Roco Proteins and the Parkinson’s Disease-Associated LRRK2
Abstract
1. Introduction
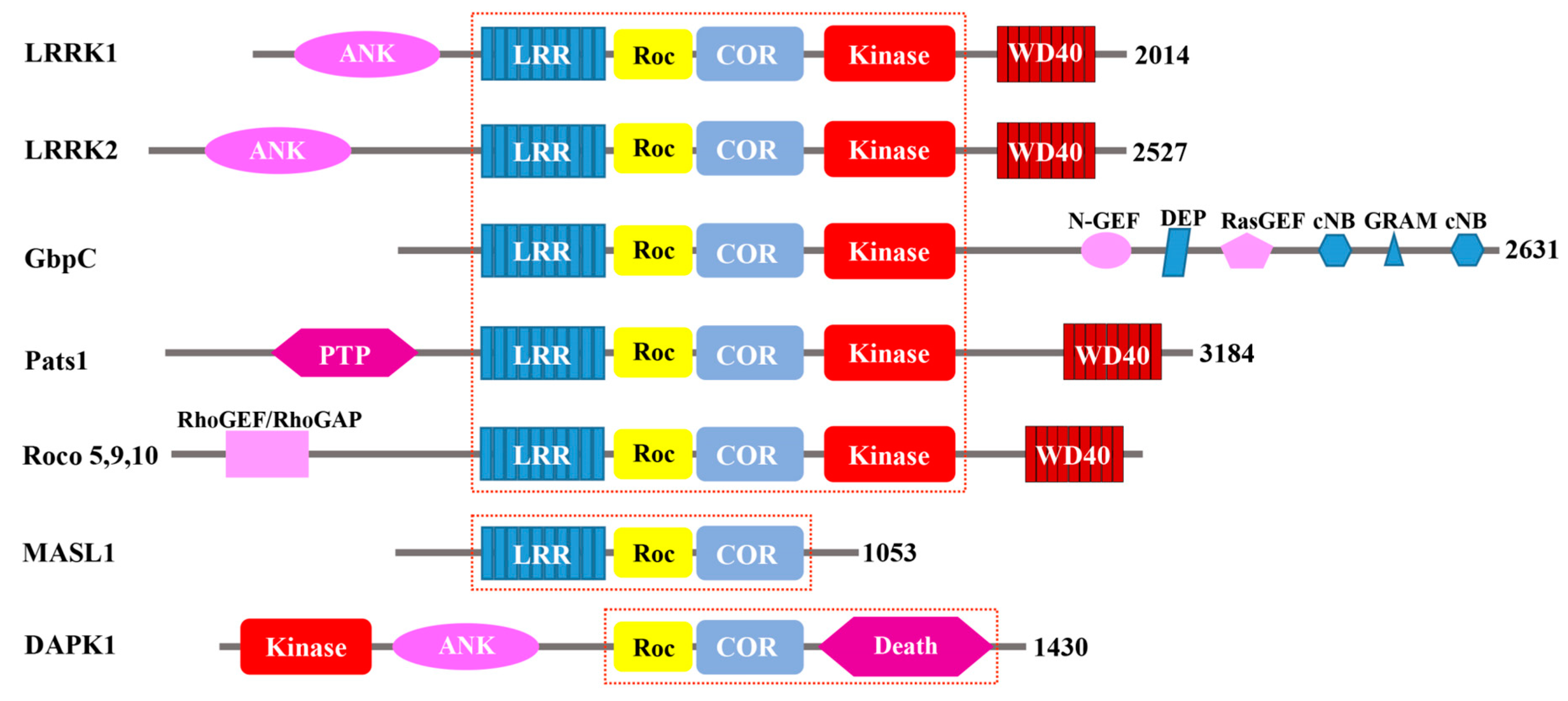
2. Biological Functions of Roco Proteins
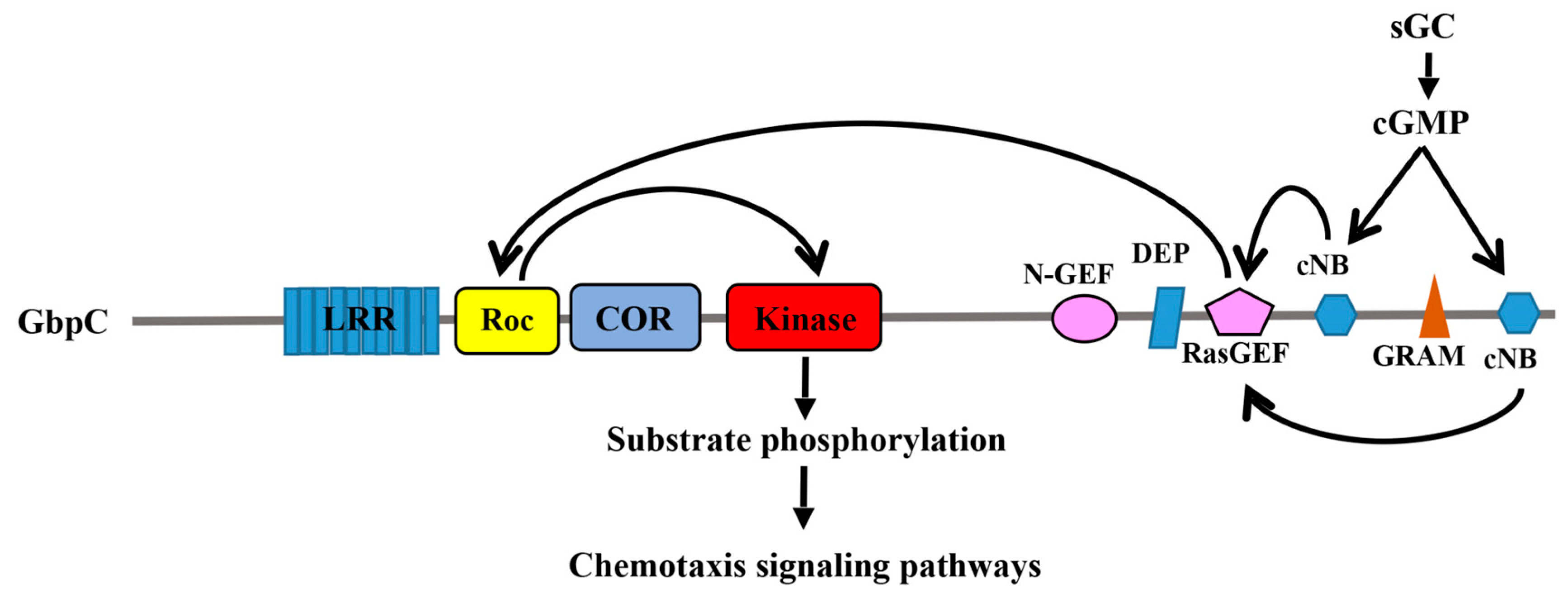
2.1. GbpC and Pats1 in D. Discoideum
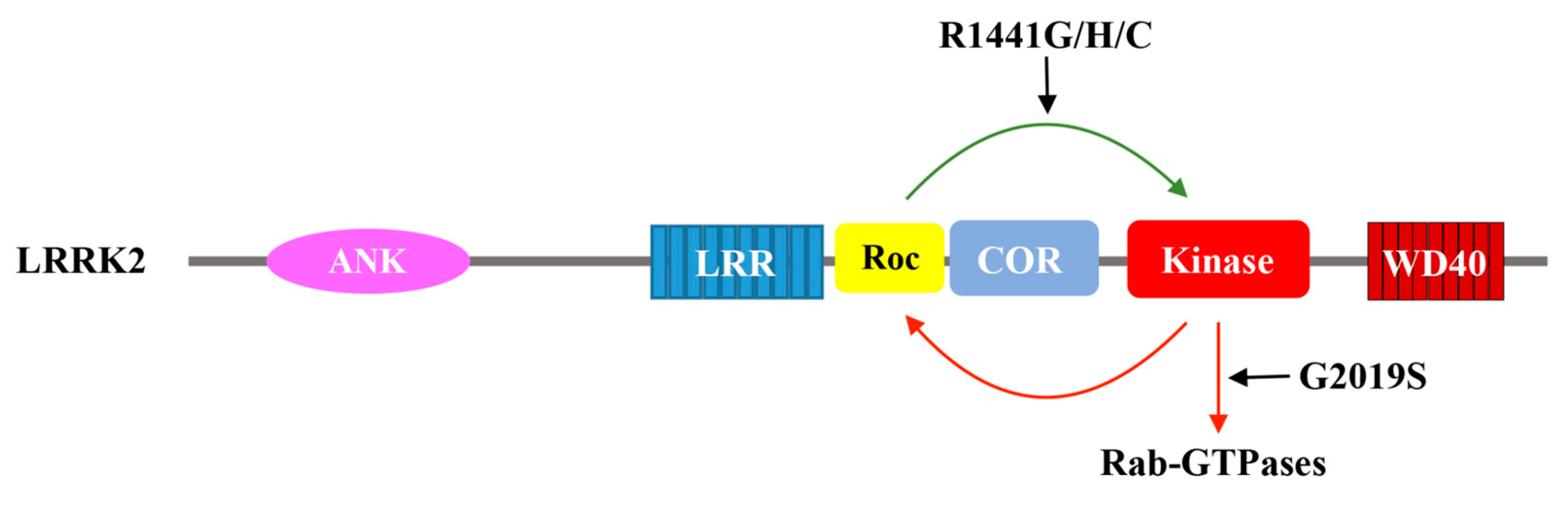
2.2. Human Roco Proteins: DAPK1, LRRK1/2, MASL1
3. Biochemical Activity of Roc and Its Regulatory Mechanisms
3.1. Roco Proteins are Functional GTPases
3.2. Regulatory Mechanisms of Roc Activity
4. The Mechanism of Roc Transmitting Its Signal Downstream
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| GEFs | Guanine nucleotide exchange factors |
| GAPs | GTPase-activating proteins |
| Roc | Ras of complex proteins |
| GbpC | cGMP binding protein C |
| LRR | Leucine-rich repeat |
| COR | C-terminal of Roc |
| LRRK1/2 | Leucine-rich repeat kinase 1/2 |
| MASL1 | Malignant fibrous histiocytoma amplified sequence 1 (MFHAS1) |
| DAPK1 | Death-associated protein kinase 1 |
| GRAM | Glucosyltransferases Rab-like GTPase activators and Myotubularins |
| PD | Parkinson’s disease |
| AD | Alzheimer’s disease |
| OSMD | Osteosclerotic metaphyseal dysplasia |
| CMA | Chaperone-mediated autophagy |
| GADs | G-proteins activated by nucleotide-dependent dimerization |
| CK1 | Casein kinase 1 alpha |
| PP1 | Protein phosphatase 1 |
| PP2A | Protein phosphatase 2A |
References
- Bosgraaf, L.; Van Haastert, P.J. Roc, a Ras/GTPase domain in complex proteins. Biochim. Biophys. Acta 2003, 1643, 5–10. [Google Scholar] [CrossRef]
- Goldberg, J.M.; Bosgraaf, L.; Van Haastert, P.J.; Smith, J.L. Identification of four candidate cGMP targets in Dictyostelium. Proc. Natl. Acad. Sci. USA 2002, 99, 6749–6754. [Google Scholar] [CrossRef]
- Kobe, B.; Kajava, A.V. The leucine-rich repeat as a protein recognition motif. Curr. Opin. Struct. Biol. 2001, 11, 725–732. [Google Scholar] [CrossRef]
- Cohen, O.; Feinstein, E.; Kimchi, A. DAP-kinase is a Ca2+/calmodulin-dependent, cytoskeletal-associated protein kinase, with cell death-inducing functions that depend on its catalytic activity. EMBO J. 1997, 16, 998–1008. [Google Scholar] [CrossRef]
- Itoh, N.; Nagata, S. A novel protein domain required for apoptosis. Mutational analysis of human Fas antigen. J. Biol. Chem. 1993, 268, 10932–10937. [Google Scholar] [PubMed]
- Aravind, L.; Dixit, V.M.; Koonin, E.V. Apoptotic molecular machinery: Vastly increased complexity in vertebrates revealed by genome comparisons. Science 2001, 291, 1279–1284. [Google Scholar] [CrossRef]
- Deiss, L.P.; Feinstein, E.; Berissi, H.; Cohen, O.; Kimchi, A. Identification of a novel serine/threonine kinase and a novel 15-kD protein as potential mediators of the gamma interferon-induced cell death. Genes Dev. 1995, 9, 15–30. [Google Scholar] [CrossRef] [PubMed]
- Marin, I.; van Egmond, W.N.; van Haastert, P.J. The Roco protein family: A functional perspective. FASEB J. 2008, 22, 3103–3110. [Google Scholar] [CrossRef] [PubMed]
- Paisan-Ruiz, C.; Jain, S.; Evans, E.W.; Gilks, W.P.; Simon, J.; van der Brug, M.; Lopez de Munain, A.; Aparicio, S.; Gil, A.M.; Khan, N.; et al. Cloning of the gene containing mutations that cause PARK8-linked Parkinson’s disease. Neuron 2004, 44, 595–600. [Google Scholar] [CrossRef]
