SK-216, a Novel Inhibitor of Plasminogen Activator Inhibitor-1, Suppresses Lung Metastasis of Human Osteosarcoma
Abstract
1. Introduction
2. Results
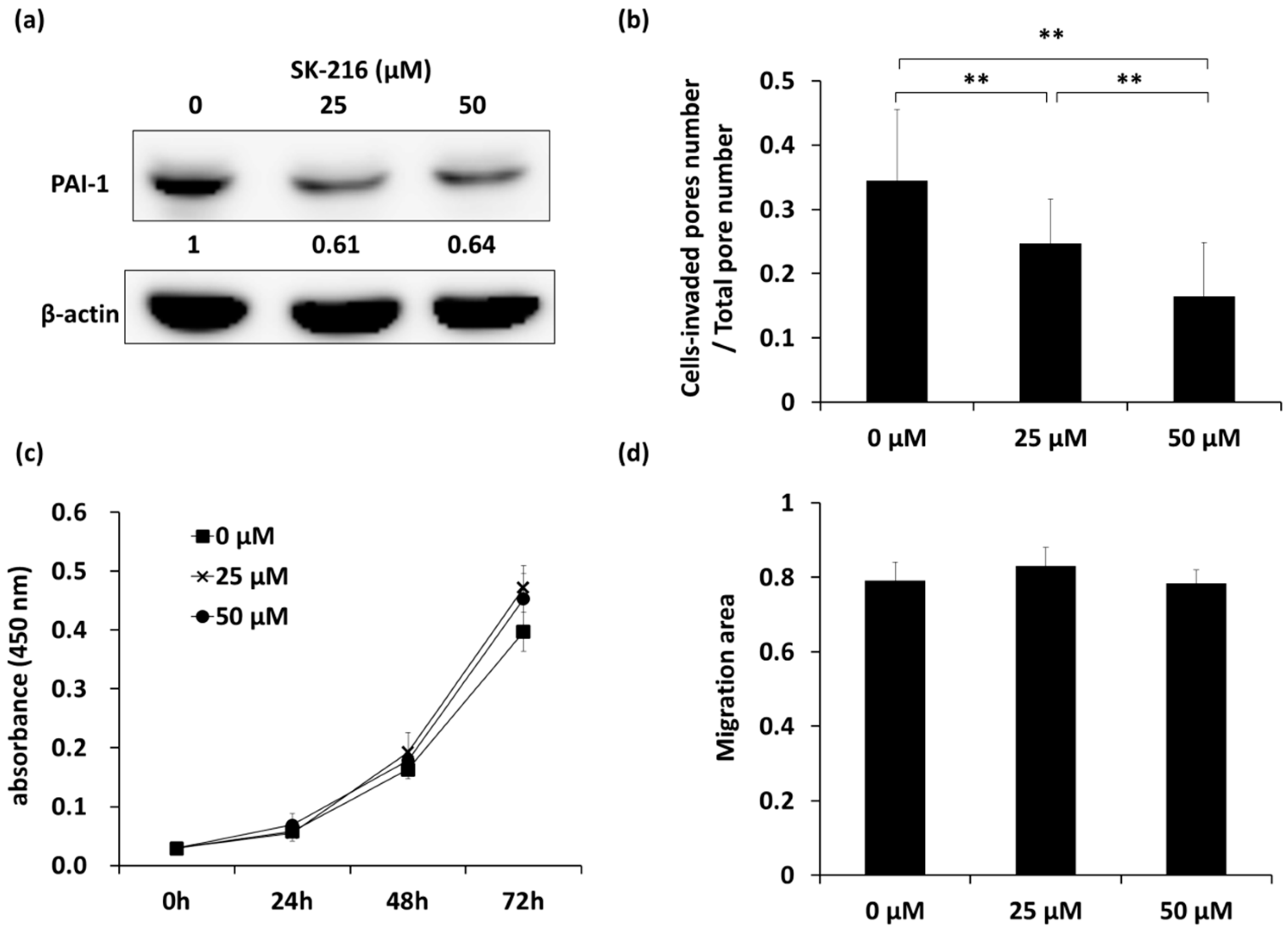
2.1. SK-216 Inhibits the Invasion of 143B Cells In Vitro
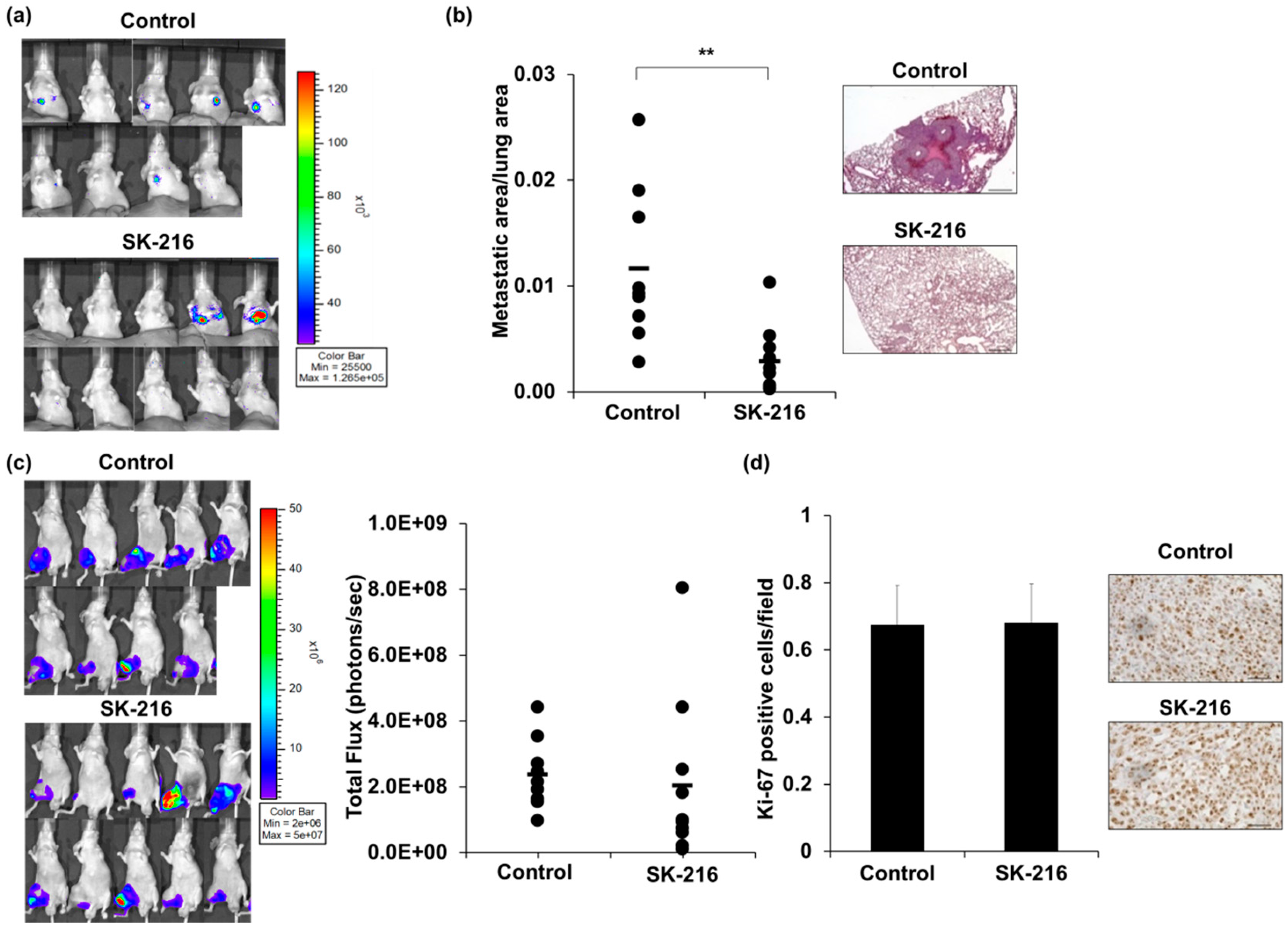
2.2. SK-216 Suppresses Lung Metastasis of Osteosarcoma Cells In Vivo
2.3. SK-216 Suppresses PAI-1 Expression of Osteosarcoma Cells In Vivo
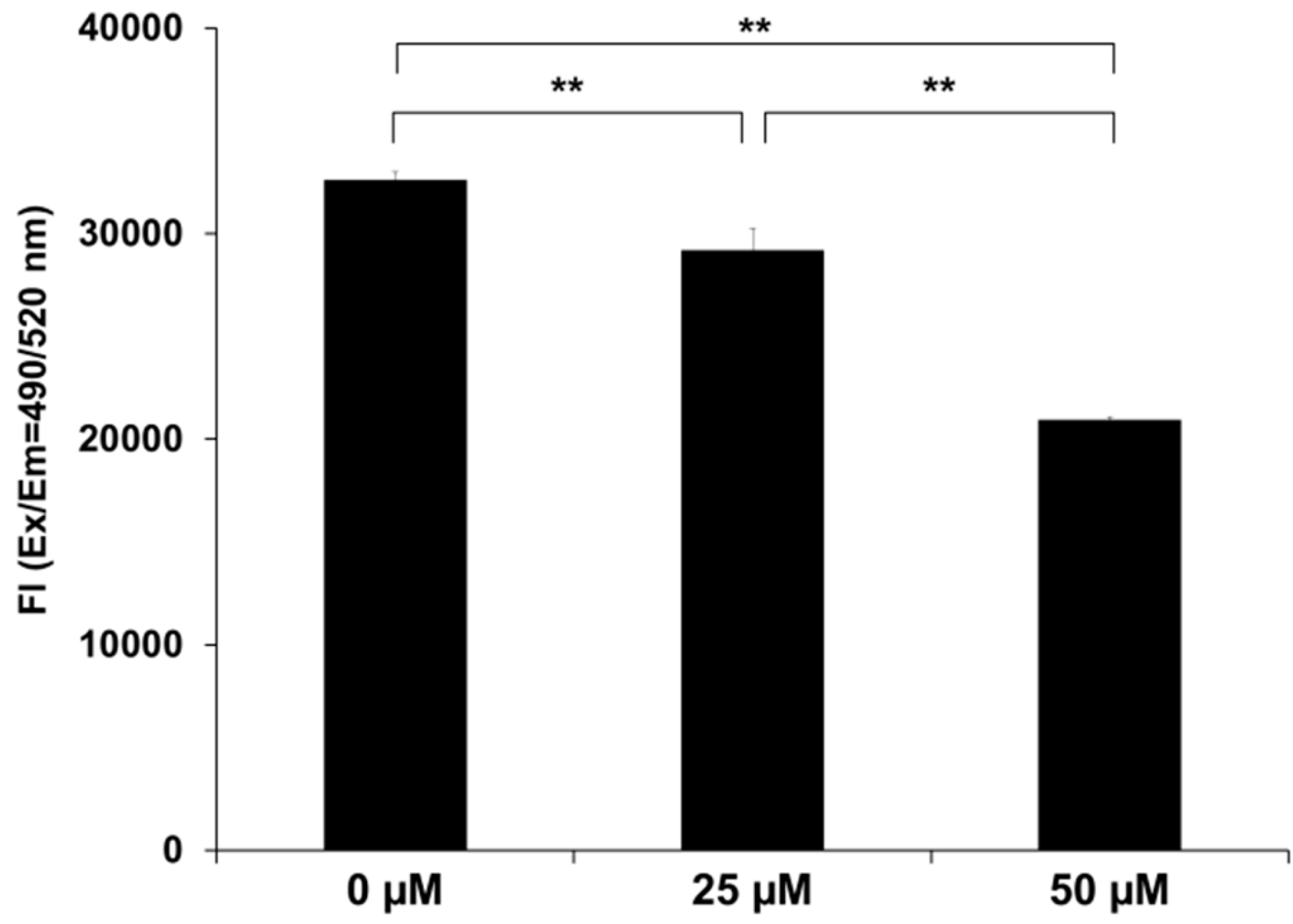
2.4. SK-216 Suppresses MMP-13 Secretion
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Cell Culture and Transfection
4.3. Cell Proliferation Assay
4.4. Cell Migration Assay
4.5. Cell Invasion Assay
4.6. Western Blotting
4.7. Measurement of Secreted Matrix Metalloproteinase-13 (MMP-13) Protein
4.8. Animal Model
4.9. Histological Analysis of Tumors and Lung Metastases
4.10. Statistical Analyses
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Allison, D.C.; Carney, S.C.; Ahlmann, E.R.; Hendifar, A.; Chawla, S.; Fedenko, A.; Angeles, C.; Menendez, L.R. A meta-analysis of osteosarcoma outcomes in the modern medical era. Sarcoma 2012, 2012, 704872. [Google Scholar] [CrossRef] [PubMed]
- Duchman, K.; Gao, Y.; Miller, B.J. Prognostic factors for survival in patients with high-grade osteosarcoma using the Surveillance, Epidemiology, and End Results (SEER) Program database. Cancer Epidemiol. 2015, 39, 593–599. [Google Scholar] [CrossRef] [PubMed]
- Osaki, M.; Takeshita, F.; Sugimoto, Y.; Kosaka, N.; Yamamoto, Y.; Yoshioka, Y.; Kobayashi, E.; Yamada, T.; Kawai, A.; Inoue, T.; et al. MicroRNA-143 regulates human osteosarcoma metastasis by regulating matrix metalloprotease-13 expression. Mol. Ther. 2011, 19, 1123–1130. [Google Scholar] [CrossRef] [PubMed]
