UBC® Rapid Test—A Urinary Point-of-Care (POC) Assay for Diagnosis of Bladder Cancer with a focus on Non-Muscle Invasive High-Grade Tumors: Results of a Multicenter-Study
Abstract
1. Introduction
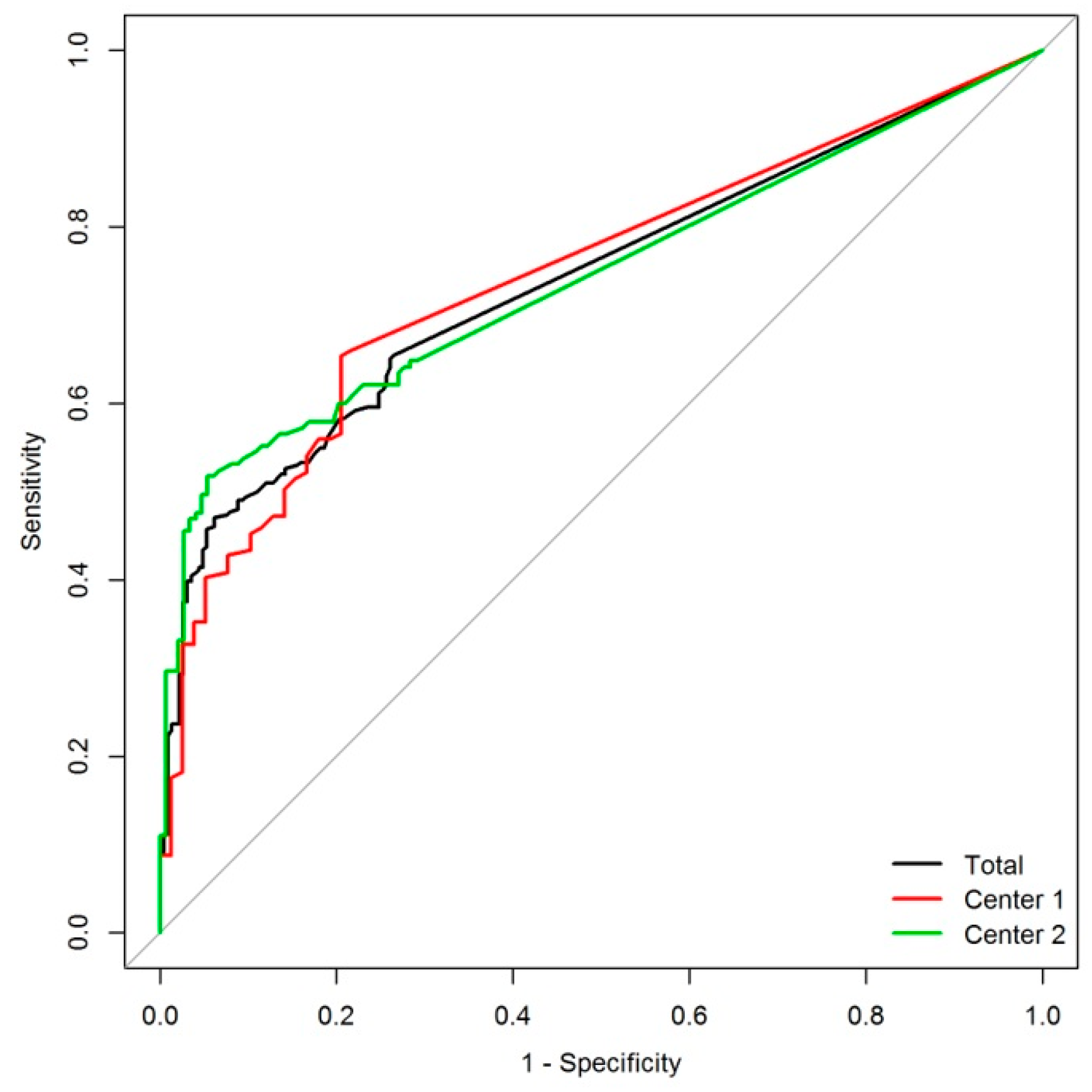
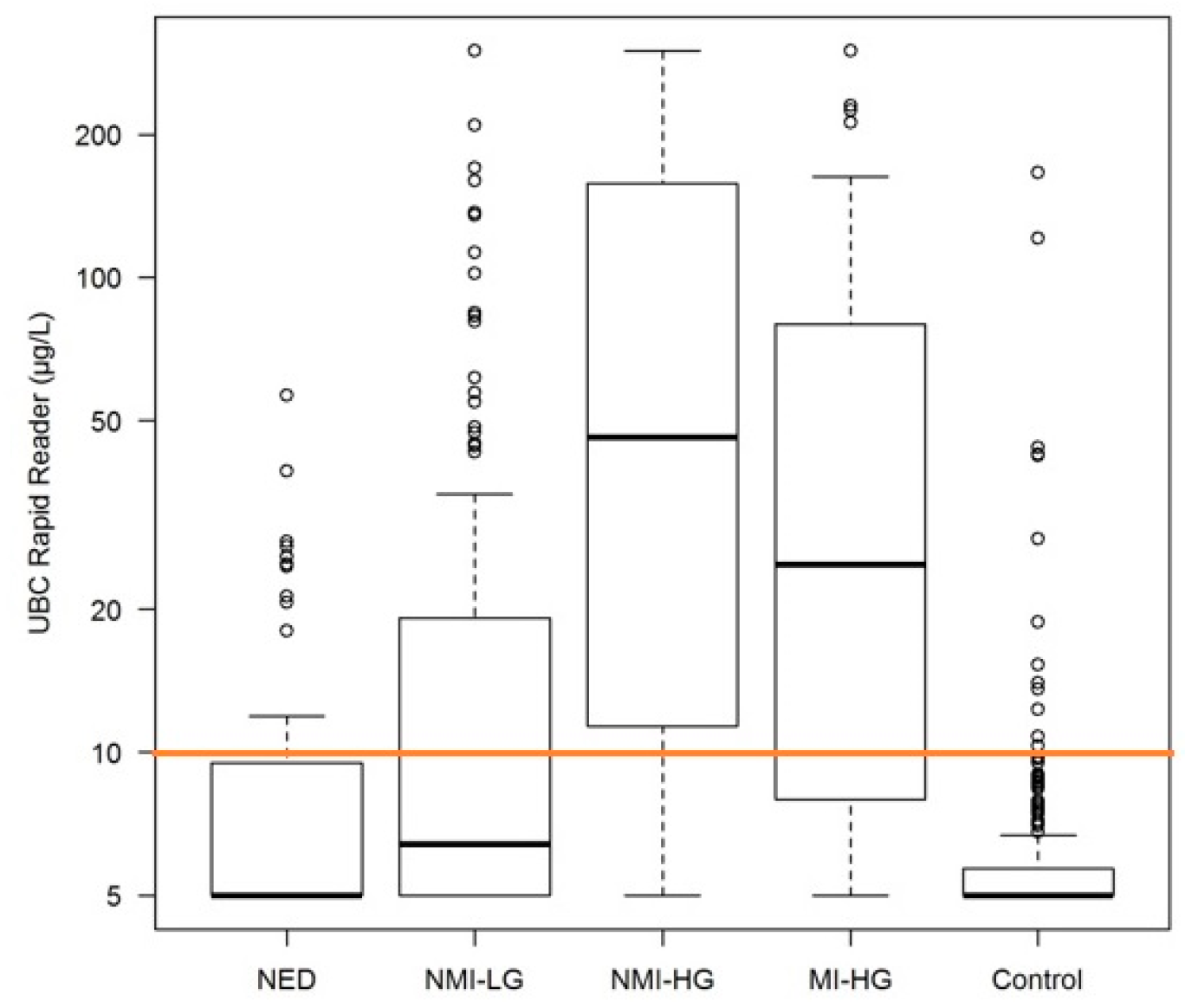
2. Results
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Procedure
4.3. Statistical Analysis
4.4. Ethics
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ferlay, J.; Steliarova-Foucher, E.; Lortet-Tieulent, J.; Rosso, S.; Coebergh, J.W.; Comber, H.; Forman, D.; Bray, F. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries in 2012. Eur. J. Cancer 2013, 49, 1374–1403. [Google Scholar] [CrossRef] [PubMed]
- Witjes, J.A.; Comperat, E.; Cowan, N.C.; de Santis, M.; Gakis, G.; Lebret, T.; Ribal, M.J.; Van der Heijden, A.G.; Sherif, A. EAU guidelines on muscle-invasive and metastatic bladder cancer: Summary of the 2013 guidelines. Eur. Urol. 2014, 65, 778–792. [Google Scholar] [CrossRef] [PubMed]
- Lotan, Y.; Roehrborn, C.G. Sensitivity and specificity of commonly available bladder tumor markers versus cytology: Results of a comprehensive literature review and meta-analyses. Urology 2003, 61, 109–118. [Google Scholar] [CrossRef]
- Southgate, J.; Harnden, P.; Trejdosiewicz, L.K. Cytokeratin expression patterns in normal and malignant urothelium: A review of the biological and diagnostic implications. Histol. Histopathol. 1999, 14, 657–664. [Google Scholar] [PubMed]
- Lokeshwar, V.B.; Habuchi, T.; Grossman, H.B.; Murphy, W.M.; Hautmann, S.H.; Hemstreet, G.P., III; Bono, A.V.; Getzenberg, R.H.; Goebell, P.; Schmitz-Drager, B.J.; et al. Bladder tumor markers beyond cytology: International Consensus Panel on bladder tumor markers. Urology 2005, 66, 35–63. [Google Scholar] [CrossRef] [PubMed]