- Zimprich, A.; Biskup, S.; Leitner, P.; Lichtner, P.; Farrer, M.; Lincoln, S.; Kachergus, J.; Hulihan, M.; Uitti, R.J.; Calne, D.B.; et al. Mutations in LRRK2 cause autosomal-dominant parkinsonism with pleomorphic pathology. Neuron 2004, 44, 601–607. [Google Scholar] [CrossRef]
- Greggio, E.; Jain, S.; Kingsbury, A.; Bandopadhyay, R.; Lewis, P.; Kaganovich, A.; van der Brug, M.P.; Beilina, A.; Blackinton, J.; Thomas, K.J.; et al. Kinase activity is required for the toxic effects of mutant LRRK2/dardarin. Neurobiol. Dis. 2006, 23, 329–341. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.; Wu, C.X.; Burlak, C.; Zhang, S.; Sahm, H.; Wang, M.; Zhang, Z.Y.; Vogel, K.W.; Federici, M.; Riddle, S.M.; et al. Parkinson disease-associated mutation R1441H in LRRK2 prolongs the “active state” of its GTPase domain. Proc. Natl. Acad. Sci. USA 2014, 111, 4055–4060. [Google Scholar] [CrossRef] [PubMed]
- Takai, Y.; Sasaki, T.; Matozaki, T. Small GTP-binding proteins. Physiol. Rev. 2001, 81, 153–208. [Google Scholar] [CrossRef] [PubMed]
- Lewis, P.A. The function of ROCO proteins in health and disease. Biol. Cell 2009, 101, 183–191. [Google Scholar] [CrossRef]
- Bosgraaf, L.; Van Haastert, P.J. A model for cGMP signal transduction in Dictyostelium in perspective of 25 years of cGMP research. J. Muscle Res. Cell Motil. 2002, 23, 781–791. [Google Scholar] [CrossRef] [PubMed]
- Bosgraaf, L.; Russcher, H.; Smith, J.L.; Wessels, D.; Soll, D.R.; Van Haastert, P.J. A novel cGMP signalling pathway mediating myosin phosphorylation and chemotaxis in Dictyostelium. EMBO J. 2002, 21, 4560–4570. [Google Scholar] [CrossRef]
- Van Egmond, W.N.; Kortholt, A.; Plak, K.; Bosgraaf, L.; Bosgraaf, S.; Keizer-Gunnink, I.; van Haastert, P.J. Intramolecular activation mechanism of the Dictyostelium LRRK2 homolog Roco protein GbpC. J. Biol. Chem. 2008, 283, 30412–30420. [Google Scholar] [CrossRef]
- Kortholt, A.; van Egmond, W.N.; Plak, K.; Bosgraaf, L.; Keizer-Gunnink, I.; van Haastert, P.J. Multiple regulatory mechanisms for the Dictyostelium Roco protein GbpC. J. Biol. Chem. 2012, 287, 2749–2758. [Google Scholar] [CrossRef]
- Abysalh, J.C.; Kuchnicki, L.L.; Larochelle, D.A. The identification of pats1, a novel gene locus required for cytokinesis in Dictyostelium discoideum. Mol. Biol. Cell 2003, 14, 14–25. [Google Scholar] [CrossRef]
- Inbal, B.; Cohen, O.; Polak-Charcon, S.; Kopolovic, J.; Vadai, E.; Eisenbach, L.; Kimchi, A. DAP kinase links the control of apoptosis to metastasis. Nature 1997, 390, 180–184. [Google Scholar] [CrossRef]
- Greggio, E.; Lewis, P.A.; van der Brug, M.P.; Ahmad, R.; Kaganovich, A.; Ding, J.; Beilina, A.; Baker, A.K.; Cookson, M.R. Mutations in LRRK2/dardarin associated with Parkinson disease are more toxic than equivalent mutations in the homologous kinase LRRK1. J. Neurochem. 2007, 102, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Girisha, K.M.; Iida, A.; Hebbar, M.; Shukla, A.; Shah, H.; Nishimura, G.; Matsumoto, N.; Nismath, S.; Miyake, N.; et al. Identification of a novel LRRK1 mutation in a family with osteosclerotic metaphyseal dysplasia. J. Hum. Genet. 2017, 62, 437–441. [Google Scholar] [CrossRef] [PubMed]
- Gasser, T. Molecular pathogenesis of Parkinson disease: Insights from genetic studies. Expert Rev. Mol. Med. 2009, 11, e22. [Google Scholar] [CrossRef] [PubMed]
