Low HIF-1α and low EGFR mRNA Expression Significantly Associate with Poor Survival in Soft Tissue Sarcoma Patients; the Proteins React Differently
Abstract
1. Background
2. Results
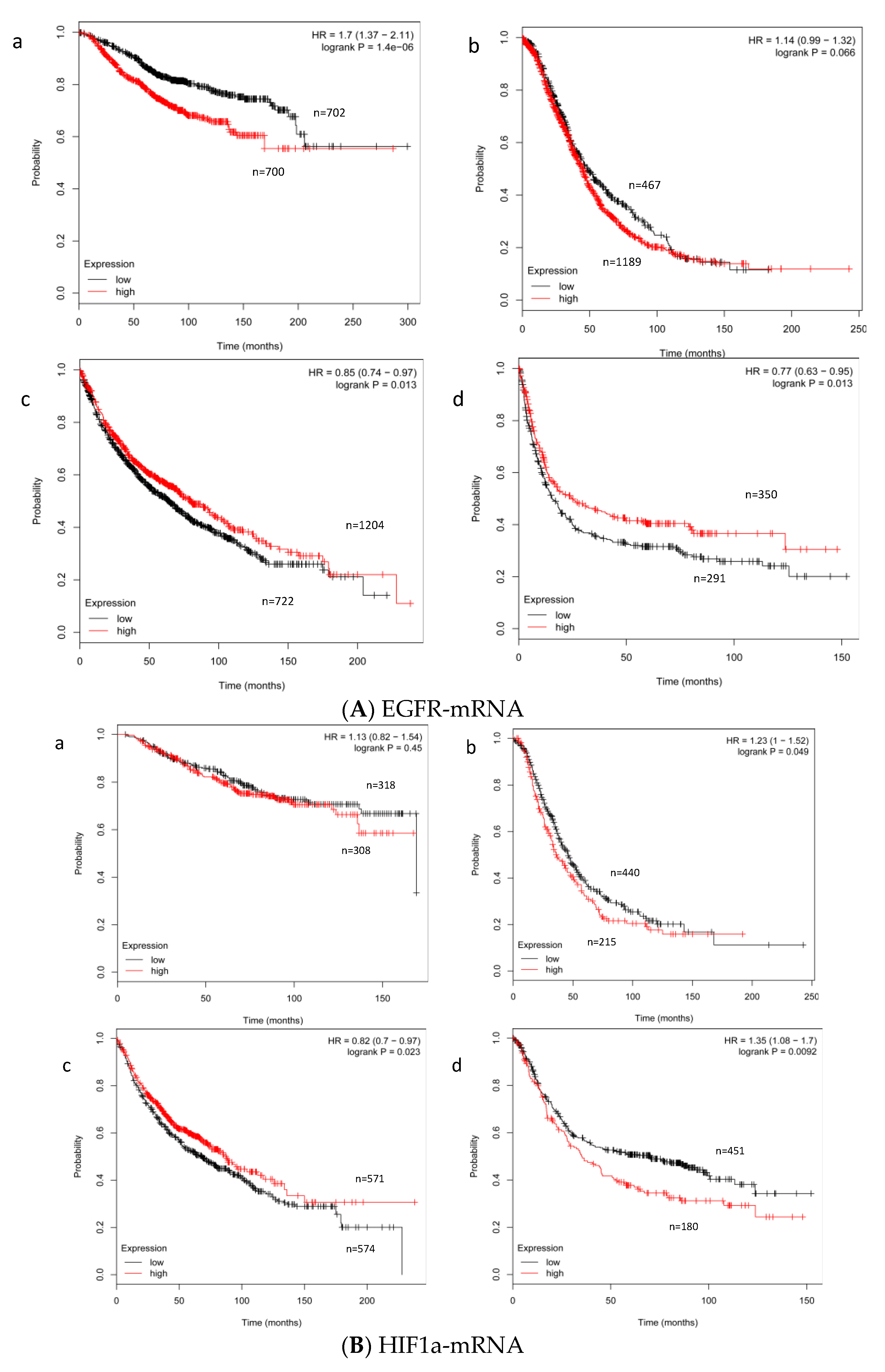
2.1. Expression Level of EGFR- and HIF-1α mRNA in STS
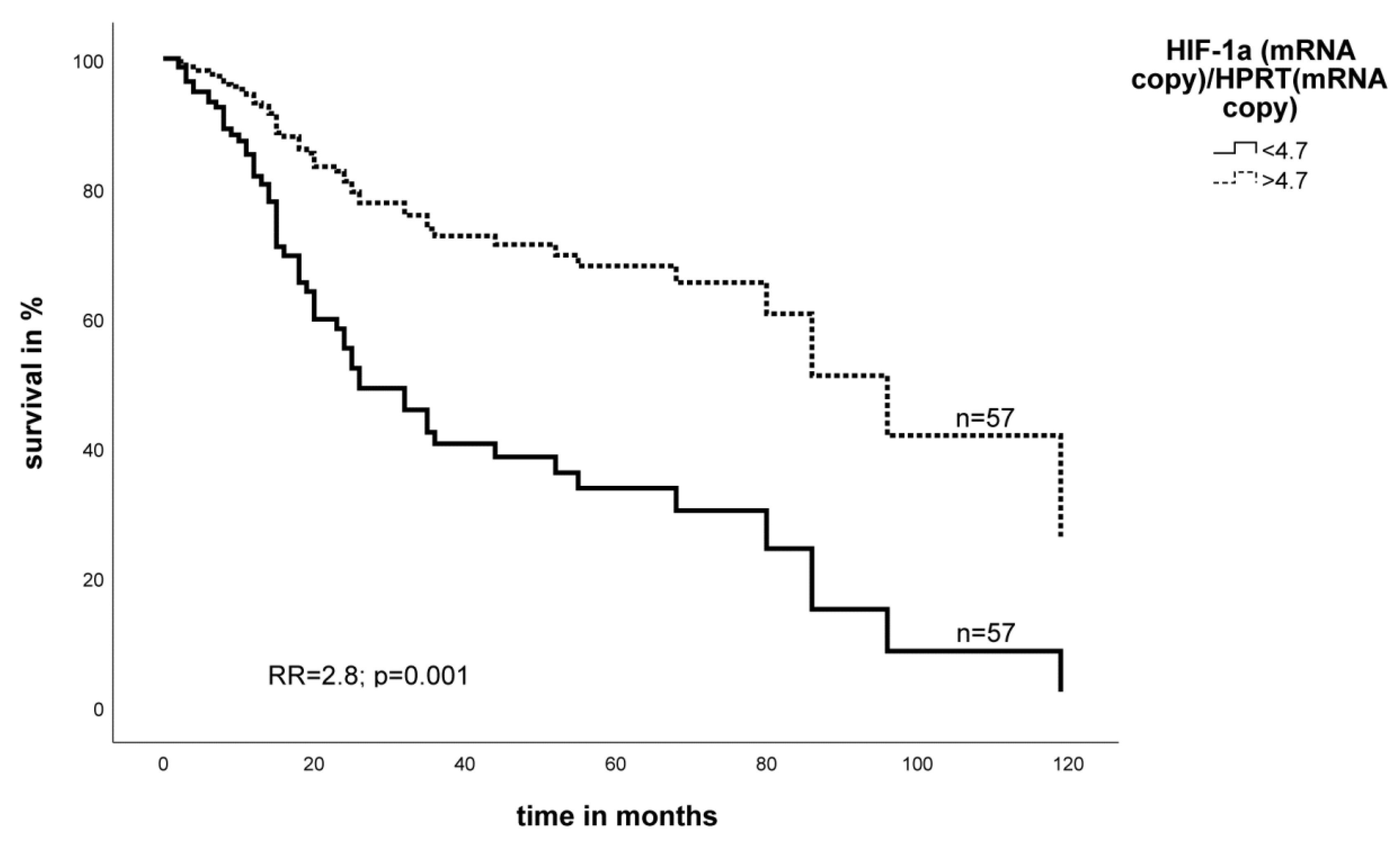
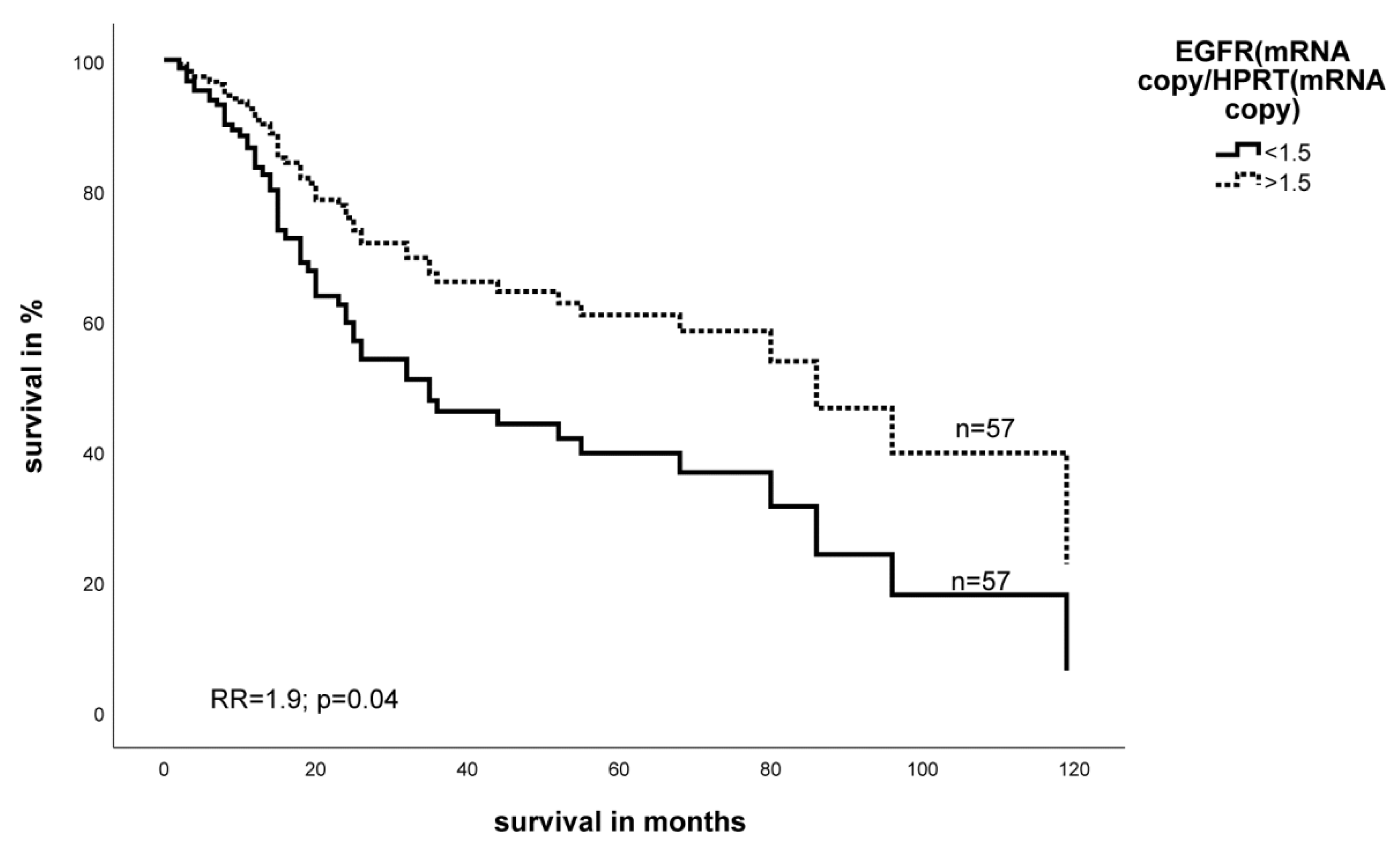
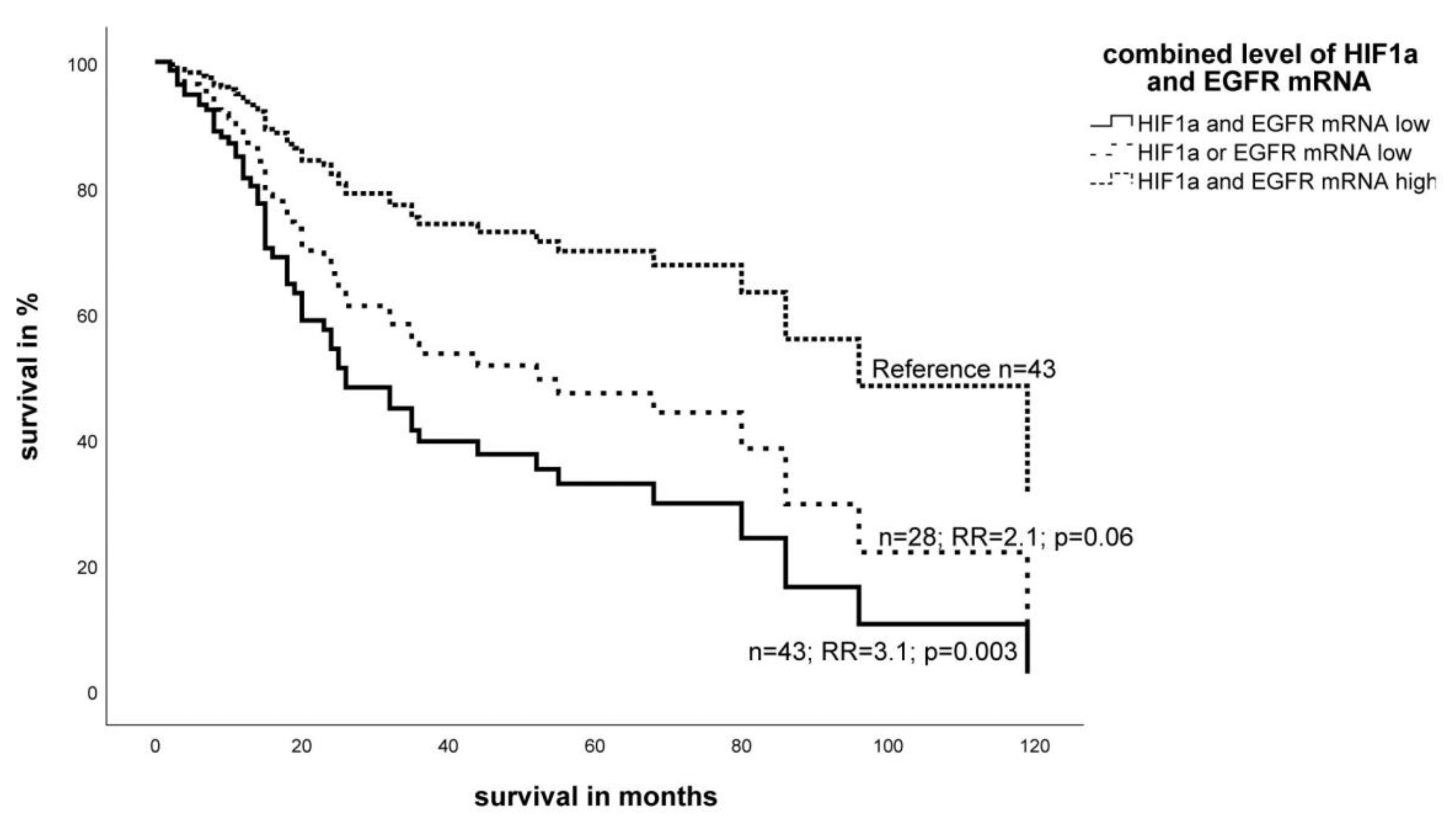
2.2. Effect of the Expression Level of EGFR- and HIF-1α mRNA on Survival
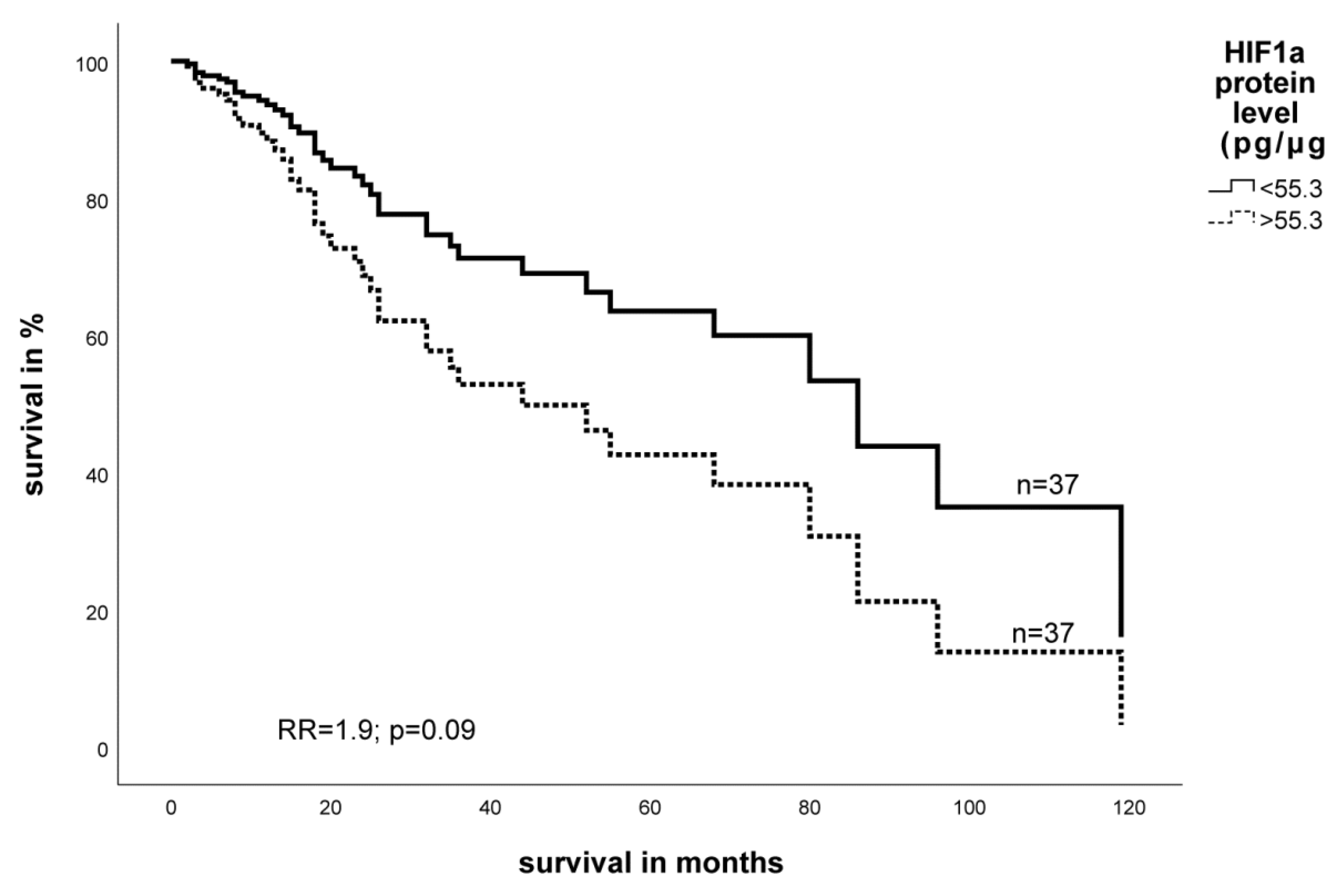
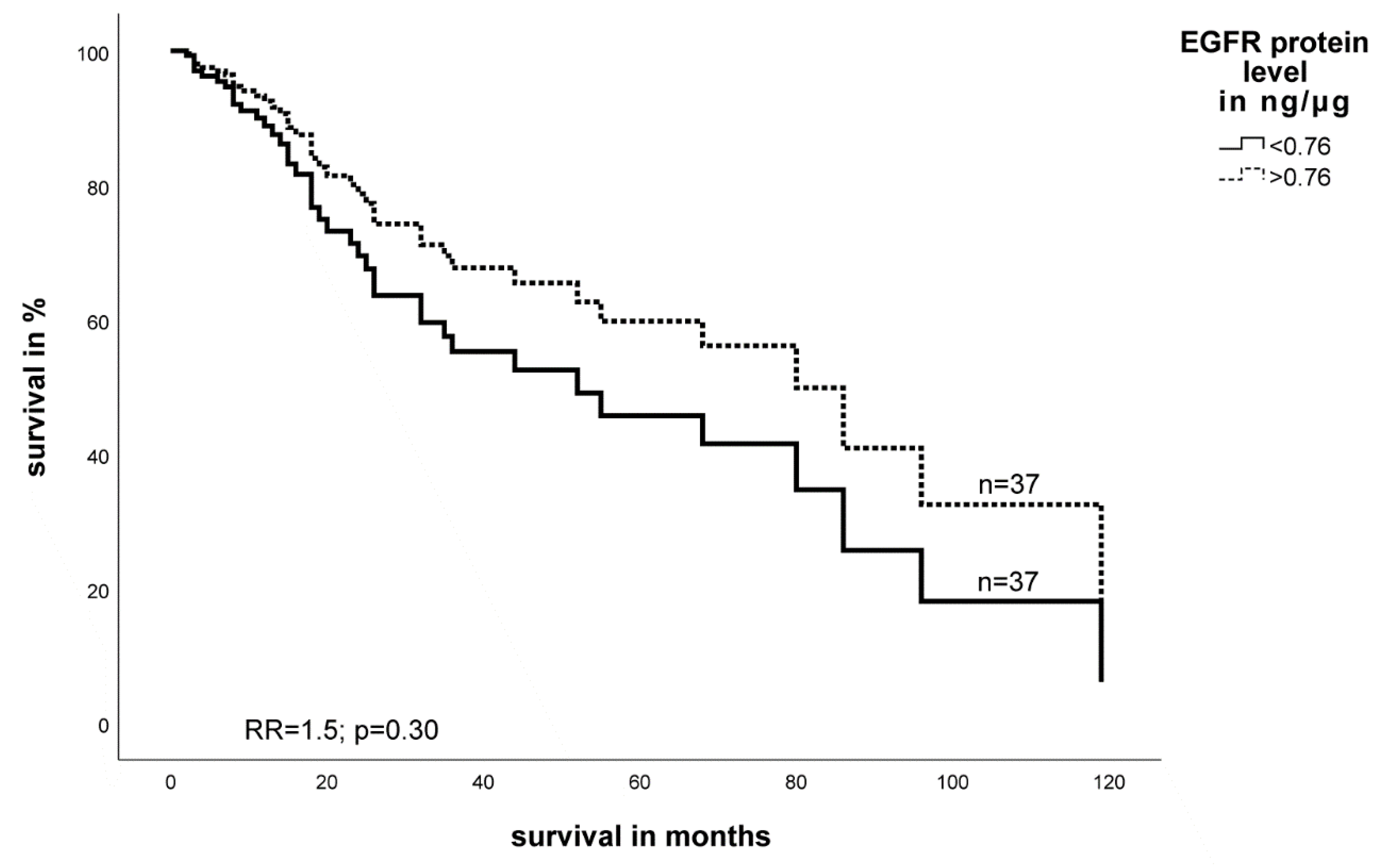
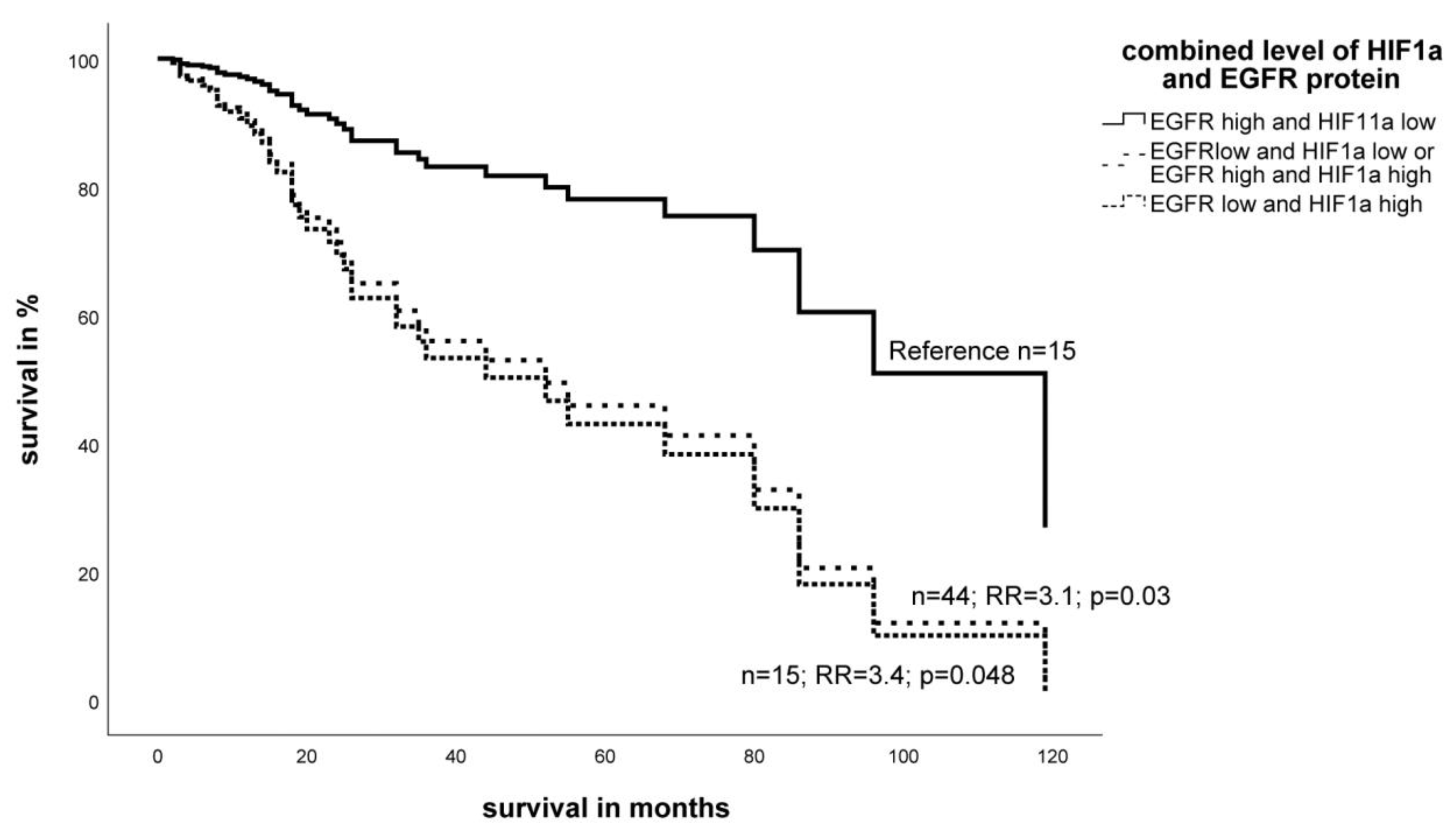
2.3. Expression Level of EGFR- and HIF-1α Protein in STS and Impact on Survival
2.4. Correlation of Biomarker Expression and Clinico-Pathological Data
3. Discussion
4. Material and Methods
4.1. Tissue Samples and Histopathologic Data
4.2. RNA Preparation, cDNA Synthesis and Transcript Analysis by Multiplex Quantitative Fluorescence PCR
4.3. Protein Isolation and ELISA Procedure
5. Statistical Analysis
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- WHO. WHO Classification of Tumours of Soft Tissue and Bone, 4th ed.; This Book Reflects the Views of a Working Group That Convened for a Consensus and Editorial Meeting at the University of Zurich, Switzerland, 18–20 April 2012; International Agency for Research on Cancer: Lyon, French, 2013. [Google Scholar]
- Goldblum, J.R.; Folpe, A.L.; Weiss, S.W.; Enzinger, F.M. Enzinger and Weiss’s Soft Tissue Tumors, 6th ed.; Elsevier: Philadelphia, PA, USA, 2014. [Google Scholar]
- Semenza, G.L. Targeting HIF-1 for cancer therapy. Nat. Rev. Cancer 2003, 3, 721–732. [Google Scholar] [CrossRef] [PubMed]
- Birner, P.; Schindl, M.; Obermair, A.; Plank, C.; Breitenecker, G.; Oberhuber, G. Overexpression of hypoxia-inducible factor 1alpha is a marker for an unfavorable prognosis in early-stage invasive cervical cancer. Cancer Res. 2000, 60, 4693–4696. [Google Scholar] [PubMed]
- Koukourakis, M.I.; Giatromanolaki, A.; Sivridis, E.; Simopoulos, C.; Turley, H.; Talks, K.; Gatter, K.C.; Harris, A.L. Hypoxia-inducible factor (HIF1A and HIF2A), angiogenesis, and chemoradiotherapy outcome of squamous cell head-and-neck cancer. Int. J. Radiat. Oncol. Boil. Phys. 2002, 53, 1192–1202. [Google Scholar] [CrossRef]
- Aebersold, D.M.; Burri, P.; Beer, K.T.; Laissue, J.; Djonov, V.; Greiner, R.H.; Semenza, G.L. Expression of hypoxia-inducible factor-1alpha: A novel predictive and prognostic parameter in the radiotherapy of oropharyngeal cancer. Cancer Res. 2001, 61, 2911–2916. [Google Scholar]