- Siracusano, S.; Niccolini, B.; Knez, R.; Tiberio, A.; Benedetti, E.; Bonin, S.; Ciciliato, S.; Pappagallo, G.L.; Belgrano, E.; Stanta, G. The simultaneous use of telomerase, cytokeratin 20 and CD4 for bladder cancer detection in urine. Eur. Urol. 2005, 47, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Hodges, K.B.; Lopez-Beltran, A.; Davidson, D.D.; Montironi, R.; Cheng, L. Urothelial dysplasia and other flat lesions of the urinary bladder: Clinicopathologic and molecular features. Hum. Pathol. 2010, 41, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, G.L.; Lorenzo-Gomez, M.F.; Hautmann, S.H.; Friedrich, M.G.; Ekici, S.; Huland, H.; Lokeshwar, V. A side by side comparison of cytology and biomarkers for bladder cancer detection. J. Urol. 2004, 172, 1123–1126. [Google Scholar] [CrossRef] [PubMed]
- Ritter, R.; Hennenlotter, J.; Kuhs, U.; Hofmann, U.; Aufderklamm, S.; Blutbacher, P.; Deja, A.; Hohneder, A.; Gerber, V.; Gakis, G.; et al. Evaluation of a new quantitative point-of-care test platform for urine-based detection of bladder cancer. Urol. Oncol. 2014, 32, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Ecke, T.H.; Weiss, S.; Stephan, C.; Hallmann, S.; Barski, D.; Otto, T.; Gerullis, H. UBC® Rapid Test for detection of carcinoma in situ for bladder cancer. Tumour Biol. 2017, 39, 1010428317701624. [Google Scholar] [CrossRef] [PubMed]
- Shariat, S.F.; Casella, R.; Wians, F.H., Jr.; Ashfaq, R.; Balko, J.; Sulser, T.; Gasser, T.C.; Sagalowsky, A.I. Risk stratification for bladder tumor recurrence, stage and grade by urinary nuclear matrix protein 22 and cytology. Eur. Urol. 2004, 45, 304–313, author reply 313. [Google Scholar] [CrossRef] [PubMed]
