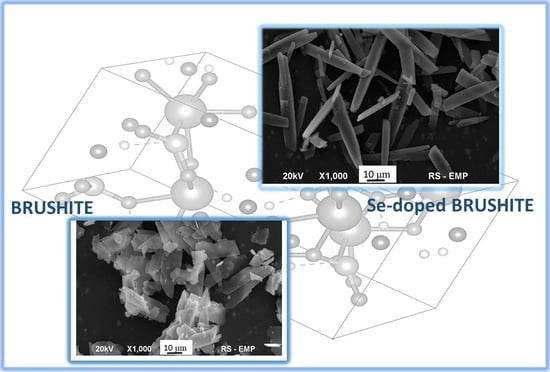
Selenium-Enriched Brushite: A Novel Biomaterial for Potential Use in Bone Tissue Engineering
Abstract
1. Introduction
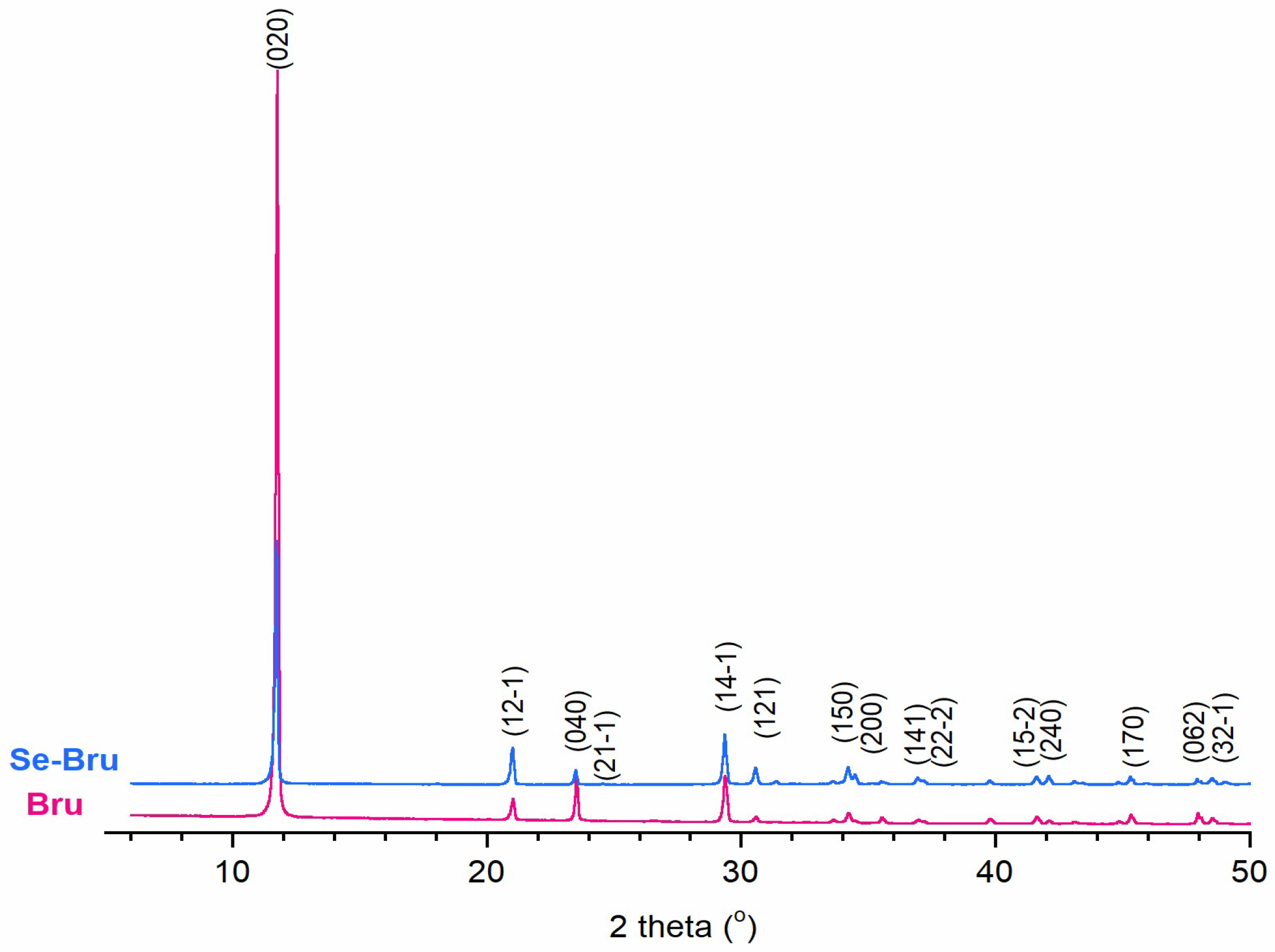
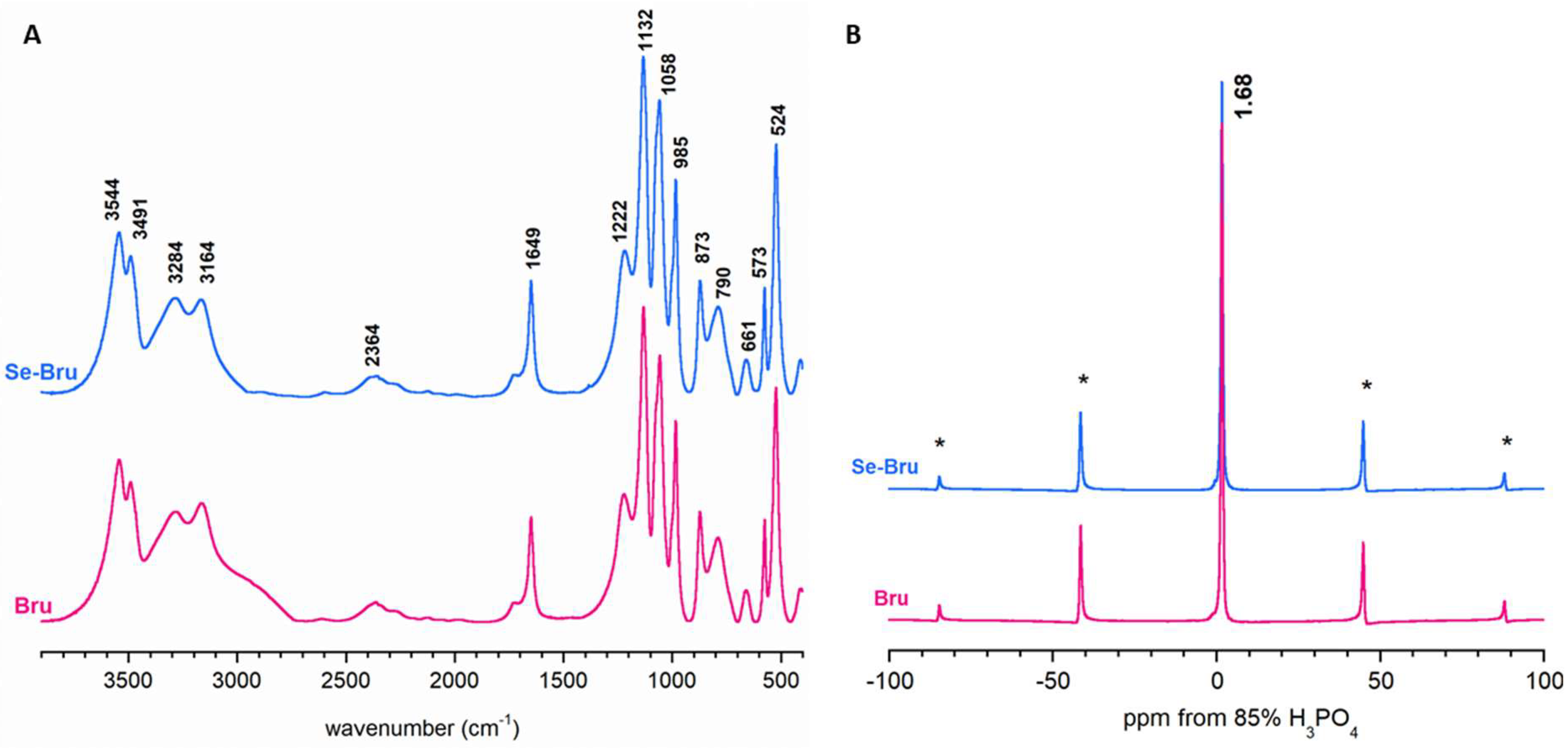
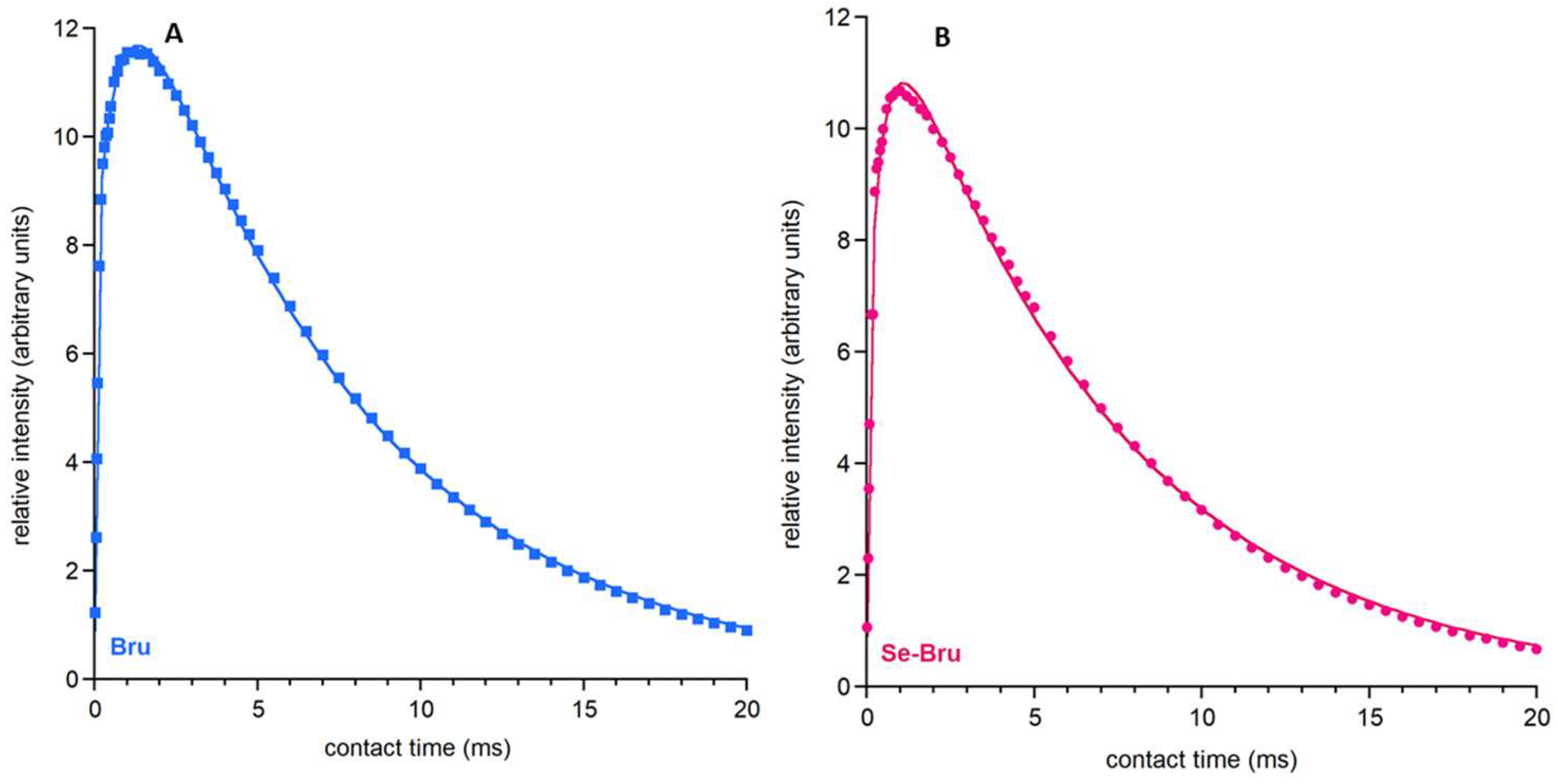
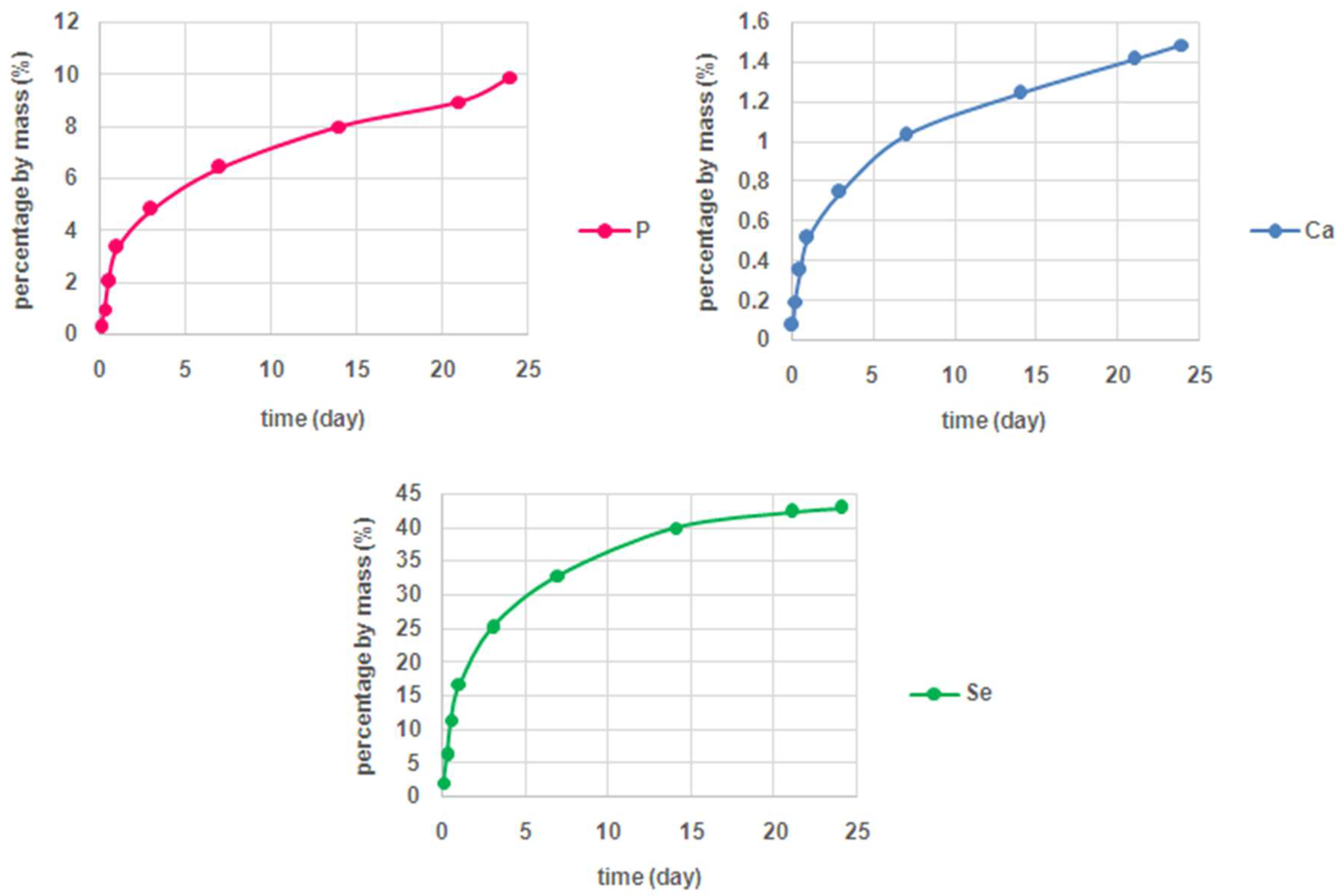
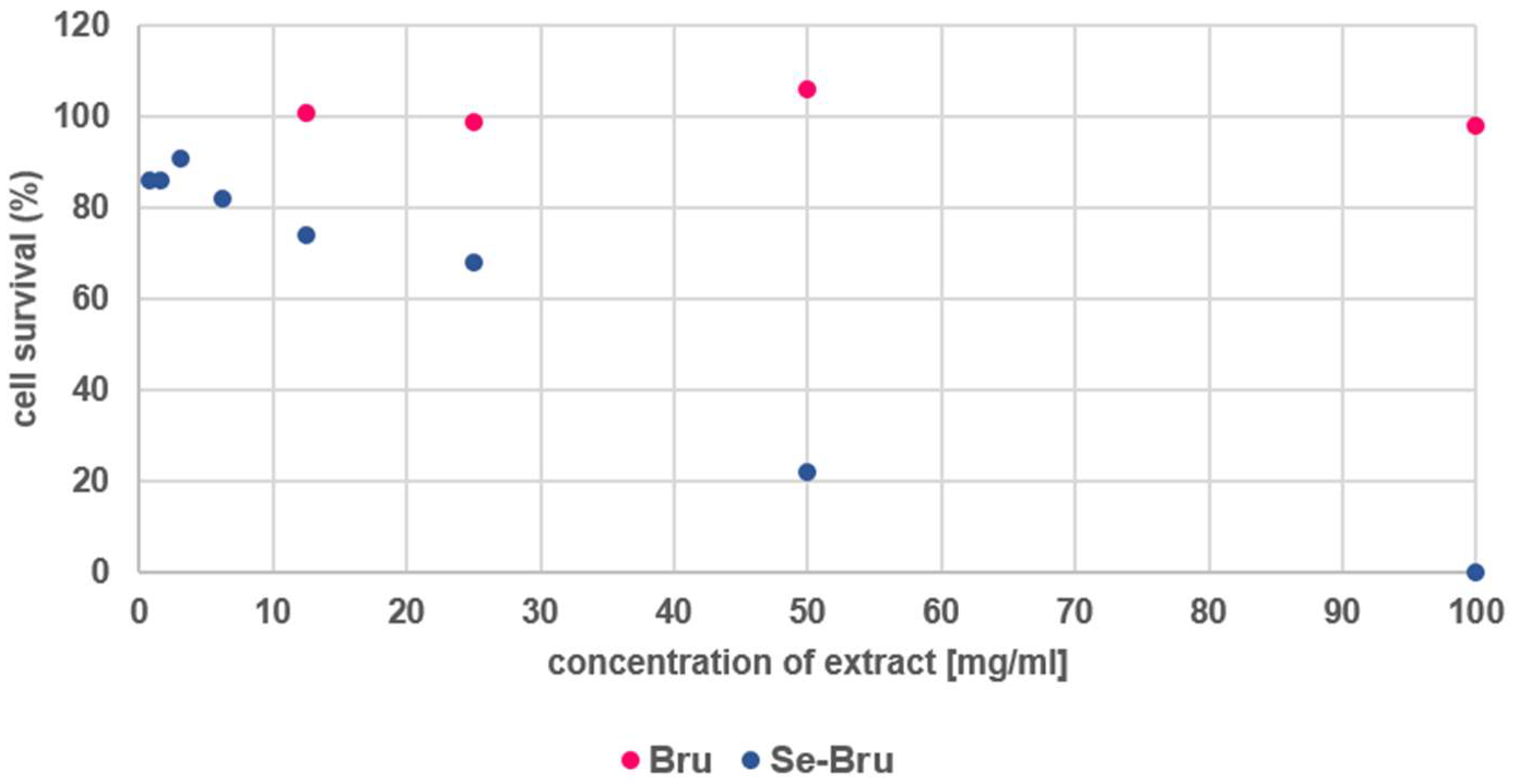
2. Results and Discussion
3. Materials and Methods
3.1. Sample Preparation
3.2. Characterization
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Peak Position-2 Theta (°) | ||
|---|---|---|
| Peak Index | Bru | Se-Bru |
| 020 | 11.75 | 11.70 |
| 12-1 | 21.00 | 20.99 |
| 040 | 23.50 | 23.47 |
| 21-1 | 24.56 | 24.53 |
| 14-1 | 29.35 | 29.33 |
| 121 | 30.56 | 30.54 |
| 150 | 34.19 | 34.18 |
| 200 | 34.45 | 34.45 |
| 141 | 37.06 | 36.90 |
| 22-2 | 37.17 | 37.14 |
| 15-2 | 41.61 | 41.60 |
| 240 | 42.09 | 42.08 |
| 170 | 45.30 | 45.27 |
| 062 | 47.95 | 47.92 |
| 32-1 | 48.61 | 48.60 |
Appendix B
| Wavenumbers (CM−1) | Vibration Modes | |
|---|---|---|
| Bru | Se-Bru | |
| 3544-3491 | 3544-3491 | ν3 H2O (lattice water molecules) |
| 3283-3163 | 3285-3167 | ν1 H2O (lattice water molecules) |
| 2943 | 2945 | PO-H stretching |