- Volm, M.; Koomägi, R. Hypoxia-inducible factor (HIF-1) and its relationship to apoptosis and proliferation in lung cancer. Anticancer. Res. 2000, 20, 1527–1533. [Google Scholar] [PubMed]
- Fillies, T.; Werkmeister, R.; van Diest, P.J.; Brandt, B.; Joos, U.; Buerger, H. HIF1-alpha overexpression indicates a good prognosis in early stage squamous cell carcinomas of the oral floor. BMC Cancer 2005, 5, 84. [Google Scholar] [CrossRef] [PubMed]
- Lidgren, A.; Hedberg, Y.; Grankvist, K.; Rasmuson, T.; Vasko, J.; Ljungberg, B. The expression of hypoxia-inducible factor 1alpha is a favorable independent prognostic factor in renal cell carcinoma. Clin. Cancer Res. 2005, 11, 1129–1135. [Google Scholar] [PubMed]
- Kim, J.I.; Choi, K.U.; Lee, I.S.; Choi, Y.J.; Kim, W.T.; Shin, D.H.; Kim, K.; Lee, J.H.; Kim, J.Y.; Sol, M.Y. Expression of hypoxic markers and their prognostic significance in soft tissue sarcoma. Oncol. Lett. 2015, 9, 1699–1706. [Google Scholar] [CrossRef]
- Forker, L.; Gaunt, P.; Sioletic, S.; Shenjere, P.; Potter, R.; Roberts, D.; Irlam, J.; Valentine, H.; Hughes, D.; Hughes, A.; et al. The hypoxia marker CAIX is prognostic in the UK phase III VorteX-Biobank cohort: An important resource for translational research in soft tissue sarcoma. Br. J. Cancer 2018, 118, 698–704. [Google Scholar] [CrossRef]
- Smeland, E.; Kilvaer, T.K.; Sorbye, S.; Valkov, A.; Andersen, S.; Bremnes, R.M.; Busund, L.-T.; Donnem, T. Prognostic impacts of hypoxic markers in soft tissue sarcoma. Sarcoma 2012, 2012, 541650. [Google Scholar] [CrossRef]
- Eisinger-Mathason, T.S.K.; Zhang, M.; Qiu, Q.; Skuli, N.; Nakazawa, M.S.; Karakasheva, T.; Mucaj, V.; Shay, J.E.S.; Stangenberg, L.; Sadri, N.; et al. Hypoxia-dependent modification of collagen networks promotes sarcoma metastasis. Cancer Discov. 2013, 3, 1190–1205. [Google Scholar] [CrossRef] [PubMed]
- Francis, P.; Namløs, H.M.; Müller, C.; Edén, P.; Fernebro, J.; Berner, J.-M.; Bjerkehagen, B.; Akerman, M.; Bendahl, P.-O.; Isinger, A.; et al. Diagnostic and prognostic gene expression signatures in 177 soft tissue sarcomas: Hypoxia-induced transcription profile signifies metastatic potential. BMC Genom. 2007, 8, 73. [Google Scholar] [CrossRef]
- Rankin, E.B.; Giaccia, A.J. Hypoxic control of metastasis. Science 2016, 352, 175–180. [Google Scholar] [CrossRef]
- Shintani, K.; Matsumine, A.; Kusuzaki, K.; Matsubara, T.; Satonaka, H.; Wakabayashi, T.; Hoki, Y.; Uchida, A. Expression of hypoxia-inducible factor (HIF)-1alpha as a biomarker of outcome in soft-tissue sarcomas. Virchows Arch. Int. J. Pathol. 2006, 449, 673–681. [Google Scholar] [CrossRef]
