Royal Jelly Abrogates Cadmium-Induced Oxidative Challenge in Mouse Testes: Involvement of the Nrf2 Pathway
Abstract
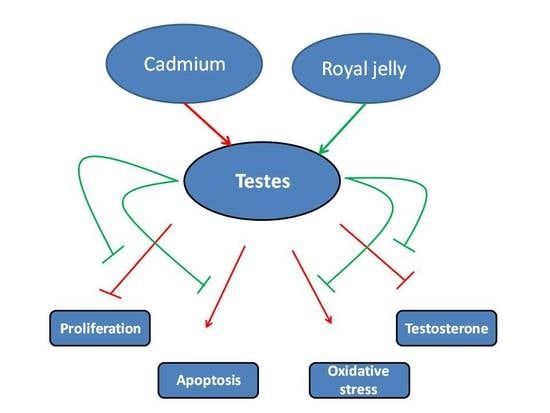
1. Introduction
2. Results
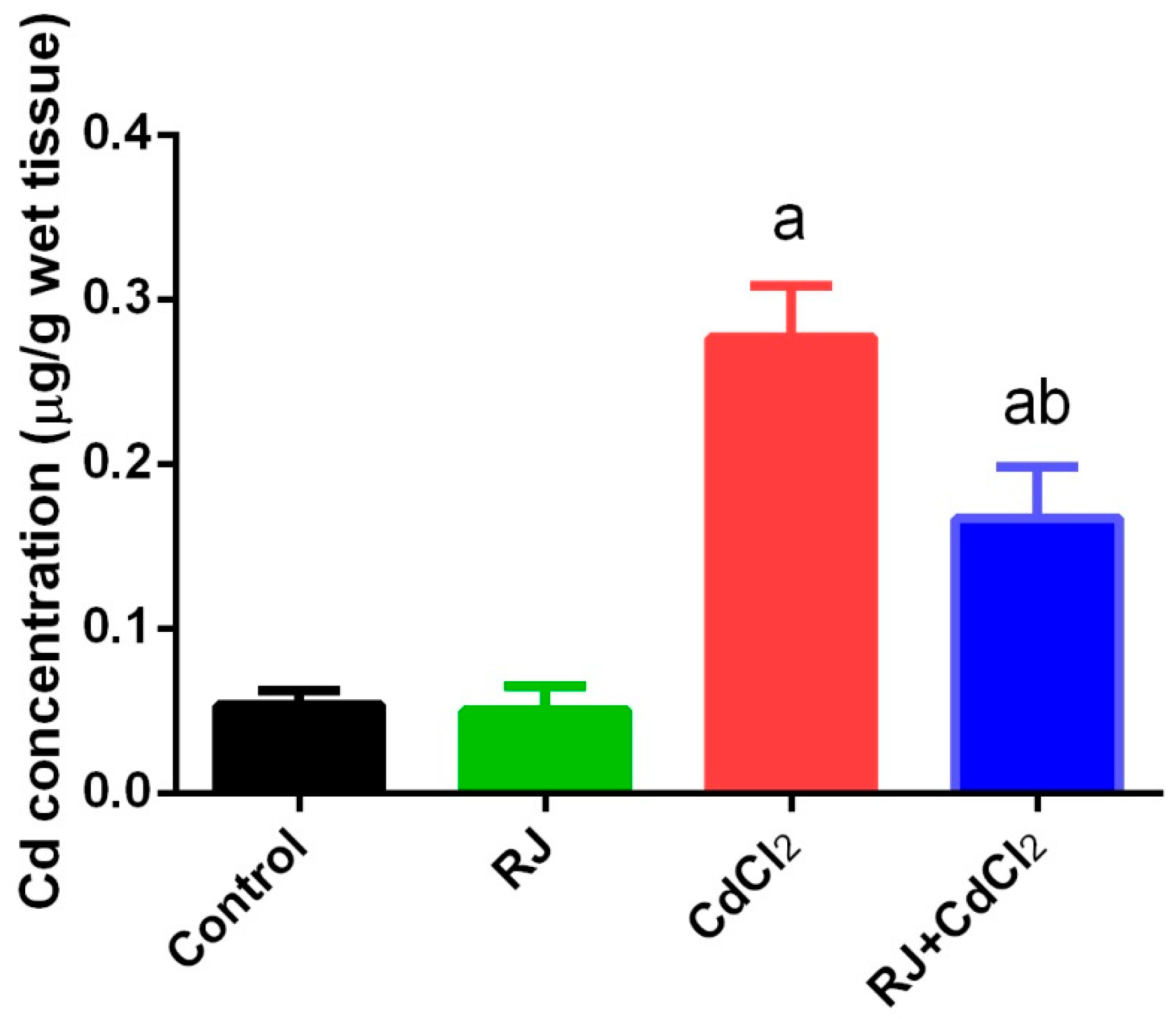
2.1. Effect of RJ on Cd Accumulation in the Testicular Tissue
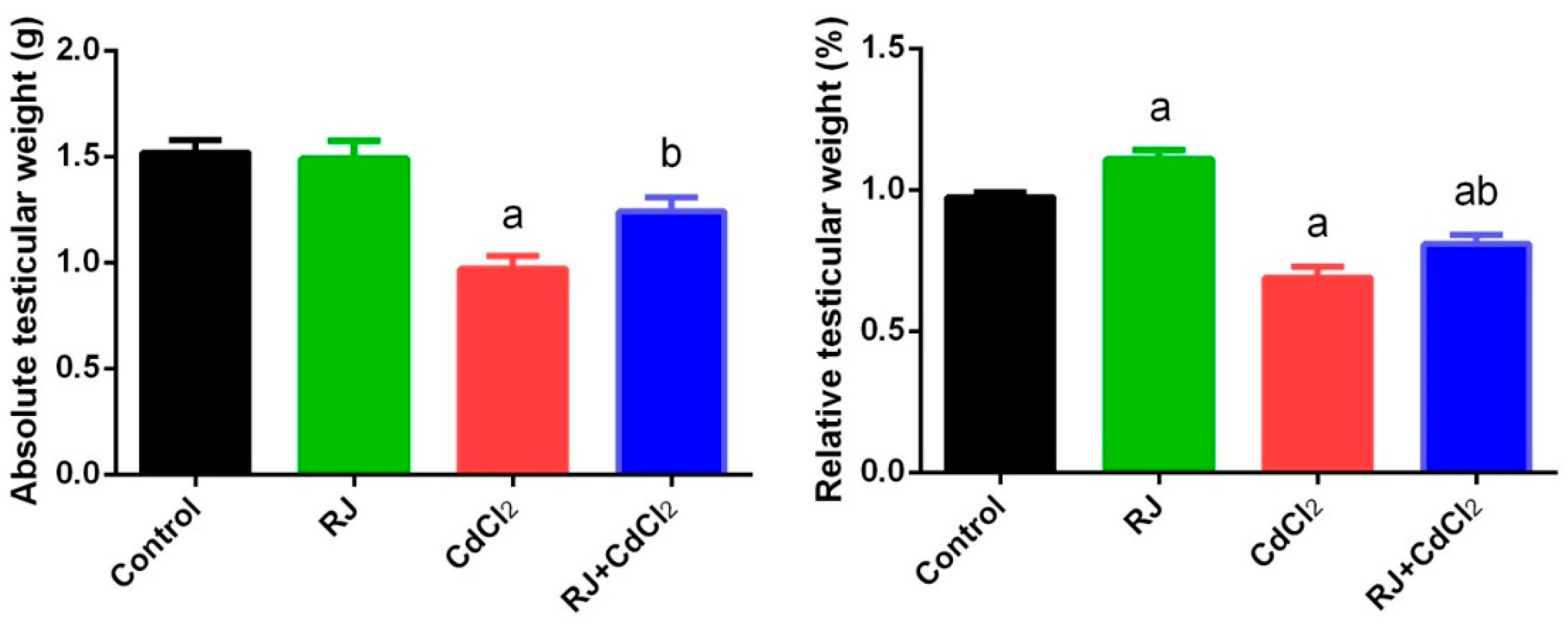
2.2. Effect of RJ and Cd on the Absolute and Relative Weight of Testes
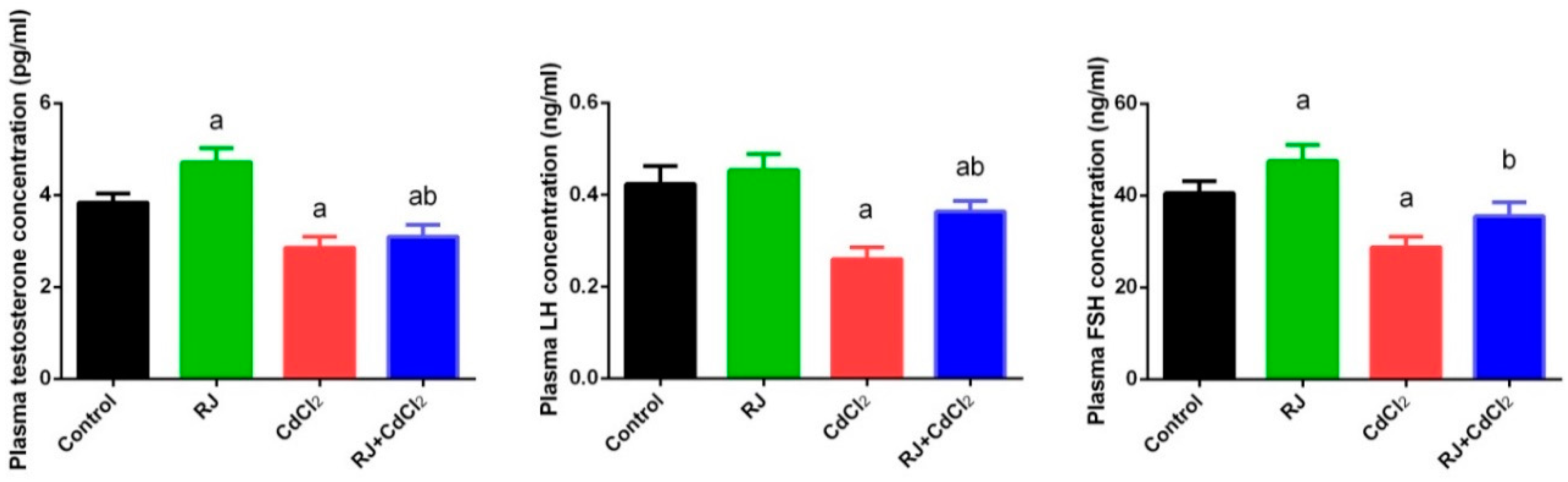
2.3. Effect of RJ and Cd on Serum Levels of Testosterone, LH, and FSH
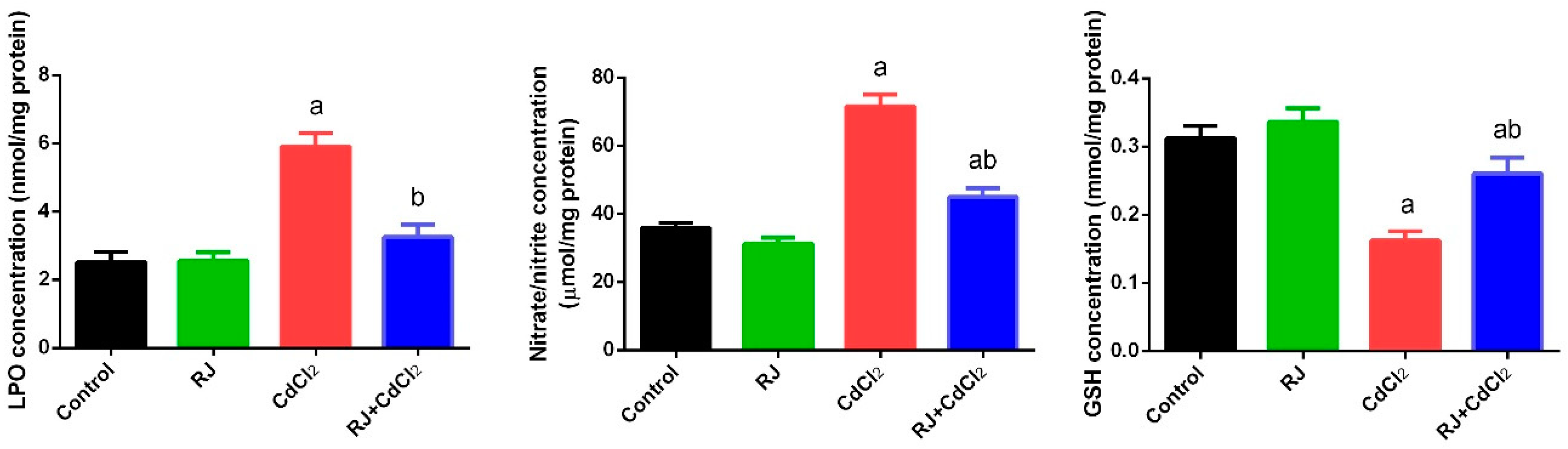
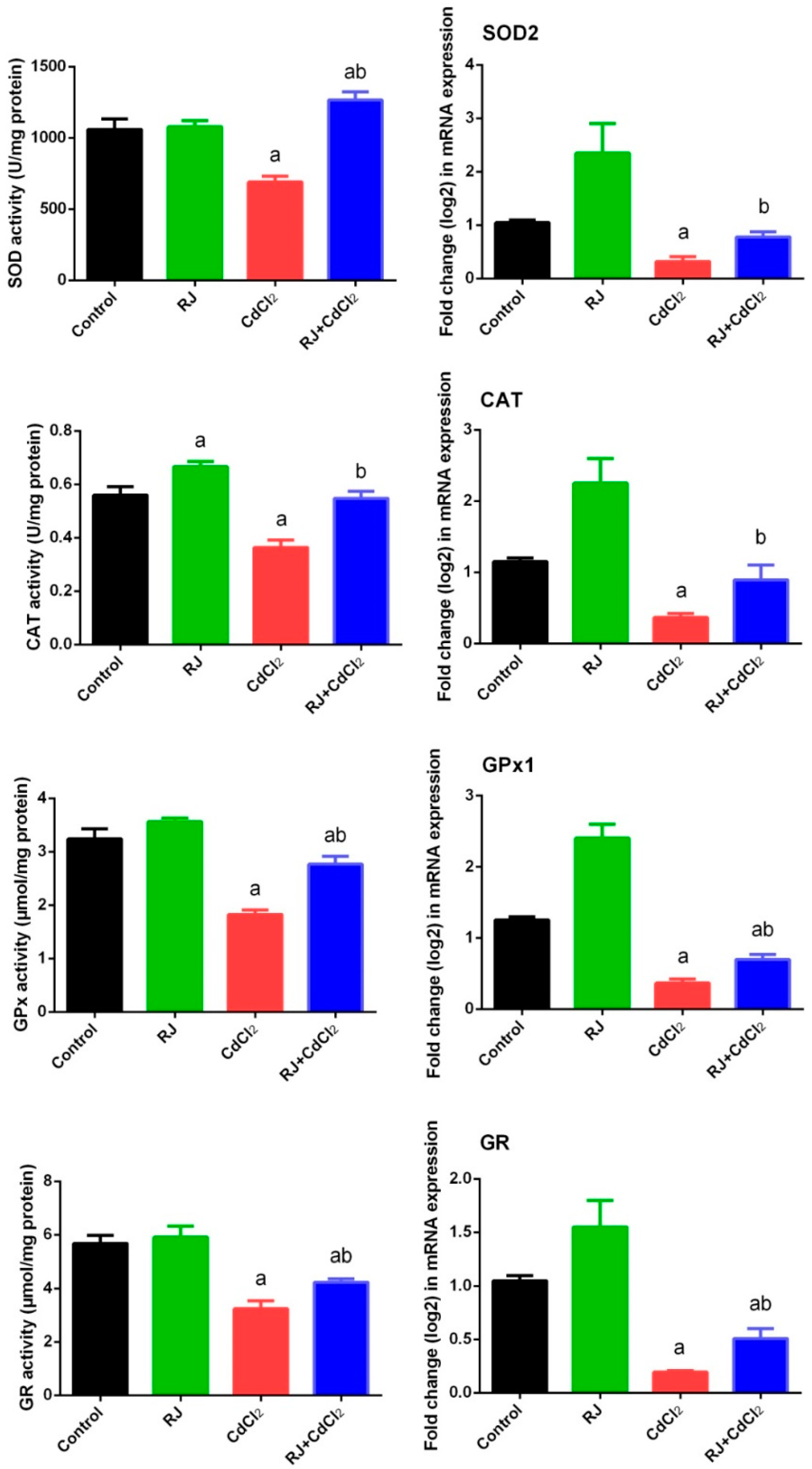
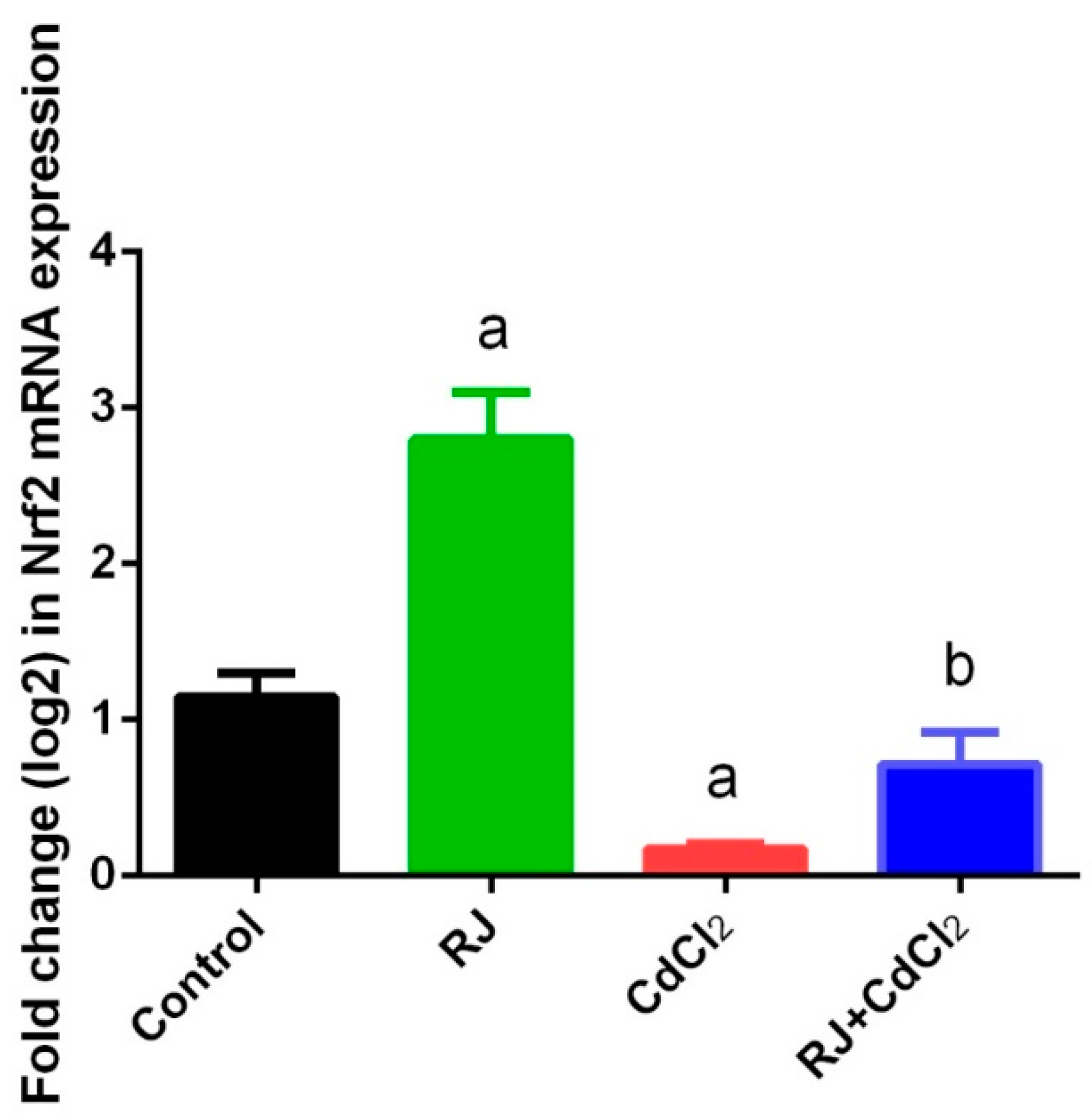
2.4. Effect of RJ on Cd-Induced Oxidative Stress in the Testicular Tissue
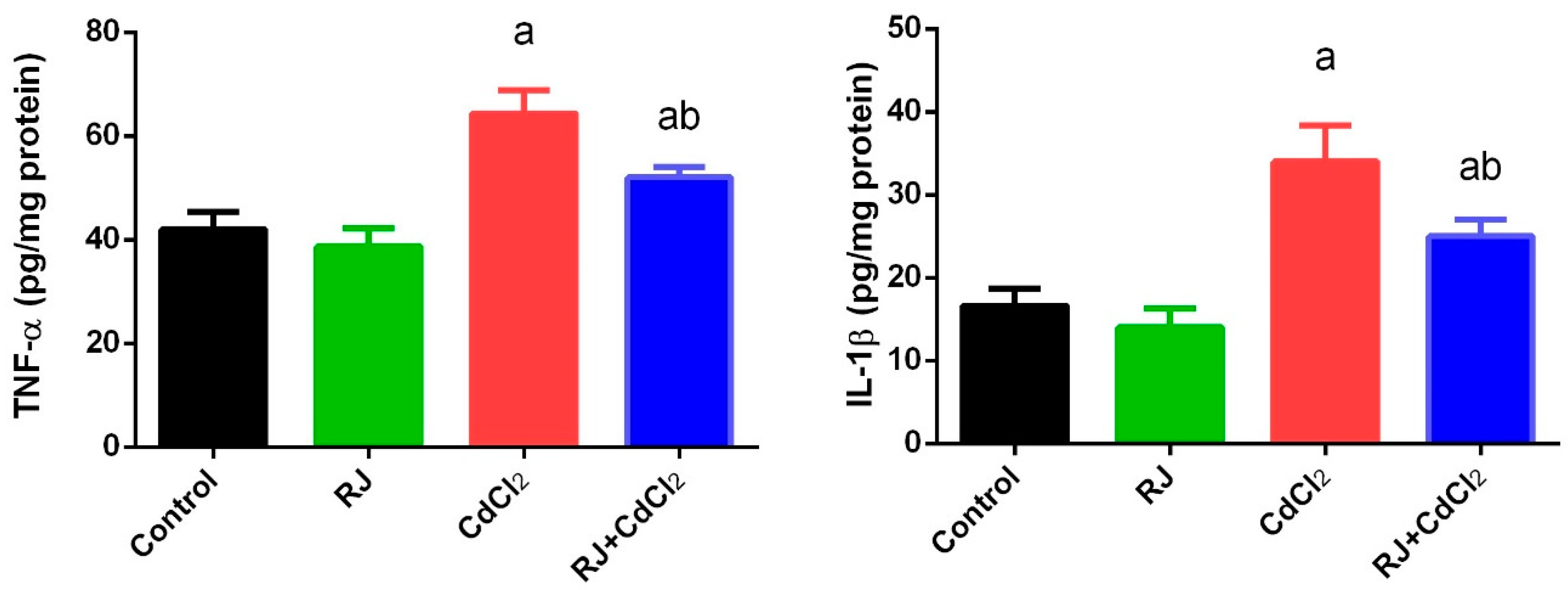
2.5. Effect of RJ on Cd induced-Inflammatory Status in the Testicular Tissue
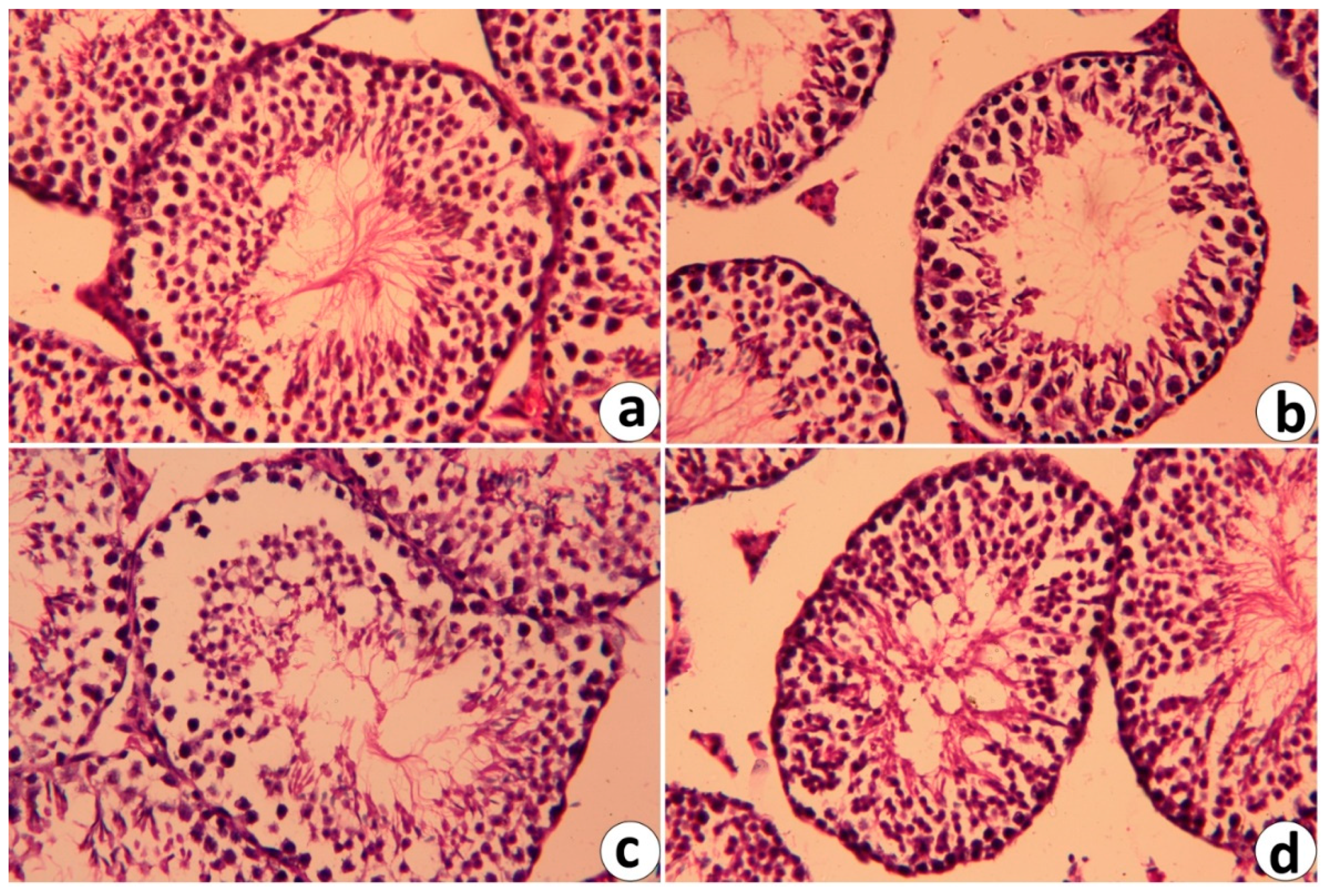
2.6. Histopathological Changes in Testicular Tissue Following RJ and/or CdCl2
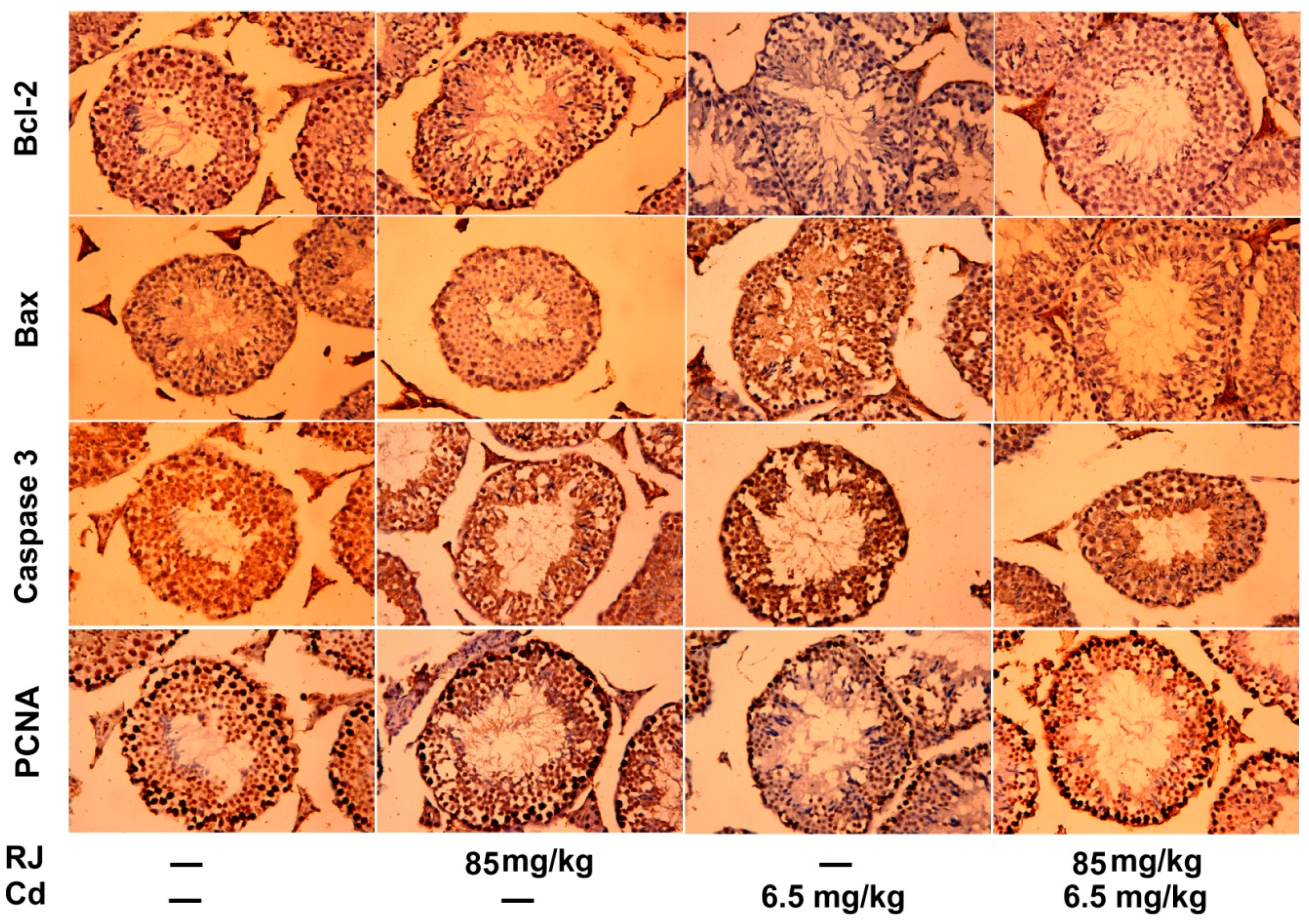
2.7. Effect of RJ on Cd-Triggered Apoptosis and Cytotoxicity in the Testicular Tissue
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Animals and Experimental Design
4.3. Estimation of Cadmium Concentration in Testes
4.4. Testicular Index
4.5. Biochemical Analyses
4.5.1. Hormones Measurements
4.5.2. Lipid Peroxidation (LPO)
4.5.3. Nitrate/Nitrite Level
4.5.4. Reduced Glutathione (GSH)
4.6. Catalase (CAT) Activity
4.7. Superoxide Dismutase (SOD) Activity
4.8. Glutathione Peroxidase (GPx) Activity
4.9. Glutathione Reductase (GR) Activity
4.10. Determination of Testicular Levels of IL-1ß and TNF-α Levels
4.11. Real Time-PCR
4.12. Histopathological Investigation
4.13. Immunohistochemical Investigations
4.14. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Elkhadragy, M.F.; Kassab, R.B.; Metwally, D.M.; Almeer, R.; Abdel-Gaber, R.; Al-Olayan, E.M.; Essawy, E.A.; Amin, H.K.; Abdel Moneim, A.E. Protective effects of fragaria ananassa methanolic extract in a rat model of cadmium chloride-induced neurotoxicity. Biosci. Rep. 2018, 38, BSR20180861. [Google Scholar] [CrossRef] [PubMed]
