Evaluation of Polyphenol Anthocyanin-Enriched Extracts of Blackberry, Black Raspberry, Blueberry, Cranberry, Red Raspberry, and Strawberry for Free Radical Scavenging, Reactive Carbonyl Species Trapping, Anti-Glycation, Anti-β-Amyloid Aggregation, and Microglial Neuroprotective Effects
Abstract
:1. Introduction
2. Results
2.1. Total Phenolic and Anthocyanins Contents of Berry Extracts
2.2. Berry Extracts Scavenge Free Radicals in the DPPH Assay
2.3. Berry Extracts Scavenge Reactive Carbonyl Species (RCS)
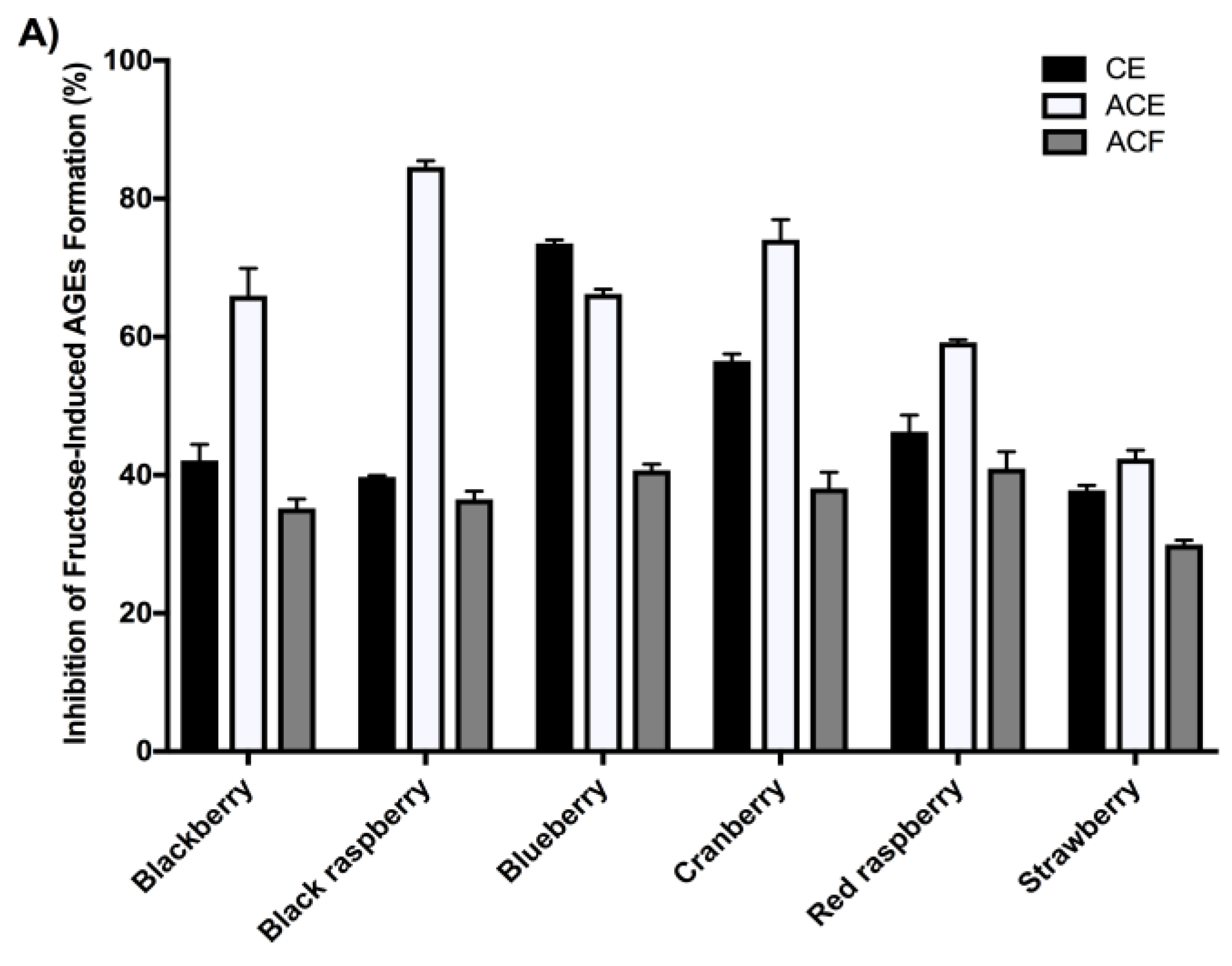
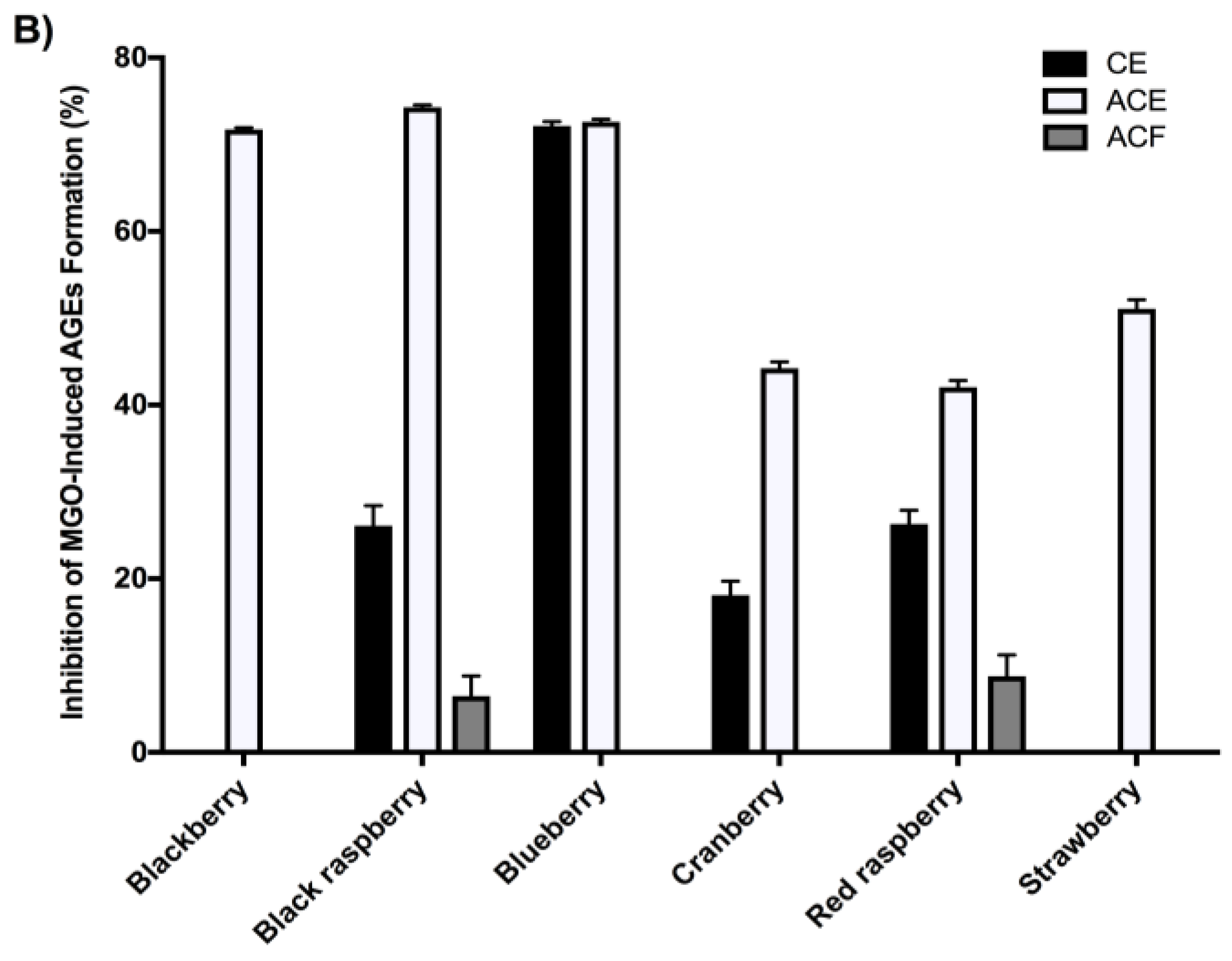
2.4. Berry Extracts Inhibit the Formation of AGEs
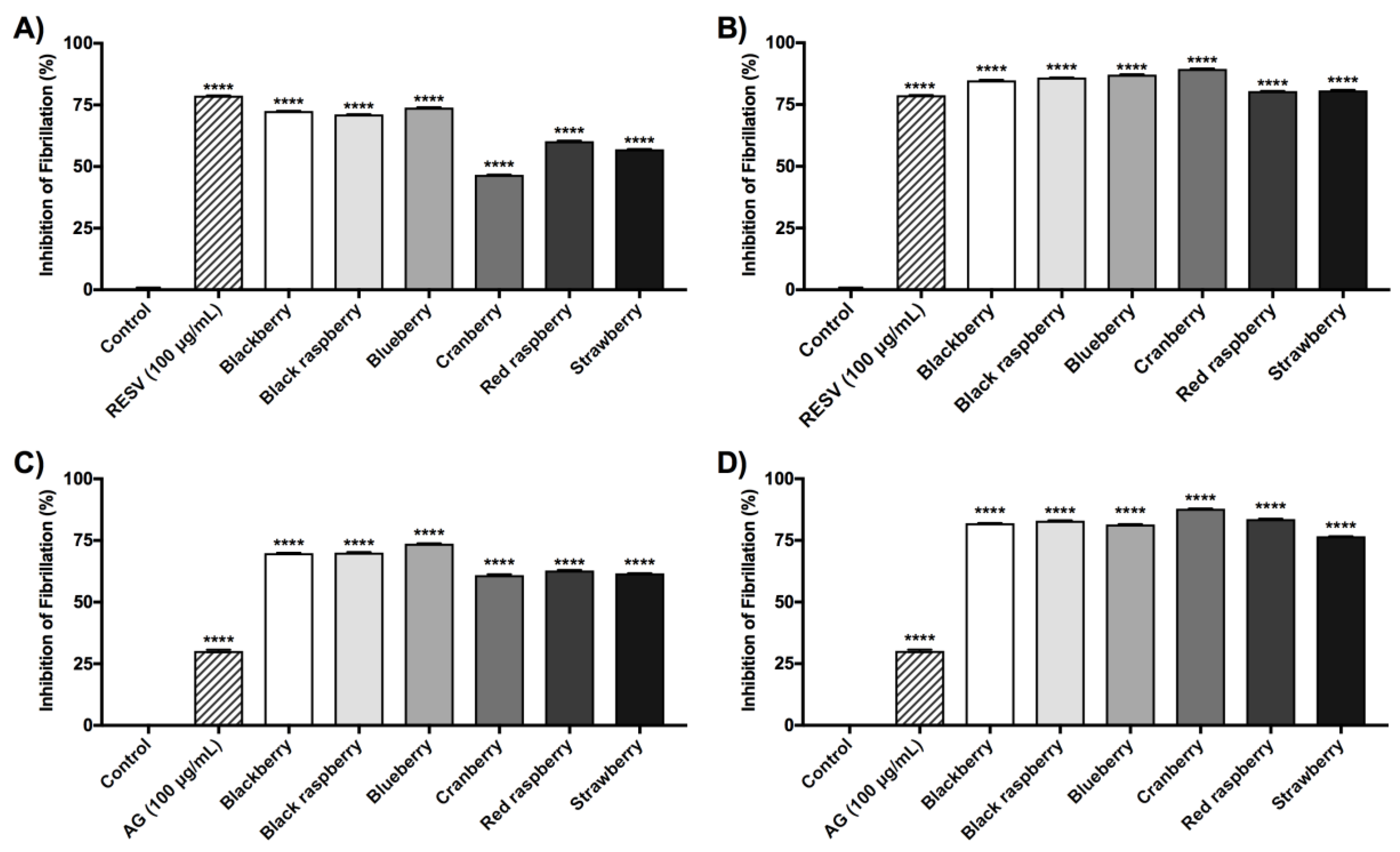
2.5. Berry Anthocyanin-Enriched Extracts (ACEs) Inhibit Aβ Fibrillation
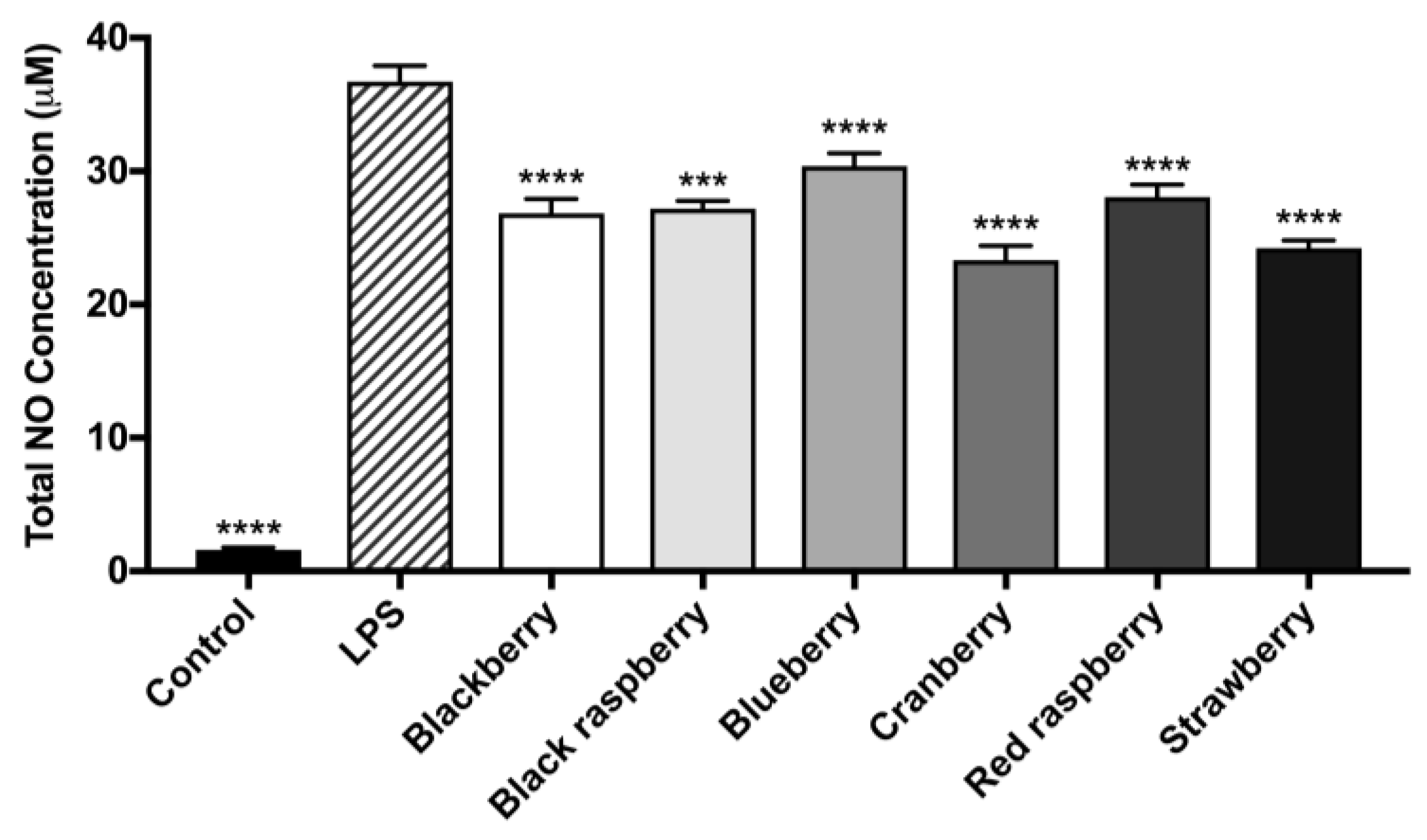
2.6. Berry Anthocyanin-Enriched Extracts (ACEs) Reduce LPS-Induced Inflammation in BV-2 Microglia
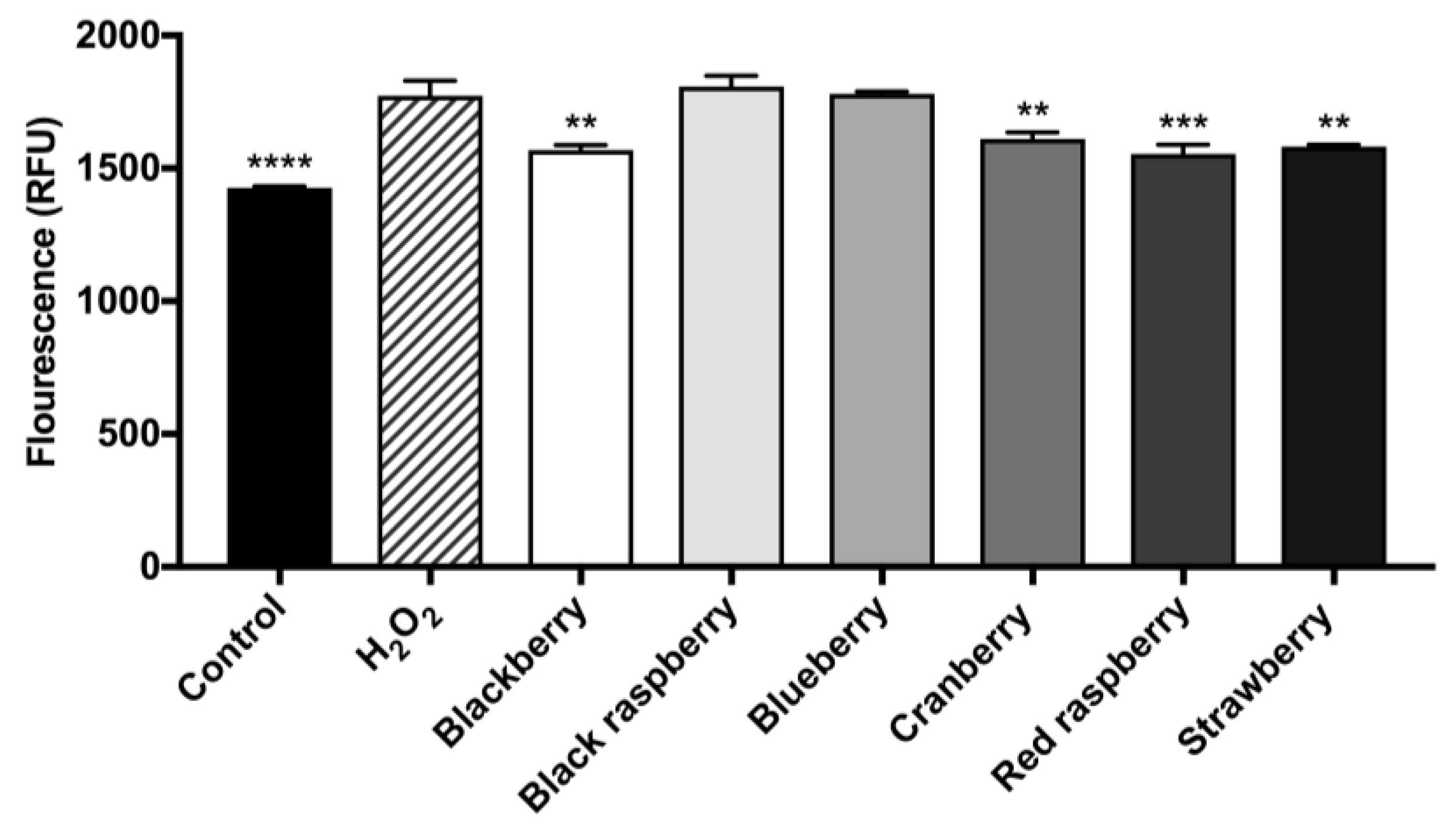
2.7. Berry Anthocyanin-Enriched Extracts (ACEs) H2O2-Induced Oxidative Stress in BV-2 Microglia
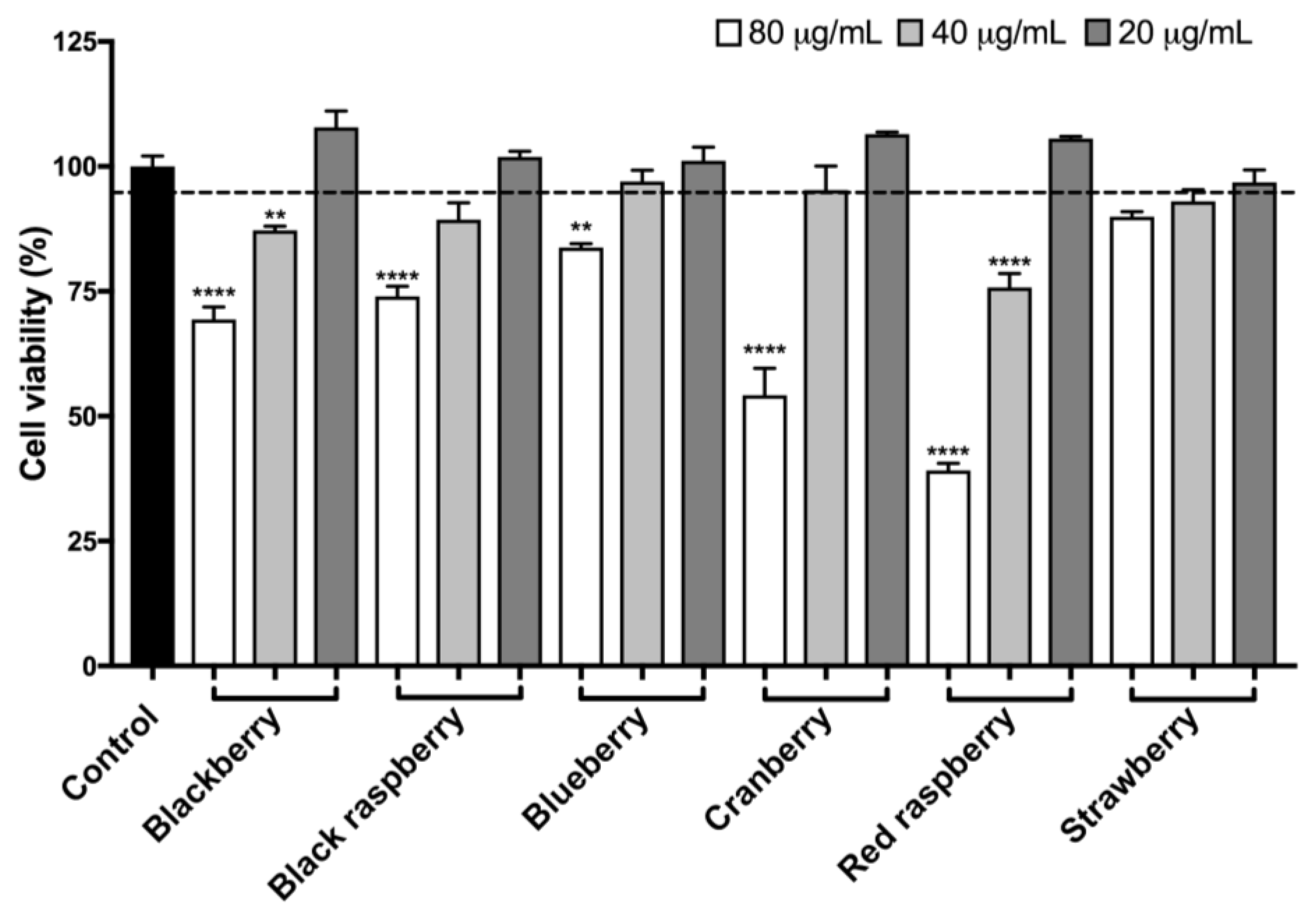
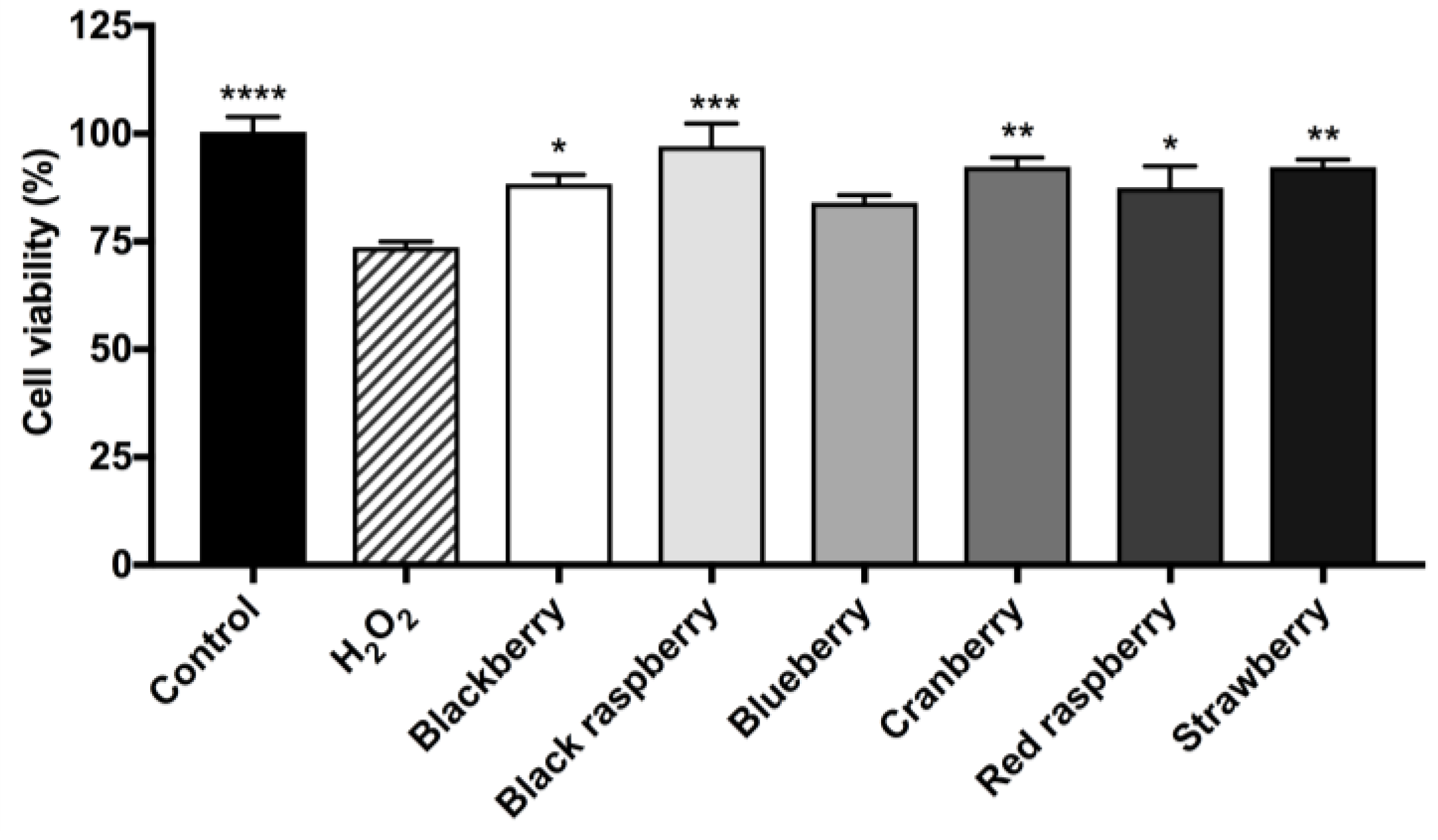
2.8. Berry Anthocyanin-Enriched Extracts (ACEs) Protect BV-2 Microglia against H2O2-Induced Cytotoxicity
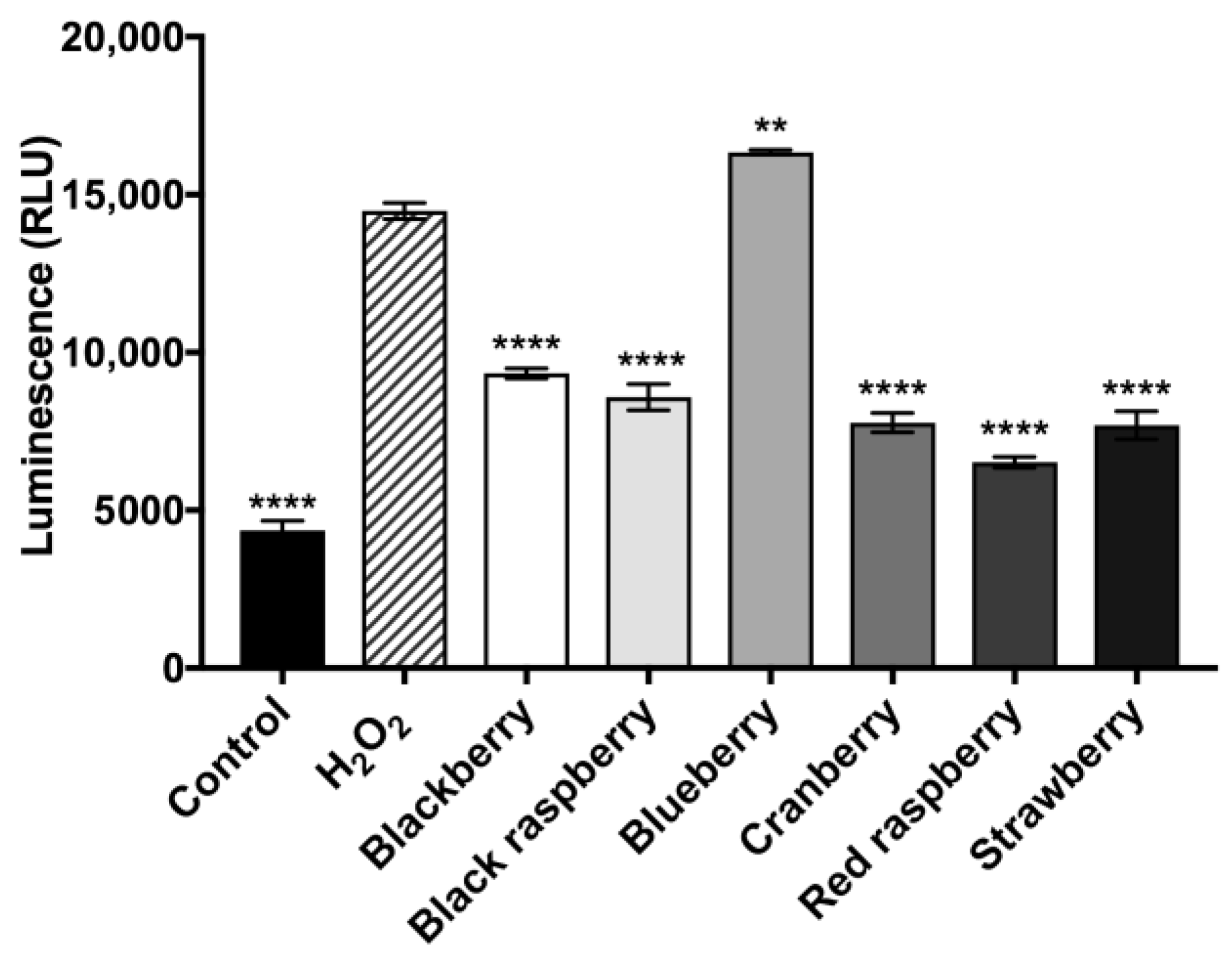
2.9. Berry Anthocyanin-Enriched Extracts (ACEs) Decrease H2O2-Induced Activity of Caspase-3/7 in BV-2 Microglia
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Berry Materials
4.3. Preparation and Fractionation of Berry Extracts
4.4. Total Phenolic and Anthocyanins Content
4.5. 2,2-Diphenyl-1-Picrylhydrazyl (DPPH) Free Radical Scavenging Assay
4.6. Reactive Carbonyl Species (Methylglyoxal; MGO) Trapping Assay
4.7. Inhibition of the Formation of Advanced Glycation Endproducts (AGEs)
4.8. Anti-Aβ Fibrillation Assay
4.9. Cell Culture
4.10. Effects of Berry ACEs on BV-2 Microglia Viability
4.11. Measurement of Total Nitric Oxide Species (NOS) in BV-2 Microglia by Griess Assay
4.12. Measurement of Reactive Oxygen Species (ROS) in BV-2 Microglia
4.13. Measurement of Caspase-3/7 Activity in BV-2 Microglia after H2O2 Exposure
4.14. Measurement of BV-2 Microglia Viability after Exposure to Hydrogen Peroxide (H2O2)
4.15. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| AGEs | advanced glycation endproducts |
| Aβ | beta amyloid |
| AD | Alzheimer’s disease |
| ACE | anthocyanins-enriched extract |
| ACF | anthocyanins-free extract |
| AG | aminoguanidine |
| BHT | butylated hydroxytoluene |
| BSA | bovine serum albumin |
| CE | crude extract |
| GAE | gallic acid equivalent |
| LPS | lipopolysaccharide |
| MGO | methylglyoxal |
| NOS | nitric oxide species |
| RCS | reactive carbonyl species |
| ROS | reactive oxygen species |
References
- Vicente Miranda, H.; El-Agnaf, O.; Outeiro, T.F. Glycation in Parkinson’s disease and Alzheimer’s disease. Mov. Disord. 2016, 31, 782–790. [Google Scholar] [CrossRef] [PubMed]