- Peng, X.-H.; Karna, P.; Cao, Z.; Jiang, B.-H.; Zhou, M.; Yang, L. Cross-talk between epidermal growth factor receptor and hypoxia-inducible factor-1alpha signal pathways increases resistance to apoptosis by up-regulating survivin gene expression. J. Boil. Chem. 2006, 281, 25903–25914. [Google Scholar] [CrossRef] [PubMed]
- Furlan, D.; Sahnane, N.; Carnevali, I.; Cerutti, R.; Bertoni, F.; Kwee, I.; Uccella, S.; Bertolini, V.; Chiaravalli, A.M.; Capella, C. Up-regulation of the hypoxia-inducible factor-1 transcriptional pathway in colorectal carcinomas. Hum. Pathol. 2008, 39, 1483–1494. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, T.O.; Hsu, F.D.; O’Connell, J.X.; Gilks, C.B.; Sorensen, P.H.; Linn, S.; West, R.B.; Liu, C.L.; Botstein, D.; Brown, P.O.; et al. Tissue Microarray Validation of Epidermal Growth Factor Receptor and SALL2 in Synovial Sarcoma with Comparison to Tumors of Similar Histology. Am. J. Pathol. 2003, 163, 1449–1456. [Google Scholar] [CrossRef]
- Ren, W.; Korchin, B.; Zhu, Q.-S.; Wei, C.; Dicker, A.; Heymach, J.; Lazar, A.; Pollock, R.E.; Lev, D. Epidermal growth factor receptor blockade in combination with conventional chemotherapy inhibits soft tissue sarcoma cell growth in vitro and in vivo. Clin. Cancer Res. 2008, 14, 2785–2795. [Google Scholar] [CrossRef]
- Braun, A.C.; de Mello, C.A.L.; Corassa, M.; Abdallah, E.A.; Urvanegia, A.C.; Alves, V.S.; Flores, B.C.T.C.P.; Díaz, M.; Nicolau, U.R.; Silva, V.S.E.; et al. EGFR expression in circulating tumor cells from high-grade metastatic soft tissue sarcomas. Cancer Boil. Ther. 2018, 19, 454–460. [Google Scholar] [CrossRef] [PubMed]
- Henderson, T.; Chen, M.; Darrow, M.A.; Li, C.-S.; Chiu, C.-L.; Monjazeb, A.M.; Murphy, W.J.; Canter, R.J. Alterations in cancer stem-cell marker CD44 expression predict oncologic outcome in soft-tissue sarcomas. J. Surg. Res. 2018, 223, 207–214. [Google Scholar] [CrossRef]
- Hoffmann, A.-C.; Hoffman, A.-C.; Danenberg, K.D.; Taubert, H.; Danenberg, P.V.; Wuerl, P. A three-gene signature for outcome in soft tissue sarcoma. Clin. Cancer Res. 2009, 15, 5191–5198. [Google Scholar] [CrossRef] [PubMed]
- Matsuyama, T.; Nakanishi, K.; Hayashi, T.; Yoshizumi, Y.; Aiko, S.; Sugiura, Y.; Tanimoto, T.; Uenoyama, M.; Ozeki, Y.; Maehara, T. Expression of hypoxia-inducible factor-1alpha in esophageal squamous cell carcinoma. Cancer Sci. 2005, 96, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Carroll, P.A.; Kenerson, H.L.; Yeung, R.S.; Lagunoff, M. Latent Kaposi’s sarcoma-associated herpesvirus infection of endothelial cells activates hypoxia-induced factors. J. Virol. 2006, 80, 10802–10812. [Google Scholar] [CrossRef] [PubMed]