- Kumari, U.; Tan, E.K. LRRK2 in Parkinson’s disease: Genetic and clinical studies from patients. FEBS J. 2009, 276, 6455–6463. [Google Scholar] [CrossRef] [PubMed]
- Bialik, S.; Kimchi, A. The death-associated protein kinases: Structure, function, and beyond. Annu. Rev. Biochem. 2006, 75, 189–210. [Google Scholar] [CrossRef] [PubMed]
- Raval, A.; Tanner, S.M.; Byrd, J.C.; Angerman, E.B.; Perko, J.D.; Chen, S.S.; Hackanson, B.; Grever, M.R.; Lucas, D.M.; Matkovic, J.J.; et al. Downregulation of death-associated protein kinase 1 (DAPK1) in chronic lymphocytic leukemia. Cell 2007, 129, 879–890. [Google Scholar] [CrossRef] [PubMed]
- Henshall, D.C.; Schindler, C.K.; So, N.K.; Lan, J.Q.; Meller, R.; Simon, R.P. Death-associated protein kinase expression in human temporal lobe epilepsy. Ann. Neurol. 2004, 55, 485–494. [Google Scholar] [CrossRef]
- Carlessi, R.; Levin-Salomon, V.; Ciprut, S.; Bialik, S.; Berissi, H.; Albeck, S.; Peleg, Y.; Kimchi, A. GTP binding to the ROC domain of DAP-kinase regulates its function through intramolecular signalling. EMBO Rep. 2011, 12, 917–923. [Google Scholar] [CrossRef]
- Jebelli, J.D.; Dihanich, S.; Civiero, L.; Manzoni, C.; Greggio, E.; Lewis, P.A. GTP binding and intramolecular regulation by the ROC domain of Death Associated Protein Kinase 1. Sci. Rep. 2012, 2, 695. [Google Scholar] [CrossRef]
- Sakabe, T.; Shinomiya, T.; Mori, T.; Ariyama, Y.; Fukuda, Y.; Fujiwara, T.; Nakamura, Y.; Inazawa, J. Identification of a novel gene, MASL1, within an amplicon at 8p23.1 detected in malignant fibrous histiocytomas by comparative genomic hybridization. Cancer Res. 1999, 59, 511–515. [Google Scholar]
- Kumkhaek, C.; Aerbajinai, W.; Liu, W.; Zhu, J.; Uchida, N.; Kurlander, R.; Hsieh, M.M.; Tisdale, J.F.; Rodgers, G.P. MASL1 induces erythroid differentiation in human erythropoietin-dependent CD34+ cells through the Raf/MEK/ERK pathway. Blood 2013, 121, 3216–3227. [Google Scholar] [CrossRef] [PubMed]
- Xing, W.; Goodluck, H.; Zeng, C.; Mohan, S. Erratum: Role and mechanism of action of leucine-rich repeat kinase 1 in bone. Bone Res. 2017, 5, 17029. [Google Scholar] [CrossRef] [PubMed]
- Zeng, C.; Goodluck, H.; Qin, X.; Liu, B.; Mohan, S.; Xing, W. Leucine-rich repeat kinase-1 regulates osteoclast function by modulating RAC1/Cdc42 Small GTPase phosphorylation and activation. Am. J. Physiol. Endocrinol. Metab. 2016, 311, E772–E780. [Google Scholar] [CrossRef] [PubMed]
- Lewis, P.A.; Manzoni, C. LRRK2 and human disease: A complicated question or a question of complexes? Sci. Signal. 2012, 5, pe2. [Google Scholar] [CrossRef] [PubMed]
- Cookson, M.R. The role of leucine-rich repeat kinase 2 (LRRK2) in Parkinson’s disease. Nat. Rev. Neurosci. 2010, 11, 791–797. [Google Scholar] [CrossRef] [PubMed]
- Kalogeropulou, A.F.; Zhao, J.; Bolliger, M.F.; Memou, A.; Narasimha, S.; Molitor, T.P.; Wilson, W.H.; Rideout, H.J.; Nichols, R.J. P62/SQSTM1 is a novel leucine-rich repeat kinase 2 (LRRK2) substrate that enhances neuronal toxicity. Biochem. J. 2018, 475, 1271–1293. [Google Scholar] [CrossRef] [PubMed]
- Tong, Y.; Yamaguchi, H.; Giaime, E.; Boyle, S.; Kopan, R.; Kelleher, R.J., 3rd; Shen, J. Loss of leucine-rich repeat kinase 2 causes impairment of protein degradation pathways, accumulation of alpha-synuclein, and apoptotic cell death in aged mice. Proc. Natl. Acad. Sci. USA 2010, 107, 9879–9884. [Google Scholar] [CrossRef] [PubMed]