| 2364 | 2359 | |
| 1725 | 1727 | Combination (bending) and rotation of residual free water |
| 1649 | 1650 | H-O-H bending of lattice water molecules |
| 1222 | 1219 | δ (PO-H) |
| 1133 | 1134 | νd(P-OH) |
| 1058 | 1060 | |
| 985 | 985 | νs(P-OH) |
| 874 | 873 | ν(P-O(H)) |
| 790 | 790 | δ(P-O(H)) |
| 660 | 660 | water libration |
| 576 | 577 | δ(O-P-O(H)) |
| 524 | 523 | δ(O-P-O(H)) |
| 410 | 412 | δ(O-P-O(H)) |
References
- Dorozhkin, S.V. Calcium Orthophosphates in Nature, Biology and Medicine. Materials 2009, 2, 399–498. [Google Scholar] [CrossRef]
- Mandel, S.; Tas, A.C. Brushite (CaHPO4·2H2O) to octacalcium phosphate (Ca8(HPO4)2(PO4)4·5H2O) transformation in DMEM solution at 36.5 °C. Mater. Sci. Eng. C 2010, 30, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Boanini, E.; Ganzano, M.; Bigi, A. Ionic substitutions in calcium phosphates synthesized at low temperature. Acta Biomater. 2010, 6, 1882–1894. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.W.; Shin, M.C.; Kim, Y.N.; Oh, J.M. Brushite ceramics coatings for dental brace brackets fabricated via aerosol deposition. Ceram. Int. 2017, 43, 1044–1051. [Google Scholar] [CrossRef]