- Bernhoft, R.A. Cadmium toxicity and treatment. Sci. World J. 2013, 2013, 394652. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Du, Y. Cadmium and its neurotoxic effects. Oxidat. Med. Cell. Longev. 2013, 2013, 898034. [Google Scholar] [CrossRef] [PubMed]
- Bernard, A. Cadmium & its adverse effects on human health. Indian J. Med. Res. 2008, 128, 557–564. [Google Scholar] [PubMed]
- Ahmed, M.M.; El-Shazly, S.A.; Alkafafy, M.E.; Mohamed, A.A.; Mousa, A.A. Protective potential of royal jelly against cadmium-induced infertility in male rats. Andrologia 2018, 50, e12996. [Google Scholar] [CrossRef]
- Elmallah, M.I.Y.; Elkhadragy, M.F.; Al-Olayan, E.M.; Abdel Moneim, A.E. Protective effect of fragaria ananassa crude extract on cadmium-induced lipid peroxidation, antioxidant enzymes suppression, and apoptosis in rat testes. Int. J. Mol. Sci. 2017, 18, 975. [Google Scholar] [CrossRef]
- Yang, S.H.; Yu, L.H.; Li, L.; Guo, Y.; Zhang, Y.; Long, M.; Li, P.; He, J.B. Protective mechanism of sulforaphane on cadmium-induced sertoli cell injury in mice testis via nrf2/are signaling pathway. Molecules 2018, 23, 1774. [Google Scholar] [CrossRef]
- Al Omairi, N.E.; Radwan, O.K.; Alzahrani, Y.A.; Kassab, R.B. Neuroprotective efficiency of mangifera indica leaves extract on cadmium-induced cortical damage in rats. Metab. Brain Dis. 2018, 33, 1121–1130. [Google Scholar] [CrossRef]
- Almeer, R.S.; Alarifi, S.; Alkahtani, S.; Ibrahim, S.R.; Ali, D.; Moneim, A. The potential hepatoprotective effect of royal jelly against cadmium chloride-induced hepatotoxicity in mice is mediated by suppression of oxidative stress and upregulation of nrf2 expression. Biomed. Pharmacother. 2018, 106, 1490–1498. [Google Scholar] [CrossRef]
- Ramanathan, A.N.K.G.; Nair, A.J.; Sugunan, V.S. A review on royal jelly proteins and peptides. J. Funct. Foods 2018, 44, 255–264. [Google Scholar] [CrossRef]
- Fratini, F.; Cilia, G.; Mancini, S.; Felicioli, A. Royal jelly: An ancient remedy with remarkable antibacterial properties. Microbiol. Res. 2016, 192, 130–141. [Google Scholar] [CrossRef]
- Okamoto, I.; Taniguchi, Y.; Kunikata, T.; Kohno, K.; Iwaki, K.; Ikeda, M.; Kurimoto, M. Major royal jelly protein 3 modulates immune responses in vitro and in vivo. Life Sci. 2003, 73, 2029–2045. [Google Scholar] [CrossRef]
