Use of Metabolomics as a Complementary Omic Approach to Implement Risk Criteria for First-Degree Relatives of Gastric Cancer Patients
Abstract
:1. Introduction
2. Results
2.1. Individual Characteristics
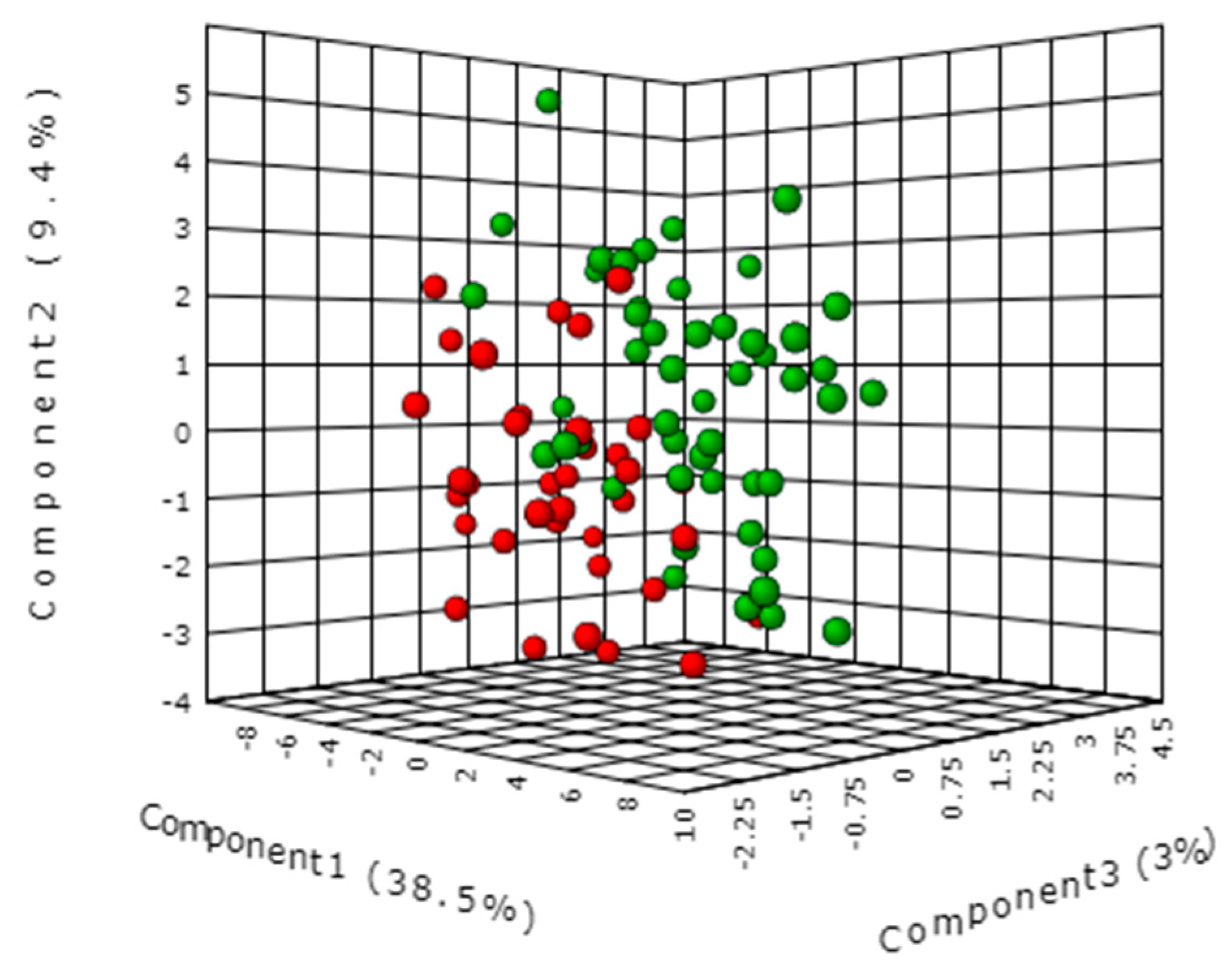
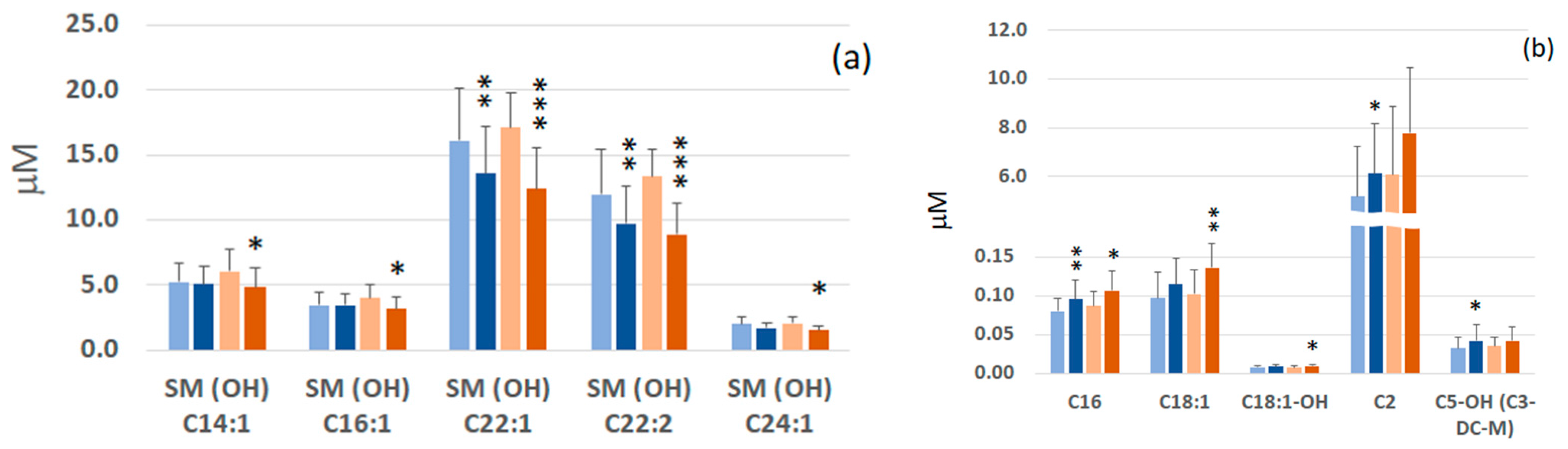
2.2. Comparison of Serum Metabolomic Profiles of GC and FDR
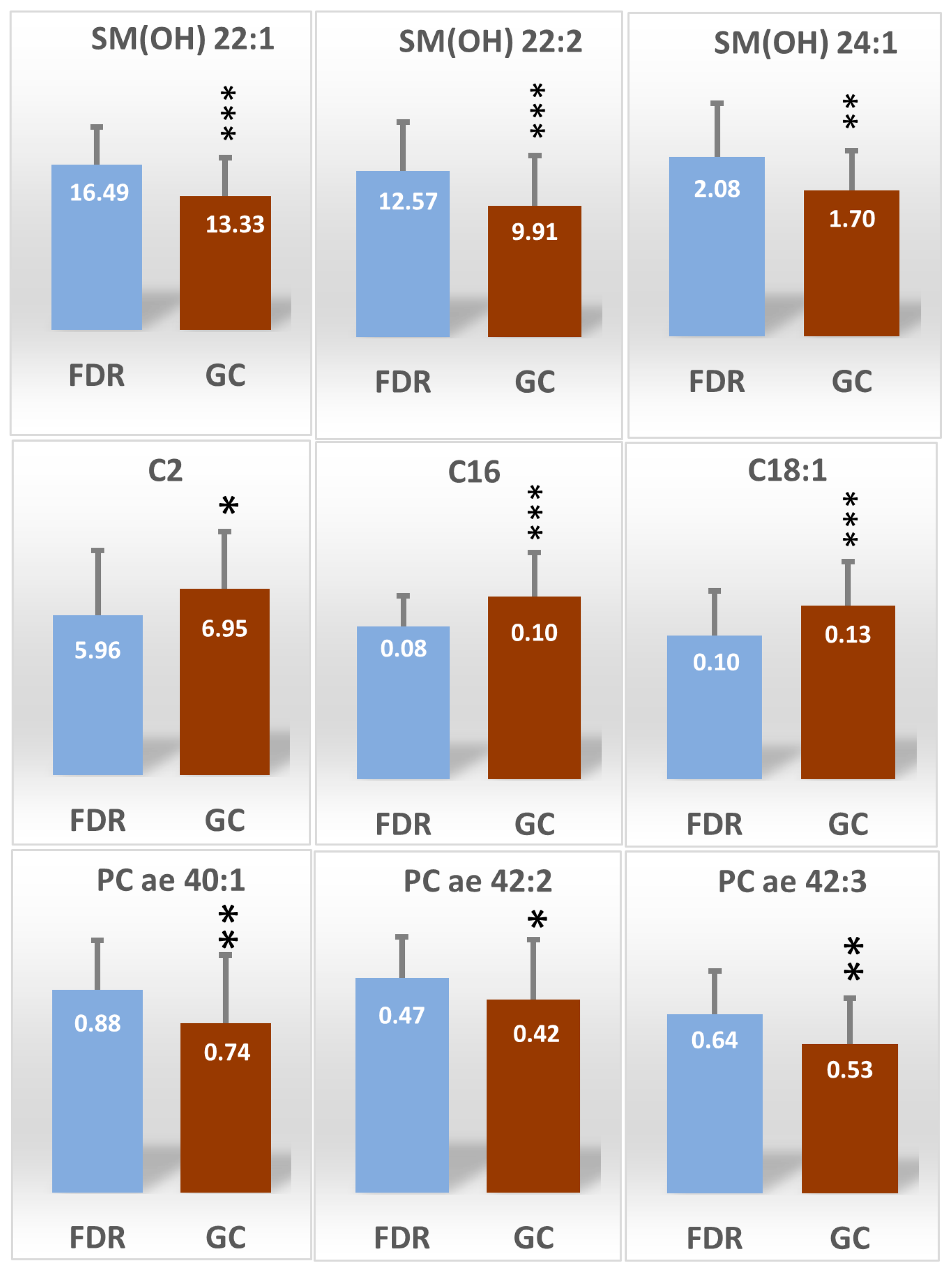
2.3. Identification and Selection of the Most Significant Metabolites
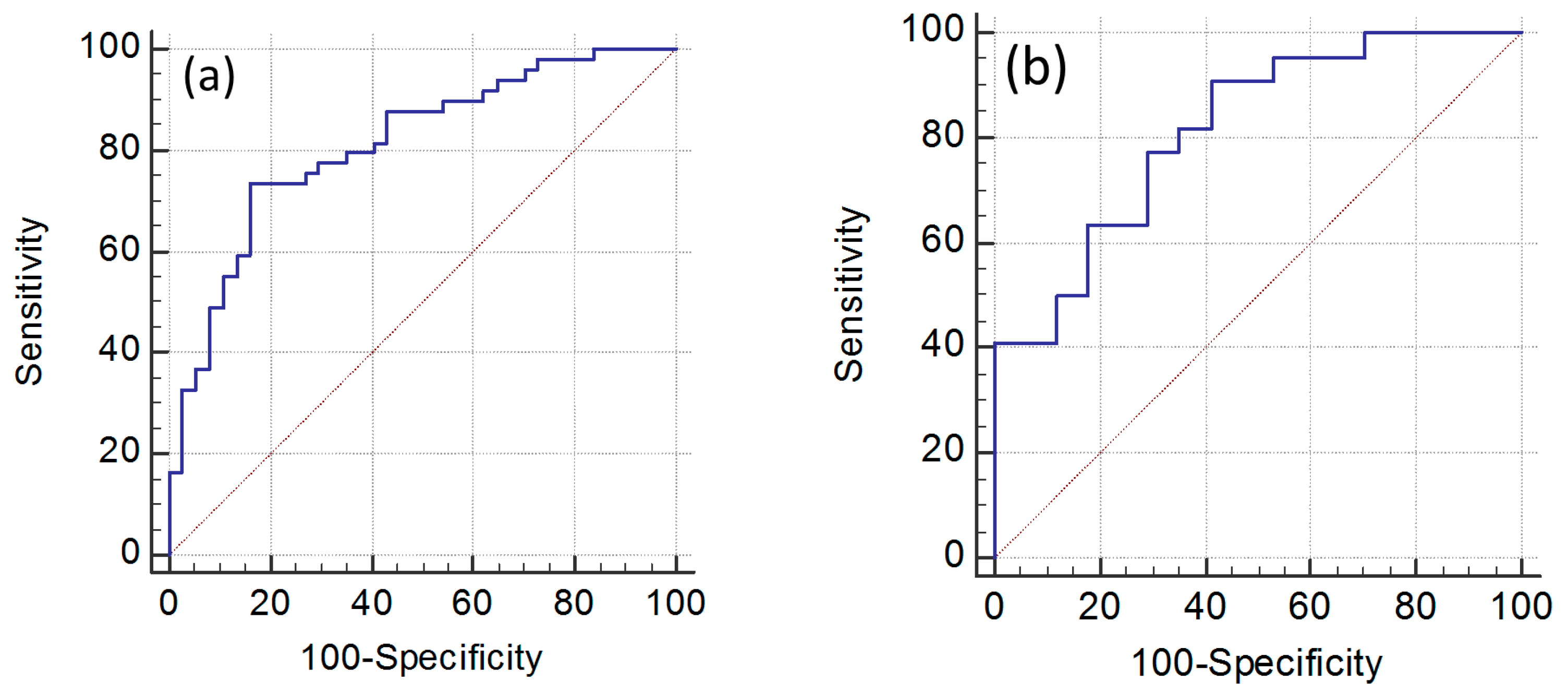
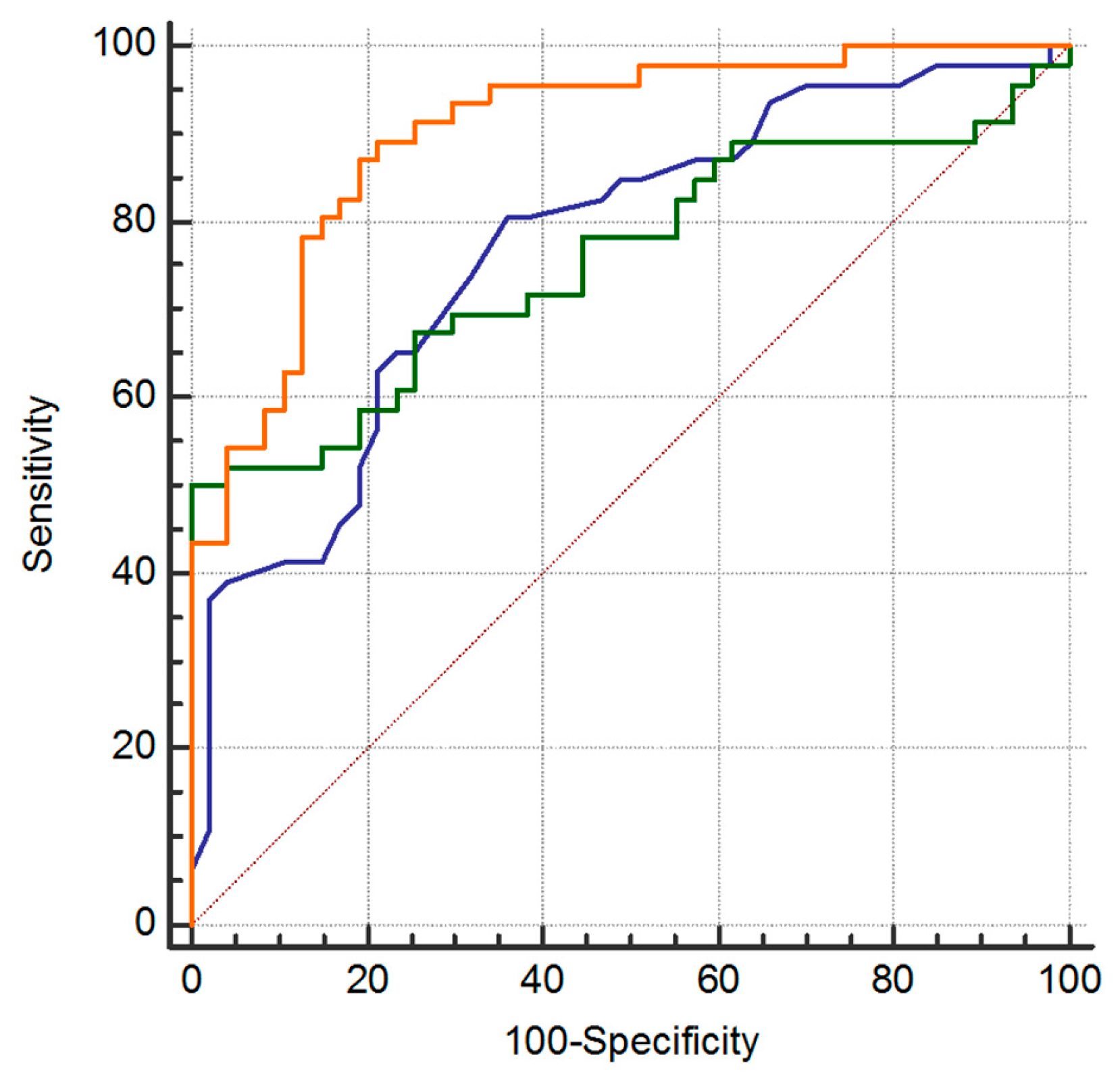
2.4. Model Performance for Metabolites
2.5. Effect of H. pylori Infection on Levels of Sphingomyelins and Acylcarnitines
2.6. Age Effect on the Serum Levels of the SM(OH)22:1 and C16 Metabolites
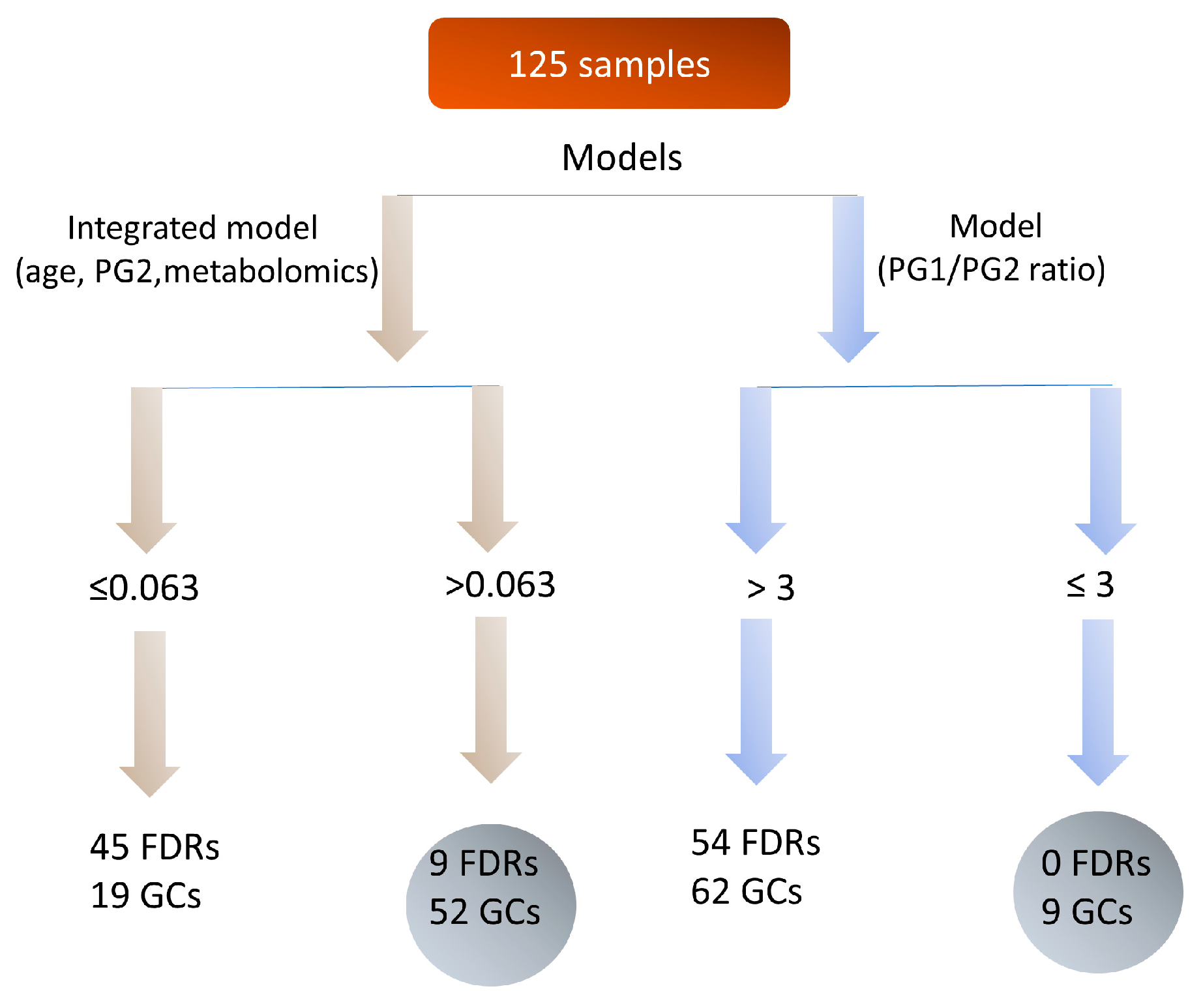
2.7. Integrated Metabolomics Model
3. Discussion
4. Experimental Section
4.1. Participants
4.2. Sample Collection
4.3. Design of the Study
4.4. Metabolomics Investigation
