Effect of Long-Term Strontium Exposure on the Content of Phytoestrogens and Allantoin in Soybean
Abstract
1. Introduction
2. Results and Discussion
2.1. Impact of Strontium Ions on Soybean Growth
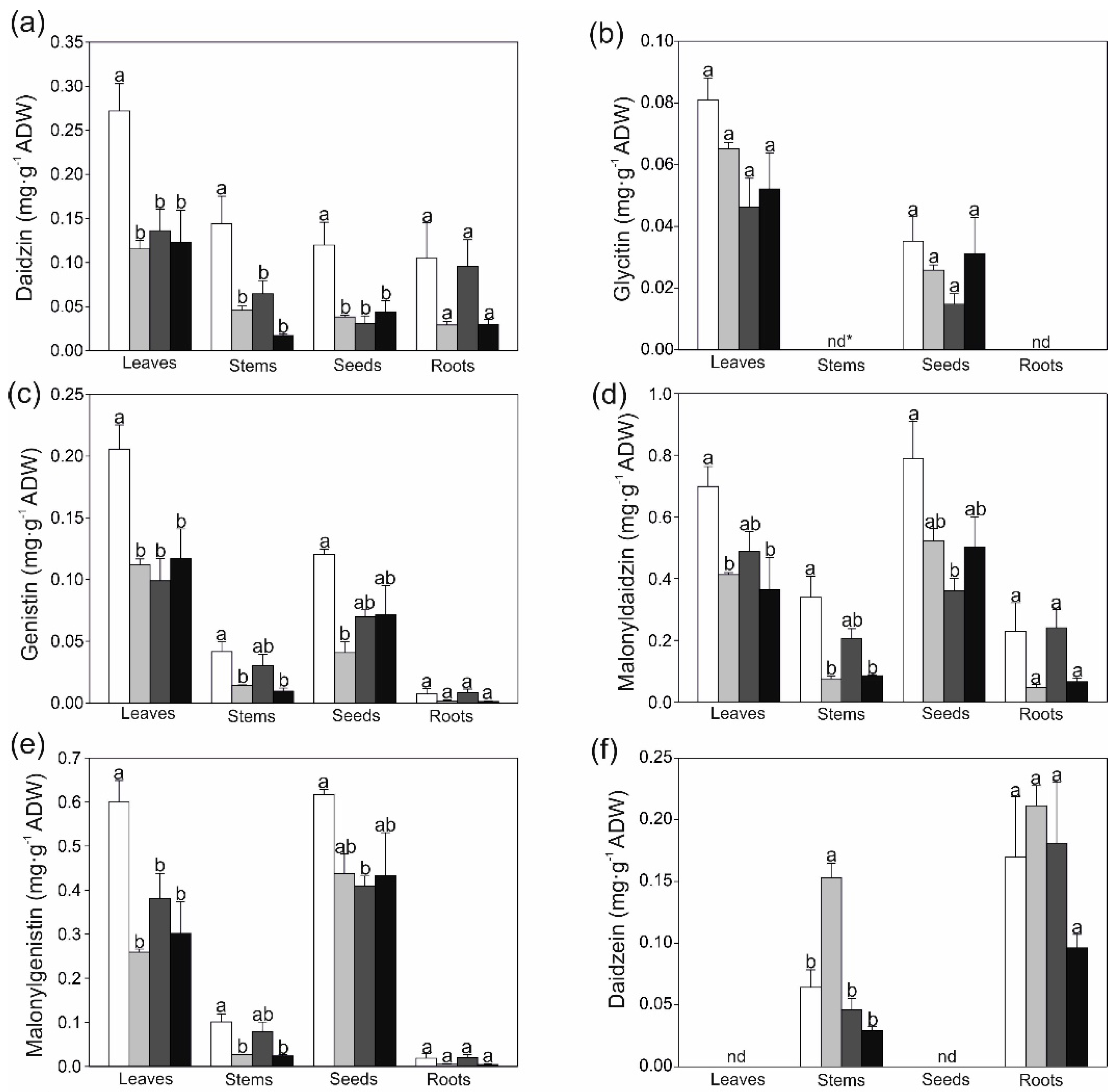
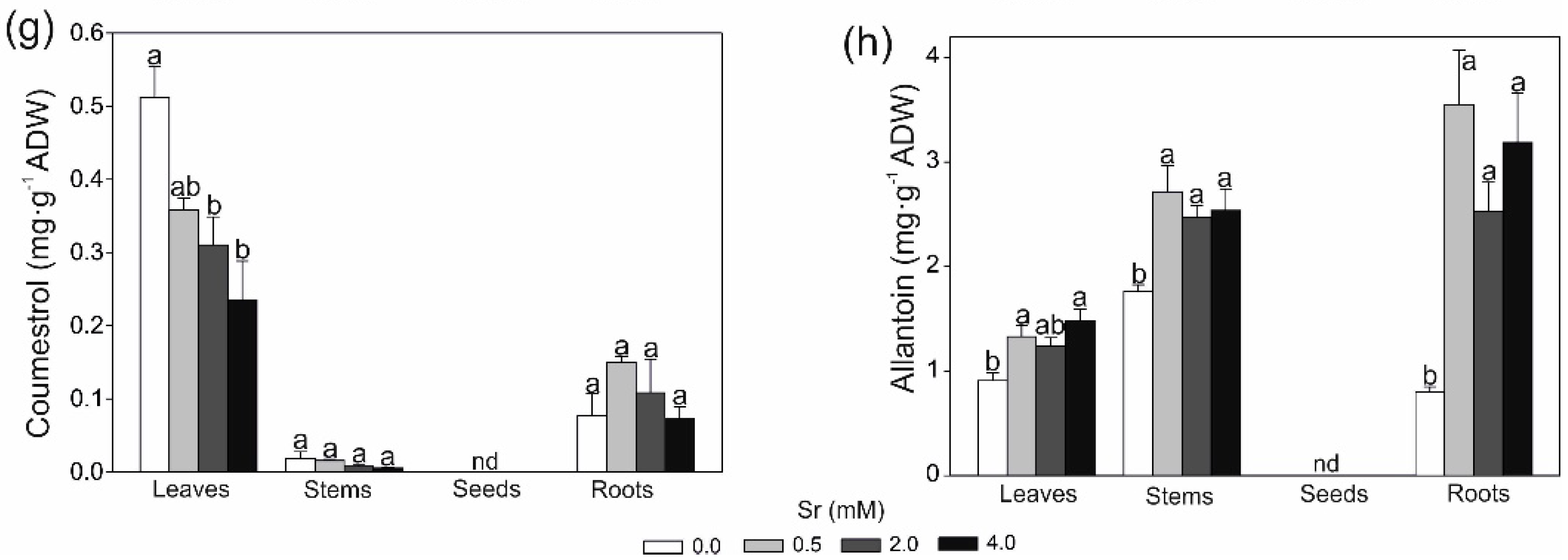
2.2. Changes in Secondary Metobolites and Antioxidant Capacity in Response to Strontium
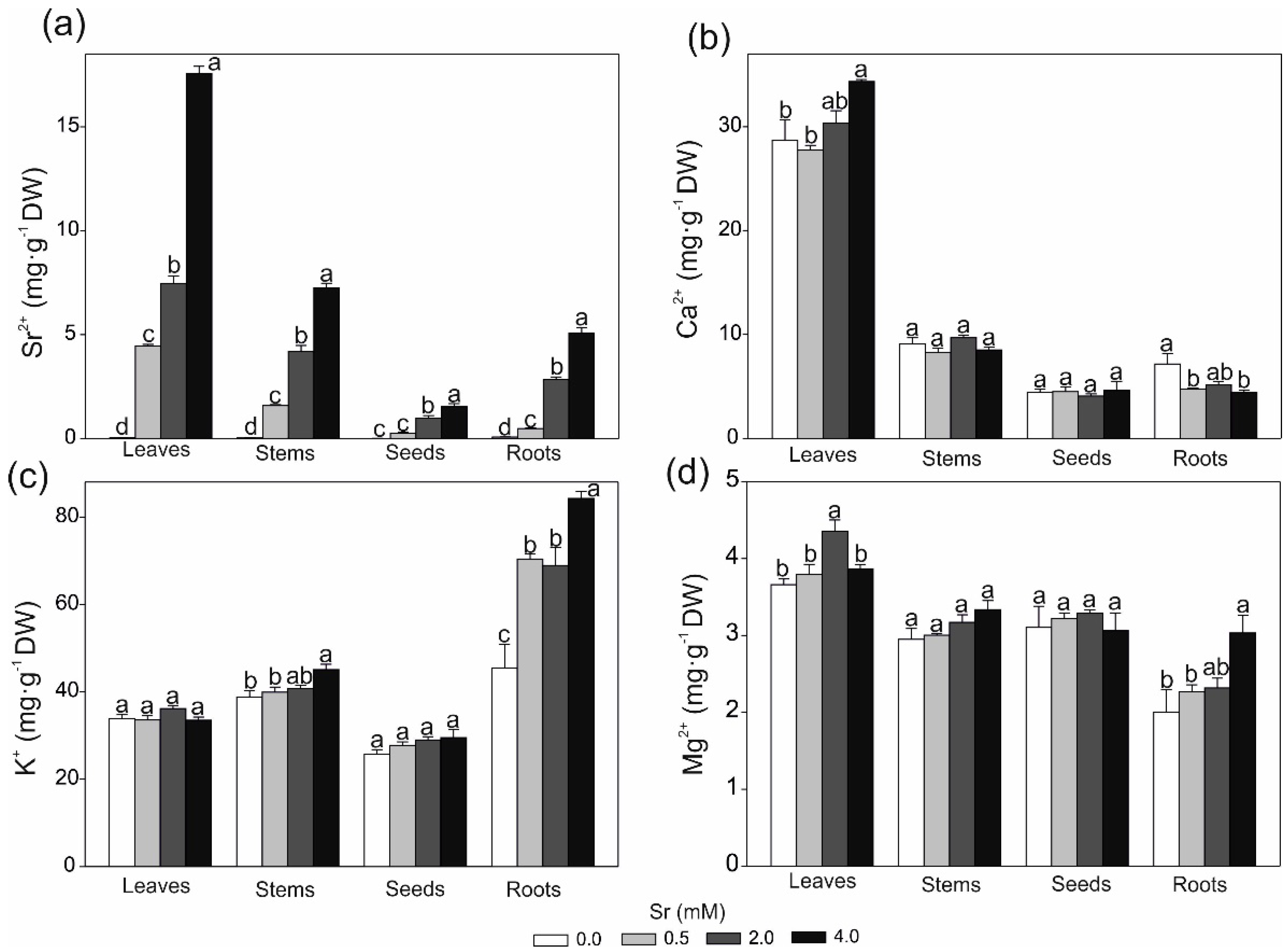
2.3. Strontium and Some Macro- and Microlement Concentrations
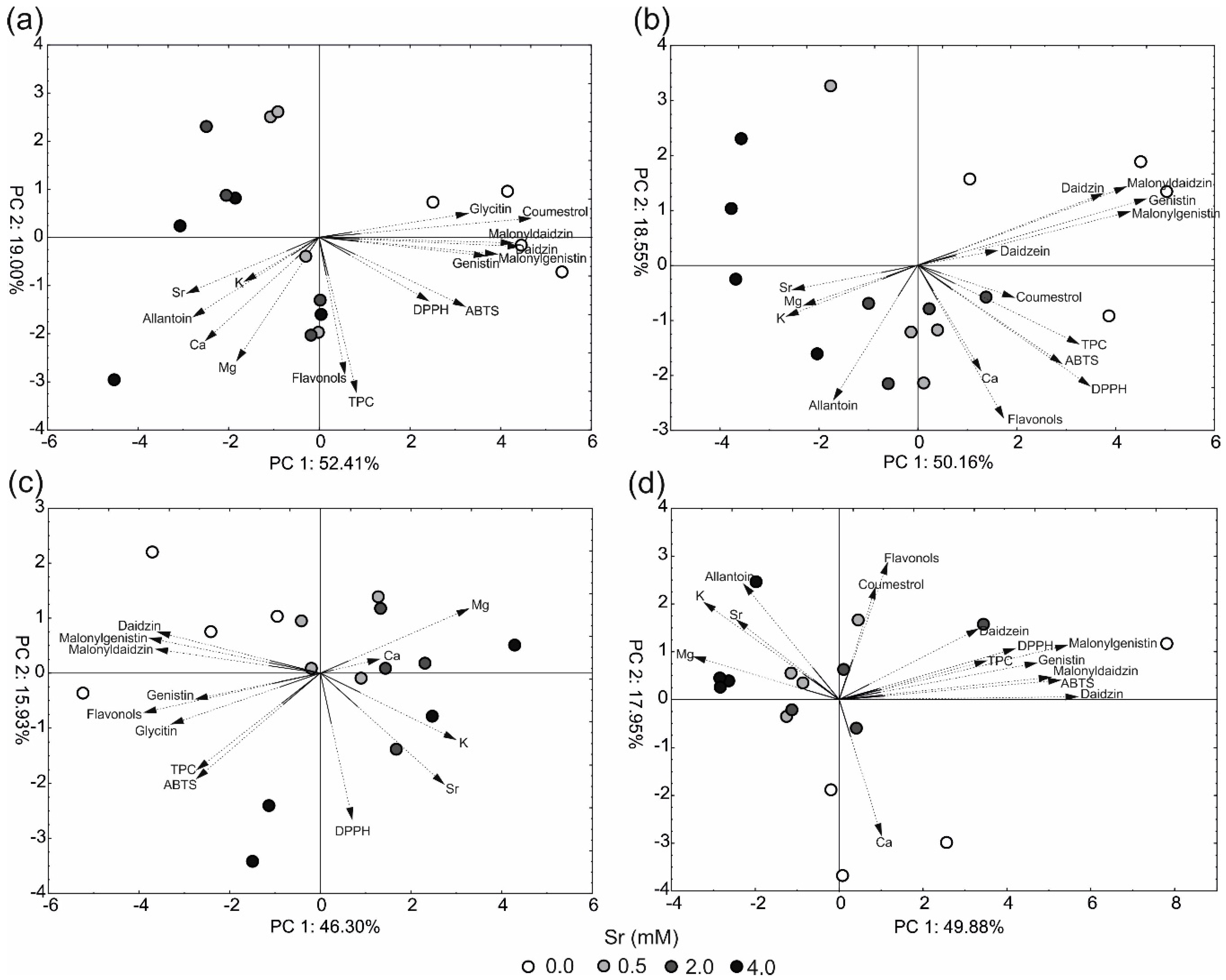
2.4. Principal Component Analysis
3. Materials and Methods
3.1. Plant Material
3.2. Sample Preparation
3.3. HPLC Analysis of Phytoestrogens
3.4. Allantoin Determination Using Capillary Electrophoresis
3.5. Determination of Total Phenolics, Soluble Flavonols, and Antioxidant Capacity
3.6. Determination of Strontium and Macro- and Microelements
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ABTS | 2-azino-bis-3-ethylbenzthiazoline-6-sulphonic acid |
| ADW | air-dry weight |
| ANOVA | analysis of variance |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| PC | principle component |
| PCA | principle component analysis |
| TPC | total phenolic content |
References
- Dixon, R.A.; Paiva, N.L. Stress-induced phenylpropanoid metabolism. Plant Cell 1995, 7, 1085–1097. [Google Scholar] [CrossRef] [PubMed]
- Kováčik, J.; Klejdus, B. Dynamics of phenolic acids and lignin accumulation in metal-treated Matricaria chamomilla roots. Plant Cell Rep. 2008, 27, 605–615. [Google Scholar] [CrossRef] [PubMed]