- Melliou, E.; Chinou, I. Chemistry and bioactivity of royal jelly from greece. J. Agric. Food Chem. 2005, 53, 8987–8992. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.C.; Chou, W.M.; Widowati, D.A.; Lin, I.P.; Peng, C.C. 10-hydroxy-2-decenoic acid of royal jelly exhibits bactericide and anti-inflammatory activity in human colon cancer cells. BMC Complement. Altern. Med. 2018, 18, 202. [Google Scholar] [CrossRef]
- Zhang, S.; Shao, Q.; Geng, H.; Su, S. The effect of royal jelly on the growth of breast cancer in mice. Oncol. Lett. 2017, 14, 7615–7621. [Google Scholar] [CrossRef] [PubMed]
- Ghanbari, E.; Nejati, V.; Khazaei, M. Antioxidant and protective effects of royal jelly on histopathological changes in testis of diabetic rats. Int. J. Reprod. Biomed. 2016, 14, 519–526. [Google Scholar] [CrossRef]
- Fan, P.; Han, B.; Feng, M.; Fang, Y.; Zhang, L.; Hu, H.; Hao, Y.; Qi, Y.; Zhang, X.; Li, J. Functional and proteomic investigations reveal major royal jelly protein 1 associated with anti-hypertension activity in mouse vascular smooth muscle cells. Sci. Rep. 2016, 6, 30230. [Google Scholar] [CrossRef] [PubMed]
- Guendouz, M.; Haddi, A.; Grar, H.; Kheroua, O.; Saidi, D.; Kaddouri, H. Preventive effects of royal jelly against anaphylactic response in a murine model of cow’s milk allergy. Pharm. Biol. 2017, 55, 2145–2152. [Google Scholar] [CrossRef] [PubMed]
- Satarug, S.; Baker, J.R.; Urbenjapol, S.; Haswell-Elkins, M.; Reilly, P.E.; Williams, D.J.; Moore, M.R. A global perspective on cadmium pollution and toxicity in non-occupationally exposed population. Toxicol. Lett. 2003, 137, 65–83. [Google Scholar] [CrossRef]
- Fan, R.; Hu, P.C.; Wang, Y.; Lin, H.Y.; Su, K.; Feng, X.S.; Wei, L.; Yang, F. Betulinic acid protects mice from cadmium chloride-induced toxicity by inhibiting cadmium-induced apoptosis in kidney and liver. Toxicol. Lett. 2018, 299, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, O.; Oishi, S. Testicular toxicity of dietary 2,2-bis(4-hydroxyphenyl)propane (bisphenol a) in f344 rats. Arch. Toxicol. 2001, 75, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Hachfi, L.; Sakly, R. Effect of cd transferred via food product on spermatogenesis in the rat. Andrologia 2010, 42, 62–64. [Google Scholar] [CrossRef] [PubMed]