- Hirahata, M.; Osaki, M.; Kanda, Y.; Sugimoto, Y.; Yoshioka, Y.; Kosaka, N.; Takeshita, F.; Fujiwara, T.; Kawai, A.; Ito, H.; et al. PAI-1, a target gene of miR-143, regulates invasion and metastasis by upregulating MMP-13 expression of human osteosarcoma. Cancer Med. 2016, 5, 892–902. [Google Scholar] [CrossRef] [PubMed]
- Dawson, S.; Henney, A. The status of PAI-1 as a risk factor for arterial and thrombotic disease: A review. Atherosclerosis 1992, 95, 105–117. [Google Scholar] [CrossRef]
- Binder, B.R.; Mihaly, J. The plasminogen activator inhibitor “paradox” in cancer. Immunol. Lett. 2008, 118, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Mutoh, M.; Niho, N.; Komiya, M.; Takahashi, M.; Ohtsubo, R.; Nakatogawa, K.; Ueda, K.; Sugimura, T. Plasminogen activator inhibitor-1 (Pai-1) blockers suppress intestinal polyp formation in Min mice. Carcinogenesis 2008, 29, 824–829. [Google Scholar] [CrossRef] [PubMed]
- Masuda, T.; Hattori, N.; Senoo, T.; Akita, S.; Ishikawa, N.; Fujitaka, K.; Haruta, Y.; Murai, H.; Kohno, N. SK-216, an inhibitor of plasminogen activator inhibitor-1, limits tumor progression and angiogenesis. Mol. Cancer Ther. 2013, 12, 2378–2388. [Google Scholar] [CrossRef] [PubMed]
- Luukkaa, M.; Vihinen, P.; Kronqvist, P.; Vahlberg, T.; Pyrhönen, S.; Kähäri, V.M.; Grénman, R. Association between high collagenase-3 expression levels and poor prognosis in patients with head and neck cancer. Head Neck 2006, 28, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.P.; Shen, G.H.; Ko, J.L. Matrix metalloproteinase-13 expression is associated with bone marrow microinvolvement and prognosis in non-small cell lung cancer. Lung Cancer 2006, 52, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Leeman, M.F.; McKay, J.A.; Murray, G.I. Matrix metalloproteinase 13 activity is associated with poor prognosis in colorectal cancer. J. Clin. Pathol. 2002, 55, 758–762. [Google Scholar] [CrossRef] [PubMed]
