Biochemical Mechanism of Rhododendrol-Induced Leukoderma
Abstract
1. Introduction
2. Early Events of RD Metabolism Catalyzed by Tyrosinase
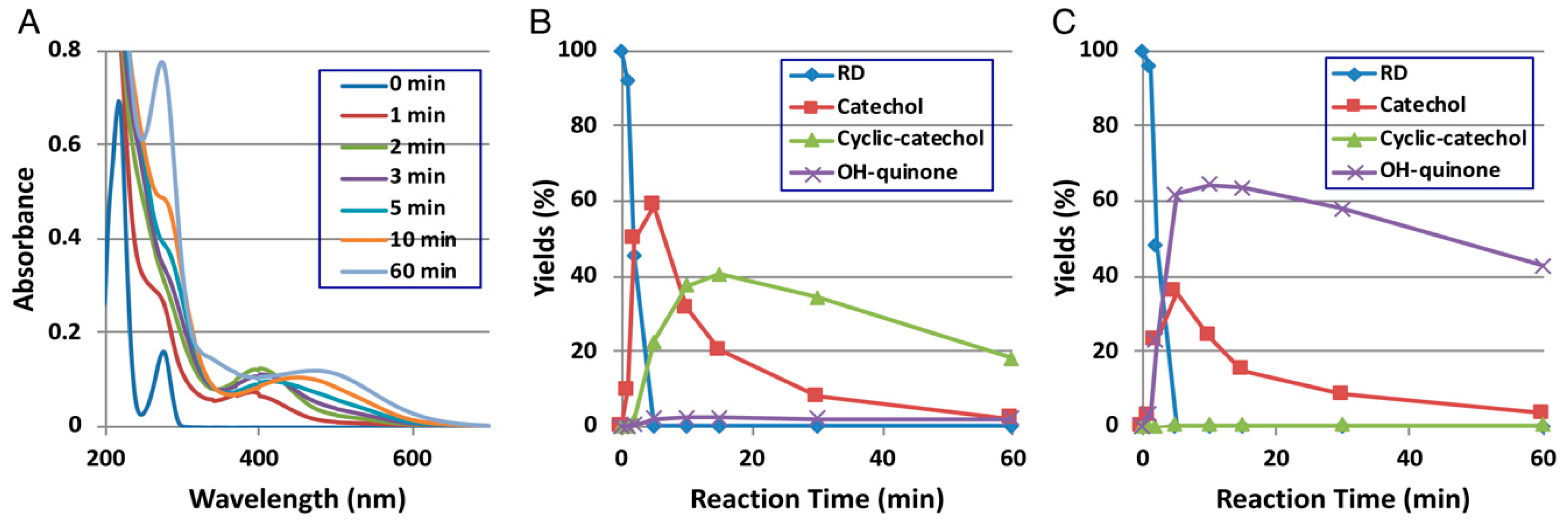
3. RD Metabolism In Vitro and In Vivo
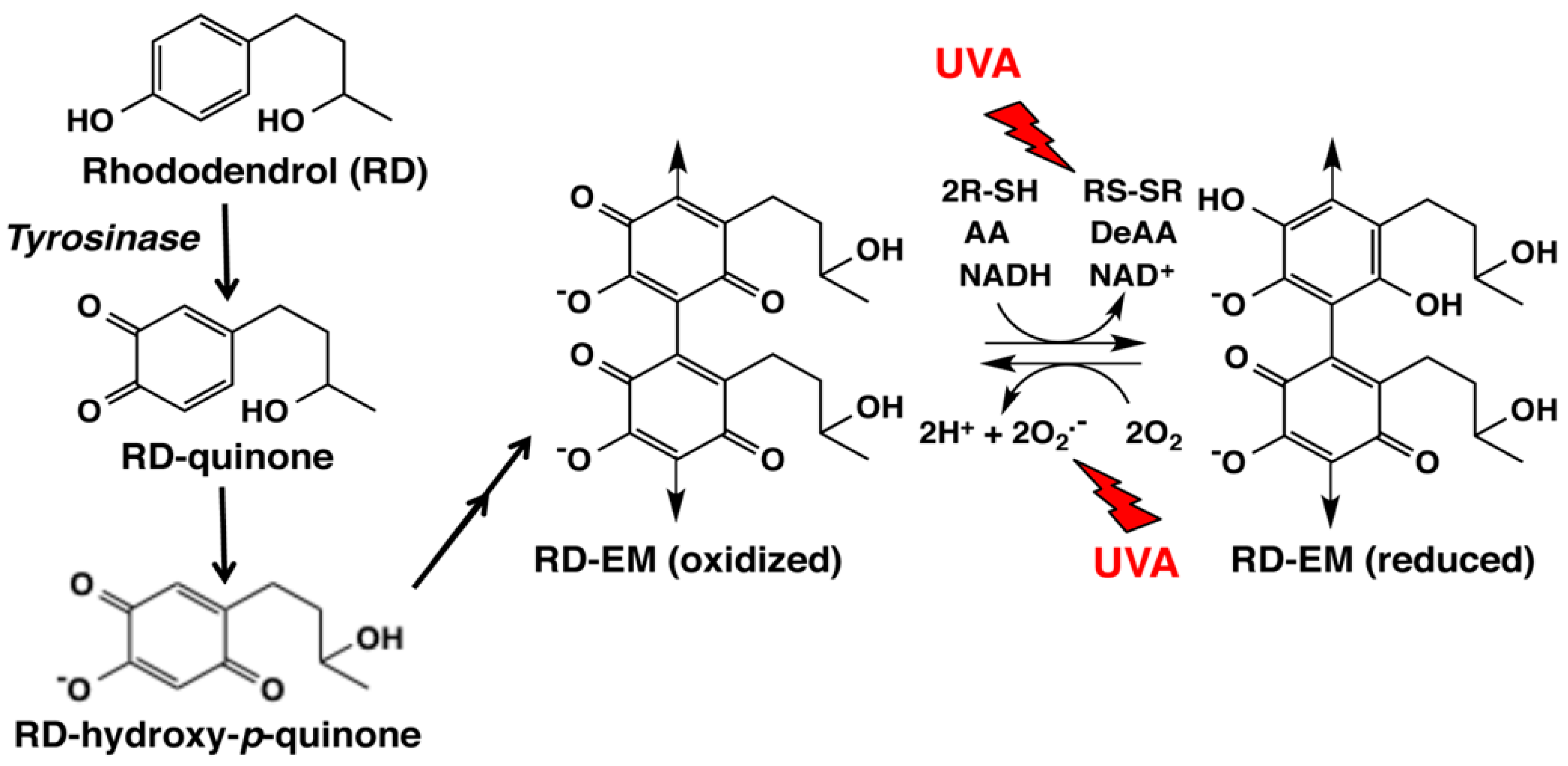
4. Late Events of RD Metabolism in Relation to the Pro-Oxidant Activity
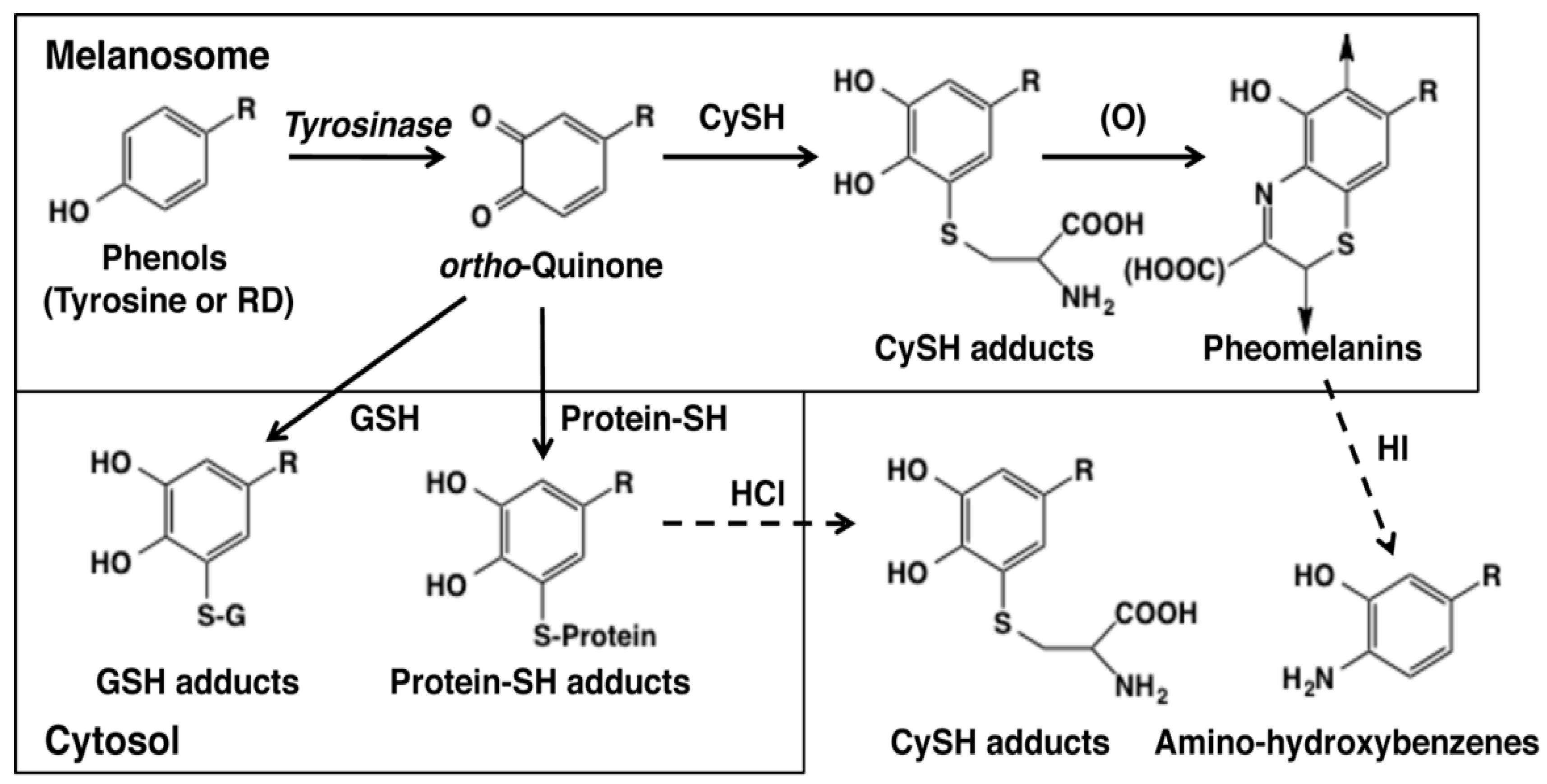
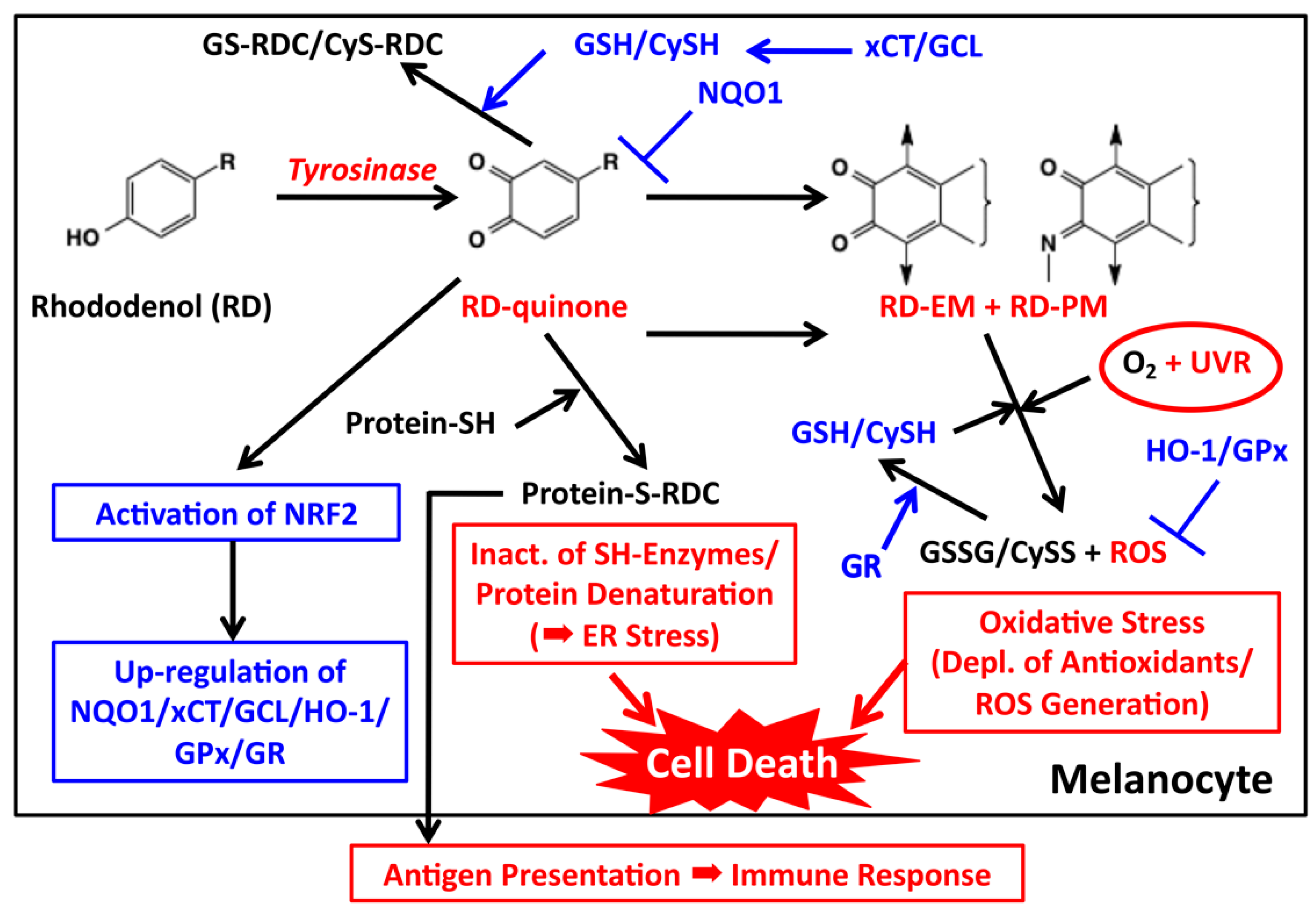
5. Melanocyte Toxicity of RD and the Detoxifying Mechanism
5.1. Roles of Tyrosinase
5.2. Cytotoxicity of o-Quinones (RD-Quinone and RD-Cyclic Quinone)
5.3. Cytotoxicity of Catechols (RD-Catechol and RS-RD-Catechol)
5.4. Cytotoxicity by the Pro-Oxidant Activity of RD-Eumelanin and RD-Pheomelanin
6. Immunological Mechanisms
7. Concluding Remarks
Acknowledgements
Author Contributions
Conflicts of Interest
References
- Tallent, W.H. d-Betuligenol from Rhododendron maximum L. J. Org. Chem. 1964, 29, 988–989. [Google Scholar] [CrossRef]
- Inoue, T.; Ishidate, Y.; Fujita, M.; Kubo, M.; Fukushima, M.; Nagai, M. Studies on the constituents of Aceraceae plants. I. Constituents in the leaves and the stem bark of Acer nikoense Maxim. Yakugaku Zasshi 1978, 98, 41–46. (In Japanese) [Google Scholar] [CrossRef] [PubMed]
- Fuchino, H.; Honishi, S.; Satoh, T.; Yagi, A.; Saitsu, K.; Tatsumi, T.; Tanaka, N. Chemical evaluation of Betula species in Japan. II. Constituents of Betula platyphylia var. japonica. Chem. Pharm. Bull. 1996, 44, 1033–1038. [Google Scholar] [CrossRef]
- Akazawa, H.; Ahihisa, T.; Taguchi, Y.; Banno, N.; Yoneima, R.; Yasukawa, K. Melanogenesis inhibitory and free radical scavenging activities of diarylheptanoids and other phenolic compounds from the bark of Acer nikoense. Biol. Pharm. Bull. 2006, 29, 1970–1972. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, T.; Kim, G.; Kaneko, M. Studies on constituents of Rhdodendron fauriei leaves. Yakugaku Zasshi 1942, 62, 4–6. (In Japanese) [Google Scholar]
- Tanemura, A.; Yang, L.; Yang, F.; Nagata, Y.; Wataya-Kaneda, M.; Fukai, K.; Tsuruta, D.; Ohe, R.; Yamakawa, M.; Suzuki, T.; et al. An immune pathological and ultrastructural skin analysis for rhododenol-induced leukoderma patients. J. Dermatol. Sci. 2015, 77, 185–188. [Google Scholar] [CrossRef] [PubMed]
- Aoyama, Y.; Ito, A.; Suzuki, K.; Suzuki, T.; Tanemura, A.; Nishigori, K.; Ito, M.; Katayama, I.; Sugiura, S.; Matsunaga, K. The first epidemiological report on rhododenol-induced leukoderma in Japan based on a nationwide survey. Jpn. J. Dermatol. 2014, 124, 2095–2109. (In Japanese) [Google Scholar]