- Herzig, M.C.; Kolly, C.; Persohn, E.; Theil, D.; Schweizer, T.; Hafner, T.; Stemmelen, C.; Troxler, T.J.; Schmid, P.; Danner, S.; et al. LRRK2 protein levels are determined by kinase function and are crucial for kidney and lung homeostasis in mice. Hum. Mol. Genet. 2011, 20, 4209–4223. [Google Scholar] [CrossRef]
- Orenstein, S.J.; Kuo, S.H.; Tasset, I.; Arias, E.; Koga, H.; Fernandez-Carasa, I.; Cortes, E.; Honig, L.S.; Dauer, W.; Consiglio, A.; et al. Interplay of LRRK2 with chaperone-mediated autophagy. Nat. Neurosci. 2013, 16, 394–406. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, P.N.; Wang, X.; Zhu, X.; Chen, S.G.; Wilson-Delfosse, A.L. The Roc domain of leucine-rich repeat kinase 2 is sufficient for interaction with microtubules. J. Neurosci. Res. 2008, 86, 1711–1720. [Google Scholar] [CrossRef]
- Meixner, A.; Boldt, K.; Van Troys, M.; Askenazi, M.; Gloeckner, C.J.; Bauer, M.; Marto, J.A.; Ampe, C.; Kinkl, N.; Ueffing, M. A QUICK screen for Lrrk2 interaction partners—leucine-rich repeat kinase 2 is involved in actin cytoskeleton dynamics. Mol. Cell Proteom. 2011, 10, M110-001172. [Google Scholar] [CrossRef]
- Jaleel, M.; Nichols, R.J.; Deak, M.; Campbell, D.G.; Gillardon, F.; Knebel, A.; Alessi, D.R. LRRK2 phosphorylates moesin at threonine-558: Characterization of how Parkinson’s disease mutants affect kinase activity. Biochem. J. 2007, 405, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Chan, D.; Citro, A.; Cordy, J.M.; Shen, G.C.; Wolozin, B. Rac1 protein rescues neurite retraction caused by G2019S leucine-rich repeat kinase 2 (LRRK2). J. Biol. Chem. 2011, 286, 16140–16149. [Google Scholar] [CrossRef] [PubMed]
- Parisiadou, L.; Xie, C.; Cho, H.J.; Lin, X.; Gu, X.L.; Long, C.X.; Lobbestael, E.; Baekelandt, V.; Taymans, J.M.; Sun, L.; et al. Phosphorylation of ezrin/radixin/moesin proteins by LRRK2 promotes the rearrangement of actin cytoskeleton in neuronal morphogenesis. J. Neurosci. 2009, 29, 13971–13980. [Google Scholar] [CrossRef] [PubMed]
- Civiero, L.; Cogo, S.; Biosa, A.; Greggio, E. The role of LRRK2 in cytoskeletal dynamics. Biochem. Soc Trans. 2018. [Google Scholar] [CrossRef]
- Biskup, S.; Moore, D.J.; Celsi, F.; Higashi, S.; West, A.B.; Andrabi, S.A.; Kurkinen, K.; Yu, S.W.; Savitt, J.M.; Waldvogel, H.J.; et al. Localization of LRRK2 to membranous and vesicular structures in mammalian brain. Ann. Neurol. 2006, 60, 557–569. [Google Scholar] [CrossRef]
- Alegre-Abarrategui, J.; Christian, H.; Lufino, M.M.; Mutihac, R.; Venda, L.L.; Ansorge, O.; Wade-Martins, R. LRRK2 regulates autophagic activity and localizes to specific membrane microdomains in a novel human genomic reporter cellular model. Hum. Mol. Genet. 2009, 18, 4022–4034. [Google Scholar] [CrossRef]
- MacLeod, D.A.; Rhinn, H.; Kuwahara, T.; Zolin, A.; Di Paolo, G.; McCabe, B.D.; Marder, K.S.; Honig, L.S.; Clark, L.N.; Small, S.A.; et al. RAB7L1 interacts with LRRK2 to modify intraneuronal protein sorting and Parkinson’s disease risk. Neuron 2013, 77, 425–439. [Google Scholar] [CrossRef]