- Nannuru, K.C.; Futakuchi, M.; Varney, M.L.; Vincent, T.M.; Marcusson, E.G.; Singh, R.K. Matrix metalloproteinase (MMP)-13 regulates mammary tumor-induced osteolysis by activating MMP9 and transforming growth factor-beta signaling at the tumor-bone interface. Cancer Res. 2010, 70, 3494–3504. [Google Scholar] [CrossRef] [PubMed]
- Akech, J.; Wixted, J.J.; Bedard, K.; van der Deen, M.; Hussain, S.; Guise, T.A.; van Wijnen, A.J.; Stein, J.L.; Languino, L.R.; Altieri, D.C.; et al. Runx2 association with progression of prostate cancer in patients: Mechanisms mediating bone osteolysis and osteoblastic metastatic lesions. Oncogene 2010, 29, 811–821. [Google Scholar] [CrossRef] [PubMed]
- Omori, K.; Hattori, N.; Senoo, T.; Takayama, Y.; Masuda, T.; Nakashima, T.; Iwamoto, H.; Fujitaka, K.; Hamada, H.; Kohno, N. Inhibition of plasminogen activator inhibitor-1 attenuates transforming growth factor-β-dependent epithelial mesenchymal transition and differentiation of fibroblasts to myofibroblasts. PLoS ONE 2016, 11, e0148969. [Google Scholar] [CrossRef] [PubMed]
- Gorlatova, N.V.; Cale, J.M.; Elokdah, H.; Li, D.; Fan, K.; Warnock, M. Mechanism of inactivation of plasminogen activator inhibitor-1 by a small molecule inhibitor. J. Biol. Chem. 2007, 282, 9288–9296. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.; Placencio, V.R.; DeClerck, Y.A. Protumorigenic activity of plasminogen activator inhibitor-1 through an antiapoptotic function. J. Natl. Cancer Inst. 2012, 104, 1470–1484. [Google Scholar] [CrossRef] [PubMed]