- Lafuente, A. The hypothalamic-pituitary-gonadal axis is target of cadmium toxicity. An update of recent studies and potential therapeutic approaches. Food Chem. Toxicol. 2013, 59, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Morita, H.; Ikeda, T.; Kajita, K.; Fujioka, K.; Mori, I.; Okada, H.; Uno, Y.; Ishizuka, T. Effect of royal jelly ingestion for six months on healthy volunteers. Nutr. J. 2012, 11, 77. [Google Scholar] [CrossRef]
- Moutsatsou, P.; Papoutsi, Z.; Kassi, E.; Heldring, N.; Zhao, C.; Tsiapara, A.; Melliou, E.; Chrousos, G.P.; Chinou, I.; Karshikoff, A.; et al. Fatty acids derived from royal jelly are modulators of estrogen receptor functions. PLoS ONE 2010, 5, e15594. [Google Scholar] [CrossRef] [PubMed]
- Mishima, S.; Suzuki, K.M.; Isohama, Y.; Kuratsu, N.; Araki, Y.; Inoue, M.; Miyata, T. Royal jelly has estrogenic effects in vitro and in vivo. J. Ethnopharmacol. 2005, 101, 215–220. [Google Scholar] [CrossRef]
- Fallah, A.; Mohammad-Hasani, A.; Colagar, A.H. Zinc is an essential element for male fertility: A review of zn roles in men’s health, germination, sperm quality, and fertilization. J. Reprod. Infertil. 2018, 19, 69–81. [Google Scholar]
- Tamler, R.; Mechanick, J.I. Dietary supplements and nutraceuticals in the management of andrologic disorders. Endocrinol. Metab. Clin. N. Am. 2007, 36, 533–552. [Google Scholar] [CrossRef]
- Abarikwu, S.O.; Adebayo, O.L.; Otuechere, C.A.; Iserhienrhien, B.O.; Badejo, T.A. Selenium and rutin alone or in combination do not have stronger protective effects than their separate effects against cadmium-induced renal damage. Pharm. Biol. 2016, 54, 896–904. [Google Scholar] [CrossRef]
- Tang, B.; Zhang, L.; Geng, Y. Determination of the antioxidant capacity of different food natural products with a new developed flow injection spectrofluorimetry detecting hydroxyl radicals. Talanta 2005, 65, 769–775. [Google Scholar] [CrossRef]