4.5. Data Processing and Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Torre, L.A.; Siegel, R.L.; Ward, E.M.; Jemal, A. Global Cancer Incidence and Mortality Rates and Trends—An Update. Cancer Epidemiol. Biomarker Prev. 2016, 25, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef] [PubMed]
- Parsonnet, J.; Friedman, G.D.; Vandersteen, D.P.; Chang, Y.; Vogelman, J.H.; Orentreich, N.; Sibley, R.K. Helicobacter pylori infection and the risk of gastric carcinoma. N. Engl. J. Med. 1991, 325, 1127–1131. [Google Scholar] [CrossRef] [PubMed]
- Corso, G.; Figueiredo, J.; Biffi, R.; Trentin, C.; Bonanni, B.; Feroce, I.; Serrano, D.; Cassano, E.; Annibale, B.; Melo, S.; et al. E-cadherin germline mutation carriers: Clinical management and genetic implications. Cancer Metastasis Rev. 2014, 33, 1081–1094. [Google Scholar] [CrossRef] [PubMed]
- Asaka, M.; Mabe, K.; Matsushima, R.; Tsuda, M. Helicobacter pylori Eradication to Eliminate Gastric Cancer: The Japanese Strategy. Gastroenterol. Clin. N. Am. 2015, 44, 639–648. [Google Scholar] [CrossRef] [PubMed]