- Dresler, S.; Rutkowska, E.; Bednarek, W.; Stanisławski, G.; Kubrak, T.; Bogucka-Kocka, A.; Wójcik, M. Selected secondary metabolites in Echium vulgare L. populations from nonmetalliferous and metalliferous areas. Phytochemistry 2017, 133, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Chennupati, P.; Seguin, P.; Chamoun, R.; Jabaji, S. Effects of high-temperature stress on soybean isoflavone concentration and expression of key genes involved in isoflavone synthesis. J. Agric. Food Chem. 2012, 60, 12421–12427. [Google Scholar] [CrossRef] [PubMed]
- Szymczak, G.; Wójciak-Kosior, M.; Sowa, I.; Zapała, K.; Strzemski, M.; Kocjan, R. Evaluation of isoflavone content and antioxidant activity of selected soy taxa. J. Food Comp. Anal. 2017, 57, 40–48. [Google Scholar] [CrossRef]
- Gueven, A.; Knorr, D. Isoflavonoid production by soy plant callus suspension culture. J. Food Eng. 2011, 103, 237–243. [Google Scholar] [CrossRef]
- Theboral, J.; Sivanandhan, G.; Subramanyam, K.; Arun, M.; Selvaraj, N.; Manickavasagam, M.; Ganapathi, A. Enhanced production of isoflavones by elicitation in hairy root cultures of Soybean. Plant Cell Tiss. Org. 2014, 117, 477–481. [Google Scholar] [CrossRef]
- Chebrolu, K.K.; Fritschi, F.B.; Ye, S.; Krishnan, H.B.; Smith, J.R.; Gillman, J.D. Impact of heat stress during seed development on soybean seed metabolome. Metabolomics 2016, 12, 1–14. [Google Scholar] [CrossRef]
- Da Graça, J.P.; Ueda, T.E.; Janegitz, T.; Vieira, S.S.; Salvador, M.C.; de Oliveira, M.C.N.; Zingaretti, S.M.; Powers, S.J.; Pickett, J.A.; Birkett, M.A.; et al. The natural plant stress elicitor cis-jasmone causes cultivar-dependent reduction in growth of the stink bug, Euschistus heros and associated changes in flavonoid concentrations in soybean, Glycine max. Phytochemistry 2016, 131, 84–91. [Google Scholar] [CrossRef]