- Kolmas, J.; Groszyk, E.; Kwiatkowska-Różycka, D. Substituted Hydroxyapatites with Antibacterial Properties. Biomed. Res. Int. 2014, 2014, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Rayman, M.P. The importance of Selenium to Human Health. Lancet 2000, 356, 233–241. [Google Scholar] [CrossRef]
- Rayman, M.P. Selenium in cancer prevention: A review of the evidence and mechanism of action. Proc. Nutr. Soc. 2005, 64, 527–542. [Google Scholar] [CrossRef]
- Zeng, H.; Cao, J.J.; Combs, G.F., Jr. Selenium in Bone Health: Roles in Antioxidant Protection and Cell Proliferation. Nutrients 2013, 5, 97–110. [Google Scholar] [CrossRef]
- Fernandes, A.P.; Gandin, V. Selenium compounds as therapeutic agents in cancer. Biochim. Biophys. Acta 2015, 1850, 1642–1660. [Google Scholar] [CrossRef]
- Blackburn, G.; Scott, T.G.; Bayer, I.S. Bionanomaterials for bone tumor engineering and tumor destruction. J. Mater. Chem. B 2013, 1, 1519. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, J.; Zhou, L.; Chen, J.; Liu, Y.; Qiu, Z.; Zhang, S. Dual functional selenium-substituted hydroxyapatite. Interface Focus 2012, 2, 378–386. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hang, H.; Yan, L.; Zhang, S. Selenium-substituted hydroxyapatite nanoparticles and their in vivo antitumor effect on hepatocellular carcinoma. Colloids Surf. B Biointerfaces 2016, 140, 297–306. [Google Scholar]