- Beilina, A.; Rudenko, I.N.; Kaganovich, A.; Civiero, L.; Chau, H.; Kalia, S.K.; Kalia, L.V.; Lobbestael, E.; Chia, R.; Ndukwe, K.; et al. Unbiased screen for interactors of leucine-rich repeat kinase 2 supports a common pathway for sporadic and familial Parkinson disease. Proc. Natl. Acad. Sci. USA 2014, 111, 2626–2631. [Google Scholar] [CrossRef]
- Purlyte, E.; Dhekne, H.S.; Sarhan, A.R.; Gomez, R.; Lis, P.; Wightman, M.; Martinez, T.N.; Tonelli, F.; Pfeffer, S.R.; Alessi, D.R. Rab29 activation of the Parkinson’s disease-associated LRRK2 kinase. EMBO J. 2018, 37, 1–18. [Google Scholar] [CrossRef]
- Steger, M.; Tonelli, F.; Ito, G.; Davies, P.; Trost, M.; Vetter, M.; Wachter, S.; Lorentzen, E.; Duddy, G.; Wilson, S.; et al. Phosphoproteomics reveals that Parkinson’s disease kinase LRRK2 regulates a subset of Rab GTPases. eLife 2016, 5, e12813. [Google Scholar] [CrossRef]
- Steger, M.; Diez, F.; Dhekne, H.S.; Lis, P.; Nirujogi, R.S.; Karayel, O.; Tonelli, F.; Martinez, T.N.; Lorentzen, E.; Pfeffer, S.R.; et al. Systematic proteomic analysis of LRRK2-mediated Rab GTPase phosphorylation establishes a connection to ciliogenesis. eLife 2017, 6, e31012. [Google Scholar] [CrossRef] [PubMed]
- Dhekne, H.S.; Yanatori, I.; Gomez, R.C.; Tonelli, F.; Diez, F.; Schule, B.; Steger, M.; Alessi, D.R.; Pfeffer, S.R. A pathway for Parkinson’s Disease LRRK2 kinase to block primary cilia and Sonic hedgehog signaling in the brain. eLife 2018, 7, e40202. [Google Scholar] [CrossRef] [PubMed]
- Ito, G.; Okai, T.; Fujino, G.; Takeda, K.; Ichijo, H.; Katada, T.; Iwatsubo, T. GTP binding is essential to the protein kinase activity of LRRK2, a causative gene product for familial Parkinson’s disease. Biochemistry 2007, 46, 1380–1388. [Google Scholar] [CrossRef]
- Gotthardt, K.; Weyand, M.; Kortholt, A.; Van Haastert, P.J.; Wittinghofer, A. Structure of the Roc-COR domain tandem of C. tepidum, a prokaryotic homologue of the human LRRK2 Parkinson kinase. EMBO J. 2008, 27, 2239–2249. [Google Scholar] [CrossRef] [PubMed]
- Dihanich, S.; Civiero, L.; Manzoni, C.; Mamais, A.; Bandopadhyay, R.; Greggio, E.; Lewis, P.A. GTP binding controls complex formation by the human ROCO protein MASL1. FEBS J. 2014, 281, 261–274. [Google Scholar] [CrossRef]
- Korr, D.; Toschi, L.; Donner, P.; Pohlenz, H.D.; Kreft, B.; Weiss, B. LRRK1 protein kinase activity is stimulated upon binding of GTP to its Roc domain. Cell Signal. 2006, 18, 910–920. [Google Scholar] [CrossRef] [PubMed]
- Biosa, A.; Trancikova, A.; Civiero, L.; Glauser, L.; Bubacco, L.; Greggio, E.; Moore, D.J. GTPase activity regulates kinase activity and cellular phenotypes of Parkinson’s disease-associated LRRK2. Hum. Mol. Genet. 2013, 22, 1140–1156. [Google Scholar] [CrossRef] [PubMed]
- Terheyden, S.; Ho, F.Y.; Gilsbach, B.K.; Wittinghofer, A.; Kortholt, A. Revisiting the Roco G-protein cycle. Biochem. J. 2015, 465, 139–147. [Google Scholar] [CrossRef]
- Xiong, Y.; Coombes, C.E.; Kilaru, A.; Li, X.; Gitler, A.D.; Bowers, W.J.; Dawson, V.L.; Dawson, T.M.; Moore, D.J. GTPase activity plays a key role in the pathobiology of LRRK2. PLoS Genet. 2010, 6, e1000902. [Google Scholar] [CrossRef]
