Luteolin Inhibits Tumorigenesis and Induces Apoptosis of Non-Small Cell Lung Cancer Cells via Regulation of MicroRNA-34a-5p
Abstract
:1. Introduction
2. Results
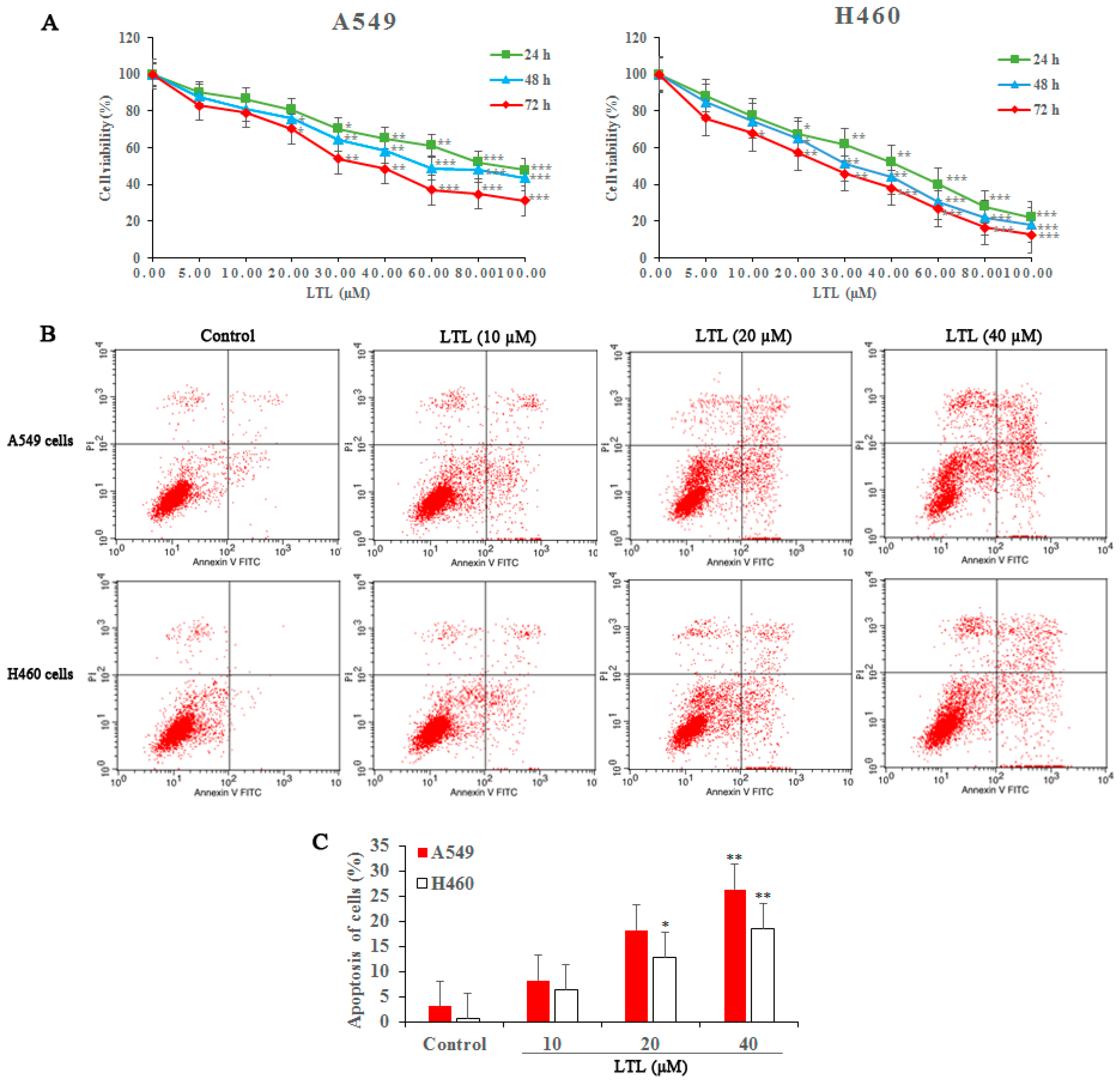
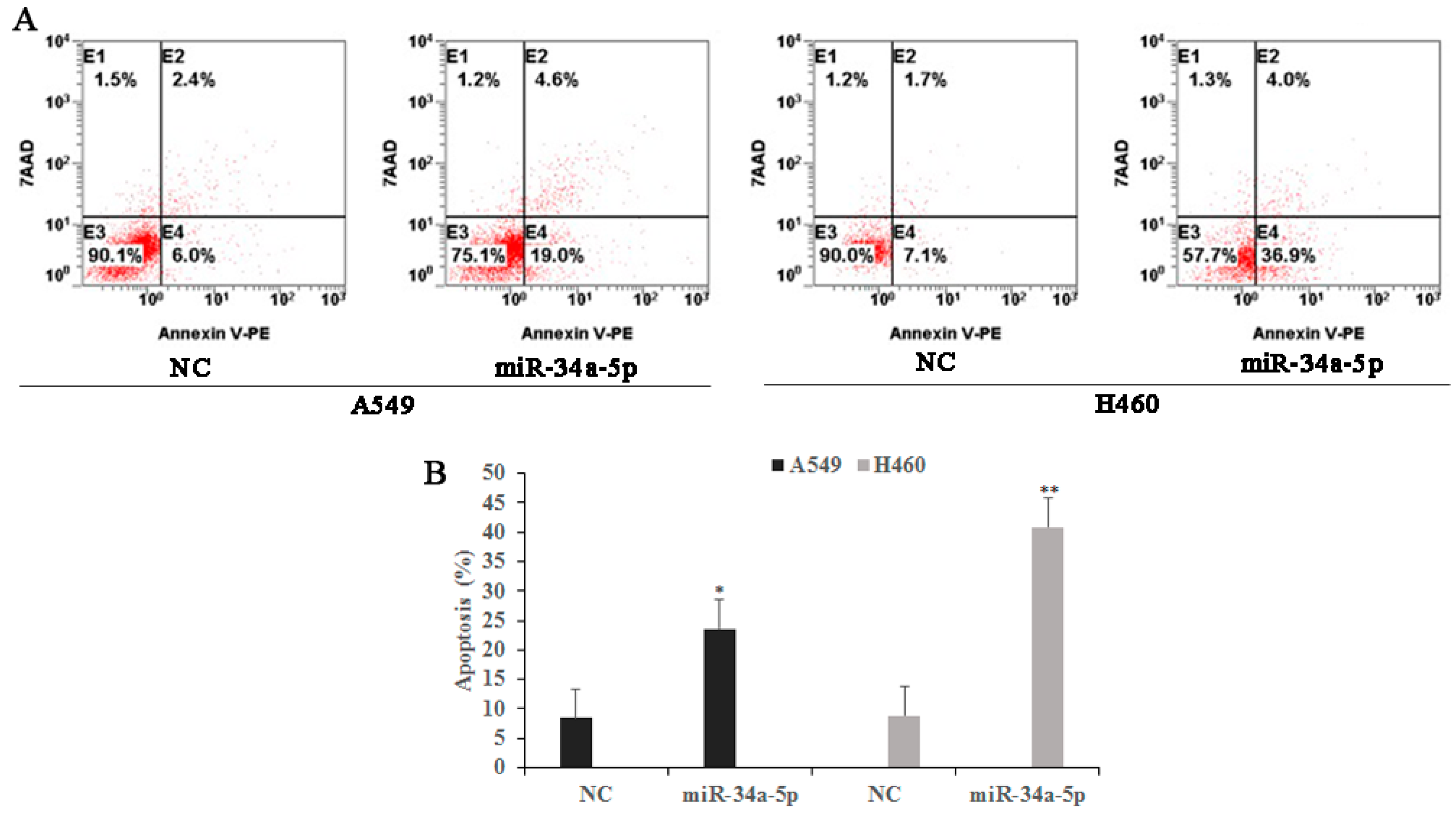
2.1. LTL (Luteolin) Inhibits the Proliferation of and Induces Apoptosis in A549 and H460 Cancer Cells In Vitro
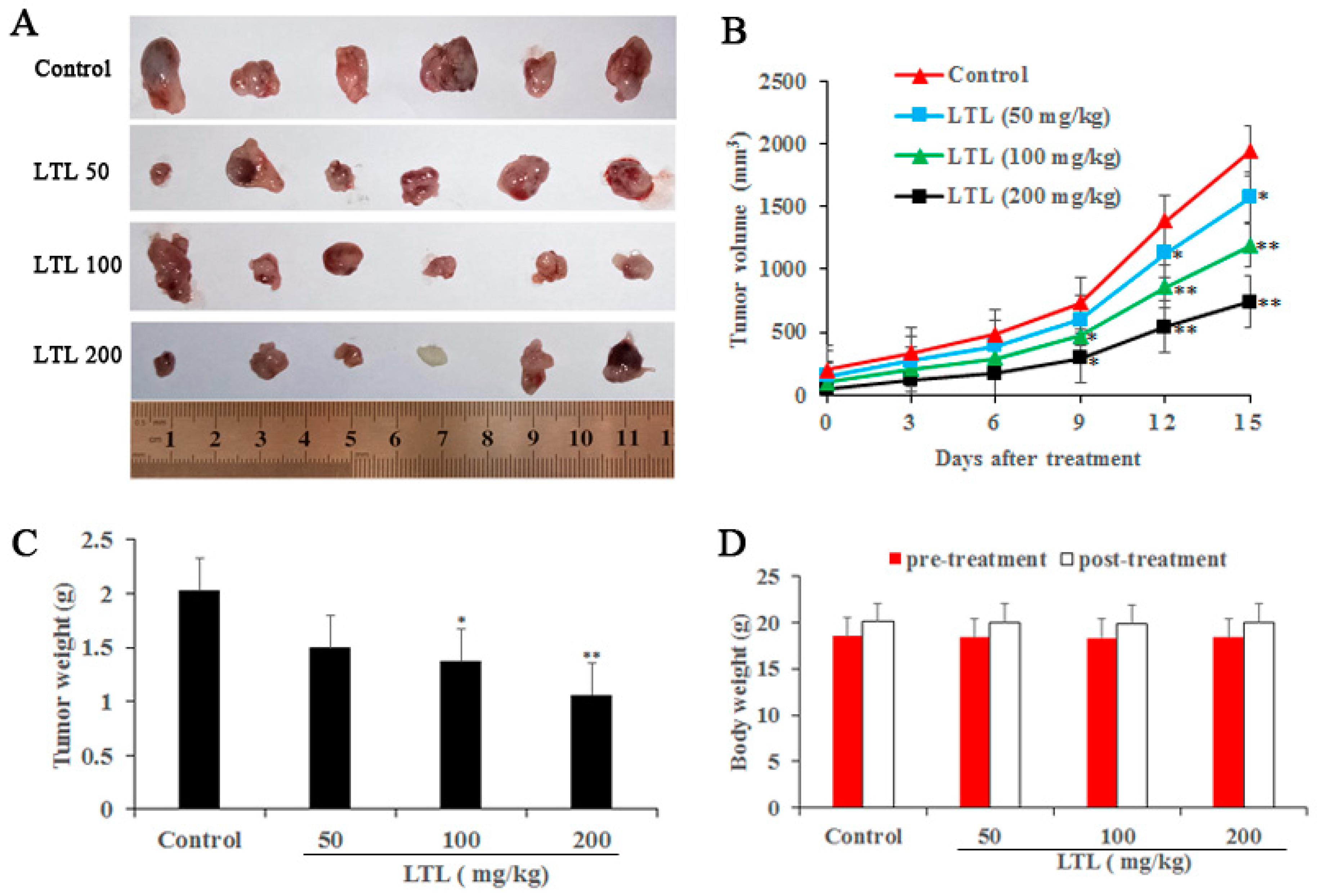
2.2. LTL Inhibits Tumor Growth in the H460 Xenografts Mice Model
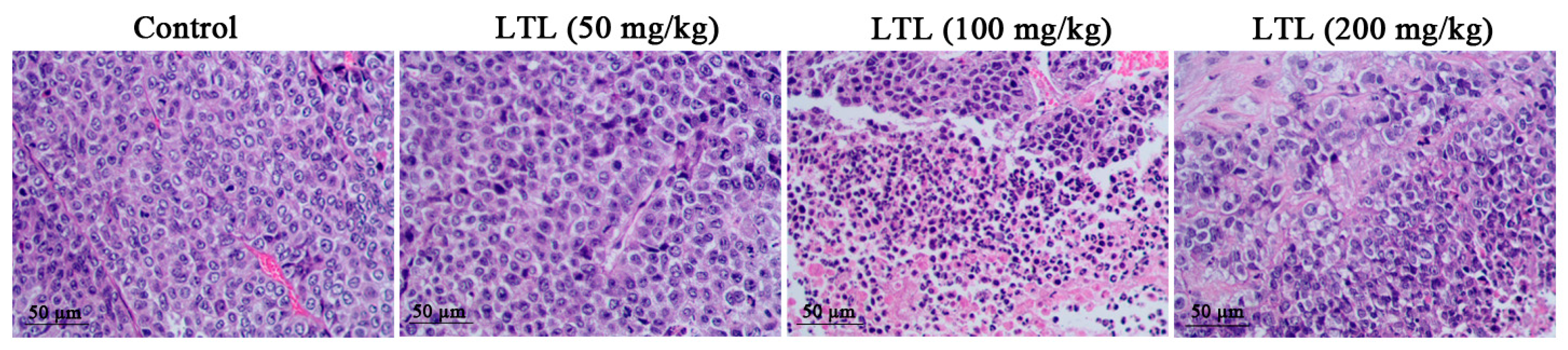
2.3. Effect of LTL on Lung Histology
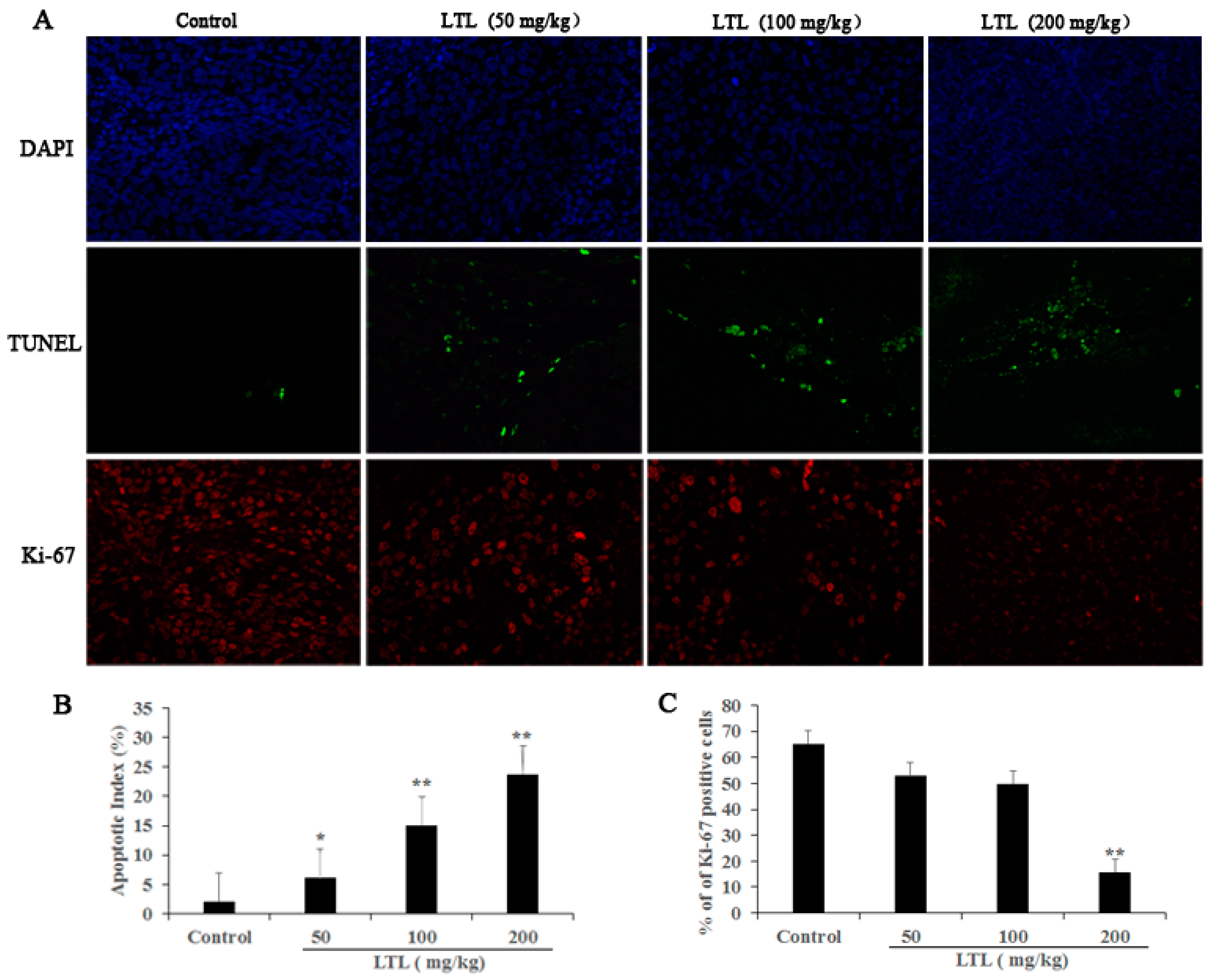
2.4. LTL Treatment Promotes Apoptotic Cell Death and Inhibits Cell Proliferation
2.5. Expression of MiRNAs Changes in Response to LTL in H460 Tumor Xenografts
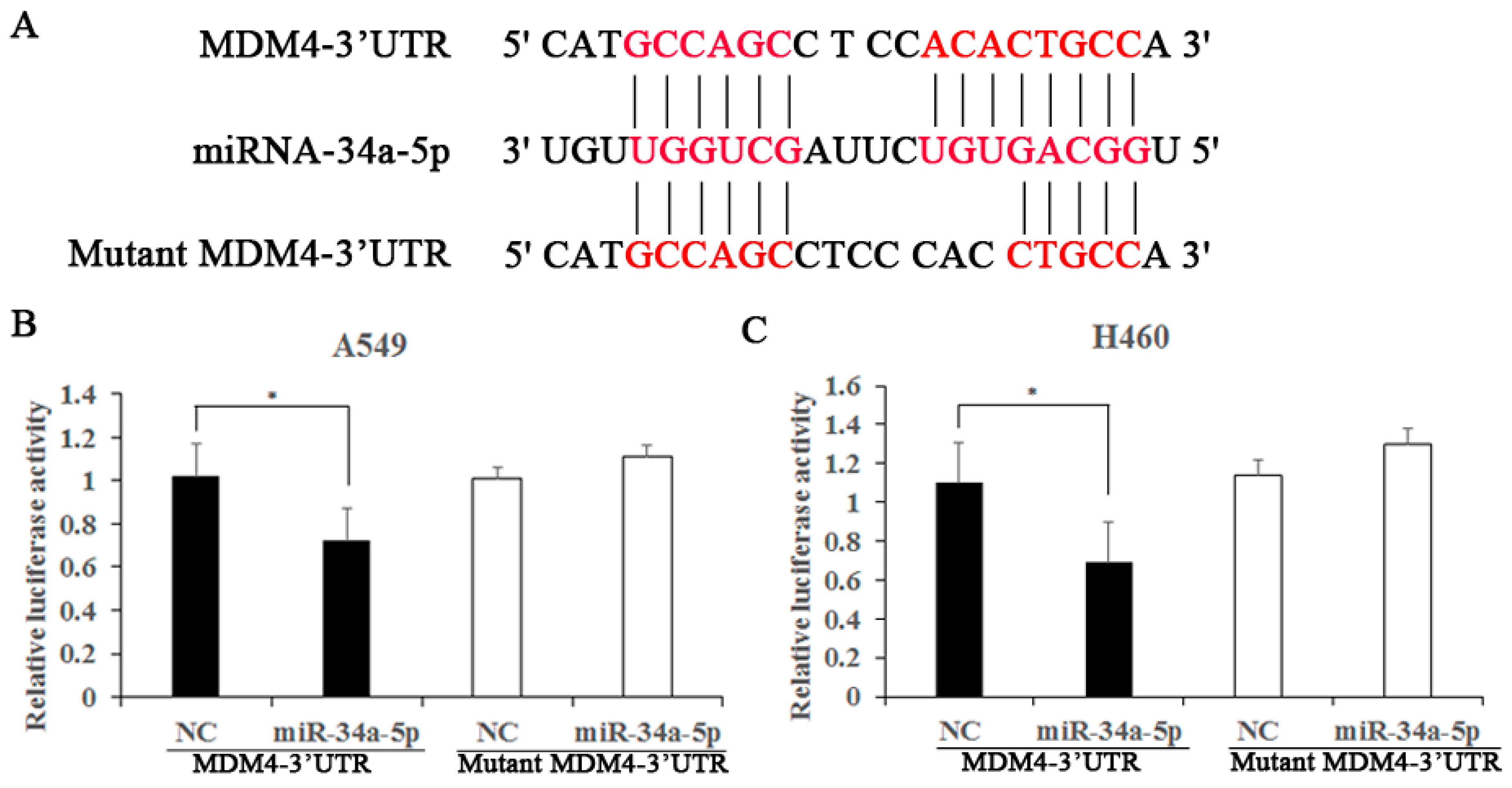
2.6. MDM4 Is a Direct Downstream Target of miR-34a-5p
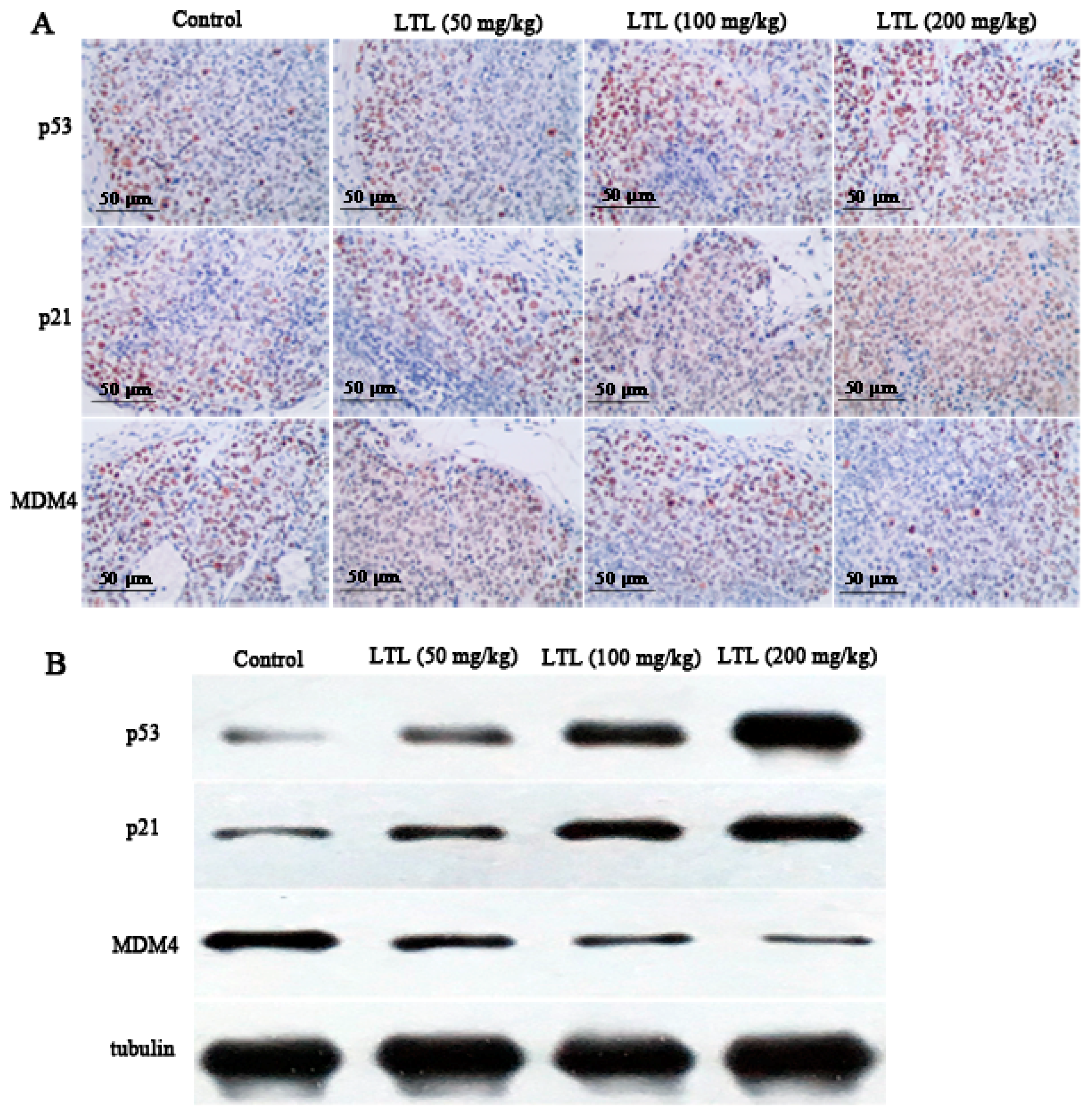
2.7. LTL Increases the Expression of P53 and P21 and Decreases the Expression of MDM4 In Vivo
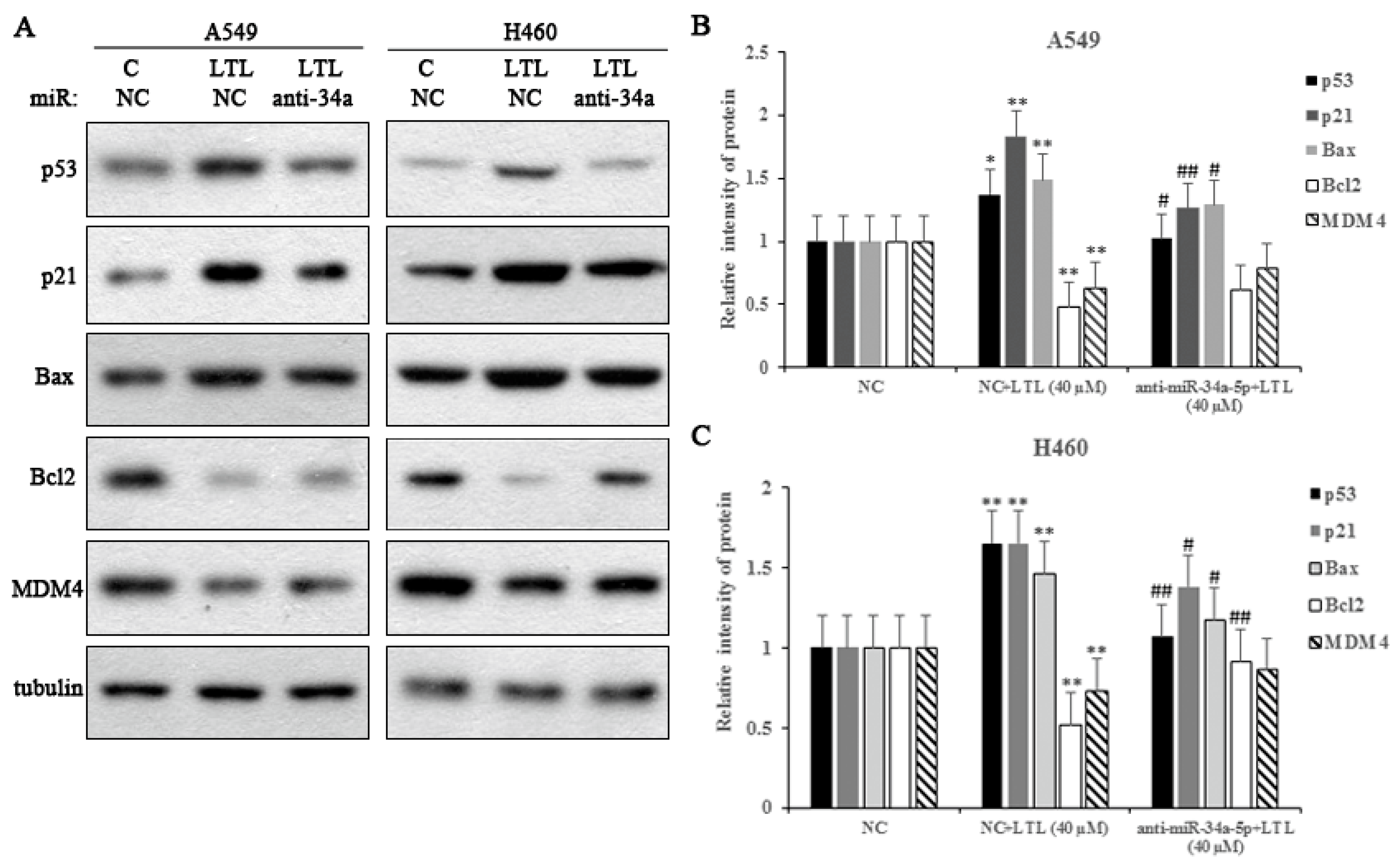
2.8. miR-34a-5p Participates in LTL-Induced Apoptotic Effects by Regulating Its Downstream Target Protein Expressions In Vitro
2.9. miR-34a-5p Participates in LTL-Induced Apoptotic Effects through Regulation of Caspase-3 and Caspase-9 Activities
3. Discussion
4. Materials and Methods
4.1. Reagents and Antibodies
4.2. Cell Culture
4.3. Cell Viability Assay
4.4. Apoptosis Assay
4.5. Tumor Xenograft Model in Mice
4.6. Histological Evaluation, Immunohistochemistry, and Western Blot Analysis
4.7. In Situ Terminal Deoxynucleotidyl Transferase dUTP Nick End Labeling (TUNEL) Assay and Evaluation of Ki-67 Proliferation
4.8. Microarray Analysis
4.9. Quantitative Real-Time PCR
4.10. Luciferase Reporter Gene Assay
4.11. Microrna Inhibition, Transfection, and Western Blot Analysis In Vitro