- Wang, Y.; Wang, J.; Hao, H.; Cai, M.; Wang, S.; Ma, J.; Li, Y.; Mao, C.; Zhang, S. In Vitro and In Vivo Mechanism of Bone Tumor Inhibition by Selenium-Doped Bone Mineral Nanoparticles. ACS Nano. 2018, 10, 9927–9937. [Google Scholar] [CrossRef] [PubMed]
- Uskokovic, V.; Iyer, M.A.; Wu, V.M. One Ion to Rule Them All: Combined Antibacterial, Osteoinductive and Anticancer Properties of Selenite-Incorporated Hydroxyapatite. J. Mater. Chem. B 2017, 5, 1430–1445. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Chai, Y.; Cao, N.; Wang, Y. Synthesis and characterization of selenium substituted hydroxyapatite via hydrothermal method. Mater. Lett. 2014, 134, 123–125. [Google Scholar] [CrossRef]
- Kolmas, J.; Groszyk, E.; Piotrowska, U. Nanocrystalline hydroxyapatite enriched in selenite and manganese ions: Physicochemical and antibacterial properties. Nanoscale Res. Lett. 2015, 10, 278. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, B. Surface Characterization and Biocompatibility of Selenium-Doped Hydroxyapatite Coating on Titanium Alloy. Int. J. Appl. Ceram. Technol. 2016, 13, 1059–1068. [Google Scholar] [CrossRef]