- Miki, K. Gastric cancer screening by combined assay for serum anti-Helicobacter pylori IgG antibody and serum pepsinogen levels—“ABC method”. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2011, 87, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, Y.; Nagata, Y.; Hiratsuka, R.; Kawase, Y.; Tominaga, T.; Takeuchi, S.; Sakagami, S.; Ishida, S. Gastric Cancer Screening by Combined Assay for Serum Anti-Helicobacter pylori IgG Antibody and Serum Pepsinogen Levels—The ABC Method. Digestion 2016, 93, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Corona, G.; Rizzolio, F.; Giordano, A.; Toffoli, G. Pharmaco-metabolomics: An emerging “omics” tool for the personalization of anticancer treatments and identification of new valuable therapeutic targets. J. Cell Physiol. 2012, 227, 2827–2831. [Google Scholar] [CrossRef] [PubMed]
- Gowda, G.A.; Zhang, S.; Gu, H.; Asiago, V.; Shanaiah, N.; Raftery, D. Metabolomics-based methods for early disease diagnostics. Expert. Rev. Mol. Diagn. 2008, 8, 617–633. [Google Scholar] [CrossRef] [PubMed]
- Houten, S.M.; Wanders, R.J. A general introduction to the biochemistry of mitochondrial fatty acid beta-oxidation. J. Inherit. Metab. Dis. 2010, 33, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Noland, R.C.; Koves, T.R.; Seiler, S.E.; Lum, H.; Lust, R.M.; Ilkayeva, O.; Stevens, R.D.; Hegardt, F.G.; Muoio, D.M. Carnitine insufficiency caused by aging and overnutrition compromises mitochondrial performance and metabolic control. J. Biol. Chem. 2009, 284, 22840–22852. [Google Scholar] [CrossRef] [PubMed]
- Muller, F.L.; Lustgarten, M.S.; Jang, Y.; Richardson, A.; Van, R.H. Trends in oxidative aging theories. Free Radic. Biol. Med. 2007, 43, 477–503. [Google Scholar] [CrossRef] [PubMed]
- Kurabe, N.; Suzuki, M.; Inoue, Y.; Kahyo, T.; Iwaizumi, M.; Konno, H.; Setou, M.; Sugimura, H. Abstract 394A: Phosphatidylcholine-34:2 and -36:4 have tumor suppressive function for gastric cancer. Cancer Res. 2016, 76 (Suppl. S14), 394A. [Google Scholar] [CrossRef]
- Kurabe, N.; Igarashi, H.; Ohnishi, I.; Tajima, S.; Inoue, Y.; Takahashi, Y.; Setou, M.; Sugimura, H. Visualization of sphingolipids and phospholipids in the fundic gland mucosa of human stomach using imaging mass spectrometry. World J. Gastrointest. Pathophysiol. 