- Cuypers, A.; Plusquin, M.; Remans, T.; Jozefczak, M.; Keunen, E.; Gielen, H.; Opdenakker, K.; Nair, A.R.; Munters, E.; Artois, T.J.; et al. Cadmium stress: An oxidative challenge. Biometals 2010, 23, 927–940. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Qu, W.; Kadiiska, M.B. Role of oxidative stress in cadmium toxicity and carcinogenesis. Toxicol. Appl. Pharmacol. 2009, 238, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Jihen el, H.; Imed, M.; Fatima, H.; Abdelhamid, K. Protective effects of selenium (se) and zinc (zn) on cadmium (cd) toxicity in the liver of the rat: Effects on the oxidative stress. Ecotoxicol. Environ. Saf. 2009, 72, 1559–1564. [Google Scholar] [CrossRef] [PubMed]
- Casalino, E.; Calzaretti, G.; Sblano, C.; Landriscina, C. Molecular inhibitory mechanisms of antioxidant enzymes in rat liver and kidney by cadmium. Toxicology 2002, 179, 37–50. [Google Scholar] [CrossRef]
- Jurczuk, M.; Brzoska, M.M.; Moniuszko-Jakoniuk, J.; Galazyn-Sidorczuk, M.; Kulikowska-Karpinska, E. Antioxidant enzymes activity and lipid peroxidation in liver and kidney of rats exposed to cadmium and ethanol. Food Chem. Toxicol. 2004, 42, 429–438. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Ekusa, A.; Iwai, K.; Yonekura, M.; Takahata, Y.; Morimatsu, F. Royal jelly peptides inhibit lipid peroxidation in vitro and in vivo. J. Nutr. Sci. Vitaminol. 2008, 54, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Kamakura, M.; Maebuchi, M.; Ozasa, S.; Komori, M.; Ogawa, T.; Sakaki, T.; Moriyama, T. Influence of royal jelly on mouse hepatic gene expression and safety assessment with a DNA microarray. J. Nutr. Sci. Vitaminol. 2005, 51, 148–155. [Google Scholar] [CrossRef]
- Silici, S.; Ekmekcioglu, O.; Eraslan, G.; Demirtas, A. Antioxidative effect of royal jelly in cisplatin-induced testes damage. Urology 2009, 74, 545–551. [Google Scholar] [CrossRef]
- Tamura, S.; Kono, T.; Harada, C.; Yamaguchi, K.; Moriyama, T. Estimation and characterisation of major royal jelly proteins obtained from the honeybee apis merifera. Food Chem. 2009, 114, 1491–1497. [Google Scholar] [CrossRef]
- Pourmoradian, S.; Mahdavi, R.; Mobasseri, M.; Faramarzi, E.; Mobasseri, M. Effects of royal jelly supplementation on glycemic control and oxidative stress factors in type 2 diabetic female: A randomized clinical trial. Chin. J. Integr. Med. 2014, 20, 347–352. [Google Scholar] [CrossRef]