- Yang, L.; Yang, F.; Wataya-Kaneda, M.; Tanemura, A.; Tsuruta, D.; Katayama, I. 4-(4-Hydroroxyphenyl)-2-butanol (rhododendrol) activates the autophagy-lysosome pathway in melanocytes: Insights into the mechanisms of rhododendrol-induced leukoderma. J. Dermatol. Sci. 2015, 77, 182–185. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Yamashita, T.; Ojika, M.; Wakamatsu, K. Tyrosinase-catalyzed oxidation of rhododendrol produces 2-methylchromane-6,7-dione, the putative ultimate toxic metabolite: Implications for melanocyte toxicity. Pigment Cell Melanoma Res. 2014, 27, 744–753. [Google Scholar] [CrossRef] [PubMed]
- Kishida, R.; Kasai, H.; Aspera, S.M.; Arevalo, R.L.; Nakanishi, H. Density functional theory-based First principles calculations of rhododendrol-quinone reactions: Preference to thiol binding over cyclization. J. Phys. Soc. Jpn. 2017, 86, 024804. [Google Scholar] [CrossRef]
- Palumbo, A.; d’Ischia, M.; Misuraca, G.; Prota, G. Skin depigmentation by hydroquinone: A chemical and biochemical insight. Pigment Cell Melanoma Res. 1992, 3, 299–303. [Google Scholar] [CrossRef]
- De Lucia, M.; Panzella, A.; Napolitano, A.; d’Ischia, M. Oxidative chemistry of the natural antioxidant hydroxytyrosol: Hydrogen peroxide-dependent hydroxylation and hydroquinone/o-quinone coupling pathways. Tetrahedron 2006, 62, 1273–1278. [Google Scholar] [CrossRef]
- Cooksey, C.J.; Garratt, P.J.; Land, E.J.; Pavel, S.; Ramsden, C.A.; Riley, P.A.; Smit, N.P.M. Evidence of the indirect formation of the catecholic intermediate substrate responsible for the autoactivation kinetics of tyrosinase. J. Biol. Chem. 1997, 272, 26226–26235. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.; Tobin, D.J.; Shibahara, S.; Wortsman, J. Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol. Rev. 2004, 84, 1155–1228. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Wakamatsu, K. Chemistry of mixed melanogenesis—Pivotal roles of dopaquinone. Photochem. Photobiol. 2008, 84, 582–592. [Google Scholar] [CrossRef] [PubMed]
- Hearing, V.J. Determination of melanin synthetic pathways. J. Investig. Dermatol. 2011, 131, E8–E11. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.; Zmijewski, M.A.; Pawelek, J. l-Tyrosine and l-dihydroxyphenylalanine as hormone-like regulators of melanocyte functions. Pigment Cell Melanoma Res. 2012, 25, 14–27. [Google Scholar] [CrossRef] [PubMed]
- d’Ischia, M.; Wakamatsu, K.; Napolitano, A.; Briganti, S.; Garcia-Borron, J.-C.; Kovacs, D.; Meredith, P.; Pezzella, A.; Picardo, M.; Sarna, T.; et al. Melanins and melanogenesis: Methods, standards, protocols. Pigment Cell Melanoma Res. 2013, 26, 616–633. [Google Scholar] [CrossRef] [PubMed]
- Ramsden, C.A.; Riley, R.A. Tyrosinase: The four oxidation states of the active site and their relevance to enzymatic activation, oxidation and inactivation. Bioorg. Med. Chem. 2014, 22, 2388–2395. [Google Scholar] [CrossRef] [PubMed]
- Graham, D.G.; Tiffany, S.M.; Bell, W.R., Jr.; Gutknecht, W.F. Autoxidation versus covalent binding of quinones as the mechanism of toxicity of dopamine, 6-hydroxydopamine, and related compounds toward C1300 neuroblastoma cells in vitro. Mol. Pharmacol. 1978, 14, 644–653. [Google Scholar] [PubMed]