2016, 7, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Breslow, D.K.; Weissman, J.S. Membranes in balance: Mechanisms of sphingolipid homeostasis. Mol. Cell 2010, 40, 267–279. [Google Scholar] [CrossRef] [PubMed]
- Morad, S.A.; Cabot, M.C. Ceramide-orchestrated signalling in cancer cells. Nat. Rev. Cancer 2013, 13, 51–65. [Google Scholar] [CrossRef] [PubMed]
- Hendrich, A.B.; Michalak, K. Lipids as a target for drugs modulating multidrug resistance of cancer cells. Curr. Drug Targets 2003, 4, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Hama, H. Fatty acid 2-Hydroxylation in mammalian sphingolipid biology. Biochim. Biophys. Acta 2010, 1801, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Kota, V.; Hama, H. 2′-Hydroxy ceramide in membrane homeostasis and cell signaling. Adv. Biol. Regul. 2014, 54, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Venkatesh, H.; Monje, M. Neuronal Activity in Ontogeny and Oncology. Trends Cancer 2017, 3, 89–112. [Google Scholar] [CrossRef] [PubMed]
- Hayakawa, Y.; Sakitani, K.; Konishi, M.; Asfaha, S.; Niikura, R.; Tomita, H.; Renz, B.W.; Tailor, Y.; Macchini, M.; Middelhoff, M.; et al. Nerve Growth Factor Promotes Gastric Tumorigenesis through Aberrant Cholinergic Signaling. Cancer Cell 2017, 31, 21–34. [Google Scholar] [CrossRef] [PubMed]
- De Re, V.; Orzes, E.; Canzonieri, V.; Maiero, S.; Fornasarig, M.; Alessandrini, L.; Cervo, S.; Steffan, A.; Zanette, G.; Mazzon, C.; et al. Pepsinogens to Distinguish Patients With Gastric Intestinal Metaplasia and Helicobacter pylori Infection Among Populations at Risk for Gastric Cancer. Clin. Transl. Gastroenterol. 2016, 7, e183. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Wishart, D.S. Using MetaboAnalyst 3.0 for Comprehensive Metabolomics Data Analysis. Curr. Protoc. Bioinform. 2016, 55, 14.10.1–14.10.91. [Google Scholar] [CrossRef]


| (a) | |||
| GC | FDR | p a | |
| N | 49 | 37 | NS |
| M/F b | 27/22 | 10/27 | NS |
| Age c | 61 (19–85) | 53 (30–69) | 0.00009 |
| H. pylori (−) d,# | 32 | 25 | NS |
| H. pylori (+) e,# | 14 | 10 | NS |
| PG-I (ng/mL) f | 118.2 (2.7–706.4) | 97.2 (3.1–658.4) | NS |