- Ma, Q. Role of nrf2 in oxidative stress and toxicity. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 401–426. [Google Scholar] [CrossRef] [PubMed]
- Jenkhetkana, W.; Thitiorulb, S.; Jansomc, C.; Ratanavalachai, T. Genoprotective effects of thai royal jelly against doxorubicin in human lymphocytes in vitro. Nat. Prod. Commun. 2018, 13, 79–84. [Google Scholar]
- Inoue, Y.; Hara, H.; Mitsugi, Y.; Yamaguchi, E.; Kamiya, T.; Itoh, A.; Adachi, T. 4-hydroperoxy-2-decenoic acid ethyl ester protects against 6-hydroxydopamine-induced cell death via activation of nrf2-are and eif2alpha-atf4 pathways. Neurochem. Int. 2018, 112, 288–296. [Google Scholar] [CrossRef] [PubMed]
- Garlanda, C.; Dinarello, C.A.; Mantovani, A. The interleukin-1 family: Back to the future. Immunity 2013, 39, 1003–1018. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Lim, K.T. Preventive effect of phytoglycoprotein (27 kda) on inflammatory factors at liver injury in cadmium chloride-exposed icr mice. J. Cell. Biochem. 2011, 112, 694–703. [Google Scholar] [CrossRef]
- Freitas, M.; Fernandes, E. Zinc, cadmium and nickel increase the activation of nf-kappab and the release of cytokines from thp-1 monocytic cells. Metallomics: Integr. Biometal Sci. 2011, 3, 1238–1243. [Google Scholar] [CrossRef]
- Abdel-Hafez, S.M.N.; Rifaai, R.A.; Abdelzaher, W.Y. Possible protective effect of royal jelly against cyclophosphamide induced prostatic damage in male albino rats; a biochemical, histological and immuno-histo-chemical study. Biomed. Pharmacother. 2017, 90, 15–23. [Google Scholar] [CrossRef]
- Zargar, H.R.; Hemmati, A.A.; Ghafourian, M.; Arzi, A.; Rezaie, A.; Javad-Moosavi, S.A. Long-term treatment with royal jelly improves bleomycin-induced pulmonary fibrosis in rats. Can. J. Physiol. Pharmacol. 2017, 95, 23–31. [Google Scholar] [CrossRef]
- Turner, T.T.; Lysiak, J.J. Oxidative stress: A common factor in testicular dysfunction. J. Androl. 2008, 29, 488–498. [Google Scholar] [CrossRef]
- Rafat, N.; Monfared, A.S.; Shahidi, M.; Pourfallah, T.A. The modulating effect of royal jelly consumption against radiation-induced apoptosis in human peripheral blood leukocytes. J. Med. Phys. 2016, 41, 52–57. [Google Scholar]