- Bolton, J.L.; Trush, M.A.; Penning, T.M.; Dryhurst, G.; Monks, T.J. Role of quinones in toxicology. Chem. Res. Toxicol. 2000, 13, 135–160. [Google Scholar] [CrossRef] [PubMed]
- Hariharan, V.; Klarquist, J.; Reust, M.J.; Koshoffer, A.; McKee, M.D.; Boissy, R.E.; Le Poole, I.C. Monobenzyl ether of hydroquinone and 4-tertiary butyl phenol activate markedly different physiological responses in melanocytes: Relevance to skin depigmentation. J. Investig. Dermatol. 2010, 130, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Thörneby-Andersson, K.; Sterner, O.; Hansson, C. Tyrosinase-mediated formation of a reactive quinone from the depigmenting agents, 4-tert-butylphenol and 4-tert-butylcatechol. Pigment Cell Res. 2000, 13, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Naish, S.; Cooksey, C.J.; Riley, P.A. Initial mushroom tyrosinase-catalysed oxidation product of 4-hydroxyanisole is 4-methoxy-ortho-benzoquinone. Pigment Cell Res. 1988, 1, 379–381. [Google Scholar] [CrossRef] [PubMed]
- Manini, P.; Napolitano, A.; Westerhof, W.; Riley, P.A.; d’Ischia, M.A. Reactive ortho-quinone generated by tyrosinase-catalyzed oxidation of the skin depigmenting agent monobenzone: Self-coupling and thiol-conjugation reactions and possible implications for melanocyte toxicity. Chem. Res. Toxicol. 2009, 22, 1398–1405. [Google Scholar] [CrossRef] [PubMed]
- Van den Boorn, J.G.; Picavet, D.I.; van Swieten, P.F.; van Veen, H.A.; Konijnenberg, D.; van Veelen, P.A.; van Capel, T.; Jong, E.C.; Reits, E.A.; Drijhout, J.W.; et al. Skin-depigmenting agent monobenzone induces potent T-cell autoimmunity toward pigmented cells by tyrosinase haptenation and melanosome autophagy. J. Investig. Dermatol. 2011, 131, 1240–1251. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Wakamatsu, K. A convenient screening method to differentiate phenolic skin whitening tyrosinase inhibitors from leukoderma-inducing phenols. J. Dermatol. Sci. 2015, 80, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Tandon, M.; Thomas, P.D.; Shokravi, M.; Singh, S.; Chang, D.; Jimbow, K. Synthesis and antitumor effect of the melanogenesis-based antimelanoma agent, N-propionyl-4-S-cysteaminylphenol. Biochem. Pharmacol. 1998, 55, 2023–2029. [Google Scholar] [CrossRef]
- Ishii-Osai, Y.; Yamashita, T.; Tamura, Y.; Sato, N.; Ito, A.; Honda, H.; Wakamatsu, K.; Ito, S.; Nakayaka, E.; Okura, M.; et al. N-Propionyl-4-S-cysteaminylphenol induces apoptosis in B16F1 cells and mediates tumor-specific T-cell immune responses in a mouse melanoma model. J. Dermatol. Sci. 2012, 67, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Nishigaki, A.; Ishii-Osai, Y.; Ojika, M.; Wakamatsu, K.; Yamashita, T.; Tamura, Y.; Ito, A.; Honda, H.; Nakayama, A.; et al. Mechanism of putative neo-antigen formation from N-propionyl-4-S-cysteaminylphenol, a tyrosinase substrate, in melanoma models. Biochem. Pharmacol. 