- Lüth, H.-J.; Ogunlade, V.; Kuhla, B.; Kientsch-Engel, R.; Stahl, P.; Webster, J.; Arendt, T.; Münch, G. Age-and stage-dependent accumulation of advanced glycation end products in intracellular deposits in normal and Alzheimer’s disease brains. Cereb. Cortex 2005, 15, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Beeri, M.S.; Moshier, E.; Schmeidler, J.; Godbold, J.; Uribarri, J.; Reddy, S.; Sano, M.; Grossman, H.T.; Cai, W.; Vlassara, H. Serum concentration of an inflammatory glycotoxin, methylglyoxal, is associated with increased cognitive decline in elderly individuals. Mech. Ageing Dev. 2011, 132, 583–587. [Google Scholar] [CrossRef] [PubMed]
- Angeloni, C.; Zambonin, L.; Hrelia, S. Role of methylglyoxal in Alzheimer’s disease. BioMed Res. Int. 2014. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, H.; Barger, S.; Barnum, S.; Bradt, B.; Bauer, J.; Cole, G.M.; Cooper, N.R.; Eikelenboom, P.; Emmerling, M.; Fiebich, B.L. Inflammation and Alzheimer’s disease. Neurobiol. Aging 2000, 21, 383–421. [Google Scholar] [CrossRef]
- Liu, W.; Ma, H.; Frost, L.; Yuan, T.; Dain, J.A.; Seeram, N.P. Pomegranate phenolics inhibit formation of advanced glycation endproducts by scavenging reactive carbonyl species. Food & Funct. 2014, 5, 2996–3004. [Google Scholar]
- Ma, H.; Liu, W.; Frost, L.; Wang, L.; Kong, L.; Dain, J.A.; Seeram, N.P. The hydrolyzable gallotannin, penta-O-galloyl-β-d-glucopyranoside, inhibits the formation of advanced glycation endproducts by protecting protein structure. Mol. BioSyst. 2015, 11, 1338–1347. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ma, H.; Liu, W.; Yuan, T.; Seeram, N. New Antiglycative Compounds from Cumin (Cuminum cyminum) Spice. J. Agric. Food Chem. 2015, 63, 10097–10102. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Liu, W.; Frost, L.; Kirschenbaum, L.J.; Dain, J.A.; Seeram, N.P. Glucitol-core containing gallotannins inhibit the formation of advanced glycation end-products mediated by their antioxidant potential. Food Funct. 2016, 7, 2213–2222. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Liu, W.; Ma, H.; Marais, J.P.; Khoo, C.; Dain, J.A.; Rowley, D.C.; Seeram, N.P. Effect of cranberry (Vaccinium macrocarpon) oligosaccharides on the formation of advanced glycation end-products. J. Berry Res. 2016, 6, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Wei, Z.; Ma, H.; Cai, A.; Liu, Y.; Sun, J.; DaSilva, N.; Johnson, S.; Kirschenbaum, L.; Cho, B. Anti-glycation and anti-oxidative effects of a phenolic-enriched maple syrup extract and its protective effects on normal human colon cells. Food Funct. 2017, 8, 757–766. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Ma, H.; DaSilva, N.A.; Rose, K.N.; Johnson, S.L.; Zhang, L.; Wan, C.; Dain, J.A.; Seeram, N.P. Development of a neuroprotective potential algorithm for medicinal plants. Neurochem. Int. 2016, 100, 164–177. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Wang, P.; Luo, Y.; Zhao, M.; Chen, F. Health benefits of anthocyanins and molecular mechanisms: Update from recent decade. Crit. Rev. Food Sci. Nutr. 2015, 57, 1729–1741. [Google Scholar] [CrossRef] [PubMed]