- Ecke, T.H.; Arndt, C.; Stephan, C.; Hallmann, S.; Lux, O.; Otto, T.; Ruttloff, J.; Gerullis, H. Preliminary Results of a Multicentre Study of the UBC Rapid Test for Detection of Urinary Bladder Cancer. Anticancer Res. 2015, 35, 2651–2655. [Google Scholar] [PubMed]
- Gleichenhagen, J.; Arndt, C.; Casjens, S.; Meinig, C.; Gerullis, H.; Raiko, I.; Bruning, T.; Ecke, T.; Johnen, G. Evaluation of a New Survivin ELISA and UBC® Rapid for the Detection of Bladder Cancer in Urine. Int. J. Mol. Sci. 2018, 19, 226. [Google Scholar] [CrossRef] [PubMed]
- Styrke, J.; Henriksson, H.; Ljungberg, B.; Hasan, M.; Silfverberg, I.; Einarsson, R.; Malmstrom, P.U.; Sherif, A. Evaluation of the diagnostic accuracy of UBC® Rapid in bladder cancer: A Swedish multicentre study. Scand. J. Urol. 2017, 51, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Carbayo, M.; Herrero, E.; Megias, J.; Mira, A.; Soria, F. Initial evaluation of the new urinary bladder cancer rapid test in the detection of transitional cell carcinoma of the bladder. Urology 1999, 54, 656–661. [Google Scholar] [CrossRef]
- Mian, C.; Lodde, M.; Haitel, A.; Vigl, E.E.; Marberger, M.; Pycha, A. Comparison of the monoclonal UBC-ELISA test and the NMP22 ELISA test for the detection of urothelial cell carcinoma of the bladder. Urology 2000, 55, 223–226. [Google Scholar] [CrossRef]
- Hakenberg, O.W.; Fuessel, S.; Richter, K.; Froehner, M.; Oehlschlaeger, S.; Rathert, P.; Meye, A.; Wirth, M.P. Qualitative and quantitative assessment of urinary cytokeratin 8 and 18 fragments compared with voided urine cytology in diagnosis of bladder carcinoma. Urology 2004, 64, 1121–1126. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Carbayo, M.; Herrero, E.; Megias, J.; Mira, A.; Espasa, A.; Chinchilla, V.; Soria, F. Initial evaluation of the diagnostic performance of the new urinary bladder cancer antigen test as a tumor marker for transitional cell carcinoma of the bladder. J. Urol. 1999, 161, 1110–1115. [Google Scholar] [CrossRef]