2012, 84, 646–653. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Gerwat, W.; Kolbe, L.; Yamashita, T.; Ojika, M.; Wakamatsu, K. Human tyrosinase is able to oxidize both enantiomers of rhododendrol. Pigment Cell Melanoma Res. 2014, 27, 1149–1153. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Okura, M.; Nakanishi, Y.; Ojika, M.; Wakamatsu, K.; Yamashita, T. Tyrosinase-catalyzed metabolism of rhododendrol (RD) in B16 melanoma cells: Production of RD-pheomelanin and covalent binding with thiol proteins. Pigment Cell Melanoma Res. 2015, 28, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Okura, M.; Wakamatsu, K.; Yamashita, T. The potent pro-oxidant activity of rhododendrol-eumelanin induces cysteine depletion in B16 melanoma cells. Pigment Cell Melanoma Res. 2017, 30, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Agata, M.; Okochi, K.; Wakamatsu, K. The potent pro-oxidant activity of rhododendrol-eumelanin is enhanced by ultraviolet A radiation. Pigment Cell Melanoma Res. 2018. accepted for publication. [Google Scholar]
- Tokura, Y.; Fujiyama, T.; Ikeya, S.; Tatsuno, K.; Aoshima, M.; Kasuya, A.; Ito, T. Biochemical, cytological, and immunological mechanisms of rhododendrol-induced leukoderma. J. Dermatol. Sci. 2015, 77, 146–149. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, M.; Kondo, M.; Sato, K.; Umeda, M.; Kawabata, K.; Takahashi, Y.; Suzuki, T.; Matsunaga, K.; Inoue, S. Rhododendrol, a depigmentation-inducing phenolic compound, exerts melanocyte cytotoxicity via a tyrosinase-dependent mechanism. Pigment Cell Melanoma Res. 2014, 27, 754–763. [Google Scholar] [CrossRef] [PubMed]
- Tse, D.C.; McCreery, R.L.; Adams, R.N. Potential oxidative pathways of brain catecholamines. J. Med. Chem. 1976, 19, 37–40. [Google Scholar] [CrossRef] [PubMed]
- Kato, T.; Ito, S.; Fujita, K. Tyrosinase-catalyzed binding of 3,4-dihydroxyphenylalanine with proteins through the sulfhydryl group. Biochim. Biophys. Acta 1986, 881, 415–421. [Google Scholar] [CrossRef]
- Ito, S.; Kato, T.; Fujita, K. Covalent binding of catechols to proteins through the sulphydryl group. Biochem. Pharmacol. 1988, 37, 1707–1710. [Google Scholar] [CrossRef]
- Potterf, S.B.; Virador, V.; Wakamatsu, K.; Furumura, M.; Santis, C.; Ito, S.; Hearing, V.J. Cysteine transport in melanosomes from murine melanocytes. Pigment Cell Res. 1999, 12, 4–12. [Google Scholar] [CrossRef] [PubMed]
- Mitra, D.; Luo, X.; Morgan, A.; Wang, J.; Hoang, M.P.; Lo, J.; Guerrero, C.R.; Lennerz, J.K.; Mihm, M.C.; Wargo, J.A.; et al. An ultraviolet-radiation-independent pathway to melanoma carcinogenesis in the red hair/fair skin background. Nature 2012, 491, 449–453. [Google Scholar] [CrossRef] [PubMed]