- Thangthaeng, N.; Poulose, S.M.; Miller, M.G.; Shukitt-Hale, B. Preserving Brain Function in Aging: The Anti-glycative Potential of Berry Fruit. Neuromol. Med. 2016, 18, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Pribis, P.; Shukitt-Hale, B. Cognition: The new frontier for nuts and berries. Am. J. Clin. Nutr. 2014, 100, 347S–352S. [Google Scholar] [CrossRef] [PubMed]
- Lau, F.C.; Shukitt-Hale, B.; Joseph, J.A. The beneficial effects of fruit polyphenols on brain aging. Neurobiol. Aging 2005, 26, 128–132. [Google Scholar] [CrossRef] [PubMed]
- Harris, C.S.; Cuerrier, A.; Lamont, E.; Haddad, P.S.; Arnason, J.T.; Bennett, S.A.; Johns, T. Investigating wild berries as a dietary approach to reducing the formation of advanced glycation endproducts: Chemical correlates of in vitro antiglycation activity. Plant Foods Hum. Nutr. 2014, 69, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Skrovankova, S.; Sumczynski, D.; Mlcek, J.; Jurikova, T.; Sochor, J. Bioactive compounds and antioxidant activity in different types of berries. Int. J. Mol. Sci. 2015, 16, 24673–24706. [Google Scholar] [CrossRef] [PubMed]
- Siriwoharn, T.; Wrolstad, R.E.; Finn, C.E.; Pereira, C.B. Influence of cultivar, maturity, and sampling on blackberry (Rubus L. Hybrids) anthocyanins, polyphenolics, and antioxidant properties. J. Agric. Food Chem. 2004, 52, 8021–8030. [Google Scholar] [CrossRef] [PubMed]
- Bowen-Forbes, C.S.; Zhang, Y.; Nair, M.G. Anthocyanin content, antioxidant, anti-inflammatory and anticancer properties of blackberry and raspberry fruits. J. Food Comp. Anal. 2010, 23, 554–560. [Google Scholar] [CrossRef]
- Kalt, W.; McDonald, J.; Donner, H. Anthocyanins, phenolics, and antioxidant capacity of processed lowbush blueberry products. J. Food Sci. 2000, 65, 390–393. [Google Scholar] [CrossRef]
- Prior, R.L.; Lazarus, S.A.; Cao, G.; Muccitelli, H.; Hammerstone, J.F. Identification of procyanidins and anthocyanins in blueberries and cranberries (Vaccinium spp.) using high-performance liquid chromatography/mass spectrometry. J. Agric. Food Chem. 2001, 49, 1270–1276. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, F.L.; Escribano-Bailón, M.T.; Alonso, J.J.P.; Rivas-Gonzalo, J.C.; Santos-Buelga, C. Anthocyanin pigments in strawberry. LWT-Food Sci. Technol. 2007, 40, 374–382. [Google Scholar] [CrossRef]
- Liu, R.H. Dietary bioactive compounds and their health implications. J. Food Sci. 2013, 78, A18-25. [Google Scholar] [CrossRef] [PubMed]