- Habig, K.; Walter, M.; Poths, S.; Riess, O.; Bonin, M. RNA interference of LRRK2-microarray expression analysis of a Parkinson’s disease key player. Neurogenetics 2008, 9, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Haebig, K.; Gloeckner, C.J.; Miralles, M.G.; Gillardon, F.; Schulte, C.; Riess, O.; Ueffing, M.; Biskup, S.; Bonin, M. ARHGEF7 (Beta-PIX) acts as guanine nucleotide exchange factor for leucine-rich repeat kinase 2. PLoS ONE 2010, 5, e13762. [Google Scholar] [CrossRef] [PubMed]
- Chia, R.; Haddock, S.; Beilina, A.; Rudenko, I.N.; Mamais, A.; Kaganovich, A.; Li, Y.; Kumaran, R.; Nalls, M.A.; Cookson, M.R. Phosphorylation of LRRK2 by casein kinase 1alpha regulates trans-Golgi clustering via differential interaction with ARHGEF7. Nat. Commun. 2014, 5, 5827. [Google Scholar] [CrossRef] [PubMed]
- Stafa, K.; Trancikova, A.; Webber, P.J.; Glauser, L.; West, A.B.; Moore, D.J. GTPase activity and neuronal toxicity of Parkinson’s disease-associated LRRK2 is regulated by ArfGAP1. PLoS Genet. 2012, 8, e1002526. [Google Scholar] [CrossRef]
- Xiong, Y.; Yuan, C.; Chen, R.; Dawson, T.M.; Dawson, V.L. ArfGAP1 is a GTPase activating protein for LRRK2: Reciprocal regulation of ArfGAP1 by LRRK2. J. Neurosci. 2012, 32, 3877–3886. [Google Scholar] [CrossRef] [PubMed]
- Dusonchet, J.; Li, H.; Guillily, M.; Liu, M.; Stafa, K.; Derada Troletti, C.; Boon, J.Y.; Saha, S.; Glauser, L.; Mamais, A.; et al. A Parkinson’s disease gene regulatory network identifies the signaling protein RGS2 as a modulator of LRRK2 activity and neuronal toxicity. Hum. Mol. Genet. 2014, 23, 4887–4905. [Google Scholar] [CrossRef] [PubMed]
- Gasper, R.; Meyer, S.; Gotthardt, K.; Sirajuddin, M.; Wittinghofer, A. It takes two to tango: Regulation of G proteins by dimerization. Nat. Rev. Mol. Cell Biol. 2009, 10, 423–429. [Google Scholar] [CrossRef]
- Sen, S.; Webber, P.J.; West, A.B. Dependence of leucine-rich repeat kinase 2 (LRRK2) kinase activity on dimerization. J. Biol. Chem. 2009, 284, 36346–36356. [Google Scholar] [CrossRef] [PubMed]
- Berger, Z.; Smith, K.A.; Lavoie, M.J. Membrane localization of LRRK2 is associated with increased formation of the highly active LRRK2 dimer and changes in its phosphorylation. Biochemistry 2010, 49, 5511–5523. [Google Scholar] [CrossRef]
- Civiero, L.; Russo, I.; Bubacco, L.; Greggio, E. Molecular Insights and Functional Implication of LRRK2 Dimerization. Adv. Neurobiol. 2017, 14, 107–121. [Google Scholar]
- Greggio, E.; Cookson, M.R. Leucine-rich repeat kinase 2 mutations and Parkinson’s disease: Three questions. ASN Neuro 2009, 1, AN20090007. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Z.; Zhang, S.; Bustos, D.; Kleinheinz, T.; Le Pichon, C.E.; Dominguez, S.L.; Solanoy, H.O.; Drummond, J.; Zhang, X.; Ding, X.; et al. Ser1292 autophosphorylation is an indicator of LRRK2 kinase activity and contributes to the cellular effects of PD mutations. Sci. Transl. Med. 2012, 4, 164ra161. [Google Scholar] [CrossRef]
- Liu, Z.; Mobley, J.A.; DeLucas, L.J.; Kahn, R.A.; West, A.B. LRRK2 autophosphorylation enhances its GTPase activity. FASEB J. 2016, 30, 336–347. [Google Scholar] [CrossRef] [PubMed]
- Deyaert, E.; Wauters, L.; Guaitoli, G.; Konijnenberg, A.; Leemans, M.; Terheyden, S.; Petrovic, A.; Gallardo, R.; Nederveen-Schippers, L.M.; Athanasopoulos, P.S.; et al. A homologue of the Parkinson’s disease-associated protein LRRK2 undergoes a monomer-dimer transition during GTP turnover. Nat. Commun. 2017, 8, 1008. [Google Scholar] [CrossRef]