- Wójciak-Kosior, M.; Sowa, I.; Blicharski, T.; Strzemski, M.; Dresler, S.; Szymczak, G.; Wnorowski, A.; Kocjan, R.; Świeboda, R. The Stimulatory effect of strontium ions on phytoestrogens content in Glycine max (L.) Merr. Molecules 2016, 21, 90. [Google Scholar] [CrossRef]
- Zavala, J.A.; Mazza, C.A.; Dillon, F.M.; Chludil, H.D.; Ballaré, C.L. Soybean resistance to stink bugs (Nezara viridula and Piezodorus guildinii) increases with exposure to solar UV-B radiation and correlates with isoflavonoid content in pods under field conditions. Plant Cell Environ. 2015, 38, 920–928. [Google Scholar] [CrossRef]
- Janas, K.M.; Cvikrová, M.; Palagiewicz, A.; Szafranska, K.; Posmyk, M.M. Constitutive elevated accumulation of phenylpropanoids in soybean roots at low temperature. Plant Sci. 2002, 163, 369–373. [Google Scholar] [CrossRef]
- Cornwell, T.; Cohick, W.; Raskin, I. Dietary phytoestrogens and health. Phytochemistry 2004, 65, 995–1016. [Google Scholar] [CrossRef]
- Delmonte, P.; Perry, J.; Rader, J.I. Determination of isoflavones in dietary supplements containing soy, Red Clover and kudzu: Extraction followed by basic or acid hydrolysis. J. Chromatogr. 2006, 1107, 59–69. [Google Scholar] [CrossRef]
- Sowa, I.; Wójciak-Kosior, M.; Strzemski, M.; Rokicka, K.; Blicharski, T.; Kocjan, R. Analysis of compounds with phytoestrogenic activity in dietary supplements with use of HPTLC-densitometry method. Acta Pol. Pharm. 2014, 71, 265–269. [Google Scholar]
- Ososki, A.L.; Kennelly, E.J. Phytoestrogens: A review of the present state of research. Phytother. Res. 2003, 17, 845–869. [Google Scholar] [CrossRef]
- Saidak, Z.; Marie, P.J. Strontium signaling: Molecular mechanisms and therapeutic implications in osteoporosis. Pharmacol. Therapeut. 2012, 136, 216–226. [Google Scholar] [CrossRef]