- Secades, P.; Rodrigo, J.P.; Hermsen, M.; Alvarez, C.; Suarez, C.; Chiara, M.-D. Increase in gene dosage is a mechanism of HIF-1alpha constitutive expression in head and neck squamous cell carcinomas. Genes Chromosom. Cancer 2009, 48, 441–454. [Google Scholar] [CrossRef]
- Eskandani, M.; Abdolalizadeh, J.; Hamishehkar, H.; Nazemiyeh, H.; Barar, J. Galbanic acid inhibits HIF-1α expression via EGFR/HIF-1α pathway in cancer cells. Fitoterapia 2015, 101, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Sato, O.; Wada, T.; Kawai, A.; Yamaguchi, U.; Makimoto, A.; Kokai, Y.; Yamashita, T.; Chuman, H.; Beppu, Y.; Tani, Y.; et al. Expression of epidermal growth factor receptor, ERBB2 and KIT in adult soft tissue sarcomas: A clinicopathologic study of 281 cases. Cancer 2005, 103, 1881–1890. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.-L.; Hannan, M.T.; Russell, P.J.; Crowe, P.J. Expression of HER1/EGFR protein in human soft tissue sarcomas. Eur. J. Surg. Oncol. 2006, 32, 466–468. [Google Scholar] [CrossRef]
- Bode, B.; Frigerio, S.; Behnke, S.; Senn, B.; Odermatt, B.; Zimmermann, D.R.; Moch, H. Mutations in the tyrosine kinase domain of the EGFR gene are rare in synovial sarcoma. Mod. Pathol. 2006, 19, 541–547. [Google Scholar] [CrossRef]
- Yang, J.-L.; Gupta, R.D.; Goldstein, D.; Crowe, P.J. Significance of Phosphorylated Epidermal Growth Factor Receptor and Its Signal Transducers in Human Soft Tissue Sarcoma. Int. J. Mol. Sci. 2017, 18, 1159. [Google Scholar] [CrossRef]
- Miserocchi, G.; de Vita, A.; Mercatali, L.; Recine, F.; Liverani, C.; Spadazzi, C.; Pieri, F.; Riva, N.; Bongiovanni, A.; Casadei, R.; et al. Characterization and Drug Sensitivity of a New High-Grade Myxofibrosarcoma Cell Line. Cells 2018, 7, 186. [Google Scholar] [CrossRef]
- Wang, X.; Goldstein, D.; Crowe, P.J.; Yang, M.; Garrett, K.; Zeps, N.; Yang, J.-L. Overcoming resistance of targeted EGFR monotherapy by inhibition of STAT3 escape pathway in soft tissue sarcoma. Oncotarget 2016, 7, 21496–21509. [Google Scholar] [CrossRef] [PubMed]
- Wichmann, H.; Güttler, A.; Bache, M.; Taubert, H.; Vetter, M.; Würl, P.; Holzhausen, H.J.; Eckert, A.W.; Kappler, M.; Vordermark, D. Inverse prognostic impact of ErbB2 mRNA and protein expression level in tumors of soft tissue sarcoma patients. Strahlenther. Onkol. 2014, 190, 912–918. [Google Scholar] [CrossRef] [PubMed]
- Dziadziuszko, R.; Witta, S.E.; Cappuzzo, F.; Park, S.; Tanaka, K.; Danenberg, P.V.; Barón, A.E.; Crino, L.; Franklin, W.A.; Bunn, P.A.; et al. Epidermal growth factor receptor messenger RNA expression, gene dosage, and gefitinib sensitivity in non-small cell lung cancer. Clin. Cancer Res. 2006, 12, 3078–3084. [Google Scholar] [CrossRef] [PubMed]