4.12. Caspase-3 and -9 Assay
4.13. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| LTL | Luteolin |
| NSCLC | Non-small cell lung cancer |
| qPCR | Quantitative PCR |
| JNK | c-Jun N-terminal kinase |
| NF-κB | Nuclear transcription factor κB |
| MEK/ERK | Mitogen-activated protein kinase/extracellular regulated protein kinase |
| CCL2 | Chemokine (CeC motif) ligand 2 |
| OC | Ovarian cancer |
| TUNEL | Terminal deoxynucleotidyl transferase dUTP nick end labeling |
| DMSO | Dimethyl sulfoxide |
| RPMI | Roswell Park Memorial Institute |
| FBS | Fetal bovine serum |
| DAB | Diaminobenzidine |
| CMC | Sodium carboxymethyl cellulose |
| CCK-8 | Cell counter kit-8 |
| ATCC | American type culture collection |
| IHC | Immunohistochemistry |
| NC | Negative control |
| PARP | Poly (ADP) ribose polymerase |
References
- Torre, L.A.; Sauer, A.M.G.; Chen, M.S.; Kagawa-Singer, M.; Jemal, A.; Siegel, R.L. Cancer statistics for Asian Americans, native hawaiians, and pacific islanders: Converging incidence in males and females. CA Cancer J. Clin. 2016, 66, 182–202. [Google Scholar] [CrossRef] [PubMed]
- Molina, J.R.; Yang, P.; Cassivi, S.D.; Schild, S.E.; Adjei, A.A. Non-small cell lung cancer: Epidemiology, risk factors, treatment, and survivorship. Mayo Clin. Proc. 2008, 83, 584–594. [Google Scholar] [CrossRef]
- Hussain, S. Nanomedicine for treatment of lung cancer. Adv. Exp. Med. Biol. 2016, 890, 137–147. [Google Scholar] [PubMed]
- Yang, J.; Lin, J.; Liu, T.; Chen, T.; Pan, S.; Huang, W.; Li, S. Analysis of lncRNA expression profiles in non-small cell lung cancers (NSCLC) and their clinical subtypes. Lung Cancer 2014, 85, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Fillmore, C.M.; Hammerman, P.S.; Kim, C.F.; Wong, K.K. Non-small-cell lung cancers: A heterogeneous set of diseases. Nat. Rev. Cancer 2014, 14, 535–546. [Google Scholar] [CrossRef] [PubMed]