- Karadeniz, A.; Simsek, N.; Karakus, E.; Yildirim, S.; Kara, A.; Can, I.; Kisa, F.; Emre, H.; Turkeli, M. Royal jelly modulates oxidative stress and apoptosis in liver and kidneys of rats treated with cisplatin. Oxidat. Med. Cell. Longev. 2011, 2011, 981793. [Google Scholar] [CrossRef] [PubMed]
- Valiollahpoor Amiri, M.; Deldar, H.; Ansari Pirsaraei, Z. Impact of supplementary royal jelly on in vitro maturation of sheep oocytes: Genes involved in apoptosis and embryonic development. Syst. Biol. Reprod. Med. 2016, 62, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.P.; Street, J.C.; Shupe, J.L.; Bourcier, D.R. Accumulation and depletion of cadmium and lead in tissues and milk of lactating cows fed small amounts of these metals. J. Dairy Sci. 1982, 65, 972–979. [Google Scholar] [CrossRef]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Sastry, K.V.; Moudgal, R.P.; Mohan, J.; Tyagi, J.S.; Rao, G.S. Spectrophotometric determination of serum nitrite and nitrate by copper-cadmium alloy. Anal. Biochem. 2002, 306, 79–82. [Google Scholar] [CrossRef] [PubMed]
- Sedlak, J.; Lindsay, R.H. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with ellman’s reagent. Anal. Biochem. 1968, 25, 192–205. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar]
- Misra, H.P.; Fridovich, I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J. Biol. Chem. 1972, 247, 3170–3175. [Google Scholar]
- Lawrence, R.A.; Burk, R.F. Glutathione peroxidase activity in selenium-deficient rat liver. 1976. Biochem. Biophys. Res. Commun. 2012, 425, 503–509. [Google Scholar] [CrossRef]
- Farias, J.G.; Puebla, M.; Acevedo, A.; Tapia, P.J.; Gutierrez, E.; Zepeda, A.; Calaf, G.; Juantok, C.; Reyes, J.G. Oxidative stress in rat testis and epididymis under intermittent hypobaric hypoxia: Protective role of ascorbate supplementation. J. Androl. 2010, 31, 314–321. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative pcr and the 2(-delta delta c(t)) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Drury, R.A.B.; Wallington, E.A. Preparation and fixation of tissues. In Carleton’s Histological Technique; Oxford University Press: Oxford, UK, 1980; pp. 41–54. [Google Scholar]
