Therapeutic and Preventive Effects of Osteoclastogenesis Inhibitory Factor on Osteolysis, Proliferation of Mammary Tumor Cell and Induction of Cancer Stem Cells in the Bone Microenvironment
Abstract
1. Introduction
2. Results
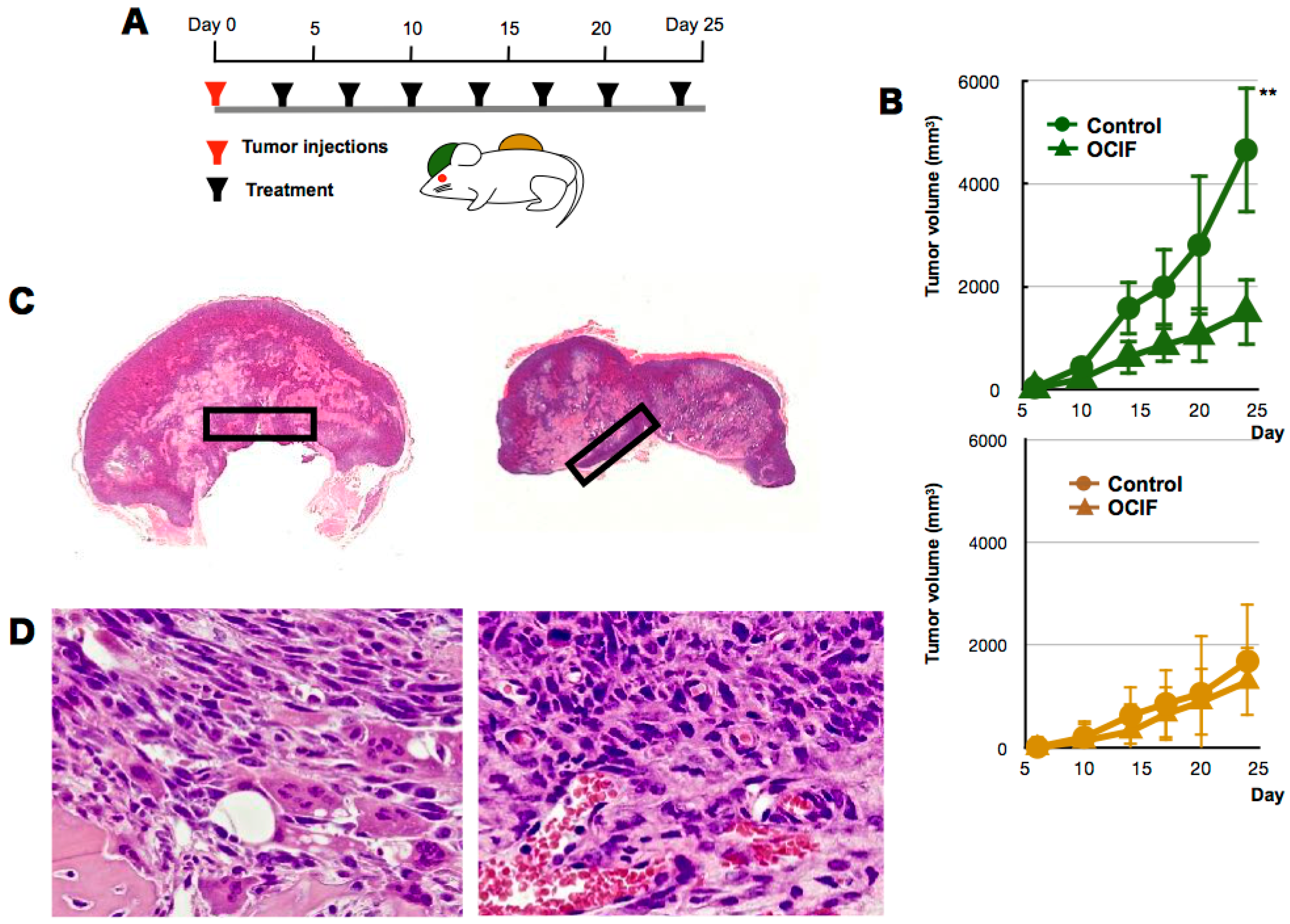
2.1. Effects of hOCIF on Tumor Growth in the Tumor Microenvironments
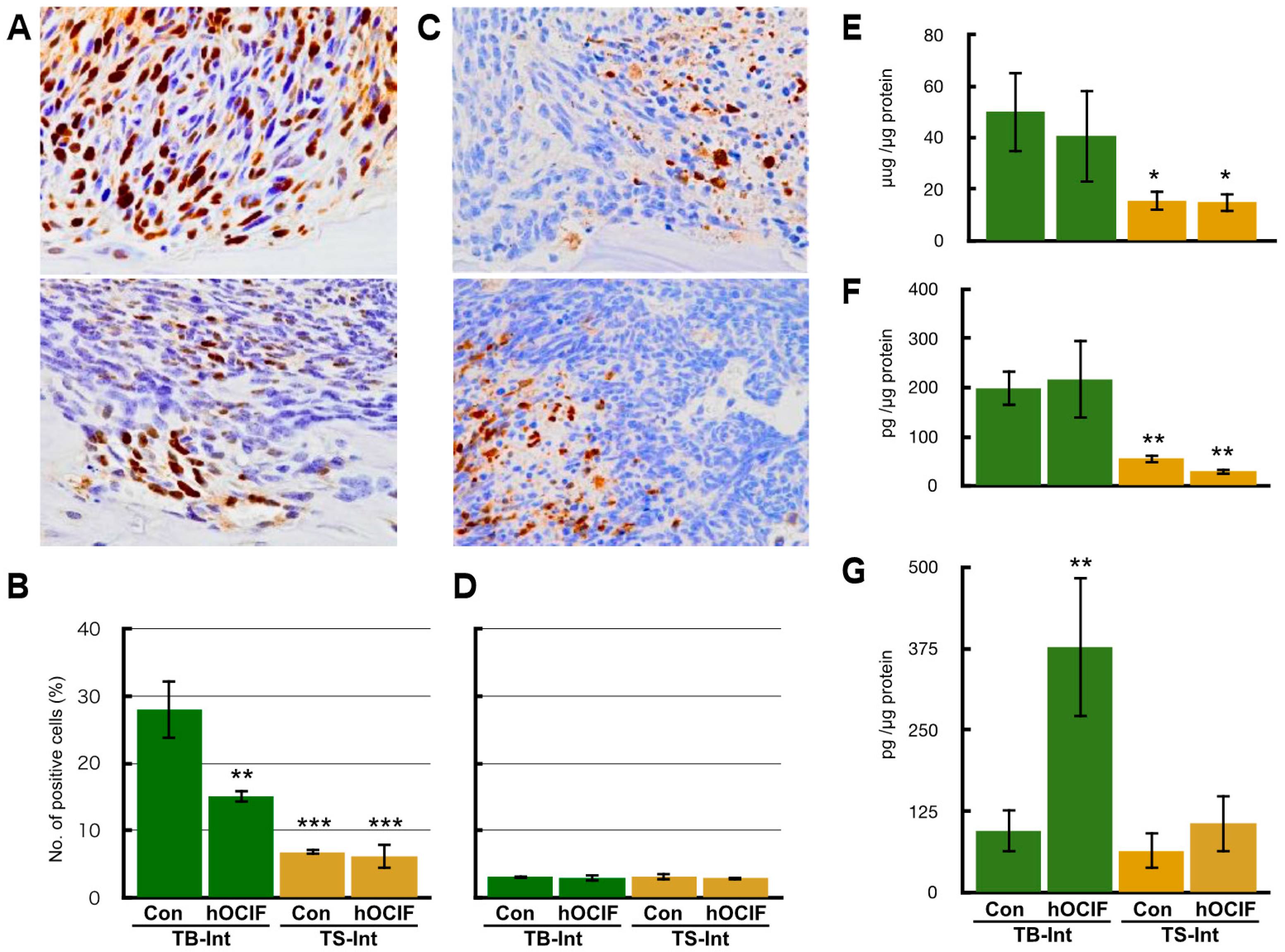
2.2. Effects of hOCIF on Osteolysis and Cytokine Levels in the Tumor Microenvironments
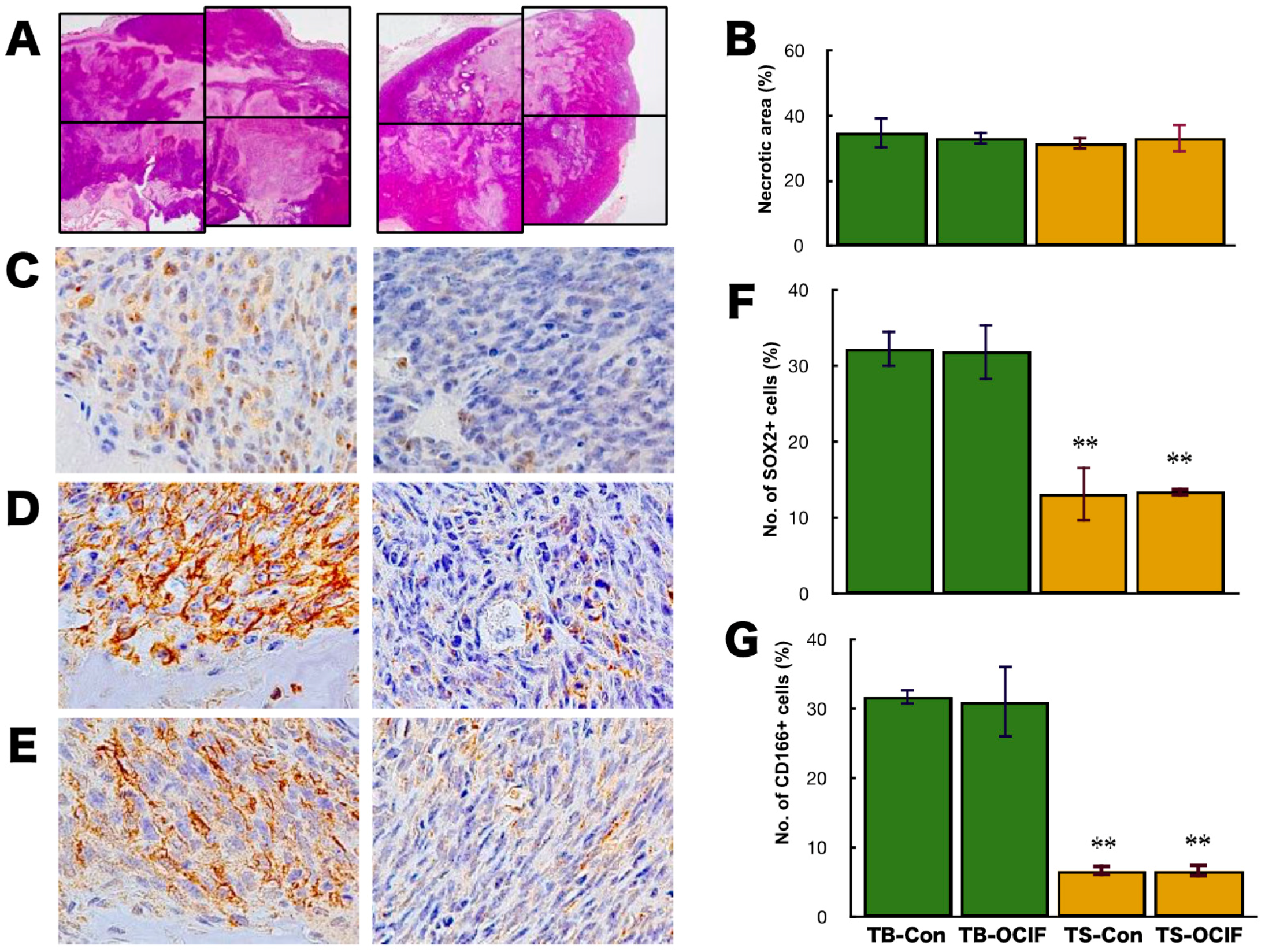
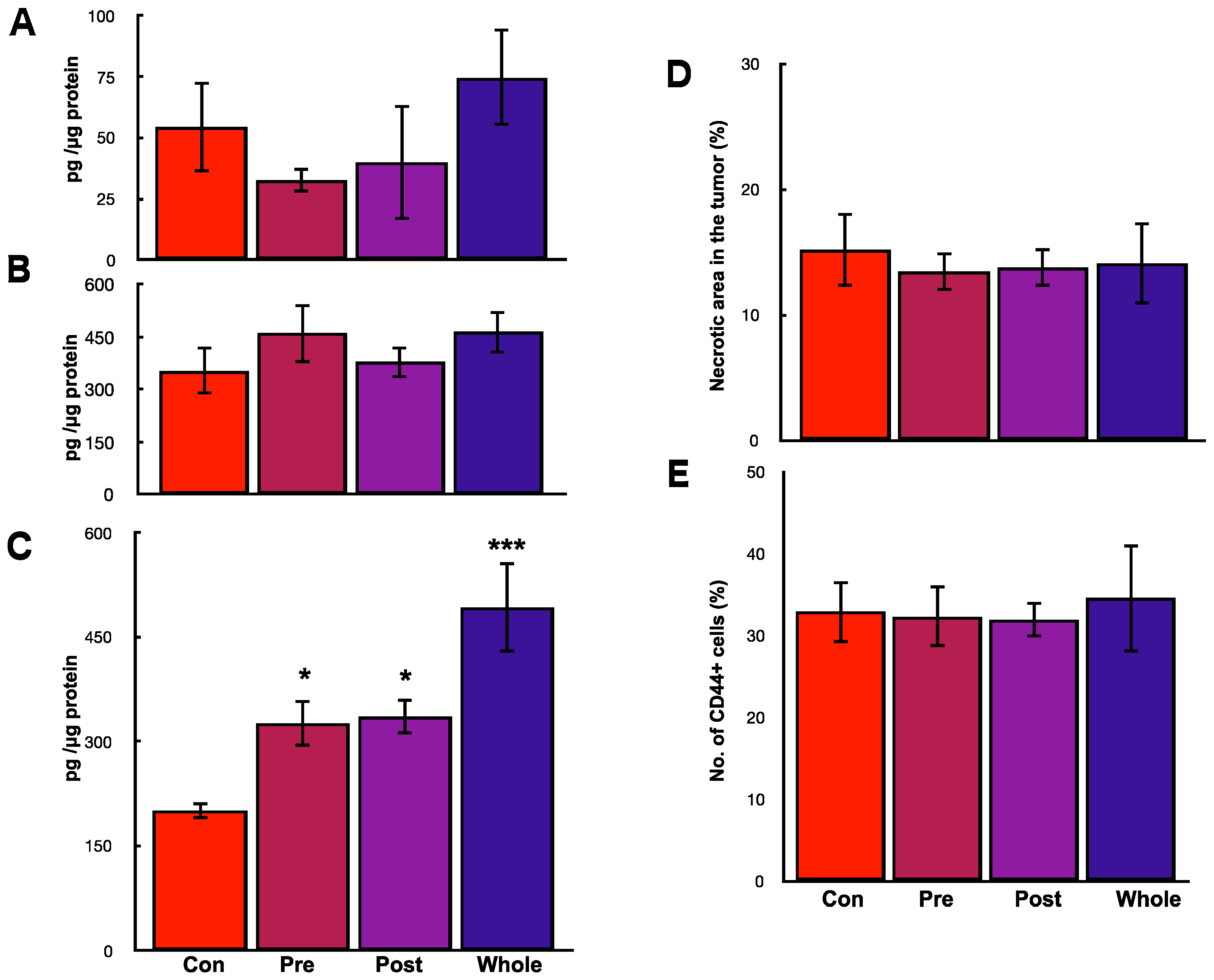
2.3. Effects of hOCIF on the Induction of Necrosis and CSCs in the Microenvironments
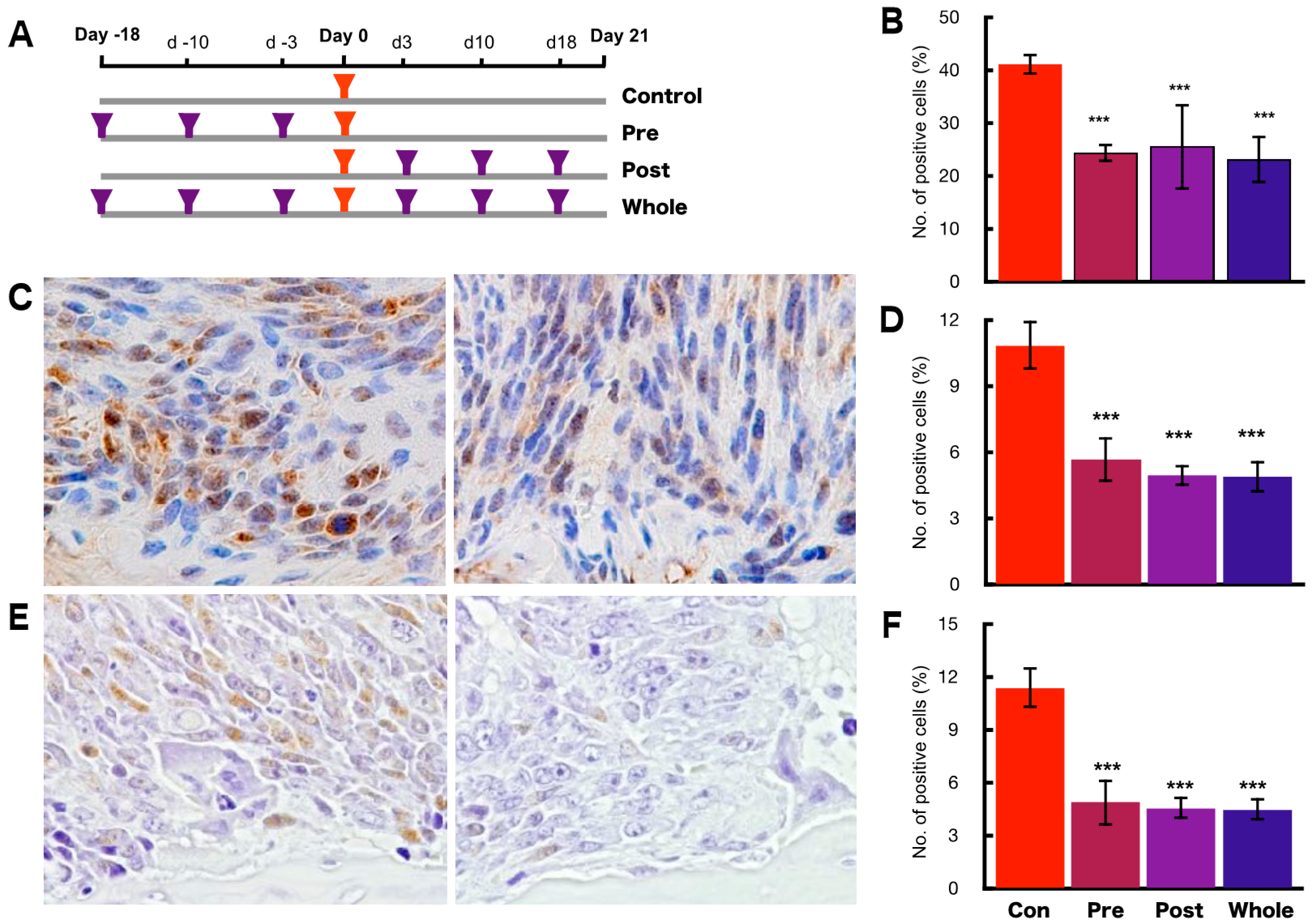
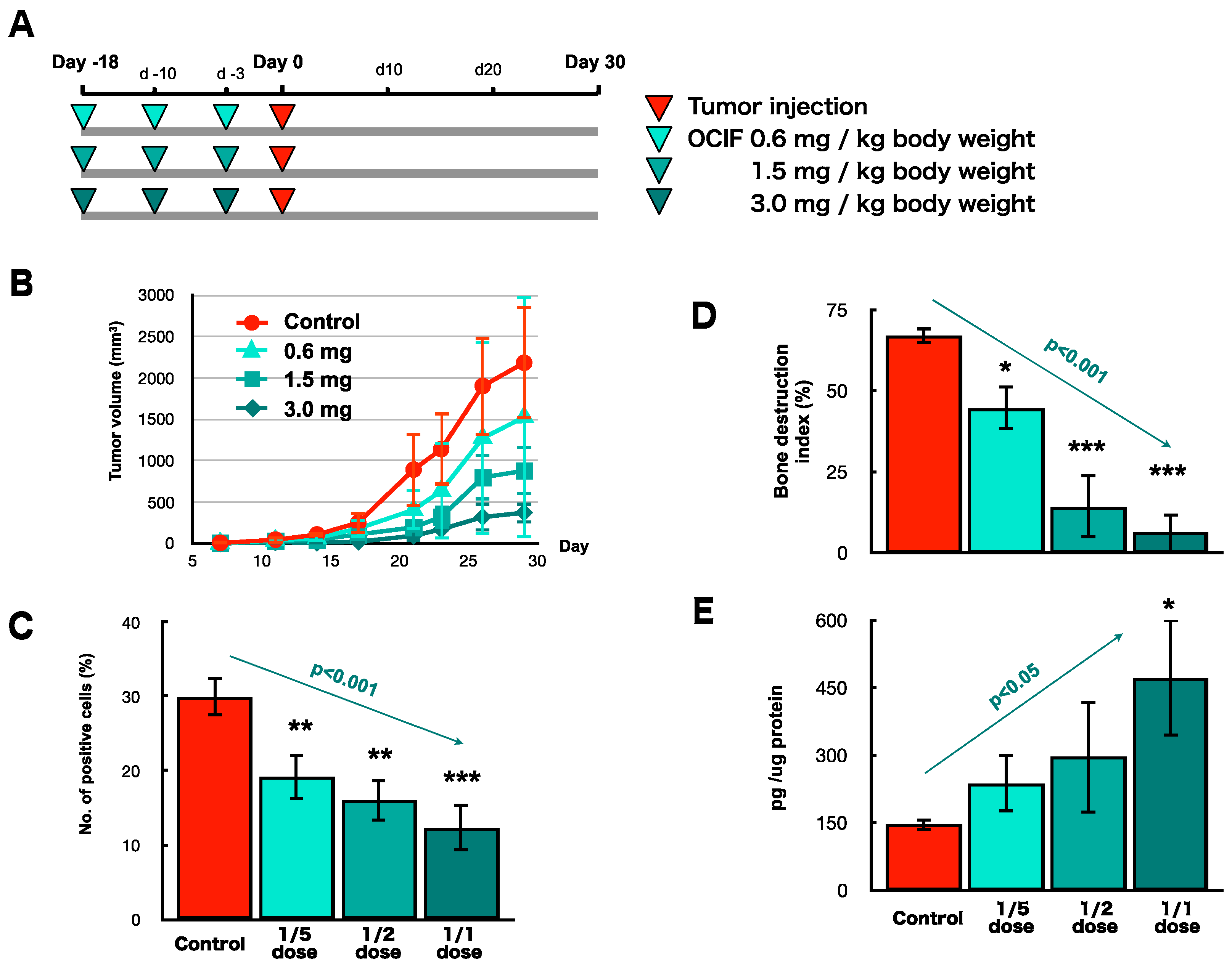
2.4. Preventive Effects of hOCIF on Tumor Growth and Cell Proliferation
2.5. Preventive Effects of hOCIF on Osteolysis and Cytokine Levels in the Tumor Microenvironment
2.6. Preventive Effects of hOCIF on the Induction of Necrosis and CSCs in the Tumor Microenvironment
2.7. Preventive Effects of Low Doses of hOCIF on Osteolysis and Cytokine Levels in the Tumor Microenvironment (3)
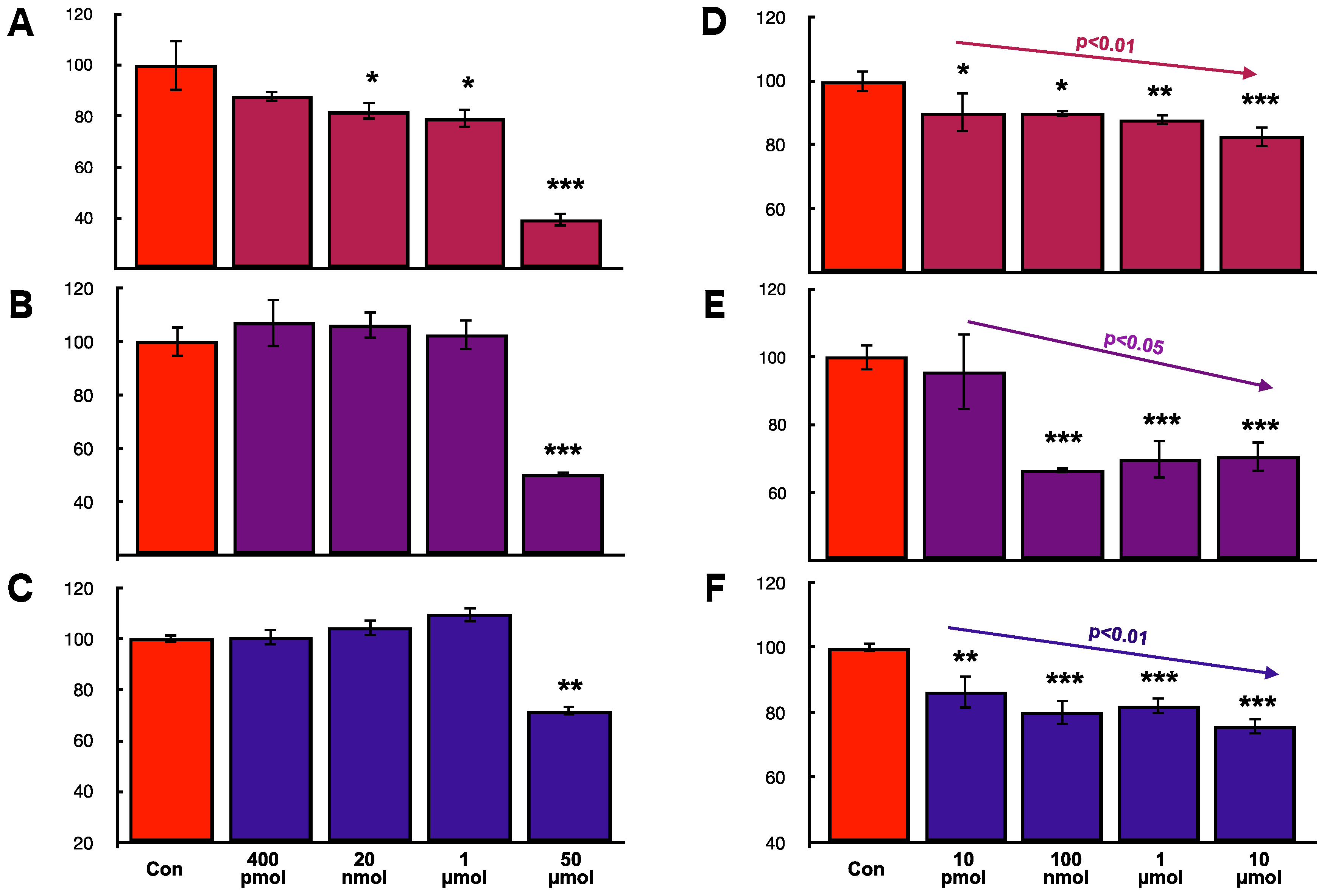
2.8. Effects of hOCIF and mOPG on Cell Proliferation of Mammary Tumor Cells In Vitro
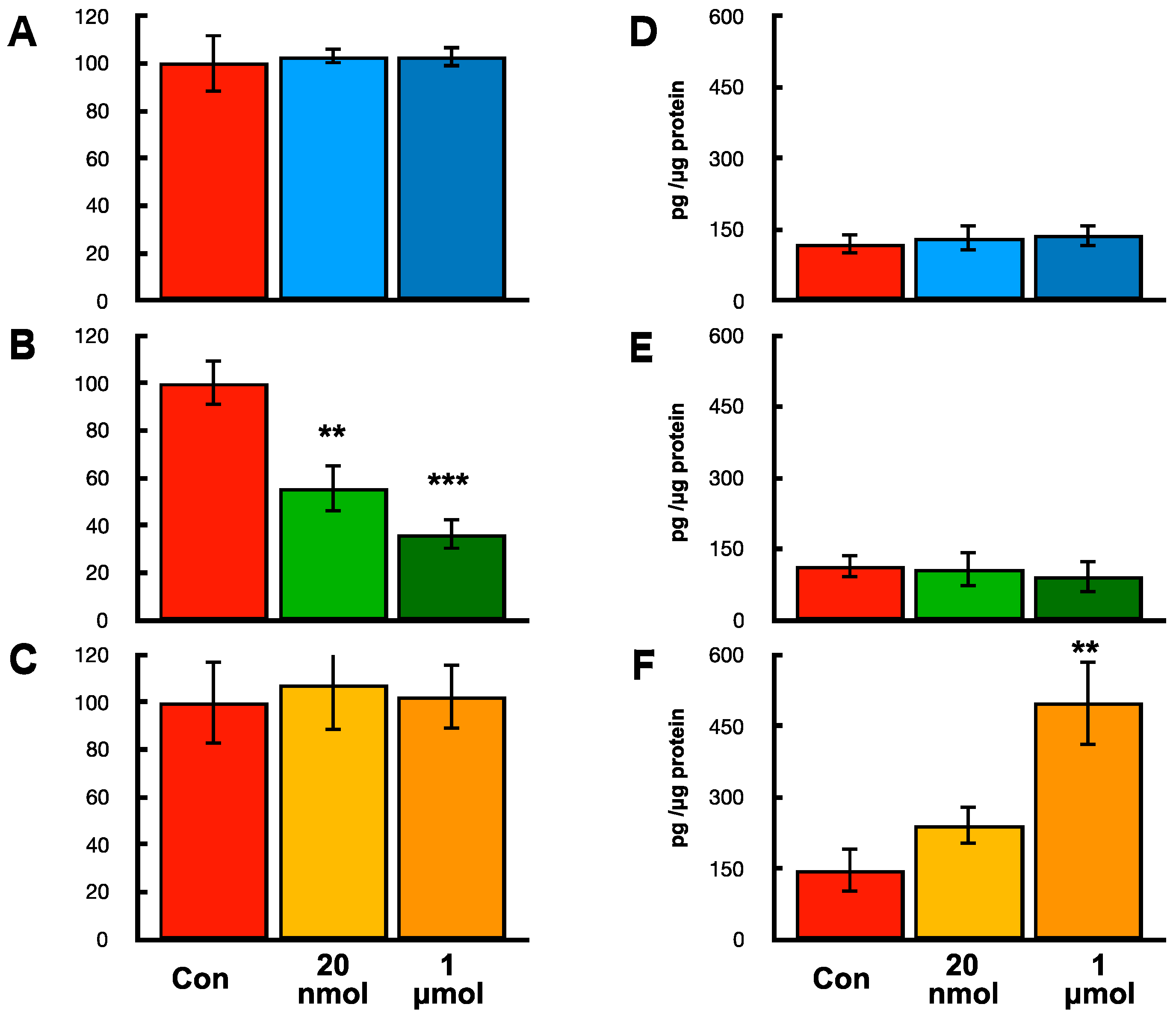
2.9. Effects of hOCIF on Proliferation and mOPG Levels of Mammary Tumors Cell, Osteoclasts, and Mouse Mesenchymal Stem Cells In Vitro
3. Discussion
4. Materials and Methods
4.1. Tumor Cell Lines and Tissue Preparation
4.2. Treatment with Human Recombinant OCIF (hOCIF)
4.3. Immunohistochemistry and Tartrate Resistant Acid Phosphatase Staining
4.4. In Vitro Cell Proliferation
4.5. Effects of hOCIF on Osteoclast and Mouse Mesenchymal Stem Cell In Vitro
4.6. Statistical Analysis
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Katanoda, K.; Hori, M. Incidence rate for breast cancer in Japanese in Japan and in the United States from the Cancer Incidence in Five Continents. Jpn. J. Clin. Oncol. 2016, 46, 883. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2017. CA Cancer J. Clin. 2017, 67, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Yi, M.; Liu, P.; Li, X.; Mittendorf, E.A.; He, J.; Ren, Y.; Nayeemuddin, K.; Hunt, K.K. Comparative analysis of clinicopathologic features, treatment, and survival of Asian women with a breast cancer diagnosis residing in the United States. Cancer 2012, 118, 4117–4125. [Google Scholar] [CrossRef] [PubMed]