- Araz, O.; Ucar, E.Y.; Meral, M.; Yalcin, A.; Acemoglu, H.; Dogan, H.; Karaman, A.; Aydin, Y.; Gorguner, M.; Akgun, M. Frequency of Class I and II HLA alleles in patients with lung cancer according to chemotherapy response and 5-year survival. Clin. Respir. J. 2015, 9, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Latimer, K.M.; Mott, T.F. Lung cancer: Diagnosis, treatment principles, and screening. Am. Fam. Physician 2015, 91, 250–256. [Google Scholar] [PubMed]
- Laskin, J.J.; Sandler, A.B. State of the art in therapy for non-small cell lung cancer. Cancer Investig. 2005, 23, 427–442. [Google Scholar] [CrossRef]
- Lin, Y.; Shi, R.; Wang, X.; Shen, H.M. Luteolin, a flavonoid with potentials for cancer prevention and therapy. Curr. Cancer Drug Targets 2008, 8, 634–646. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.P.; Cai, X.T.; Hu, T.T.; Lu, W.G.; Cao, P. Mechanism of growth inhibition effect of 3′,4′,5,7-tetrahydroxyflavone on A549 cells. Chin. J. Chin. Mater. Med. 2012, 37, 1259–1264. [Google Scholar]
- Meng, G.; Chai, K.; Li, X.; Zhu, Y.; Huang, W. Luteolin exerts pro-apoptotic effect and anti-migration effects on A549 lung adenocarcinoma cells through the activation of MEK/ERK signaling pathway. Chem. Biol. Interact. 2016, 257, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.J.; Choi, H.J.; Chung, T.W.; Ha, K.T. Luteolin inhibits recruitment of monocytes and migration of Lewis lung carcinoma cells by suppressing chemokine (C-C motif) ligand 2 expression in tumor-associated macrophage. Biochem. Biophys. Res. Commun. 2016, 470, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Ji, W.; Sun, B.; Su, C. Targeting microRNAs in cancer gene therapy. Genes (Basel) 2017, 8, E21. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Ding, Z.L.; Zheng, Y.L.; Wang, W. MiR-484 promotes non-small-cell lung cancer (NSCLC) progression through inhibiting Apaf-1 associated with the suppression of apoptosis. Biomed. Pharmacother. 2017, 96, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Xu, M.; Li, P. miRNA-221 acts as an oncogenic role by directly targeting TIMP2 in non-small-cell lung carcinoma. Gene 2017, 620, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Tang, Z.; Wang, H.; Wu, W.; Zhou, F.; Ke, H.; Lu, W.; Zhang, S.; Zhang, Y.; Yang, S.; et al. CXCL6 promotes non-small cell lung cancer cell survival and metastasis via down-regulation of miR-515-5p. Biomed. Pharmacother. 2017, 97, 1182–1188. [Google Scholar] [CrossRef] [PubMed]
- He, L.; He, X.; Lim, L.P.; de Stanchina, E.; Xuan, Z.; Liang, Y.; Xue, W.; Zender, L.; Magnus, J.; Ridzon, D.; et al. A microRNA component of the p53 tumour suppressor network. Nature 2007, 447, 1130–1134. [Google Scholar] [CrossRef] [PubMed]
- Tarasov, V.; Jung, P.; Verdoodt, B.; Lodygin, D.; Epanchintsev, A.; Menssen, A.; Meister, G.; Hermeking, H. Differential regulation of microRNAs by p53 revealed by massively parallel sequencing: MiR-34a is a p53 target that induces apoptosis and G1-arrest. Cell Cycle 2007, 6, 1586–1593. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.C.; Wentzel, E.A.; Kent, O.A.; Ramachandran, K.; Mullendore, M.; Lee, K.H.; Feldmann, G.; Yamakuchi, M.; Ferlito, M.; Lowenstein, C.J.; et al. Transactivation of miR-34a by p53 broadly influences gene expression and promotes apoptosis. Mol. Cell 2007, 26, 745–752. [Google Scholar] [CrossRef] [PubMed]