- Sowa, I.; Wójciak-Kosior, M.; Strzemski, M.; Dresler, S.; Szwerc, W.; Blicharski, T.; Szymczak, G.; Kocjan, R. Biofortification of soy (Glycine max (L.) Merr.) with strontium ions. J. Agr. Food. Chem. 2014, 62, 5248–5252. [Google Scholar] [CrossRef]
- Burger, A.; Lichtscheidl, I. Strontium in the environment: Review about reactions of plants towards stable and radioactive strontium isotopes. Sci. Total Environ. 2019, 653, 1458–1512. [Google Scholar] [CrossRef]
- Moyen, C.; Roblin, G. Uptake and translocation of strontium in hydroponically grown maize plants, and subsequent effects on tissue ion content, growth and chlorophyll a/b ratio: Comparison with Ca effects. Environ. Exp. Bot. 2010, 68, 247–257. [Google Scholar] [CrossRef]
- Seregin, I.V.; Kozhevnikova, A.D. Strontium transport, distribution, and toxic effects on maize seedling growth. Russ. J. Plant Physl. 2004, 51, 215–221. [Google Scholar] [CrossRef]
- Watanabe, T.; Okada, K. Interactive effects of Al, Ca and other cations on root elongation of rice cultivars under low pH. Ann. Bot. 2005, 95, 379–385. [Google Scholar] [CrossRef]
- Mei, L.; Xitao, X.; Renhao, X.; Zhili, L. Effects of strontium-induced stress on marine microalgae Platymonas subcordiformis (Chlorophyta: Volvocales). Chin. J. Ocean. Limnol. 2006, 24, 154–160. [Google Scholar] [CrossRef]
- Dresler, S.; Szymczak, G.; Wójcik, M. Comparison of some secondary metabolite content in the seventeen species of the Boraginaceae family. Pharm. Biol. 2017, 55, 691–695. [Google Scholar] [CrossRef]
- Thomas, R.J.; Schrader, L.E. Ureide metabolism in higher plants. Phytochemistry 1981, 20, 361–371. [Google Scholar] [CrossRef]