| PG-II (ng/mL) g | 17.2 (1.1–104.0) | 9.8 (0.2–35.5) | 0.0075 |
| G17 (pmol/L) h | 15.7 (0.9–983.0) | 3.7 (0.4–109.8) | NS |
| Histological GC Type # | |||
| Intestinal | 17 | ||
| Diffuse | 11 | ||
| Mixed | 5 | ||
| (b) | |||
| GC | FDR | p a | |
| N | 22 | 17 | |
| M/F b | 12/10 | 9/8 | NS |
| Age c | 67 (34–79) | 45 (23–78) | 0.001 |
| H. pylori (−) d,# | 12 | 12 | NS |
| H. pylori (+) e,# | 4 | 4 | NS |
| PG-I (ng/mL) f | 107.5 (3.9–341.2) | 87.9 (59.3–112.0) | NS |
| PG-II (ng/mL) g | 12.6 (2.8–45.9) | 9.0 (4.5–13.8) | 0.033 |
| G17 (pmol/L) h | 3.8 (1.5–500.0) | 4.0 (0.5–14.6) | NS |
| Histological GC Type # | |||
| Intestinal | 8 | ||
| Diffuse | 2 | ||
| Mixed | 1 | ||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Corona, G.; Cannizzaro, R.; Miolo, G.; Caggiari, L.; De Zorzi, M.; Repetto, O.; Steffan, A.; De Re, V. Use of Metabolomics as a Complementary Omic Approach to Implement Risk Criteria for First-Degree Relatives of Gastric Cancer Patients. Int. J. Mol. Sci. 2018, 19, 750. https://doi.org/10.3390/ijms19030750