- Plotegher, N.; Gratton, E.; Bubacco, L. Number and Brightness analysis of alpha-synuclein oligomerization and the associated mitochondrial morphology alterations in live cells. Biochim. Biophys. Acta 2014, 1840, 2014–2024. [Google Scholar] [CrossRef] [PubMed]
- Kamikawaji, S.; Ito, G.; Iwatsubo, T. Identification of the autophosphorylation sites of LRRK2. Biochemistry 2009, 48, 10963–10975. [Google Scholar] [CrossRef] [PubMed]
- Greggio, E.; Taymans, J.M.; Zhen, E.Y.; Ryder, J.; Vancraenenbroeck, R.; Beilina, A.; Sun, P.; Deng, J.; Jaffe, H.; Baekelandt, V.; et al. The Parkinson’s disease kinase LRRK2 autophosphorylates its GTPase domain at multiple sites. Biochem. Biophys. Res. Commun 2009, 389, 449–454. [Google Scholar] [CrossRef]
- Gloeckner, C.J.; Boldt, K.; von Zweydorf, F.; Helm, S.; Wiesent, L.; Sarioglu, H.; Ueffing, M. Phosphopeptide analysis reveals two discrete clusters of phosphorylation in the N-terminus and the Roc domain of the Parkinson-disease associated protein kinase LRRK2. J. Proteome Res. 2010, 9, 1738–1745. [Google Scholar] [CrossRef]
- Webber, P.J.; Smith, A.D.; Sen, S.; Renfrow, M.B.; Mobley, J.A.; West, A.B. Autophosphorylation in the leucine-rich repeat kinase 2 (LRRK2) GTPase domain modifies kinase and GTP-binding activities. J. Mol. Biol. 2011, 412, 94–110. [Google Scholar] [CrossRef]
- Muda, K.; Bertinetti, D.; Gesellchen, F.; Hermann, J.S.; von Zweydorf, F.; Geerlof, A.; Jacob, A.; Ueffing, M.; Gloeckner, C.J.; Herberg, F.W. Parkinson-related LRRK2 mutation R1441C/G/H impairs PKA phosphorylation of LRRK2 and disrupts its interaction with 14-3-3. Proc. Natl. Acad. Sci. USA 2014, 111, E34–43. [Google Scholar] [CrossRef]
- Lobbestael, E.; Baekelandt, V.; Taymans, J.M. Phosphorylation of LRRK2: From kinase to substrate. Biochem. Soc. Trans. 2012, 40, 1102–1110. [Google Scholar] [CrossRef] [PubMed]
- Lobbestael, E.; Zhao, J.; Rudenko, I.N.; Beylina, A.; Gao, F.; Wetter, J.; Beullens, M.; Bollen, M.; Cookson, M.R.; Baekelandt, V.; et al. Identification of protein phosphatase 1 as a regulator of the LRRK2 phosphorylation cycle. Biochem. J. 2013, 456, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Taymans, J.M. Regulation of LRRK2 by Phosphatases. Adv. Neurobiol. 2017, 14, 145–160. [Google Scholar] [PubMed]
- Athanasopoulos, P.S.; Jacob, W.; Neumann, S.; Kutsch, M.; Wolters, D.; Tan, E.K.; Bichler, Z.; Herrmann, C.; Heumann, R. Identification of protein phosphatase 2A as an interacting protein of leucine-rich repeat kinase 2. Biol. Chem. 2016, 397, 541–554. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Molitor, T.P.; Langston, J.W.; Nichols, R.J. LRRK2 dephosphorylation increases its ubiquitination. Biochem. J. 2015, 469, 107–120. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Tan, Y.C.; Poulose, S.; Olanow, C.W.; Huang, X.Y.; Yue, Z. Leucine-rich repeat kinase 2 (LRRK2)/PARK8 possesses GTPase activity that is altered in familial Parkinson’s disease R1441C/G mutants. J. NeuroChem. 2007, 103, 238–247. [Google Scholar] [PubMed]
- Smith, W.W.; Pei, Z.; Jiang, H.; Dawson, V.L.; Dawson, T.M.; Ross, C.A. Kinase activity of mutant LRRK2 mediates neuronal toxicity. Nat. Neurosci. 2006, 9, 1231–1233. [Google Scholar] [CrossRef] [PubMed]