| Name | Accession Number | Forward Primer (5’---3’) | Reverse Primer (5’---3’) |
|---|---|---|---|
| GAPDH | NM_001289726.1 | TCACCACCATGGAGAAGGC | GCTAAGCAGTTGGTGGTGCA |
| SOD2 | NM_013671.3 | GCCCAAACCTATCGTGTCCA | AGGGAACCCTAAATGCTGCC |
| CAT | NM_009804.2 | CCGACCAGGGCATCAAAA | GAGGCCATAATCCGGATCTTC |
| GSH-Px1 | NM_001329527.1 | CAGCCGGAAAGAAAGCGATG | TTGCCATTCTGGTGTCCGAA |
| GSH-R | NM_010344.4 | TGGCACTTGCGTGAATGTTG | CGAATGTTGCATAGCCGTGG |
| Nrf2 | NM_010902.4 | CCTCTGTCACCAGCTCAAGG | TTCTGGGCGGCGACTTTATT |
| iNOS | NM_001313922.1 | CGAAACGCTTCACTTCCAA | TGAGCCTATATTGCTGTGGCT |
| IL-1β | NM_008361.4 | TGCCACCTTTTGACAGTGATG | TTCTTGTGACCCTGAGCGAC |
| TNF-α | NM_013693.3 | AGAGGCACTCCCCCAAAAGA | CGATCACCCCGAAGTTCAGT |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almeer, R.S.; Soliman, D.; Kassab, R.B.; AlBasher, G.I.; Alarifi, S.; Alkahtani, S.; Ali, D.; Metwally, D.; Abdel Moneim, A.E. Royal Jelly Abrogates Cadmium-Induced Oxidative Challenge in Mouse Testes: Involvement of the Nrf2 Pathway. Int. J. Mol. Sci. 2018, 19, 3979. https://doi.org/10.3390/ijms19123979
Almeer RS, Soliman D, Kassab RB, AlBasher GI, Alarifi S, Alkahtani S, Ali D, Metwally D, Abdel Moneim AE. Royal Jelly Abrogates Cadmium-Induced Oxidative Challenge in Mouse Testes: Involvement of the Nrf2 Pathway. International Journal of Molecular Sciences. 2018; 19(12):3979. https://doi.org/10.3390/ijms19123979
Chicago/Turabian StyleAlmeer, Rafa S., Doaa Soliman, Rami B. Kassab, Gadah I. AlBasher, Saud Alarifi, Saad Alkahtani, Daoud Ali, Dina Metwally, and Ahmed E. Abdel Moneim. 2018. "Royal Jelly Abrogates Cadmium-Induced Oxidative Challenge in Mouse Testes: Involvement of the Nrf2 Pathway" International Journal of Molecular Sciences 19, no. 12: 3979. https://doi.org/10.3390/ijms19123979
APA StyleAlmeer, R. S., Soliman, D., Kassab, R. B., AlBasher, G. I., Alarifi, S., Alkahtani, S., Ali, D., Metwally, D., & Abdel Moneim, A. E. (2018). Royal Jelly Abrogates Cadmium-Induced Oxidative Challenge in Mouse Testes: Involvement of the Nrf2 Pathway. International Journal of Molecular Sciences, 19(12), 3979. https://doi.org/10.3390/ijms19123979