Corona G, Cannizzaro R, Miolo G, Caggiari L, De Zorzi M, Repetto O, Steffan A, De Re V. Use of Metabolomics as a Complementary Omic Approach to Implement Risk Criteria for First-Degree Relatives of Gastric Cancer Patients. International Journal of Molecular Sciences. 2018; 19(3):750. https://doi.org/10.3390/ijms19030750
Chicago/Turabian StyleCorona, Giuseppe, Renato Cannizzaro, Gianmaria Miolo, Laura Caggiari, Mariangela De Zorzi, Ombretta Repetto, Agostino Steffan, and Valli De Re. 2018. "Use of Metabolomics as a Complementary Omic Approach to Implement Risk Criteria for First-Degree Relatives of Gastric Cancer Patients" International Journal of Molecular Sciences 19, no. 3: 750. https://doi.org/10.3390/ijms19030750
APA StyleCorona, G., Cannizzaro, R., Miolo, G., Caggiari, L., De Zorzi, M., Repetto, O., Steffan, A., & De Re, V. (2018). Use of Metabolomics as a Complementary Omic Approach to Implement Risk Criteria for First-Degree Relatives of Gastric Cancer Patients. International Journal of Molecular Sciences, 19(3), 750. https://doi.org/10.3390/ijms19030750