- West, A.B.; Moore, D.J.; Choi, C.; Andrabi, S.A.; Li, X.; Dikeman, D.; Biskup, S.; Zhang, Z.; Lim, K.L.; Dawson, V.L.; et al. Parkinson’s disease-associated mutations in LRRK2 link enhanced GTP-binding and kinase activities to neuronal toxicity. Hum. Mol. Genet. 2007, 16, 223–232. [Google Scholar] [CrossRef]
- Greggio, E.; Zambrano, I.; Kaganovich, A.; Beilina, A.; Taymans, J.M.; Daniels, V.; Lewis, P.; Jain, S.; Ding, J.; Syed, A.; et al. The Parkinson disease-associated leucine-rich repeat kinase 2 (LRRK2) is a dimer that undergoes intramolecular autophosphorylation. J. Biol. Chem. 2008, 283, 16906–16914. [Google Scholar] [CrossRef]
- Klein, C.L.; Rovelli, G.; Springer, W.; Schall, C.; Gasser, T.; Kahle, P.J. Homo- and heterodimerization of ROCO kinases: LRRK2 kinase inhibition by the LRRK2 ROCO fragment. J. Neurochem. 2009, 111, 703–715. [Google Scholar] [CrossRef]
- James, N.G.; Digman, M.A.; Gratton, E.; Barylko, B.; Ding, X.; Albanesi, J.P.; Goldberg, M.S.; Jameson, D.M. Number and brightness analysis of LRRK2 oligomerization in live cells. Biophys. J. 2012, 102, L41-3. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Stasi, L.P.; Ho, M.H.; Zhao, B.; Wang, H.; Long, K.; Xu, Q.; Sang, Y.; Sun, C.; Hu, H.; et al. Discovery of 4-ethoxy-7H-pyrrolo[2,3-d]pyrimidin-2-amines as potent, selective and orally bioavailable LRRK2 inhibitors. Bioorg. Med. Chem. Lett. 2018, 28, 1615–1620. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhou, Y.; Tang, G.; Wong, N.K.; Yang, M.; Tan, D.; Xiao, Y. Chemoproteomics Reveals the Antiproliferative Potential of Parkinson’s Disease Kinase Inhibitor LRRK2-IN-1 by Targeting PCNA Protein. Mol. Pharm. 2018, 15, 3252–3259. [Google Scholar] [CrossRef] [PubMed]
- Reith, A.D.; Bamborough, P.; Jandu, K.; Andreotti, D.; Mensah, L.; Dossang, P.; Choi, H.G.; Deng, X.; Zhang, J.; Alessi, D.R.; et al. GSK2578215A; a potent and highly selective 2-arylmethyloxy-5-substitutent-N-arylbenzamide LRRK2 kinase inhibitor. Bioorg. Med. Chem. Lett. 2012, 22, 5625–5629. [Google Scholar] [CrossRef] [PubMed]
- Fuji, R.N.; Flagella, M.; Baca, M.; Baptista, M.A.; Brodbeck, J.; Chan, B.K.; Fiske, B.K.; Honigberg, L.; Jubb, A.M.; Katavolos, P.; et al. Effect of selective LRRK2 kinase inhibition on nonhuman primate lung. Sci. Transl. Med. 2015, 7, 273ra15. [Google Scholar] [CrossRef] [PubMed]
- Rudenko, I.N.; Chia, R.; Cookson, M.R. Is inhibition of kinase activity the only therapeutic strategy for LRRK2-associated Parkinson’s disease? BMC Med. 2012, 10, 20. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, J.; Hoang, Q.Q. Roco Proteins and the Parkinson’s Disease-Associated LRRK2. Int. J. Mol. Sci. 2018, 19, 4074. https://doi.org/10.3390/ijms19124074
Liao J, Hoang QQ. Roco Proteins and the Parkinson’s Disease-Associated LRRK2. International Journal of Molecular Sciences. 2018; 19(12):4074. https://doi.org/10.3390/ijms19124074
Chicago/Turabian StyleLiao, Jingling, and Quyen Q. Hoang. 2018. "Roco Proteins and the Parkinson’s Disease-Associated LRRK2" International Journal of Molecular Sciences 19, no. 12: 4074. https://doi.org/10.3390/ijms19124074
APA StyleLiao, J., & Hoang, Q. Q. (2018). Roco Proteins and the Parkinson’s Disease-Associated LRRK2. International Journal of Molecular Sciences, 19(12), 4074. https://doi.org/10.3390/ijms19124074