- Alamillo, J.M.; Dí, A.-L.; Sánchez-Moran, M.A.V.; Pineda, M. Molecular analysis of ureide accumulation under drought stress in Phaseolus vulgaris L. Plant Cell Environ. 2010, 33, 1828–1837. [Google Scholar] [CrossRef]
- Irani, S.; Todd, C.D. Ureide metabolism under abiotic stress in Arabidopsis thaliana. J. Plant Physil. 2016, 199, 87–95. [Google Scholar] [CrossRef]
- Irani, S.; Lobo, J.M.; Gray, G.R.; Todd, C.D. Allantoin accumulation in response to increased growth irradiance in Arabidopsis thaliana. Biol. Plantarum 2018, 62, 181–187. [Google Scholar] [CrossRef]
- Dresler, S.; Wójciak-Kosior, M.; Sowa, I.; Stanisławski, G.; Bany, I.; Wójcik, M. Effect of short-term Zn/Pb or long-term multi-metal stress on physiological and morphological parameters of metallicolous and nonmetallicolous Echium vulgare L. populations. Plant Physiol. Bioch. 2017, 115, 380–389. [Google Scholar] [CrossRef]
- Brychkova, G.; Alikulov, Z.; Fluhr, R.; Sagi, M. A critical role for ureides in dark and senescence-induced purine remobilization is unmasked in the Atxdh1 Arabidopsis mutant. Plant J. 2008, 54, 496–509. [Google Scholar] [CrossRef]
- Nourimand, M.; Todd, C.D. Allantoin increases cadmium tolerance in Arabidopsis via activation of antioxidant mechanisms. Plant Cell Physiol. 2016, 57, 2485–2496. [Google Scholar] [CrossRef]
- Kabata-Pendias, A.; Mukherjee, A.B. Trace Elements from Soil to Human; Springer-Verlag: Berlin Heidelberg, 2007; ISBN 978-3-540-32713-4. [Google Scholar]
- Dresler, S.; Bednarek, W.; Hawrylak-Nowak, B.; Wójcik, M. Morphometric and phytochemical profile of seeds of metallicolous and nonmetallicolous Echium vulgare populations. Biochem. Syst. Ecol. 2017, 70, 304–310. [Google Scholar] [CrossRef]
