Schirru, G.; Mårlind, J.; Ettorre, A.; Merlo, A.; Viti, F.; Neri, D. Design, construction, and characterization of a large synthetic human antibody phage display library. Proteomics 2005, 5, 2340–2350. [Google Scholar] [CrossRef] [PubMed]
- Böldicke, T.; Tesar, M.; Griesel, C.; Rohde, M.; Gröne, H.J.; Waltenberger, J.; Kollet, O.; Lapidot, T.; Yayon, A.; Weich, H. Anti-VEGFR-2 scFvs for Cell Isolation. Single-Chain Antibodies Recognizing the Human Vascular Endothelial Growth Factor Receptor-2 (VEGFR-2/flk-1) on the Surface of Primary Endothelial Cells and Preselected CD34+ Cells from Cord Blood. Stem Cells 2001, 19, 24–36. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.; Jimenez, X.; Zhang, H.; Bohlen, P.; Witte, L.; Zhu, Z. Selection of high affinity human neutralizing antibodies to VEGFR2 from a large antibody phage display library for antiangiogenesis therapy. Int. J. Cancer 2002, 97, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.; Shen, J.; Vil, M.D.; Zhang, H.; Jimenez, X.; Bohlen, P.; Witte, L.; Zhu, Z. Tailoring in vitro selection for a picomolar affinity human antibody directed against vascular endothelial growth factor receptor 2 for enhanced neutralizing activity. J. Biol. Chem. 2003, 278, 43496–43507. [Google Scholar] [CrossRef] [PubMed]
- Cobo, M.; Gutiérrez, V.; Villatoro, R.; Trigo, J.M.; Ramos, I.; López, O.; Ruiz, M.; Godoy, A.; López, I.; Arroyo, M. Spotlight on ramucirumab in the treatment of nonsmall cell lung cancer: Design, development, and clinical activity. Lung Cancer (Auckl.) 2017, 8, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Tabernero, J.; Yoshino, T.; Cohn, A.L.; Obermannova, R.; Bodoky, G.; Garcia-Carbonero, R.; Ciuleanu, T.-E.; Portnoy, D.C.; Van Cutsem, E.; Grothey, A.; et al. Ramucirumab versus placebo in combination with second-line FOLFIRI in patients with metastatic colorectal carcinoma that progressed during or after first-line therapy with bevacizumab, oxaliplatin, and a fluoropyrimidine (RAISE): A randomised, double-blind, multicentre, phase 3 study. Lancet Oncol. 2015, 16, 499–508. [Google Scholar] [PubMed]
- Zhou, Y.; Drummond, D.C.; Zou, H.; Hayes, M.E.; Adams, G.P.; Kirpotin, D.B.; Marks, J.D. Impact of single-chain Fv antibody fragment affinity on nanoparticle targeting of epidermal growth factor receptor-expressing tumor cells. J. Mol. Biol. 2007, 371, 934–947. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Kim, H.-Y.; Wahlberg, B.; Edwards, W.B. Selection and characterization of high affinity VEGFR1 antibodies from a novel human binary code scFv phage library. Biochem. Biophys. Rep. 2015, 3, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Young, N.M.; MacKenzie, C.R.; Narang, S.A.; Oomen, R.P.; Baenziger, J.E. Thermal stabilization of a single-chain Fv antibody fragment by introduction of a disulphide bond. FEBS Lett. 1995, 377, 135–139. [Google Scholar] [CrossRef]
- Glockshuber, R.; Schmidt, T.; Plückthun, A. The disulfide bonds in antibody variable domains: Effects on stability, folding in vitro, and functional expression in Escherichia coli. Biochemistry 1992, 31, 1270–1279. [Google Scholar] [CrossRef] [PubMed]
- Tan, P.H.; Sandmaier, B.M.; Stayton, P.S. Contributions of a highly conserved VH/VL hydrogen bonding interaction to scFv folding stability and refolding efficiency. Biophys. J. 1998, 75, 1473–1482. [Google Scholar] [CrossRef]
- Brinkmann, U.; Di Carlo, A.; Vasmatzis, G.; Kurochkina, N.; Beers, R.; Lee, B.; Pastan, I. Stabilization of a recombinant Fv fragment by base-loop interconnection and V(H)-V(L) permutation. J. Mol. Biol. 1997, 268, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Friedman, L.M.; Rinon, A.; Schechter, B.; Lyass, L.; Lavi, S.; Bacus, S.S.; Sela, M.; Yarden, Y. Synergistic down-regulation of receptor tyrosine kinases by combinations of mAbs: Implications for cancer immunotherapy. Proc. Natl. Acad. Sci. USA 2005, 102, 1915–1920. [Google Scholar] [CrossRef] [PubMed]
- Baselga, J.; Albanell, J. Mechanism of action of anti-HER2 monoclonal antibodies. Ann. Oncol. 2001, 12, S35–S41. [Google Scholar] [CrossRef] [PubMed]
- Drebin, J.A.; Link, V.C.; Stern, D.F.; Weinberg, R.A.; Greene, M.I. Down-modulation of an oncogene protein product and reversion of the transformed phenotype by monoclonal antibodies. Cell 1985, 41, 695–706. [Google Scholar] [CrossRef]