- Kolmas, J.; Oledzka, E.; Sobczak, M.; Nałęcz-Jawecki, G. Nanocrystalline hydroxyapatite doped with selenium oxyanions: A new material for potential biomedical applications. Mater. Sci. Eng. C 2014, 39, 134–142. [Google Scholar] [CrossRef]
- Trpkovska, M.; Soptrajanov, B.; Malkov, P. FTIR reinvestigation of the spectra of synthetic brushite and its partially deuterated analogues. J. Mol. Struct. 1999, 480–481, 661–666. [Google Scholar] [CrossRef]
- Rey, C.; Marsan, O.; Combes, C.; Drouet, C.; Grossin, D.; Sarda, S. Characterization of Calcium Phosphates using vibrational spectroscopies. In Advances in Calcium Phosphate Biomaterials; Ben-Nissan, B., Ed.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 229–267. [Google Scholar]
- Kaflak-Hachulska, A.; Slosarczyk, A.; Kolodziejski, W. Kinetics of NMR cross-polarization from protons to phosphorus-31 in natural brushite. Solid State Nucl. Magn. Reson. 2000, 15, 237–238. [Google Scholar] [CrossRef]
- Pajor, K.; Pajchel, L.; Kolodziejska, B.; Kolmas, J. Selenium-Doped Hydroxyapatite Nanocrystals–Synthesis, Physicochemical Properties and Biological Significance. Crystals 2018, 8, 188. [Google Scholar] [CrossRef]