- Yip, C.H.; Smith, R.A.; Anderson, B.O.; Miller, A.B.; Thomas, D.B.; Ang, E.S.; Caffarella, R.S.; Corbex, M.; Kreps, G.L.; McTiernan, A. Guideline implementation for breast healthcare in low- and middle-income countries: Early detection resource allocation. Cancer 2008, 113 (Suppl. S8), 2244–2256. [Google Scholar] [CrossRef] [PubMed]
- Jensen, A.O.; Jacobsen, J.B.; Norgaard, M.; Yong, M.; Fryzek, J.P.; Sorensen, H.T. Incidence of bone metastases and skeletal-related events in breast cancer patients: A population-based cohort study in Denmark. BMC Cancer 2011, 11, 29. [Google Scholar] [CrossRef] [PubMed]
- Mundy, G.R. Metastasis to bone: Causes, consequences and therapeutic opportunities. Nat. Rev. Cancer 2002, 2, 584–593. [Google Scholar] [CrossRef] [PubMed]
- Ohshiba, T.; Miyaura, C.; Inada, M.; Ito, A. Role of RANKL-induced osteoclast formation and MMP-dependent matrix degradation in bone destruction by breast cancer metastasis. Br. J. Cancer 2003, 88, 1318–1326. [Google Scholar] [CrossRef] [PubMed]
- Jimi, E.; Aoki, K.; Saito, H.; D’Acquisto, F.; May, M.J.; Nakamura, I.; Sudo, T.; Kojima, T.; Okamoto, F.; Fukushima, H.; et al. Selective inhibition of NF-κB blocks osteoclastogenesis and prevents inflammatory bone destruction in vivo. Nat. Med. 2004, 10, 617–624. [Google Scholar] [CrossRef] [PubMed]
- Lynch, C.C.; Hikosaka, A.; Acuff, H.B.; Martin, M.D.; Kawai, N.; Singh, R.K.; Vargo-Gogola, T.C.; Begtrup, J.L.; Peterson, T.E.; Fingleton, B.; et al. MMP-7 promotes prostate cancer-induced osteolysis via the solubilization of RANKL. Cancer Cell 2005, 7, 485–496. [Google Scholar] [CrossRef] [PubMed]
- Wilson, T.J.; Nannuru, K.C.; Futakuchi, M.; Sadanandam, A.; Singh, R.K. Cathepsin G enhances mammary tumor-induced osteolysis by generating soluble receptor activator of nuclear factor-κB ligand. Cancer Res. 2008, 68, 5803–5811. [Google Scholar] [CrossRef] [PubMed]
- Yoneda, T.; Hiraga, T. Crosstalk between cancer cells and bone microenvironment in bone metastasis. Biochem. Biophys. Res. Commun. 2005, 328, 679–687. [Google Scholar] [CrossRef] [PubMed]
- Coleman, R.E.; Rubens, R.D. The clinical course of bone metastases from breast cancer. Br. J. Cancer 1987, 55, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Coleman, R.E. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin. Cancer Res. 2006, 12, 6243s–6249s. [Google Scholar] [CrossRef] [PubMed]
- Dewar, J.A. Managing metastatic bone pain. BMJ 2004, 329, 812–813. [Google Scholar] [CrossRef] [PubMed]
- Van Poznak, C.H.; Temin, S.; Yee, G.C.; Janjan, N.A.; Barlow, W.E.; Biermann, J.S.; Bosserman, L.D.; Geoghegan, C.; Hillner, B.E.; Theriault, R.L.; et al. American Society of Clinical Oncology executive summary of the clinical practice guideline update on the role of bone-modifying agents in metastatic breast cancer. J. Clin. Oncol. 2011, 29, 1221–1227. [Google Scholar] [CrossRef] [PubMed]