- Raver-Shapira, N.; Marciano, E.; Meiri, E.; Spector, Y.; Rosenfeld, N.; Moskovits, N.; Bentwich, Z.; Oren, M. Transcriptional activation of miR-34a contributes to p53-mediated apoptosis. Mol. Cell 2007, 26, 731–743. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Lin, B.W.; Chen, X.L.; Zhang, B.L.; Xiao, X.J.; Shi, J.S.; Lin, J.D.; Chen, X. PAI-1/PIAS3/Stat3/miR-34a forms a positive feedback loop to promote EMT-mediated metastasis through Stat3 signaling in Non-small cell lung cancer. Biochem. Biophys. Res. Commun. 2017, 493, 1464–1470. [Google Scholar] [CrossRef] [PubMed]
- Han, K.; Wei, M.; Zhang, J.J.; Zhou, Y.; Wang, Y.L.; Su, Y.; Lin, S.C.; Gan, Z.H.; Sun, Y.N.; Min, D.L. Luteolin inhibited proliferation and induced apoptosis of prostate cancer cells through miR-301. Onco Targets Ther. 2016, 9, 3085–3094. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, J. Luteolin induced apoptosis in breast cancer cell by decreasing the expression of microRNA-21. Chongqing Med. 2013, 42, 1374–1376. [Google Scholar]
- Wu, H.; Huang, M.; Liu, Y.; Shu, Y.; Liu, P. Luteolin induces apoptosis by up-regulating miR-34a in human gastric cancer cells. Technol. Cancer Res. Treat. 2015, 14, 747–755. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Zhou, S.; Liu, J.; Zhu, J.; Xue, J.; Gu, L.; Chen, Y. MiR-34a inhibits the in vitro cell proliferation and migration in human esophageal cancer. Pathol. Res. Pract. 2016, 212, 444–449. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.X.; Zhang, Q.J.; Pei, S.G.; Yang, B.L. Effect and mechanism of miR-34a on proliferation, apoptosis and invasion of laryngeal carcinoma cells. Asian Pac. J. Trop. Med. 2016, 9, 494–498. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Li, N.; Dong, Y.; Li, S.; Xu, L.; Li, X.; Li, Y.; Li, Z.; Ng, S.S.; Sung, J.J.; et al. miR-34a-5p suppresses colorectal cancer metastasis and predicts recurrence in patients with stage II/III colorectal cancer. Oncogene 2015, 34, 4142–4152. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Hao, L.; Li, S.; Lin, S.; Lv, L.; Chen, Y.; Cui, H.; Zi, T.; Chu, X.; Na, L.; et al. Elevated circulating stearic acid leads to a major lipotoxic effect on mouse pancreatic beta cells in hyperlipidaemia via a miR-34a-5p-mediated PERK/p53-dependent pathway. Diabetologia 2016, 59, 1247–1257. [Google Scholar] [CrossRef] [PubMed]
- Ding, N.; Wu, H.; Tao, T.; Peng, E. NEAT1 regulates cell proliferation and apoptosis of ovarian cancer by miR-34a-5p/BCL2. Onco Targets Ther. 2017, 10, 4905–4915. [Google Scholar] [CrossRef] [PubMed]
- Vousden, K.H.; Prives, C. Blinded by the light: The growing complexity of p53. Cell 2009, 137, 413–431. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.F.; Lee, T.S.; Lin, S.Y.; Hsu, S.P.; Juan, S.H.; Hsu, Y.H.; Zhong, W.B.; Lee, W.S. Involvement of Ras/Raf-1/ERK actions in the magnolol-induced upregulation of p21 and cell-cycle arrest in colon cancer cells. Mol. Carcinog. 2007, 46, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yan, C. A small-molecule p53 activator induces apoptosis through inhibiting MDMX expression in breast cancer cells. Neoplasia 2011, 13, 611–619. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Mazumdar, M.; Mukherjee, S.; Bhattacharjee, P.; Adhikary, A.; Manna, A.; Chakraborty, S.; Khan, P.; Sen, A.; Das, T. Restoration of p53/miR-34a regulatory axis decreases survival advantage and ensures Bax-dependent apoptosis of non-small cell lung carcinoma cells. FEBS Lett. 2014, 588, 549–559. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Fan, H.; Lv, G.; Zhou, Q.; Yang, B.; Zheng, J.; Cao, W. 5-Aminolevulinic acid-mediated sonodynamic therapy induces anti-tumor effects in malignant melanoma via p53-miR-34a-Sirt1 axis. J. Dermatol. Sci. 2015, 79, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Mandke, P.; Wyatt, N.; Fraser, J.; Bates, B.; Berberich, S.J.; Markey, M.P. MicroRNA-34a modulates MDM4 expression via a target site in the open reading frame. PLoS ONE 2012, 7, e42034. [Google Scholar] [CrossRef] [PubMed]