- Wang, X.; Chen, C.; Wang, J. Phytoremediation of strontium contaminated soil by Sorghum bicolor (L.) Moench and soil microbial community-level physiological profiles (CLPPs). Environ. Sci. Pollut. Res. 2017, 24, 7668–7678. [Google Scholar] [CrossRef]
- Sasmaz, A.; Sasmaz, M. The phytoremediation potential for strontium of indigenous plants growing in a mining area. Ecotox. Environ. Safe 2009, 67, 139–144. [Google Scholar] [CrossRef]
- Leung, J.K.C.; Shang, Z.R. Uptake Of 137cs and 90sr In Rice Plants. Health Physics 2003, 84, 170–179. [Google Scholar] [CrossRef]
- Kamra, O.P. Somatic and genetic effects of incorporated strontium-90 and cesium-137 on Arabidopsis thaliana. Can. J. Bot. 1974, 52, 27–34. [Google Scholar] [CrossRef]
- Bataitiene, I.P. 90Sr distribution in system “soil-scots pine” (Pinus sylvestris L.). In Behaviour of Strontium in Plants and the Environment; Springer Nature, Springer International Publishing AG: Switzerland, 2017; pp. 93–108. [Google Scholar]
- Gupta, D.K.; Schulz, W.; Steinhauser, G.; Walther, C. Radiostrontium transport in plants and phytoremediation. Environ. Sci. Pollut. Res. 2018, 25, 29996–30008. [Google Scholar] [CrossRef]
- Kartosentono, S.; Nuraida, A.; Indrayanto, G.; Cholies Zaini, N. Phytoremediation of Sr2+ and its influence on the growth, Ca2+ and solasodine content of shoot cultures of Solanum laciniatum. Biotechnol. Lett. 2001, 23, 153–155. [Google Scholar] [CrossRef]
- Dresler, S.; Kubrak, T.; Bogucka-Kocka, A.; Szymczak, G. Determination of Shikonin and Rosmarinic Acid in Echium vulgare L. and Echium russicum J.F. Gmel. by Capillary Electrophoresis. J. Liq. Chromatogr. 2015, 38, 698–701. [Google Scholar] [CrossRef]