- Gomes-Giacoia, E.; Miyake, M.; Goodison, S.; Rosser, C.J. Targeting plasminogen activator inhibitor-1 inhibits angiogenesis and tumor growth in a human cancer xenograft model. Mol. Cancer Ther. 2013, 12, 2697–2708. [Google Scholar] [CrossRef] [PubMed]
- Mashiko, S.; Kitatani, K.; Toyoshima, M.; Ichimura, A.; Dan, T.; Usui, T.; Ishibashi, M.; Shigeta, S.; Nagase, S.; Miyata, T.; et al. Inhibition of plasminogen activator inhibitor-1 is a potential therapeutic strategy in ovarian cancer. Cancer Biol. Ther. 2015, 16, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Ichimura, A.; Matsumoto, S.; Suzuki, S.; Dan, T.; Yamaki, S.; Sato, Y.; Kiyomoto, H.; Ishii, N.; Okada, K.; Matsuo, O. A small molecule inhibitor to plasminogen activator inhibitor 1 inhibits macrophage migration. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 935–942. [Google Scholar] [CrossRef] [PubMed]
- Placencio, V.R.; Ichimura, A.; Miyata, T.; de Clerck, Y.A. Small molecule inhibitors of plasminogen activator inhibitor-1 elicit anti-tumorigenic and anti-angiogenic activity. PLoS ONE 2015, 10, e0133786. [Google Scholar] [CrossRef] [PubMed]
- Look, M.P.; van Putten, W.L.J.; Duffy, M.J.; Harbeck, N.; Christensen, I.J.; Thomssen, C.; Kates, R.; Spyratos, F.; Ferno, M.; Eppenberger-Castori, S.; et al. Pooled analysis of prognostic impact of urokinase-type plasminogen activator and its inhibitor PAI-1 in 8377 breast cancer patients. J. Natl. Cancer Inst. 2002, 94, 116–128. [Google Scholar] [CrossRef] [PubMed]