- Seeram, N.P.; Schutzki, R.; Chandra, A.; Nair, M.G. Characterization, quantification, and bioactivities of anthocyanins in Cornus species. J. Agric. Food Chem. 2002, 50, 2519–2523. [Google Scholar] [CrossRef] [PubMed]
- Seeram, N.P.; Adams, L.S.; Zhang, Y.; Lee, R.; Sand, D.; Scheuller, H.S.; Heber, D. Blackberry, black raspberry, blueberry, cranberry, red raspberry, and strawberry extracts inhibit growth and stimulate apoptosis of human cancer cells in vitro. J. Agric. Food Chem. 2006, 54, 9329–9339. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yuan, T.; Li, L.; Nahar, P.; Slitt, A.; Seeram, N.P. Chemical compositional, biological, and safety studies of a novel maple syrup derived extract for nutraceutical applications. J. Agric. Food Chem. 2014, 62, 6687–6698. [Google Scholar] [CrossRef] [PubMed]
- Del Rio, D.; Borges, G.; Crozier, A. Berry flavonoids and phenolics: Bioavailability and evidence of protective effects. Br. J. Nutr. 2010, 104, S67–S90. [Google Scholar] [CrossRef] [PubMed]
- De Souza, V.R.; Pereira, P.A.P.; da Silva, T.L.T.; de Oliveira Lima, L.C.; Pio, R.; Queiroz, F. Determination of the bioactive compounds, antioxidant activity and chemical composition of Brazilian blackberry, red raspberry, strawberry, blueberry and sweet cherry fruits. Food Chem. 2014, 156, 362–368. [Google Scholar] [CrossRef] [PubMed]
- Carey, A.N.; Fisher, D.R.; Rimando, A.M.; Gomes, S.M.; Bielinski, D.F.; Shukitt-Hale, B. Stilbenes and anthocyanins reduce stress signaling in BV-2 mouse microglia. J. Agric. Food Chem. 2013, 61, 5979–5986. [Google Scholar] [CrossRef] [PubMed]
- Poulose, S.M.; Fisher, D.R.; Larson, J.; Bielinski, D.F.; Rimando, A.M.; Carey, A.N.; Schauss, A.G.; Shukitt-Hale, B. Anthocyanin-rich açai (Euterpe oleracea Mart.) fruit pulp fractions attenuate inflammatory stress signaling in mouse brain BV-2 microglial cells. J. Agric. Food Chem. 2012, 60, 1084–1093. [Google Scholar] [CrossRef] [PubMed]
- Lau, F.C.; Bielinski, D.F.; Joseph, J.A. Inhibitory effects of blueberry extract on the production of inflammatory mediators in lipopolysaccharide-activated BV2 microglia. J. Neurosci. Res. 2007, 85, 1010–1017. [Google Scholar] [CrossRef] [PubMed]
- Burguillos, M.A.; Deierborg, T.; Kavanagh, E.; Persson, A.; Hajji, N.; Garcia-Quintanilla, A.; Cano, J.; Brundin, P.; Englund, E.; Venero, J.L.; et al. Caspase signalling controls microglia activation and neurotoxicity. Nature 2011, 472, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Williams, P.; Sorribas, A.; Howes, M.-J.R. Natural products as a source of Alzheimer’s drug leads. Nat. Prod. Rep. 2011, 28, 48–77. [Google Scholar] [CrossRef] [PubMed]
- Essa, M.M.; Vijayan, R.K.; Castellano-Gonzalez, G.; Memon, M.A.; Braidy, N.; Guillemin, G.J. Neuroprotective effect of natural products against Alzheimer’s disease. Neurochem. Res 2012, 37, 1829–1842. [Google Scholar] [CrossRef] [PubMed]