- Napolitano, A.; Panzella, L.; Monfrecola, G.; d’Ischia, M. Pheomelanin-induced oxidative stress: Bright and dark chemistry bridging red hair phenotype and melanoma. Pigment Cell Melanoma Res. 2014, 27, 721–733. [Google Scholar] [CrossRef] [PubMed]
- Panzella, L.; Leone, L.; Greco, G.; Vitiello, G.; D’Errico, G.; Napolitano, A.; d’Ischia, M. Red human hair pheomelanin is a potent pro-oxidant mediating UV-independent contributory mechanisms of melanomagenesis. Pigment Cell Melanoma Res. 2014, 27, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Wakamatsu, K.; Ito, S.; Rees, J.L. The usefulness of 4-amino-3-hydroxyphenylalanine as a specific marker of pheomelanin. Pigment Cell Res. 2002, 15, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Goto, N.; Tsujimoto, M.; Nagai, H.; Masaki, T.; Ito, S.; Wakamatsu, K.; Nishigori, C. 4-(4-Hydroxyphenyl)-2-butanol (rhododendrol)-induced melanocyte cytotoxicity is enhanced by UVB exposure through generation of oxidative stress. Exp. Dermatol. 2018. in revision. [Google Scholar]
- Kasamatsu, S.; Hachiya, A.; Nakamura, S.; Yasuda, Y.; Fujimori, T.; Takano, K.; Moriwaki, S.; Hase, T.; Suzuki, T.; Matsunaga, K. Depigmentation caused by application of the active brightening material, rhododendrol, is related to tyrosinase activity at a certain threshold. J. Dermatol. Sci. 2014, 76, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Abe, Y.; Okamura, K.; Kawaguchi, M.; Hozumi, Y.; Aoki, H.; Kunisada, T.; Ito, S.; Wakamatsu, K.; Matsunaga, K.; Suzuki, T. Rhododenol-induced leukoderma in a mouse model mimicking Japanese skin. J. Dermatol. Sci. 2016, 81, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, Y.; Takahashi, Y.; Sakaguchi, H.; Matsunaga, K.; Suzuki, T. Depigmentation of the skin induced by 4-(4-hydroxyphenyl)-2-butanol is spontaneously re-pigmented in brown and black guinea pigs. J. Toxicol. Sci. 2014, 9, 615–623. [Google Scholar] [CrossRef]
- Korytowski, W.; Kalynaraman, B.; Menon, I.A.; Sarna, T.; Sealy, R.C. Reaction of superoxide anions with melanins: Electron spin resonance and spin trapping studies. Biochim. Biophys. Acta 1986, 882, 145–153. [Google Scholar] [CrossRef]
- Korytowski, W.; Plas, B.; Sarna, T.; Kalyanaraman, B. Photoinduced generation of hydrogen peroxide and hydroxyl radicals in melanins. Photochem. Photobiol. 1987, 45, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Panzella, L.; Micillo, R.; Bentley, W.E.; Napolitano, A.; Payne, G.F. Reverse engineering applied to red human hair pheomelanin reveals redox-buffering as a pro-oxidant mechanism. Sci. Rep. 2015, 5, 18447. [Google Scholar] [CrossRef] [PubMed]