- Sakakibara, T.; Hibi, K.; Koike, M.; Fujiwara, M.; Kodera, Y.; Ito, K.; Nakao, A. Plasminogen activator inhibitor-1 as a potential marker for the malignancy of colorectal cancer. Br. J. Cancer 2005, 93, 799–803. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Angenete, E.; Langenskiöld, M.; Palmgren, I.; Falk, P.; Oresland, T.; Ivarsson, M.L. uPA and PAI-1 in rectal cancer—Relationship to radiotherapy and clinical outcome. J. Surg. Res. 2009, 153, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Nekarda, H.; Schmitt, M.; Ulm, K.; Wenninger, A.; Vogelsang, H.; Becker, K.; Roder, J.D.; Fink, U.; Siewert, J.R. Prognostic impact of urokinase-type plasminogen activator and its inhibitor PAI-1 in completely resected gastric cancer. Cancer Res. 1994, 54, 2900–2907. [Google Scholar] [PubMed]
- Hofmann, R.; Lehmer, A.; Buresch, M.; Hartung, R.; Ulm, K. Clinical relevance of urokinase plasminogen activator, its receptor, and its inhibitor in patients with renal cell carcinoma. Cancer 1996, 78, 487–492. [Google Scholar] [CrossRef]
- Pedersen, H.; Grøndahl-Hansen, J.; Francis, D.; Osterlind, K.; Hansen, H.H.; Danø, K.; Brünner, N. Urokinase and plasminogen activator inhibitor type 1 in pulmonary adenocarcinoma. Cancer Res. 1994, 54, 120–123. [Google Scholar] [PubMed]
- Hazelbag, S.; Kenter, G.G.; Gorter, A.; Fleuren, G.J. Prognostic relevance of TGF-beta1 and PAI-1 in cervical cancer. Int. J. Cancer 2004, 112, 1020–1028. [Google Scholar] [CrossRef] [PubMed]
- Chin, D.; Boyle, G.M.; Williams, R.M.; Ferguson, K.; Pandeya, N.; Pedley, J.; Campbell, C.M.; Theile, D.R.; Parsons, P.G.; Coman, W.B. Novel markers for poor prognosis in head and neck cancer. Int. J. Cancer 2005, 113, 789–797. [Google Scholar] [CrossRef] [PubMed]