- Brabender, J.; Danenberg, K.D.; Metzger, R.; Schneider, P.M.; Park, J.; Salonga, D.; Hölscher, A.H.; Danenberg, P.V. Epidermal growth factor receptor and HER2-neu mRNA expression in non-small cell lung cancer Is correlated with survival. Clin. Cancer Res. 2001, 7, 1850–1855. [Google Scholar] [PubMed]
- Scrideli, C.A.; Carlotti, C.G.; Mata, J.F.; Neder, L.; Machado, H.R.; Oba-Sinjo, S.M.; Rosemberg, S.; Marie, S.K.N.; Tone, L.G. Prognostic significance of co-overexpression of the EGFR/IGFBP-2/HIF-2A genes in astrocytomas. J. Neuro-Oncol. 2007, 83, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Koutras, A.K.; Kalogeras, K.T.; Dimopoulos, M.-A.; Wirtz, R.M.; Dafni, U.; Briasoulis, E.; Pectasides, D.; Gogas, H.; Christodoulou, C.; Aravantinos, G.; et al. Evaluation of the prognostic and predictive value of HER family mRNA expression in high-risk early breast cancer: A Hellenic Cooperative Oncology Group (HeCOG) study. Br. J. Cancer 2008, 99, 1775–1785. [Google Scholar] [CrossRef] [PubMed]
- Zhong, H.; Chiles, K.; Feldser, D.; Laughner, E.; Hanrahan, C.; Georgescu, M.M.; Simons, J.W.; Semenza, G.L. Modulation of hypoxia-inducible factor 1alpha expression by the epidermal growth factor/phosphatidylinositol 3-kinase/PTEN/AKT/FRAP pathway in human prostate cancer cells: Implications for tumor angiogenesis and therapeutics. Cancer Res. 2000, 60, 1541–1545. [Google Scholar]
- Wang, Y.; Roche, O.; Yan, M.S.; Finak, G.; Evans, A.J.; Metcalf, J.L.; Hast, B.E.; Hanna, S.C.; Wondergem, B.; Furge, K.A.; et al. Regulation of endocytosis via the oxygen-sensing pathway. Nat. Med. 2009, 15, 319–324. [Google Scholar] [CrossRef]
- Mace, T.A.; Collins, A.L.; Wojcik, S.E.; Croce, C.M.; Lesinski, G.B.; Bloomston, M. Hypoxia induces the overexpression of microRNA-21 in pancreatic cancer cells. J. Surg. Res. 2013, 184, 855–860. [Google Scholar] [CrossRef]
- Blick, C.; Ramachandran, A.; McCormick, R.; Wigfield, S.; Cranston, D.; Catto, J.; Harris, A.L. Identification of a hypoxia-regulated miRNA signature in bladder cancer and a role for miR-145 in hypoxia-dependent apoptosis. Br. J. Cancer 2015, 113, 634–644. [Google Scholar] [CrossRef]
- Zhao, Q.; Li, Y.; Tan, B.-B.; Fan, L.-Q.; Yang, P.-G.; Tian, Y. HIF-1α Induces Multidrug Resistance in Gastric Cancer Cells by Inducing MiR-27a. PLoS ONE 2015, 10, e0132746. [Google Scholar] [CrossRef] [PubMed]
- Cho, W.C.S.; Chow, A.S.C.; Au, J.S.K. MiR-145 inhibits cell proliferation of human lung adenocarcinoma by targeting EGFR and NUDT1. RNA Boil. 2011, 8, 125–131. [Google Scholar] [CrossRef]