- Wang, Y.; Lv, P.; Ma, Z.; Zhang, J. Enhanced Healing of Rat Calvarial Critical Size Defect with Selenium-Doped Lamellar Biocomposites. Biol. Trace Elem. Res. 2013, 155, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Zheng, X.; Li, H.; Fan, D.; Song, Z.; Ma, H.; Hua, X.; Hui, J. Monodisperse selenium-substituted hydroxyapatite: Controllable synthesis and biocompatibility. Mat. Sci. Eng. C 2017, 73, 596–602. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hang, H.; Zhang, S. Biomimetic Coprecipitation of Silk Fibrin and Calcium Phosphate: Influence of Selenite Ions. Biol. Trace Elem. Res. 2017, 178, 338–347. [Google Scholar] [CrossRef]
- Hemalatha, T.; Krithiga, G.; Kumar, B.S. Preparation and Characterization of Hydroxyapatite-Coated Selenium Nanoparticles and their Interaction with Osteosarcoma (SaOS−2) Cells. Acta Metall. Sin. (Engl. Lett.) 2014, 27, 1152–1158. [Google Scholar] [CrossRef]
- Wei, l.; Yang, H.; Hong, J.; He, Z.; Deng, C. Synthesis and structure properties of Se and Sr codoped hydroxyapatite and their biocompatibility. J. Mater Sci. 2019, 54, 2514–2525. [Google Scholar] [CrossRef]