- Berry, D.A. Biomarker studies and other difficult inferential problems: Statistical caveats. Semin. Oncol. 2007, 34, S17–S22. [Google Scholar] [CrossRef] [PubMed]
- Garraway, I.P. Targeting the RANKL pathway: Putting the brakes on prostate cancer progression in bone. J. Clin. Oncol. 2013, 31, 3838–3840. [Google Scholar] [CrossRef] [PubMed]
- Hillner, B.E.; Ingle, J.N.; Chlebowski, R.T.; Gralow, J.; Yee, G.C.; Janjan, N.A.; Cauley, J.A.; Blumenstein, B.A.; Albain, K.S.; Lipton, A.; et al. American Society of Clinical Oncology 2003 update on the role of bisphosphonates and bone health issues in women with breast cancer. J. Clin. Oncol. 2003, 21, 4042–4057. [Google Scholar] [CrossRef] [PubMed]
- Von Moos, R.; Strasser, F.; Gillessen, S.; Zaugg, K. Metastatic bone pain: Treatment options with an emphasis on bisphosphonates. Support. Care Cancer 2008, 16, 1105–1115. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Tiede, B.; Massague, J.; Kang, Y. Beyond tumorigenesis: Cancer stem cells in metastasis. Cell Res. 2007, 17, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Li, Q. Cancer stem cells and tumor metastasis (Review). Int. J. Oncol. 2014, 44, 1806–1812. [Google Scholar] [CrossRef] [PubMed]
- Donnenberg, V.S.; Donnenberg, A.D. Multiple drug resistance in cancer revisited: The cancer stem cell hypothesis. J. Clin. Pharmacol. 2005, 45, 872–877. [Google Scholar] [CrossRef] [PubMed]
- Reya, T.; Morrison, S.J.; Clarke, M.F.; Weissman, I.L. Stem cells, cancer, and cancer stem cells. Nature 2001, 414, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Futakuchi, M.; Nannuru, K.C.; Varney, M.L.; Sadanandam, A.; Nakao, K.; Asai, K.; Shirai, T.; Sato, S.Y.; Singh, R.K. Transforming growth factor-β signaling at the tumor-bone interface promotes mammary tumor growth and osteoclast activation. Cancer Sci. 2009, 100, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Boumahdi, S.; Driessens, G.; Lapouge, G.; Rorive, S.; Nassar, D.; Le Mercier, M.; Delatte, B.; Caauwe, A.; Lenglez, S.; Nkusi, E.; et al. SOX2 controls tumour initiation and cancer stem-cell functions in squamous-cell carcinoma. Nature 2014, 511, 246–250. [Google Scholar] [CrossRef] [PubMed]
- Horimoto, Y.; Arakawa, A.; Sasahara, N.; Tanabe, M.; Sai, S.; Himuro, T.; Saito, M. Combination of Cancer Stem Cell Markers CD44 and CD24 Is Superior to ALDH1 as a Prognostic Indicator in Breast Cancer Patients with Distant Metastases. PLoS ONE 2016, 11, e0165253. [Google Scholar] [CrossRef] [PubMed]