- Wang, W.; Cheng, B.; Miao, L.; Mei, Y.; Wu, M. Mutant p53-R273H gains new function in sustained activation of EGFR signaling via suppressing miR-27a expression. Cell Death Dis. 2013, 4, e574. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Ren, Y.; Moore, L.; Mei, M.; You, Y.; Xu, P.; Wang, B.; Wang, G.; Jia, Z.; Pu, P.; et al. Downregulation of miR-21 inhibits EGFR pathway and suppresses the growth of human glioblastoma cells independent of PTEN status. Lab. Investig. A J. Tech. Methods Pathol. 2010, 90, 144–155. [Google Scholar] [CrossRef] [PubMed]
- Varshney, J.; Subramanian, S. MicroRNAs as potential target in human bone and soft tissue sarcoma therapeutics. Front. Mol. Biosci. 2015, 2, 31. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Shen, J.K.; Choy, E.; Yu, Z.; Hornicek, F.J.; Duan, Z. The emerging roles and therapeutic potential of microRNAs (miRs) in liposarcoma. Discov. Med. 2015, 20, 311–324. [Google Scholar] [PubMed]
- Subramanian, S.; Kartha, R.V. MicroRNA-mediated gene regulations in human sarcomas. Cell. Mol. Life Sci. 2012, 69, 3571–3585. [Google Scholar] [CrossRef]
- Min, L.; Garbutt, C.; Tu, C.; Hornicek, F.; Duan, Z. Potentials of Long Noncoding RNAs (LncRNAs) in Sarcoma: From Biomarkers to Therapeutic Targets. Int. J. Mol. Sci. 2017, 18, 731. [Google Scholar] [CrossRef]
- Cerniglia, G.J.; Pore, N.; Tsai, J.H.; Schultz, S.; Mick, R.; Choe, R.; Xing, X.; Durduran, T.; Yodh, A.G.; Evans, S.M.; et al. Epidermal growth factor receptor inhibition modulates the microenvironment by vascular normalization to improve chemotherapy and radiotherapy efficacy. PLoS ONE 2009, 4, e6539. [Google Scholar] [CrossRef]
- Rho, J.K.; Choi, Y.J.; Lee, J.K.; Ryoo, B.-Y.; Na, I.I.; Yang, S.H.; Kim, C.H.; Yoo, Y.D.; Lee, J.C. Gefitinib circumvents hypoxia-induced drug resistance by the modulation of HIF-1alpha. Oncol. Rep. 2009, 21, 801–807. [Google Scholar]
- Luwor, R.B.; Lu, Y.; Li, X.; Mendelsohn, J.; Fan, Z. The antiepidermal growth factor receptor monoclonal antibody cetuximab/C225 reduces hypoxia-inducible factor-1 alpha, leading to transcriptional inhibition of vascular endothelial growth factor expression. Oncogene 2005, 24, 4433–4441. [Google Scholar] [CrossRef] [PubMed]
- Ray-Coquard, I.; Le Cesne, A.; Whelan, J.S.; Schoffski, P.; Bui, B.N.; Verweij, J.; Marreaud, S.; van Glabbeke, M.; Hogendoorn, P.; Blay, J.-Y. A phase II study of gefitinib for patients with advanced HER-1 expressing synovial sarcoma refractory to doxorubicin-containing regimens. Oncologist 2008, 13, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Kappler, M.; Köhler, T.; Kampf, C.; Diestelkötter, P.; Würl, P.; Schmitz, M.; Bartel, F.; Lautenschläger, C.; Rieber, E.P.; Schmidt, H.; et al. Increased survivin transcript levels: An independent negative predictor of survival in soft tissue sarcoma patients. Int. J. Cancer 2001, 95, 360–363. [Google Scholar] [PubMed]