- Divino da Rocha, M.; Pereira Dias Viegas, F.; Cristina Campos, H.; Carolina Nicastro, P.; Calve Fossaluzza, P.; Alberto Manssour Fraga, C.; Barreiro Eliezer, J.; Viegas, C. The role of natural products in the discovery of new drug candidates for the treatment of neurodegenerative disorders II: Alzheimer’s disease. CNS Neurol. Disord. Drug Targets 2011, 10, 251–270. [Google Scholar] [CrossRef]
- Kang, T.H.; Hur, J.Y.; Kim, H.B.; Ryu, J.H.; Kim, S.Y. Neuroprotective effects of the cyanidin-3-O-β-d-glucopyranoside isolated from mulberry fruit against cerebral ischemia. Neurosci. Lett. 2006, 391, 122–126. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, D.; McGhie, T.K.; Zhang, J.; Adaim, A.; Skinner, M. Effects of anthocyanins and other phenolics of boysenberry and blackcurrant as inhibitors of oxidative stress and damage to cellular DNA in SH-SY5Y and HL-60 cells. J. Sci. Food Agric. 2006, 86, 678–686. [Google Scholar] [CrossRef]
- Khan, M.S.; Ali, T.; Kim, M.W.; Jo, M.H.; Jo, M.G.; Badshah, H.; Kim, M.O. Anthocyanins protect against LPS-induced oxidative stress-mediated neuroinflammation and neurodegeneration in the adult mouse cortex. Neurochem. Int. 2016, 100, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.A.; Amin, F.U.; Khan, M.; Abid, M.N.; Rehman, S.U.; Kim, T.H.; Kim, M.W.; Kim, M.O. Anthocyanins abrogate glutamate-induced AMPK activation, oxidative stress, neuroinflammation, and neurodegeneration in postnatal rat brain. J. Neuroinflamm. 2016, 13, 286. [Google Scholar] [CrossRef] [PubMed]
- Meireles, M.; Marques, C.; Norberto, S.; Fernandes, I.; Mateus, N.; Rendeiro, C.; Spencer, J.P.; Faria, A.; Calhau, C. The impact of chronic blackberry intake on the neuroinflammatory status of rats fed a standard or high-fat diet. J. Nutr. Bioche. 2015, 26, 1166–1173. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.-Y.; Huang, I.-M.; Hwang, L.S.; Ho, C.-T.; Li, S.; Lo, C.-Y. Anthocyanins in blackcurrant effectively prevent the formation of advanced glycation end products by trapping methylglyoxal. J. Funct. Foods 2014, 8, 259–268. [Google Scholar] [CrossRef]
- Thilavech, T.; Ngamukote, S.; Belobrajdic, D.; Abeywardena, M.; Adisakwattana, S. Cyanidin-3-rutinoside attenuates methylglyoxal-induced protein glycation and DNA damage via carbonyl trapping ability and scavenging reactive oxygen species. BMC Complement. Altern. Med. 2016, 16, 138. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Yagiz, Y.; Buran, T.J.; Nunes, C.D.N.; Gu, L. Phytochemicals from berries and grapes inhibited the formation of advanced glycation end-products by scavenging reactive carbonyls. Food Res. Int. 2011, 44, 2666–2673. [Google Scholar] [CrossRef]