- Lugli, A.; Iezzi, G.; Hostettler, I.; Muraro, M.G.; Mele, V.; Tornillo, L.; Carafa, V.; Spagnoli, G.; Terracciano, L.; Zlobec, I. Prognostic impact of the expression of putative cancer stem cell markers CD133, CD166, CD44s, EpCAM, and ALDH1 in colorectal cancer. Br. J. Cancer 2010, 103, 382–390. [Google Scholar] [CrossRef] [PubMed]
- Murphy, B.O.; Joshi, S.; Kessinger, A.; Reed, E.; Sharp, J.G. A murine model of bone marrow micrometastasis in breast cancer. Clin. Exp. Metastasis 2002, 19, 561–569. [Google Scholar] [CrossRef] [PubMed]
- Nemeth, J.A.; Harb, J.F.; Barroso, U., Jr.; He, Z.; Grignon, D.J.; Cher, M.L. Severe combined immunodeficient-hu model of human prostate cancer metastasis to human bone. Cancer Res. 1999, 59, 1987–1993. [Google Scholar] [PubMed]
- Iguchi, H.; Tanaka, S.; Ozawa, Y.; Kashiwakuma, T.; Kimura, T.; Hiraga, T.; Ozawa, H.; Kono, A. An experimental model of bone metastasis by human lung cancer cells: The role of parathyroid hormone-related protein in bone metastasis. Cancer Res. 1996, 56, 4040–4043. [Google Scholar] [PubMed]
- Paget, S. The distribution of secondary growths in cancer of the breast. Cancer Metastasis Rev. 1989, 8, 98–101. [Google Scholar] [CrossRef]
- Patel, S.A.; Dave, M.A.; Murthy, R.G.; Helmy, K.Y.; Rameshwar, P. Metastatic breast cancer cells in the bone marrow microenvironment: Novel insights into oncoprotection. Oncol. Rev. 2011, 5, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Siclari, V.A.; Guise, T.A.; Chirgwin, J.M. Molecular interactions between breast cancer cells and the bone microenvironment drive skeletal metastases. Cancer Metastasis Rev. 2006, 25, 621–633. [Google Scholar] [CrossRef] [PubMed]
- Sosnoski, D.M.; Krishnan, V.; Kraemer, W.J.; Dunn-Lewis, C.; Mastro, A.M. Changes in Cytokines of the Bone Microenvironment during Breast Cancer Metastasis. Int. J. Breast Cancer 2012, 2012, 160265. [Google Scholar] [CrossRef] [PubMed]
- Futakuchi, M.; Singh, R.K. Animal model for mammary tumor growth in the bone microenvironment. Breast Cancer 2013, 20, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Sato, S.; Futakuchi, M.; Ogawa, K.; Asamoto, M.; Nakao, K.; Asai, K.; Shirai, T. Transforming growth factor β derived from bone matrix promotes cell proliferation of prostate cancer and osteoclast activation-associated osteolysis in the bone microenvironment. Cancer Sci. 2008, 99, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Simonet, W.S.; Lacey, D.L.; Dunstan, C.R.; Kelley, M.; Chang, M.S.; Luthy, R.; Nguyen, H.Q.; Wooden, S.; Bennett, L.; Boone, T.; et al. Osteoprotegerin: A novel secreted protein involved in the regulation of bone density. Cell 1997, 89, 309–319. [Google Scholar] [CrossRef]