- Ou, X.; Lu, Y.; Liao, L.; Li, D.; Liu, L.; Liu, H.; Xu, H. Nitidine chloride induces apoptosis in human hepatocellular carcinoma cells through a pathway involving p53, p21, Bax and Bcl-2. Oncol. Rep. 2015, 33, 1264–1274. [Google Scholar] [CrossRef] [PubMed]
- Brentnall, M.; Rodriguez-Menocal, L.; de Guevara, R.L.; Cepero, E.; Boise, L.H. Caspase-9, caspase-3 and caspase-7 have distinct roles during intrinsic apoptosis. BMC Cell Biol. 2013, 14, 32. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Han, M.B.; Gao, Y.; Wang, H.; Dai, L.; Wen, Y.; Na, L.X. Curcumin triggers apoptosis via upregulation of Bax/Bcl-2 ratio and caspase activation in SW872 human adipocytes. Mol. Med. Rep. 2015, 12, 1151–1156. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Ye, T.; Liu, C.; Lu, W.; Lu, M.; Zhang, J.; Wang, M.; Cao, P. Luteolin induced G2 phase cell cycle arrest and apoptosis on non-small cell lung cancer cells. Toxicol. In Vitro 2011, 25, 1385–1391. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.Q.; Ma, Y.X.; Li, M.H.; Zhan, X.Q.; Zhang, X.; Wang, M.Y. 5-Hydroxymethylfurfural protects against ER stress-induced apoptosis in GalN/TNF-α-injured L02 hepatocytes through regulating the PERK-eIF2α signaling pathway. Chin. J. Nat. Med. 2015, 13, 896–905. [Google Scholar] [CrossRef]


| Name of miRNA | Fold Change | p Value |
|---|---|---|
| miR-100-5p | ↑2.92 | 0.0135 |
| miR-668-3p | ↑3.98 | 0.0119 |
| let-7i-5p | ↑5.169 | 0.0199 |
| let-7b-5p | ↑4.80 | 0.0146 |
| let-7a-5p | ↑3.977 | 0.0086 |
| let-7f-5p | ↑5.443 | 0.0203 |