- Liu, H.; Liu, H.; Wang, W.; Khoo, C.; Taylor, J.; Gu, L. Cranberry phytochemicals inhibit glycation of human hemoglobin and serum albumin by scavenging reactive carbonyls. Food Funct. 2011, 2, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Bastianetto, S.; Yao, Z.X.; Papadopoulos, V.; Quirion, R. Neuroprotective effects of green and black teas and their catechin gallate esters against β-amyloid-induced toxicity. Eur. J. Neurosci. 2006, 23, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, N.; Wang, K.; Wegiel, J.; Malik, M.N. Walnut extract inhibits the fibrillization of amyloid beta-protein, and also defibrillizes its preformed fibrils. Curr. Alzheimer Res. 2004, 1, 183–188. [Google Scholar] [CrossRef] [PubMed]
- McGhie, T.K.; Walton, M.C. The bioavailability and absorption of anthocyanins: Towards a better understanding. Mol. Nutr. Food Res. 2007, 51, 702–713. [Google Scholar] [CrossRef] [PubMed]
- McDougall, G.J.; Dobson, P.; Smith, P.; Blake, A.; Stewart, D. Assessing potential bioavailability of raspberry anthocyanins using an in vitro digestion system. J. Agric. Food Chem. 2005, 53, 5896–5904. [Google Scholar] [CrossRef] [PubMed]
- Schroeter, H.; Spencer, J.P.; Rice-Evans, C.; Williams, R.J. Flavonoids protect neurons from oxidized low-density-lipoprotein-induced apoptosis involving c-Jun N-terminal kinase (JNK), c-Jun and caspase-3. Biochem. J. 2001, 358, 547–557. [Google Scholar] [CrossRef] [PubMed]
- Prior, R.L.; Wu, X. Anthocyanins: Structural characteristics that result in unique metabolic patterns and biological activities. Free Rad. Res. 2006, 40, 1014–1028. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Arseneault, M.; Sanderson, T.; Murthy, V.; Ramassamy, C. Challenges for research on polyphenols from foods in Alzheimer’s disease: Bioavailability, metabolism, and cellular and molecular mechanisms. J. Agric. Food Chem. 2008, 56, 4855–4873. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, A.; Schluesener, H. Natural polyphenols against neurodegenerative disorders: Potentials and pitfalls. Ageing Res. Rev. 2012, 11, 329–345. [Google Scholar] [CrossRef] [PubMed]
- Seeram, N.; Momin, R.; Nair, M.; Bourquin, L. Cyclooxygenase inhibitory and antioxidant cyanidin glycosides in cherries and berries. Phytomedicine 2001, 8, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Bae, M.; Park, Y.; Ma, H.; Yuan, T.; Seeram, N.; Lee, J. Blackcurrant anthocyanins stimulated cholesterol transport via post-transcriptional induction of LDL receptor in Caco-2 cells. Eur. J. Nutr. 2017. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Seeram, N.P.; Lee, R.; Feng, L.; Heber, D. Isolation and identification of strawberry phenolics with antioxidant and human cancer cell antiproliferative properties. J. Agric. Food Chem. 2008, 56, 670–675. [Google Scholar] [CrossRef] [PubMed]