- Greither, T.; Wedler, A.; Rot, S.; Keßler, J.; Kehlen, A.; Holzhausen, H.-J.; Bache, M.; Würl, P.; Taubert, H.; Kappler, M. CMG2 Expression Is an Independent Prognostic Factor for Soft Tissue Sarcoma Patients. Int. J. Mol. Sci. 2017, 18, 2648. [Google Scholar] [CrossRef] [PubMed]
| Total (n = 114) | Low EGFR (n = 57) | High EGFR (n = 57) | Low HIF-1α (n = 57) | High HIF-1α (n = 57) | |
|---|---|---|---|---|---|
| p = 0.25 | p = 0.70 | ||||
| Men/Women | 49/65 | 28/29 | 21/36 | 26/31 | 23/34 |
| Tumor type | p = 0.62 | p = 0.80 | |||
| liposarcoma | 26 | 15 | 11 | 15 | 11 |
| fibrosarcoma | 7 | 2 | 5 | 3 | 4 |
| UPS | 27 | 10 | 17 | 11 | 16 |
| NS | 11 | 6 | 5 | 6 | 5 |
| RMS | 8 | 5 | 3 | 5 | 3 |
| LMS | 19 | 11 | 8 | 10 | 9 |
| Other STS | 16 | 8 | 8 | 7 | 9 |
| Tumor grade | p = 0.51 | p = 0.88 | |||
| I | 19 | 10 | 9 | 10 | 9 |
| II | 49 | 26 | 23 | 23 | 26 |
| III | 46 | 21 | 25 | 24 | 22 |
| Tumor stage | p = 0.91 | p = 0.79 | |||
| I | 16 | 7 | 9 | 8 | 8 |
| II | 47 | 26 | 21 | 24 | 23 |
| II | 39 | 16 | 23 | 20 | 19 |
| IV | 12 | 8 | 4 | 5 | 7 |
| Tumor localization | p = 0.69 | p = 0.67 | |||
| extremities | 73 | 35 | 38 | 37 | 36 |
| thorax | 11 | 7 | 4 | 7 | 4 |
| head | 4 | 2 | 2 | 2 | 2 |
| abdomen | 24 | 12 | 12 | 10 | 14 |
| multiple | 2 | 1 | 1 | 1 | 1 |
| Relapses | p = 0.004 | p = 0.18 | |||
| no | 68 | 42 | 26 | 38 | 30 |
| yes | 46 | 15 | 31 | 19 | 27 |
| Lymph node status | p = 0.44 | p = 1.00 | |||
| N0 | 107 | 52 | 55 | 54 | 53 |
| N1 or higher | 7 | 5 | 2 | 3 | 4 |
| Distant metastasis | p = 0.49 | p = 1.00 | |||
| M0 | 105 | 51 | 54 | 53 | 52 |
| M1 | 9 | 6 | 3 | 4 | 5 |
| Tumor resection | p = 0.16 | p = 0.84 | |||
| radical (R0) | 78 | 43 | 35 | 40 | 38 |
| not radical (R1) | 36 | 14 | 22 | 17 | 19 |
| Patients at follow-up | p = 0.71 | p = 0.13 | |||
| alive | 57 | 27 | 30 | 24 | 33 |
| dead | 57 | 30 | 27 | 33 | 24 |
| Total (n = 74) | Low EGFR (n = 37) | High EGFR (n = 37) | Low HIF-1α (n = 37) | High HIF-1α (n = 37) | |
|---|---|---|---|---|---|
| p = 0.64 | p = 0.35 | ||||
| Men/Women | 37/37 | 20/17 | 17/20 | 21/16 | 16/21 |
| Tumor type | p = 0.04 | p = 0.45 | |||
| liposarcoma | 19 | 14 | 5 | 12 | 7 |
| fibrosarcoma | 2 | 0 | 2 | 1 | 1 |
| UPS | 16 | 7 | 9 | 6 | 10 |
| NS | 5 | 3 | 2 | 2 | 3 |
| RMS | 6 | 5 | 1 | 3 | 3 |
| LMS | 14 | 4 | 10 | 9 | 5 |
| Other STS | 12 | 4 | 8 | 4 | 8 |
| Tumor grade | p = 0.65 | p = 0.61 | |||