- Kováčik, J.; Bačkor, M.; Kaduková, J. Physiological responses of Matricaria chamomilla to cadmium and copper excess. Environ. Toxicol. 2008, 23, 123–130. [Google Scholar] [CrossRef]
- Deng, H.; Van, B. Electrospray mass spectrometry and UV/visible spectrophotometry studies of aluminum(III)-flavonoid complexes. J. Mass Spectrom. 1998, 33, 1080–1087. [Google Scholar] [CrossRef]

| 0.0 Sr | 0.5 Sr | 2.0 Sr | 4.0 Sr | |
|---|---|---|---|---|
| Shoot biomass (g per plants) | 4.40 ± 0.42 a | 3.74 ± 0.13 a | 4.04 ± 0.33 a | 3.88 ± 0.33 a |
| Root biomass (g per plants) | 2.59 ± 0.11 b | 2.34 ± 0.14 b | 3.03 ± 0.19 ab | 3.37 ± 0.31 a |
| Total Phenolics | Soluble Flavonols | Antioxidant Capacity | ||
|---|---|---|---|---|
| ABTS | DPPH | |||
| Leaves | ||||
| 0.0 Sr | 8.18 ± 0.74 a | 4.48 ± 0.34 a | 21.1 ± 0.77 a | 1.666 ± 0.113 a |
| 0.5 Sr | 7.67 ± 0.88 a | 4.52 ± 1.25 a | 17.6 ± 1.20 ab | 1.509 ± 0.157 ab |
| 2.0 Sr | 8.01 ± 0.83 a | 4.30 ± 0.25 a | 16.7 ± 0.89 ab | 1.314 ± 0.081 ab |
| 4.0 Sr | 9.27 ± 0.73 a | 4.88 ± 0.46 a | 15.3 ± 1.05 b | 1.086 ± 0.119 b |
| Stems | ||||
| 0.0 Sr | 3.14 ± 0.22 a | 0.64 ± 0.05 a | 2.5 ± 0.09 a | 0.635 ± 0.009 a |
| 0.5 Sr | 2.13 ± 0.40 ab | 0.60 ± 0.09 a | 2.1 ± 0.22 ab | 0.481 ± 0.069 ab |
| 2.0 Sr | 2.35 ± 0.20 ab | 0.65 ± 0.04 a | 2.2 ± 0.10 a | 0.565 ± 0.022 a |
| 4.0 Sr | 1.59 ± 0.24 b | 0.53 ± 0.07 a | 1.6 ± 0.06 b | 0.403 ± 0.037 b |
| Seeds | ||||
| 0.0 Sr | 1.96 ± 0.06 a | 0.21 ± 0.01 a | 4.7 ± 0.13 a | 0.417 ± 0.031 a |
| 0.5 Sr | 1.77 ± 0.04 a | 0.19 ± 0.01 a | 4.6 ± 0.12 a | 0.455 ± 0.021 a |
| 2.0 Sr | 1.54 ± 0.13 a | 0.17 ± 0.00 a | 4.5 ± 0.06 a | 0.498 ± 0.032 a |