- Udagawa, N.; Takahashi, N.; Yasuda, H.; Mizuno, A.; Itoh, K.; Ueno, Y.; Shinki, T.; Gillespie, M.T.; Martin, T.J.; Higashio, K.; et al. Osteoprotegerin produced by osteoblasts is an important regulator in osteoclast development and function. Endocrinology 2000, 141, 3478–3484. [Google Scholar] [CrossRef] [PubMed]
- Takai, H.; Kanematsu, M.; Yano, K.; Tsuda, E.; Higashio, K.; Ikeda, K.; Watanabe, K.; Yamada, Y. Transforming growth factor-β stimulates the production of osteoprotegerin/osteoclastogenesis inhibitory factor by bone marrow stromal cells. J. Biol. Chem. 1998, 273, 27091–27096. [Google Scholar] [CrossRef] [PubMed]
- Yonou, H.; Horiguchi, Y.; Ohno, Y.; Namiki, K.; Yoshioka, K.; Ohori, M.; Hatano, T.; Tachibana, M. Prostate-specific antigen stimulates osteoprotegerin production and inhibits receptor activator of nuclear factor-κB ligand expression by human osteoblasts. Prostate 2007, 67, 840–848. [Google Scholar] [CrossRef] [PubMed]
- Viereck, V.; Grundker, C.; Blaschke, S.; Siggelkow, H.; Emons, G.; Hofbauer, L.C. Phytoestrogen genistein stimulates the production of osteoprotegerin by human trabecular osteoblasts. J. Cell Biochem. 2002, 84, 725–735. [Google Scholar] [CrossRef] [PubMed]
- Yano, K.; Nakagawa, N.; Yasuda, H.; Tsuda, E.; Higashio, K. Synovial cells from a patient with rheumatoid arthritis produce osteoclastogenesis inhibitory factor/osteoprotegerin: Reciprocal regulation of the production by inflammatory cytokines and basic fibroblast growth factor. J. Bone Miner. Metab. 2001, 19, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, M.; Sudo, T.; Saito, T.; Osada, H.; Tsujimoto, M. Involvement of p38 mitogen-activated protein kinase signaling pathway in osteoclastogenesis mediated by receptor activator of NF-κB ligand (RANKL). J. Biol. Chem. 2000, 275, 31155–31161. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Zhang, J.; Shao, H.; Liu, J.; Jin, M.; Chen, J.; Huang, Y. Transforming Growth Factor β1/Smad4 Signaling Affects Osteoclast Differentiation via Regulation of miR-155 Expression. Mol. Cells 2017, 40, 211–221. [Google Scholar] [PubMed]
- Ruan, M.; Pederson, L.; Bradley, E.W.; Bamberger, A.M.; Oursler, M.J. Transforming growth factor-β coordinately induces suppressor of cytokine signaling 3 and leukemia inhibitory factor to suppress osteoclast apoptosis. Endocrinology 2010, 151, 1713–1722. [Google Scholar] [CrossRef] [PubMed]