| I | 13 | 9 | 4 | 8 | 5 |
| II | 32 | 13 | 19 | 15 | 17 |
| III | 29 | 15 | 14 | 14 | 15 |
| Tumor stage | p = 0.94 | p = 0.60 | |||
| I | 10 | 8 | 2 | 7 | 3 |
| II | 31 | 12 | 19 | 14 | 17 |
| II | 24 | 10 | 14 | 11 | 13 |
| IV | 9 | 7 | 2 | 5 | 4 |
| Tumor localization | p = 0.22 | p = 0.12 | |||
| extremities | 47 | 25 | 22 | 20 | 27 |
| thorax | 6 | 5 | 1 | 4 | 2 |
| head | 2 | 2 | 0 | 1 | 1 |
| abdomen | 17 | 4 | 13 | 11 | 6 |
| multiple | 2 | 1 | 1 | 1 | 1 |
| Relapses | p = 0.34 | p = 0.63 | |||
| no | 45 | 25 | 20 | 24 | 21 |
| yes | 29 | 12 | 17 | 13 | 16 |
| Lymph node status | p = 0.11 | p = 0.62 | |||
| N0 | 70 | 33 | 37 | 34 | 36 |
| N1 or higher | 4 | 4 | 0 | 3 | 1 |
| Distant metastasis | p = 0.43 | p = 1.00 | |||
| M0 | 67 | 32 | 35 | 33 | 34 |
| M1 | 7 | 5 | 2 | 4 | 3 |
| Tumor resection | p = 0.14 | p = 1.00 | |||
| radical (R0) | 49 | 28 | 21 | 24 | 25 |
| not radical (R1) | 25 | 9 | 16 | 13 | 12 |
| Patients at follow-up | p = 1.00 | p = 0.48 | |||
| alive | 34 | 17 | 17 | 19 | 15 |
| dead | 40 | 20 | 20 | 18 | 22 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rot, S.; Taubert, H.; Bache, M.; Greither, T.; Würl, P.; Holzhausen, H.-J.; Eckert, A.W.; Vordermark, D.; Kappler, M. Low HIF-1α and low EGFR mRNA Expression Significantly Associate with Poor Survival in Soft Tissue Sarcoma Patients; the Proteins React Differently. Int. J. Mol. Sci. 2018, 19, 3842. https://doi.org/10.3390/ijms19123842
Rot S, Taubert H, Bache M, Greither T, Würl P, Holzhausen H-J, Eckert AW, Vordermark D, Kappler M. Low HIF-1α and low EGFR mRNA Expression Significantly Associate with Poor Survival in Soft Tissue Sarcoma Patients; the Proteins React Differently. International Journal of Molecular Sciences. 2018; 19(12):3842. https://doi.org/10.3390/ijms19123842
Chicago/Turabian StyleRot, Swetlana, Helge Taubert, Matthias Bache, Thomas Greither, Peter Würl, Hans-Jürgen Holzhausen, Alexander W. Eckert, Dirk Vordermark, and Matthias Kappler. 2018. "Low HIF-1α and low EGFR mRNA Expression Significantly Associate with Poor Survival in Soft Tissue Sarcoma Patients; the Proteins React Differently" International Journal of Molecular Sciences 19, no. 12: 3842. https://doi.org/10.3390/ijms19123842
APA StyleRot, S., Taubert, H., Bache, M., Greither, T., Würl, P., Holzhausen, H.-J., Eckert, A. W., Vordermark, D., & Kappler, M. (2018). Low HIF-1α and low EGFR mRNA Expression Significantly Associate with Poor Survival in Soft Tissue Sarcoma Patients; the Proteins React Differently. International Journal of Molecular Sciences, 19(12), 3842. https://doi.org/10.3390/ijms19123842