- Hudziak, R.M.; Lewis, G.D.; Winget, M.; Fendly, B.M.; Shepard, H.M.; Ullrich, A. p185HER2 monoclonal antibody has antiproliferative effects in vitro and sensitizes human breast tumor cells to tumor necrosis factor. Mol. Cell. Biol. 1989, 9, 1165–1172. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Okollie, B.; Artemov, D. Controlled internalization of Her-2/ neu receptors by cross-linking for targeted delivery. Cancer Biol. Ther. 2007, 6, 1960–1966. [Google Scholar] [CrossRef] [PubMed]
- Bertelsen, V.; Stang, E. The Mysterious Ways of ErbB2/HER2 Trafficking. Membranes (Basel) 2014, 4, 424–446. [Google Scholar] [CrossRef] [PubMed]
- Sarabipour, S.; Ballmer-Hofer, K.; Hristova, K. VEGFR-2 conformational switch in response to ligand binding. eLife 2016, 5, e13876. [Google Scholar] [CrossRef] [PubMed]
- Nellis, D.F.; Ekstrom, D.L.; Kirpotin, D.B.; Zhu, J.; Andersson, R.; Broadt, T.L.; Ouellette, T.F.; Perkins, S.C.; Roach, J.M.; Drummond, D.C.; et al. Preclinical manufacture of an anti-HER2 scFv-PEG-DSPE, liposome-inserting conjugate. 1. Gram-scale production and purification. Biotechnol. Prog. 2005, 21, 205–220. [Google Scholar] [CrossRef] [PubMed]
- Viardot, A.; Goebeler, M.-E.; Hess, G.; Neumann, S.; Pfreundschuh, M.; Adrian, N.; Zettl, F.; Libicher, M.; Sayehli, C.; Stieglmaier, J.; et al. Phase 2 study of the bispecific T-cell engager (BiTE) antibody blinatumomab in relapsed/refractory diffuse large B-cell lymphoma. Blood 2016, 127, 1410–1416. [Google Scholar] [CrossRef] [PubMed]
- Goebeler, M.-E.; Knop, S.; Viardot, A.; Kufer, P.; Topp, M.S.; Einsele, H.; Noppeney, R.; Hess, G.; Kallert, S.; Mackensen, A.; et al. Bispecific T-Cell Engager (BiTE) Antibody Construct Blinatumomab for the Treatment of Patients with Relapsed/Refractory Non-Hodgkin Lymphoma: Final Results From a Phase I Study. J. Clin. Oncol. 2016, 34, 1104–1111. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.; Cortes, J.; Hurvitz, S.A.; Krop, I.E.; Tripathy, D.; Verma, S.; Riahi, K.; Reynolds, J.G.; Wickham, T.J.; Molnar, I.; et al. HERMIONE: A randomized Phase 2 trial of MM-302 plus trastuzumab versus chemotherapy of physician’s choice plus trastuzumab in patients with previously treated, anthracycline-naïve, HER2-positive, locally advanced/metastatic breast cancer. BMC Cancer 2016, 16, 320. [Google Scholar] [CrossRef] [PubMed]
- Pini, A.; Viti, F.; Santucci, A.; Carnemolla, B.; Zardi, L.; Neri, P.; Neri, D. Design and use of a phage display library. Human antibodies with subnanomolar affinity against a marker of angiogenesis eluted from a two-dimensional gel. J. Biol. Chem. 1998, 273, 21769–21776. [Google Scholar] [CrossRef] [PubMed]
- Rizk, A.; Paul, G.; Incardona, P.; Bugarski, M.; Mansouri, M.; Niemann, A.; Ziegler, U.; Berger, P.; Sbalzarini, I.F. Segmentation and quantification of subcellular structures in fluorescence microscopy images using Squassh. Nat. Protoc. 2014, 9, 586–596. [Google Scholar] [CrossRef] [PubMed]
| ScFv Clones | CDR3 VH | CDR3 VL | ScFv Library | VEGFR-2 Domain Specificity | Binding Affinity Kd (nM) |
|---|---|---|---|---|---|
| G3 | P G F S A | D P R G A H | DP47DPL16 | Domain 6 | 137 |
| F1 | P G A S A | A P G G A Y | DP47DPL16 | Domain 7 | 6800 |
| A7 | GLWGGMDY | QQWNTYPYT | Mouse synthetic V-gene | Domains 2–3 | 92.6 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ballmer-Hofer, K.; A.C. Hyde, C.; Schleier, T.; Avramovic, D. ScFvs as Allosteric Inhibitors of VEGFR-2: Novel Tools to Harness VEGF Signaling. Int. J. Mol. Sci. 2018, 19, 1334. https://doi.org/10.3390/ijms19051334
Ballmer-Hofer K, A.C. Hyde C, Schleier T, Avramovic D. ScFvs as Allosteric Inhibitors of VEGFR-2: Novel Tools to Harness VEGF Signaling. International Journal of Molecular Sciences. 2018; 19(5):1334. https://doi.org/10.3390/ijms19051334
Chicago/Turabian StyleBallmer-Hofer, Kurt, Caroline A.C. Hyde, Thomas Schleier, and Dragana Avramovic. 2018. "ScFvs as Allosteric Inhibitors of VEGFR-2: Novel Tools to Harness VEGF Signaling" International Journal of Molecular Sciences 19, no. 5: 1334. https://doi.org/10.3390/ijms19051334
APA StyleBallmer-Hofer, K., A.C. Hyde, C., Schleier, T., & Avramovic, D. (2018). ScFvs as Allosteric Inhibitors of VEGFR-2: Novel Tools to Harness VEGF Signaling. International Journal of Molecular Sciences, 19(5), 1334. https://doi.org/10.3390/ijms19051334