- Haywood, R.M.; Andrady, C.; Kassouf, N.; Sheppard, N. Intensity-dependent direct solar radiation- and UVA-induced radical damage to human skin and DNA, lipids and proteins. Photochem. Photobiol. 2011, 87, 117–130. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Hinoshita, M.; Suzuki, E.; Ojika, M.; Wakamatsu, K. Tyrosinase-catalyzed oxidation of the leukoderma-inducing agent raspberry ketone produces (E)-4-(3-oxo-1-butenyl)-1,2-benzoquinone: Implications for melanocyte toxicity. Chem. Res. Toxicol. 2017, 30, 859–868. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.S.; Joo, Y.H.; Park, M.; Kim, J.-H.; Shin, H.-J.; Park, N.-H.; Lee, J.H.; Park, Y.-H.; Shin, S.S.; Lee, H.-K. Different effects of five depigmentary compounds, rhododendrol, raspberry ketone, monobenzone, rucinol and AP736 on melanogenesis and viability of human epidermal melanocytes. Exp. Dermatol. 2016, 25, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Arase, N.; Yang, L.; Tanemura, A.; Yang, F.; Suenaga, L.; Arase, H.; Katayama, I. The effect of rhododendrol inhibition of NF-κB on melanocytes in the presence of tyrosinase. J. Dermatol. Sci. 2016, 83, 157–159. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Bailly-Maitre, B.; Reed, J.C. Endoplasmic reticulum stress: Cell life and death decisions. J. Clin. Investig. 2005, 115, 2656–2664. [Google Scholar] [CrossRef] [PubMed]
- Okubo, A.; Yasuhira, S.; Shibazaki, M.; Takahashi, K.; Akasaka, T.; Masuda, T.; Maesawa, C. NAD(P)H dehydrogenase, quinone 1 (NQO1), protects melanin-producing cells from cytotoxicity of rhododendrol. Pigment Cell Melanoma Res. 2016, 29, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Siegel, D.; Yan, C.; Ross, D. NAD(P)H:quinone oxidoreductase 1 (NQO1) in the sensitivity and resistance to antitumor quinones. Biochem. Pharmacol. 2012, 83, 1033–1040. [Google Scholar] [CrossRef] [PubMed]
- Vomund, S.; Schäfer, A.; Parnham, M.J.; Brüne, B.; von Knethen, A. Nrf2, the master regulator of anti-oxidative responses. Int. J. Mol. Sci. 2017, 18, 2772. [Google Scholar] [CrossRef] [PubMed]
- Kondo, M.; Kawabata, K.; Sato, K.; Yamaguchi, S.; Hachiya, A.; Takahashi, Y.; Inoue, S. Glutathione maintenance is crucial for survival of melanocytes after exposure to rhododendrol. Pigment Cell Melanoma Res. 2016, 29, 541–549. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Baek, H.S.; Lee, M.; Park, H.; Shin, S.S.; Choi, D.W.; Lim, K.-M. Rhododenol and raspberry ketone impair the normal proliferation of melanocytes through reactive oxygen species-dependent activation of GADD45. Toxicol. In Vitro 2016, 32, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.C. Glutathione synthesis. Biochim. Biophys. Acta 2013, 1830, 3143–3153. [Google Scholar] [CrossRef] [PubMed]
- Jian, Z.; Li, K.; Liu, L.; Zhang, Y.; Zhou, Z.; Li, C.; Gao, T. Heme oxygenase-1 protects human melanocytes from H2O2-induced oxidative stress via the Nrf2-ARE pathway. J. Investig. Dermatol. 2011, 131, 1420–1427. [Google Scholar] [CrossRef] [PubMed]