- Lam, J.; Takeshita, S.; Barker, J.E.; Kanagawa, O.; Ross, F.P.; Teitelbaum, S.L. TNF-α induces osteoclastogenesis by direct stimulation of macrophages exposed to permissive levels of RANK ligand. J. Clin. Investig. 2000, 106, 1481–1488. [Google Scholar] [CrossRef]
- Kobayashi, N.; Kadono, Y.; Naito, A.; Matsumoto, K.; Yamamoto, T.; Tanaka, S.; Inoue, J. Segregation of TRAF6-mediated signaling pathways clarifies its role in osteoclastogenesis. EMBO J. 2001, 20, 1271–1280. [Google Scholar] [CrossRef] [PubMed]
- Grigoriadis, A.E.; Wang, Z.Q.; Cecchini, M.G.; Hofstetter, W.; Felix, R.; Fleisch, H.A.; Wagner, E.F. c-Fos: A key regulator of osteoclast-macrophage lineage determination and bone remodeling. Science 1994, 266, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, F.; Nishimura, R.; Matsubara, T.; Tanaka, S.; Inoue, J.; Reddy, S.V.; Hata, K.; Yamashita, K.; Hiraga, T.; Watanabe, T.; et al. Critical roles of c-Jun signaling in regulation of NFAT family and RANKL-regulated osteoclast differentiation. J. Clin. Investig. 2004, 114, 475–484. [Google Scholar] [CrossRef] [PubMed]
- Fata, J.E.; Kong, Y.Y.; Li, J.; Sasaki, T.; Irie-Sasaki, J.; Moorehead, R.A.; Elliott, R.; Scully, S.; Voura, E.B.; Lacey, D.L.; et al. The osteoclast differentiation factor osteoprotegerin-ligand is essential for mammary gland development. Cell 2000, 103, 41–50. [Google Scholar] [CrossRef]
- Cao, Y.; Bonizzi, G.; Seagroves, T.N.; Greten, F.R.; Johnson, R.; Schmidt, E.V.; Karin, M. IKKα provides an essential link between RANK signaling and cyclin D1 expression during mammary gland development. Cell 2001, 107, 763–775. [Google Scholar] [CrossRef]
- Cao, Y.; Luo, J.L.; Karin, M. IκB kinase α kinase activity is required for self-renewal of ErbB2/Her2-transformed mammary tumor-initiating cells. Proc. Natl. Acad. Sci. USA 2007, 104, 15852–15857. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.L.; Tan, W.; Ricono, J.M.; Korchynskyi, O.; Zhang, M.; Gonias, S.L.; Cheresh, D.A.; Karin, M. Nuclear cytokine-activated IKKα controls prostate cancer metastasis by repressing Maspin. Nature 2007, 446, 690–694. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.; Zhang, W.; Strasner, A.; Grivennikov, S.; Cheng, J.Q.; Hoffman, R.M.; Karin, M. Tumour-infiltrating regulatory T cells stimulate mammary cancer metastasis through RANKL-RANK signalling. Nature 2011, 470, 548–553. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Yang, B.; Teguh, D.; Zhou, L.; Xu, J.; Rong, L. Amyloid β Peptide Enhances RANKL-Induced Osteoclast Activation through NF-κB, ERK, and Calcium Oscillation Signaling. Int. J. Mol. Sci. 2016, 17, 1683. [Google Scholar] [CrossRef] [PubMed]