- Guerra-Lopez, J.R.; Guida, J.A.; Ramos, M.A.; Punte, G. The influence of Ni(II) on brushite structure stabilization. J. Mol. Str. 2017, 720–724. [Google Scholar] [CrossRef]
- Cabrejos-Azama, J.; Alkhraisat, M.H.; Rueda, C.; Torres, J.; Pintado, C.; Blanco, L.; & López-Cabarcos, E. Magnesium substitution in brushite cements: Efficacy of a new biomaterial loaded with vancomycin for the treatment of Staphylococcus aureus infections. Mat. Sci. Eng. C 2016, 61, 72–78. [Google Scholar] [CrossRef]
- Cummings, H.; Han, W.; Vahabzadeh, S.; Elsawa, S.F. Cobalt-Doped Brushite Cement: Preparation, Characterization, and In Vitro Interaction with Osteosarcoma Cells. JOM 2017, 69, 1348–1353. [Google Scholar] [CrossRef]
- Tamimi, F.; Sheikh, Z.; Barralet, J. Dicalcium phosphate cements: Brushite and monetite. Acta Biomater. 2012, 8, 474–487. [Google Scholar] [CrossRef]
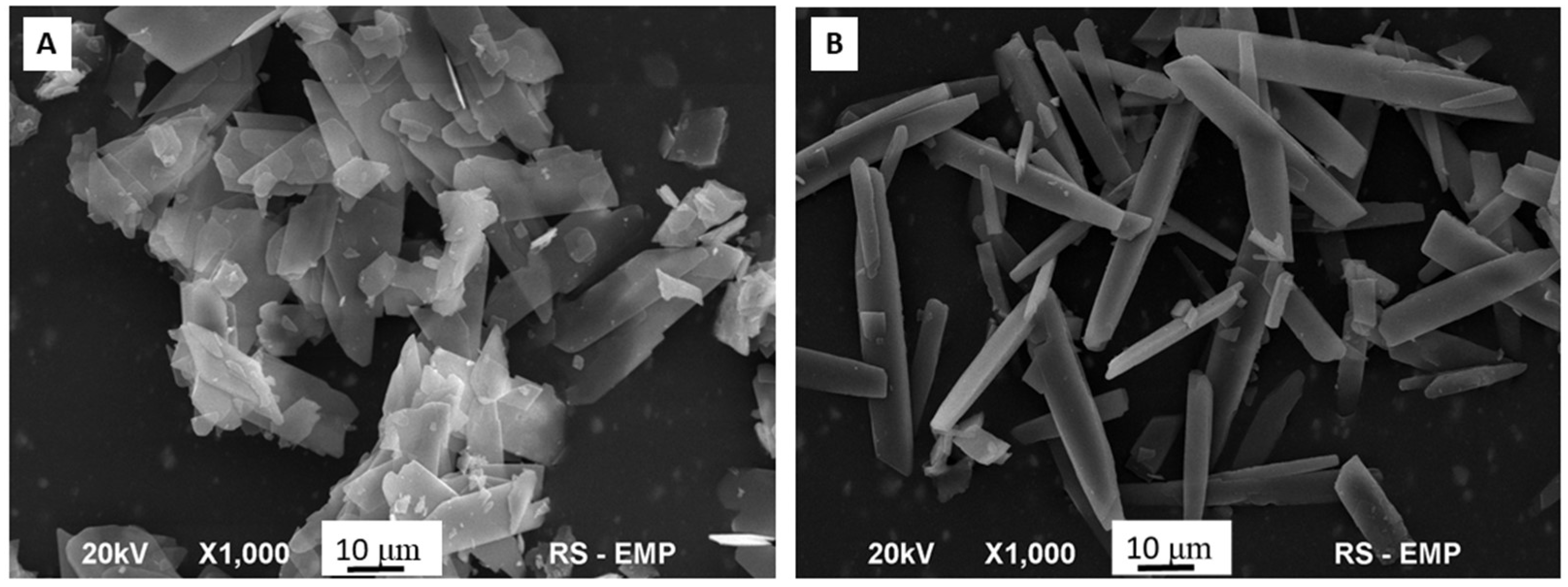
| Parameters | Bru | Se-Bru |
|---|---|---|
| Phase Composition | 100% DCPD | 100% DCPD |
| Unit Cell Parameters | ||
| a (Å) | 5.915 | 6.238 |
| b (Å) | 15.12 | 15.16 |
| c (Å) | 6.242 | 5.806 |
| β (˚) | 116.4 | 116.4 |
| Volume ((Å)3) | 500.2 | 491.7 |
| Se Content (wt%) | -------- | 0.67 ± 0.03% |