| miR-451a | ↑5.635 | 0.0336 |
| miR-34a-5p | ↑2.187 | 0.004 |
| miR-222-3p | ↑3.995 | 0.0054 |
| miR-181a-5p | ↑5.376 | 0.015 |
| miR-92a-3p | ↑6.915 | 0.0177 |
| miR-93-5p | ↑4.142 | 0.0016 |
| miR-20a-5p | ↑5.275 | 0.0019 |
| miR-17-5p | ↑5.544 | 0.001 |
| miR-106a-5p | ↑4.60 | 0.0148 |
| miR-19b-3p | ↑5.216 | 0.0085 |
| miR-1983 | ↑4.929 | 0.0039 |
| miR-208a-5p | ↑4.872 | 0.0291 |
| miR-7026-5p | ↑6.357 | 0.0052 |
| miR-6241 | ↑4.03 | 0.007 |
| miR-3470a | ↓6.97 | 0.0367 |
| miR-3470b | ↓5.459 | 0.0089 |
| miR-3472 | ↓4.82 | 0.0061 |
| miR-3105-5p | ↓3.75 | 0.0021 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, Z.-Q.; Li, M.-H.; Qin, Y.-M.; Jiang, H.-Y.; Zhang, X.; Wu, M.-H. Luteolin Inhibits Tumorigenesis and Induces Apoptosis of Non-Small Cell Lung Cancer Cells via Regulation of MicroRNA-34a-5p. Int. J. Mol. Sci. 2018, 19, 447. https://doi.org/10.3390/ijms19020447
Jiang Z-Q, Li M-H, Qin Y-M, Jiang H-Y, Zhang X, Wu M-H. Luteolin Inhibits Tumorigenesis and Induces Apoptosis of Non-Small Cell Lung Cancer Cells via Regulation of MicroRNA-34a-5p. International Journal of Molecular Sciences. 2018; 19(2):447. https://doi.org/10.3390/ijms19020447
Chicago/Turabian StyleJiang, Ze-Qun, Mu-Han Li, Yue-Mu Qin, Hai-Ying Jiang, Xu Zhang, and Mian-Hua Wu. 2018. "Luteolin Inhibits Tumorigenesis and Induces Apoptosis of Non-Small Cell Lung Cancer Cells via Regulation of MicroRNA-34a-5p" International Journal of Molecular Sciences 19, no. 2: 447. https://doi.org/10.3390/ijms19020447
APA StyleJiang, Z.-Q., Li, M.-H., Qin, Y.-M., Jiang, H.-Y., Zhang, X., & Wu, M.-H. (2018). Luteolin Inhibits Tumorigenesis and Induces Apoptosis of Non-Small Cell Lung Cancer Cells via Regulation of MicroRNA-34a-5p. International Journal of Molecular Sciences, 19(2), 447. https://doi.org/10.3390/ijms19020447