- Jean-Gilles, D.; Li, L.; Ma, H.; Yuan, T.; Chichester, C.O.; Seeram, N.P. Anti-inflammatory effects of polyphenolic-enriched red raspberry extract in an antigen-induced arthritis rat model. J. Agric. Food Chem. 2012, 60, 5755–5762. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; DaSilva, N.A.; Liu, W.; Nahar, P.P.; Wei, Z.; Liu, Y.; Pham, P.T.; Crews, R.; Vattem, D.A.; Slitt, A.L. Effects of a standardized phenolic-enriched maple syrup extract on β-amyloid aggregation, neuroinflammation in microglial and neuronal cells, and β-amyloid induced neurotoxicity in Caenorhabditis elegans. Neurochem. Res. 2016, 41, 2836–2847. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Xu, J.; DaSilva, N.A.; Wang, L.; Wei, Z.; Guo, L.; Johnson, S.L.; Lu, W.; Xu, J.; Gu, Q. Cosmetic applications of glucitol-core containing gallotannins from a proprietary phenolic-enriched red maple (Acer rubrum) leaves extract: Inhibition of melanogenesis via down-regulation of tyrosinase and melanogenic gene expression in B16F10 melanoma cells. Arch. Dermatol. Res. 2017, 309, 265–274. [Google Scholar] [PubMed]
- DaSilva, N.A.; Nahar, P.P.; Ma, H.; Eid, A.; Wei, Z.; Meschwitz, S.; Zawia, N.H.; Slitt, A.L.; Seeram, N.P. Pomegranate ellagitannin-gut microbial-derived metabolites, urolithins, inhibit neuroinflammation in vitro. Nutr. Neurosci. 2017. [Google Scholar] [CrossRef] [PubMed]
| Berry Sample | Anthocyanins |
|---|---|
| Blackberry | cyanidin-3-O-glucoside, cyanidin-3-O-arabinoside, cyanidin-3-O-xyloside, cyanidin-3-O-malonylglucoside, and cyanidin-3-O-dioxalylglucoside |
| Black raspberry | cyanidin-3-O-sambuoside, cyanidin-3-O-glucoside, cyanidin-3-O-xylosylrutinoside, and cyanidin-3-O-rutinoside |
| Blueberry | cyanidin-3-O-galactoside, petunidin-3-O-galactoside, petunidin-3-O-glucoside, peonidin-3-O-galactoside, and malvidin-3-O-glucoside |
| Cranberry | cyanidin-3-O-galactoside, cyanidin-3-O-arabinoside, peonidin-3-O-galactoside, and peonidin-3-O-arabinoside |
| Red raspberry | cyanidin-3-O-glucoside, cyanidin-3-O-arabinoside, and delphinidin-3-O-arabinoside |
| Strawberry | cyanidin-3-O-glucoside, pelargonidin-3-O-glucoside, and pelargonidin-3-O-rutinoside |
| Common Name | Species | Family | Extract a | Phenolic Content b | Anthocyanins Content c |
|---|---|---|---|---|---|
| Blackberry | Rubus sp. | Rosaceae | CE | 6.8% | 2.8% |
| ACE | 11.3% | 6.3% | |||
| ACF | 0.3% | n.d.d | |||
| Black raspberry | Rubus occidentalis | Rosaceae | CE | 4.1% | 2.8% |
| ACE | 7.5% | 5.7% | |||
| ACF | 0.3% | n.d. | |||
| Blueberry | Vaccinium angustifolium | Ericaceae | CE | 9.8% | 2.5% |
| ACE | 8.5% | 4.4% | |||
| ACF | 1.5% | n.d. | |||
| Cranberry | Vaccinium macrocarpon | Ericaceae | CE | 7.7% | 1.2% |
| ACE | 6.4% | 3.8% | |||
| ACF | 1.3% | n.d. | |||
| Red raspberry | Rubus idaeus | Rosaceae | CE | 6.4% | 4.2% |
| ACE | 5.8% | 5.6% | |||
| ACF | 0.9% | n.d. | |||
| Strawberry | Fragaria ananassa | Rosaceae | CE | 3.8% | 2.2% |