| Parameters | Bru | Se-Bru |
|---|---|---|
| T1ρH | 7.09 ± 0.05 | 6.84 ± 0.08 |
| λ | 0.51 ± 0.01 | 0.54 ± 0.02 |
| Tdf | 0.88 ± 0.03 | 0.56 ± 0.04 |
| TCP* | 0.0809 ± 0.001 | 0.101 ± 0.005 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laskus, A.; Zgadzaj, A.; Kolmas, J. Selenium-Enriched Brushite: A Novel Biomaterial for Potential Use in Bone Tissue Engineering. Int. J. Mol. Sci. 2018, 19, 4042. https://doi.org/10.3390/ijms19124042
Laskus A, Zgadzaj A, Kolmas J. Selenium-Enriched Brushite: A Novel Biomaterial for Potential Use in Bone Tissue Engineering. International Journal of Molecular Sciences. 2018; 19(12):4042. https://doi.org/10.3390/ijms19124042
Chicago/Turabian StyleLaskus, Aleksandra, Anna Zgadzaj, and Joanna Kolmas. 2018. "Selenium-Enriched Brushite: A Novel Biomaterial for Potential Use in Bone Tissue Engineering" International Journal of Molecular Sciences 19, no. 12: 4042. https://doi.org/10.3390/ijms19124042
APA StyleLaskus, A., Zgadzaj, A., & Kolmas, J. (2018). Selenium-Enriched Brushite: A Novel Biomaterial for Potential Use in Bone Tissue Engineering. International Journal of Molecular Sciences, 19(12), 4042. https://doi.org/10.3390/ijms19124042