| 4.0 Sr | 1.78 ± 0.21 a | 0.18 ± 0.02 a | 4.8 ± 0.30 a | 0.537 ± 0.047 a |
| Roots | ||||
| 0.0 Sr | 1.82 ± 0.22 a | 0.39 ± 0.08 a | 1.7 ± 0.19 a | 0.438 ± 0.067 a |
| 0.5 Sr | 1.50 ± 0.28 a | 0.53 ± 0.06 a | 1.3 ± 0.09 ab | 0.383 ± 0.035 a |
| 2.0 Sr | 2.03 ± 0.34 a | 0.53 ± 0.07 a | 1.6 ± 0.12 ab | 0.459 ± 0.049 a |
| 4.0 Sr | 1.28 ± 0.14 a | 0.45 ± 0.06 a | 1.2 ± 0.04 b | 0.321 ± 0.020 a |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dresler, S.; Wójciak-Kosior, M.; Sowa, I.; Strzemski, M.; Sawicki, J.; Kováčik, J.; Blicharski, T. Effect of Long-Term Strontium Exposure on the Content of Phytoestrogens and Allantoin in Soybean. Int. J. Mol. Sci. 2018, 19, 3864. https://doi.org/10.3390/ijms19123864
Dresler S, Wójciak-Kosior M, Sowa I, Strzemski M, Sawicki J, Kováčik J, Blicharski T. Effect of Long-Term Strontium Exposure on the Content of Phytoestrogens and Allantoin in Soybean. International Journal of Molecular Sciences. 2018; 19(12):3864. https://doi.org/10.3390/ijms19123864
Chicago/Turabian StyleDresler, Sławomir, Magdalena Wójciak-Kosior, Ireneusz Sowa, Maciej Strzemski, Jan Sawicki, Jozef Kováčik, and Tomasz Blicharski. 2018. "Effect of Long-Term Strontium Exposure on the Content of Phytoestrogens and Allantoin in Soybean" International Journal of Molecular Sciences 19, no. 12: 3864. https://doi.org/10.3390/ijms19123864
APA StyleDresler, S., Wójciak-Kosior, M., Sowa, I., Strzemski, M., Sawicki, J., Kováčik, J., & Blicharski, T. (2018). Effect of Long-Term Strontium Exposure on the Content of Phytoestrogens and Allantoin in Soybean. International Journal of Molecular Sciences, 19(12), 3864. https://doi.org/10.3390/ijms19123864