| ACE | 7.3% | 3.7% | |||
| ACF | 0.8% | n.d. |
| Berry | Extracts | Free Radical Scavenging Capacity (IC50; µg/mL) | MGO Trapping Capacity (%) |
|---|---|---|---|
| Blackberry | CE | 1968.6 ± 22.3 | 13.7 |
| ACE | 133.8 ± 11.1 | 30.3 | |
| ACF | n.d. a | 3.3 | |
| Black raspberry | CE | 2865.9 ± 62.8 | 21.8 |
| ACE | 409.6 ± 23.7 | 31.1 | |
| ACF | n.d. | 8.4 | |
| Blueberry | CE | 381.1 ± 3.1 | 15.6 |
| ACE | 454.3 ± 4.6 | 29.2 | |
| ACF | 2598.5 ± 34.7 | n.d. | |
| Cranberry | CE | 392.6 ± 2.9 | 10.1 |
| ACE | 434.5 ± 7.1 | 32.8 | |
| ACF | 2217.3 ± 11.1 | 18.4 | |
| Red raspberry | CE | 268.5 ± 8.6 | 13.7 |
| ACE | 337.7 ± 1.6 | 18.2 | |
| ACF | 2010.3 ± 60.2 | 9.4 | |
| Strawberry | CE | n.d. | 14.5 |
| ACE | 469.8 ± 2.5 | 16.2 | |
| ACF | n.d. | n.d. | |
| BHT b | 727.8 ± 11.6 | n.t.d | |
| Ascorbic acid b | 12.3 ± 1.7 | n.t. | |
| AG c | n.t. | 73.7 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, H.; Johnson, S.L.; Liu, W.; DaSilva, N.A.; Meschwitz, S.; Dain, J.A.; Seeram, N.P. Evaluation of Polyphenol Anthocyanin-Enriched Extracts of Blackberry, Black Raspberry, Blueberry, Cranberry, Red Raspberry, and Strawberry for Free Radical Scavenging, Reactive Carbonyl Species Trapping, Anti-Glycation, Anti-β-Amyloid Aggregation, and Microglial Neuroprotective Effects. Int. J. Mol. Sci. 2018, 19, 461. https://doi.org/10.3390/ijms19020461
Ma H, Johnson SL, Liu W, DaSilva NA, Meschwitz S, Dain JA, Seeram NP. Evaluation of Polyphenol Anthocyanin-Enriched Extracts of Blackberry, Black Raspberry, Blueberry, Cranberry, Red Raspberry, and Strawberry for Free Radical Scavenging, Reactive Carbonyl Species Trapping, Anti-Glycation, Anti-β-Amyloid Aggregation, and Microglial Neuroprotective Effects. International Journal of Molecular Sciences. 2018; 19(2):461. https://doi.org/10.3390/ijms19020461
Chicago/Turabian StyleMa, Hang, Shelby L. Johnson, Weixi Liu, Nicholas A. DaSilva, Susan Meschwitz, Joel A. Dain, and Navindra P. Seeram. 2018. "Evaluation of Polyphenol Anthocyanin-Enriched Extracts of Blackberry, Black Raspberry, Blueberry, Cranberry, Red Raspberry, and Strawberry for Free Radical Scavenging, Reactive Carbonyl Species Trapping, Anti-Glycation, Anti-β-Amyloid Aggregation, and Microglial Neuroprotective Effects" International Journal of Molecular Sciences 19, no. 2: 461. https://doi.org/10.3390/ijms19020461
APA StyleMa, H., Johnson, S. L., Liu, W., DaSilva, N. A., Meschwitz, S., Dain, J. A., & Seeram, N. P. (2018). Evaluation of Polyphenol Anthocyanin-Enriched Extracts of Blackberry, Black Raspberry, Blueberry, Cranberry, Red Raspberry, and Strawberry for Free Radical Scavenging, Reactive Carbonyl Species Trapping, Anti-Glycation, Anti-β-Amyloid Aggregation, and Microglial Neuroprotective Effects. International Journal of Molecular Sciences, 19(2), 461. https://doi.org/10.3390/ijms19020461