- Ishii, T.; Mann, G.E. Redox status in mammalian cells and stem cells during culture in vitro: Critical roles of Nrf2 and cystine transporter activity in the maintenance of redox balance. Redox Biol. 2014, 2, 786–794. [Google Scholar] [CrossRef] [PubMed]
- Felim, A.; Urios, A.; Neudorffer, A.; Herrera, G.; Blanco, M.; Largeron, M. Bacterial plate assays and electrochemical methods: An efficient tandem for evaluating the ability of catechol-thioether metabolites of MDMA (“ecstasy”) to induce toxic effects through redox-cycling. Chem. Res. Toxicol. 2007, 20, 685–693. [Google Scholar] [CrossRef] [PubMed]
- Okura, M.; Yamashita, T.; Ishii-Osai, Y.; Yoshikawa, M.; Sumikawa, Y.; Wakamatsu, K.; Ito, S. Effects of rhododendrol and its metabolic products on melanocytic cell growth. J. Dermatol. Sci. 2015, 80, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Monks, T.J.; Hanzlik, R.P.; Cohen, G.M.; Ross, D.; Graham, D.G. Contemporary issues in toxicology. Quinone chemistry and toxicity. Toxicol. Appl. Pharmacol. 1992, 112, 2–16. [Google Scholar] [CrossRef]
- Nagata, T.; Ito, S.; Itoga, K.; Hanazawa, H.; Masaki, H. The mechanism of melanocytes-specific cytotoxicity induced by phenol compounds having a prooxidant effect, relating to the appearance of leukoderma. Biomed. Res. Int. 2015, 2015, 479798. [Google Scholar] [CrossRef] [PubMed]
- Ye, T.; Hong, L.; Garguilo, J.; Pawlak, A.; Edwards, G.S.; Nemanich, R.J.; Sarna, T.; Simon, J.D. Photoionization thresholds of melanins obtained from free electron laser-photoelectron emission microscopy, femtosecond transient absorption spectroscopy and electron paramagnetic resonance measurements of oxygen photoconsumption. Photochem. Photobiol. 2006, 82, 733–737. [Google Scholar] [CrossRef] [PubMed]
- Satoh, T.; McKercher, S.R.; Lipton, S.A. Nrf2/ARE-mediated antioxidant actions of pro-electrophilic drugs. Free Radic. Biol. Med. 2013, 65, 645–657. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.C. Regulation of glutathione synthesis. Mol. Asp. Med. 2009, 30, 42–59. [Google Scholar] [CrossRef] [PubMed]
- Miyaji, A.; Gabe, Y.; Kohno, M.; Baba, T. Generation of hydroxyl radicals and singlet oxygen during oxidation of rhododendrol and rhododendrol-catechol. J. Clin. Biochem. Nutr. 2017, 60, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Fujiyama, T.; Ikeya, S.; Ito, T.; Tatsuno, K.; Aoshima, M.; Kasuya, A.; Sakabe, J.; Suzuki, T.; Tokura, Y. Melanocyte-specific cytotoxic T lymphocytes in patients with rhododendrol-induced leukoderma. J. Dermatol. Sci. 2015, 77, 190–192. [Google Scholar] [CrossRef] [PubMed]
- Nishioka, M.; Tanemura, A.; Yang, L.; Tanaka, A.; Arase, N.; Katayama, I. Possible involvement of CCR4+CD8+ T cells and elevated plasma CCL22 and CCL17 in patients with Rhododenol-induced leukoderma. J. Dermatol. Sci. 2015, 77, 188–190. [Google Scholar] [CrossRef] [PubMed]