- Muracciole, X.; Romain, S.; Dufour, H.; Palmari, J.; Chinot, O.; Ouafik, L.; Grisoli, F.; Figarella-Branger, D.; Martin, P.M. PAI-1 and EGFR expression in adult glioma tumors: Toward a molecular prognostic classification. Int. J. Radiat. Oncol. Biol. Phys. 2002, 52, 592–598. [Google Scholar] [CrossRef]
- Chambers, S.K.; Ivins, C.M.; Carcangiu, M.L. Plasminogen activator inhibitor-1 is an independent poor prognostic factor for survival in advanced stage epithelial ovarian cancer patients. Int. J. Cancer 1998, 79, 449–454. [Google Scholar] [CrossRef]

| IVIS | Macroscopy | |
|---|---|---|
| Control | 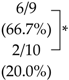 | 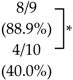 |
| SK-216 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsuge, M.; Osaki, M.; Sasaki, R.; Hirahata, M.; Okada, F. SK-216, a Novel Inhibitor of Plasminogen Activator Inhibitor-1, Suppresses Lung Metastasis of Human Osteosarcoma. Int. J. Mol. Sci. 2018, 19, 736. https://doi.org/10.3390/ijms19030736
Tsuge M, Osaki M, Sasaki R, Hirahata M, Okada F. SK-216, a Novel Inhibitor of Plasminogen Activator Inhibitor-1, Suppresses Lung Metastasis of Human Osteosarcoma. International Journal of Molecular Sciences. 2018; 19(3):736. https://doi.org/10.3390/ijms19030736
Chicago/Turabian StyleTsuge, Minori, Mitsuhiko Osaki, Ryo Sasaki, Mio Hirahata, and Futoshi Okada. 2018. "SK-216, a Novel Inhibitor of Plasminogen Activator Inhibitor-1, Suppresses Lung Metastasis of Human Osteosarcoma" International Journal of Molecular Sciences 19, no. 3: 736. https://doi.org/10.3390/ijms19030736
APA StyleTsuge, M., Osaki, M., Sasaki, R., Hirahata, M., & Okada, F. (2018). SK-216, a Novel Inhibitor of Plasminogen Activator Inhibitor-1, Suppresses Lung Metastasis of Human Osteosarcoma. International Journal of Molecular Sciences, 19(3), 736. https://doi.org/10.3390/ijms19030736