- Ang, E.; Liu, Q.; Qi, M.; Liu, H.G.; Yang, X.; Chen, H.; Zheng, M.H.; Xu, J. Mangiferin attenuates osteoclastogenesis, bone resorption, and RANKL-induced activation of NF-κB and ERK. J. Cell Biochem. 2011, 112, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Saad, F.; Eastham, J. Zoledronic Acid improves clinical outcomes when administered before onset of bone pain in patients with prostate cancer. Urology 2010, 76, 1175–1181. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Qiao, D.; Lu, Y.; Curtis, D.; Wen, X.; Yao, Y.; Zhao, H. Systematic literature review and network meta-analysis comparing bone-targeted agents for the prevention of skeletal-related events in cancer patients with bone metastasis. Oncologist 2015, 20, 440–449. [Google Scholar] [CrossRef] [PubMed]
- Coleman, R.E.; Lipton, A.; Roodman, G.D.; Guise, T.A.; Boyce, B.F.; Brufsky, A.M.; Clezardin, P.; Croucher, P.I.; Gralow, J.R.; Hadji, P.; et al. Metastasis and bone loss: Advancing treatment and prevention. Cancer Treat. Rev. 2010, 36, 615–620. [Google Scholar] [CrossRef] [PubMed]
- Sanna, G.; Zampino, M.G.; Pelosi, G.; Nole, F.; Goldhirsch, A. Jaw avascular bone necrosis associated with long-term use of biphosphonates. Ann. Oncol. 2005, 16, 1207–1208. [Google Scholar] [CrossRef] [PubMed]
- Sanna, G.; Preda, L.; Bruschini, R.; Cossu Rocca, M.; Verri, E.; Bellomi, M.; Goldhirsch, A.; Nole, F. The role of surgery in jaw bone necrosis associated with long-term use of bisphosphonates. Acta Oncol. 2008, 47, 476–478. [Google Scholar] [CrossRef] [PubMed]
- Campbell, L.L.; Polyak, K. Breast tumor heterogeneity: Cancer stem cells or clonal evolution? Cell Cycle 2007, 6, 2332–2338. [Google Scholar] [CrossRef] [PubMed]
- Clarke, M.F.; Dick, J.E.; Dirks, P.B.; Eaves, C.J.; Jamieson, C.H.; Jones, D.L.; Visvader, J.; Weissman, I.L.; Wahl, G.M. Cancer stem cells—Perspectives on current status and future directions: AACR Workshop on cancer stem cells. Cancer Res. 2006, 66, 9339–9344. [Google Scholar] [CrossRef] [PubMed]
- Sundar, S.S.; Ganesan, T.S. Role of lymphangiogenesis in cancer. J. Clin. Oncol. 2007, 25, 4298–4307. [Google Scholar] [CrossRef] [PubMed]