- Nishigori, C.; Aoyama, Y.; Ito, A.; Suzuki, K.; Suzuki, T.; Tanemura, A.; Ito, M.; Katayama, I.; Oiso, N.; Kagohashi, Y.; et al. Guide for medical professionals (i.e., dermatologists) for the management of Rhododenol-induced leukoderma. J. Dermatol. 2015, 42, 113–128. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.E. Chemical-induced vitiligo. Dermatol. Clin. 2017, 35, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Westerhof, W.; Manini, P.; Napolitano, A.; d’Ischia, M. The haptenation theory of vitiligo and melanoma rejection: A close-up. Exp. Dermatol. 2011, 20, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Sturm, R.A. Molecular genetics of human pigmentation diversity. Hum. Mol. Genet. 2009, 18, R9–R17. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.; Miyamura, Y.; Wolber, R.; Smuda, C.; Reihold, W.; Liu, H.; Kolbe, L.; Hearing, V.J. Regulation of human skin pigmentation in situ by repetitive UV exposure: Molecular characterization of responses to UVA and/or UVB. J. Investig. Dermatol. 2010, 130, 1685–1696. [Google Scholar] [CrossRef] [PubMed]

| Metabolite b | RD Not Added | RD 0.3 mM | RD 0.5 mM |
|---|---|---|---|
| Eumelanin | 17 μg | 1.8 μg | 2.1 μg |
| Pheomelanin | 0.71 μg | 0.57 μg | 0.47 μg |
| RD-pheomelanin | 0.00 μg | 0.51 μg | 0.61 μg |
| CyS-Dopa | 0.17 nmol | 0.33 nmol | 0.36 nmol |
| CyS-RD-catechol | 0.00 nmol | 0.09 nmol | 0.15 nmol |
| GS-Dopa | 0.24 nmol | 0.08 nmol | 0.12 nmol |
| GS-RD-catechol | 0.00 nmol | 0.17 nmol | 0.26 nmol |
| Protein-S-Dopa | 0.16 nmol | 0.11 nmol | 0.10 nmol |
| Protein-S-RD-catechol | 0.00 nmol | 2.2 nmol | 3.1 nmol |
| GSH | 25 nmol | 38 nmol | 59 nmol |
| CySH | 1.3 nmol | 13 nmol | 20 nmol |
| Antioxidant b | Control | RD-EM | RD-PM | Dopa-EM | Dopa-PM |
|---|---|---|---|---|---|
| GSH, 60 min | 0.7 | 67.3 | 29.7 | 33.0 | 62.3 |
| CySH, 30 min | 10.7 | 90.3 | 41.3 | 79.7 | 80.0 |
| AA, 30 min | 3.9 | 83.1 | 71.0 | 75.9 | 46.4 |
| NADH, 120 min | 5.7 | 58.7 | 37.7 | 85.5 | 84.8 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ito, S.; Wakamatsu, K. Biochemical Mechanism of Rhododendrol-Induced Leukoderma. Int. J. Mol. Sci. 2018, 19, 552. https://doi.org/10.3390/ijms19020552
Ito S, Wakamatsu K. Biochemical Mechanism of Rhododendrol-Induced Leukoderma. International Journal of Molecular Sciences. 2018; 19(2):552. https://doi.org/10.3390/ijms19020552
Chicago/Turabian StyleIto, Shosuke, and Kazumasa Wakamatsu. 2018. "Biochemical Mechanism of Rhododendrol-Induced Leukoderma" International Journal of Molecular Sciences 19, no. 2: 552. https://doi.org/10.3390/ijms19020552
APA StyleIto, S., & Wakamatsu, K. (2018). Biochemical Mechanism of Rhododendrol-Induced Leukoderma. International Journal of Molecular Sciences, 19(2), 552. https://doi.org/10.3390/ijms19020552