- Futakuchi, M.; Fukamachi, K.; Suzui, M. Heterogeneity of tumor cells in the bone microenvironment: Mechanisms and therapeutic targets for bone metastasis of prostate or breast cancer. Adv. Drug Deliv. Rev. 2016, 99 Pt B, 206–211. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, B.I.; Thommesen, L.; Stunes, A.K.; Tommeras, K.; Westbroek, I.; Waldum, H.L.; Slordahl, K.; Tamburstuen, M.V.; Reseland, J.E.; Syversen, U. Serotonin and fluoxetine modulate bone cell function in vitro. J. Cell Biochem. 2006, 98, 139–151. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Wen, Y.; Xu, J.; Wu, T.; Zhang, C.; Wang, J.; Du, J.; Wang, S. Pretreatment with Bisphosphonate Enhances Osteogenesis of Bone Marrow Mesenchymal Stem Cells. Stem. Cells Dev. 2017, 26, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Aslakson, C.J.; Miller, F.R. Selective events in the metastatic process defined by analysis of the sequential dissemination of subpopulations of a mouse mammary tumor. Cancer Res. 1992, 52, 1399–1405. [Google Scholar] [PubMed]
- Heppner, G.H.; Miller, F.R.; Shekhar, P.M. Nontransgenic models of breast cancer. Breast Cancer Res. 2000, 2, 331–334. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Futakuchi, M.; Nitanda, T.; Ando, S.; Matsumoto, H.; Yoshimoto, E.; Fukamachi, K.; Suzui, M. Therapeutic and Preventive Effects of Osteoclastogenesis Inhibitory Factor on Osteolysis, Proliferation of Mammary Tumor Cell and Induction of Cancer Stem Cells in the Bone Microenvironment. Int. J. Mol. Sci. 2018, 19, 888. https://doi.org/10.3390/ijms19030888
Futakuchi M, Nitanda T, Ando S, Matsumoto H, Yoshimoto E, Fukamachi K, Suzui M. Therapeutic and Preventive Effects of Osteoclastogenesis Inhibitory Factor on Osteolysis, Proliferation of Mammary Tumor Cell and Induction of Cancer Stem Cells in the Bone Microenvironment. International Journal of Molecular Sciences. 2018; 19(3):888. https://doi.org/10.3390/ijms19030888
Chicago/Turabian StyleFutakuchi, Mitsuru, Takao Nitanda, Saeko Ando, Harutoshi Matsumoto, Eri Yoshimoto, Katsumi Fukamachi, and Masumi Suzui. 2018. "Therapeutic and Preventive Effects of Osteoclastogenesis Inhibitory Factor on Osteolysis, Proliferation of Mammary Tumor Cell and Induction of Cancer Stem Cells in the Bone Microenvironment" International Journal of Molecular Sciences 19, no. 3: 888. https://doi.org/10.3390/ijms19030888
APA StyleFutakuchi, M., Nitanda, T., Ando, S., Matsumoto, H., Yoshimoto, E., Fukamachi, K., & Suzui, M. (2018). Therapeutic and Preventive Effects of Osteoclastogenesis Inhibitory Factor on Osteolysis, Proliferation of Mammary Tumor Cell and Induction of Cancer Stem Cells in the Bone Microenvironment. International Journal of Molecular Sciences, 19(3), 888. https://doi.org/10.3390/ijms19030888