- Babjuk, M.; Kostirova, M.; Mudra, K.; Pecher, S.; Smolova, H.; Pecen, L.; Ibrahim, Z.; Dvoracek, J.; Jarolim, L.; Novak, J.; et al. Qualitative and quantitative detection of urinary human complement factor H-related protein (BTA stat and BTA TRAK) and fragments of cytokeratins 8, 18 (UBC rapid and UBC IRMA) as markers for transitional cell carcinoma of the bladder. Eur. Urol. 2002, 41, 34–39. [Google Scholar] [CrossRef]
- Pichler, R.; Tulchiner, G.; Fritz, J.; Schaefer, G.; Horninger, W.; Heidegger, I. Urinary UBC Rapid and NMP22 Test for Bladder Cancer Surveillance in Comparison to Urinary Cytology: Results from a Prospective Single-Center Study. Int. J. Med. Sci. 2017, 14, 811. [Google Scholar] [CrossRef]
- Chou, R.; Gore, J.L.; Buckley, D.; Fu, R.; Gustafson, K.; Griffin, J.C.; Grusing, S.; Selph, S. Urinary Biomarkers for Diagnosis of Bladder Cancer: A Systematic Review and Meta-analysis. Ann. Intern. Med. 2015, 163, 922–931. [Google Scholar] [CrossRef] [PubMed]
- Van Rhijn, B.W.; van der Poel, H.G.; van der Kwast, T.H. Urine markers for bladder cancer surveillance: A systematic review. Eur. Urol. 2005, 47, 736–748. [Google Scholar] [CrossRef] [PubMed]
- Kamat, A.M.; Karam, J.A.; Grossman, H.B.; Kader, A.K.; Munsell, M.; Dinney, C.P. Prospective trial to identify optimal bladder cancer surveillance protocol: Reducing costs while maximizing sensitivity. BJU Int. 2011, 108, 1119–1123. [Google Scholar] [CrossRef] [PubMed]
- Raitanen, M.P.; Aine, R.; Rintala, E.; Kallio, J.; Rajala, P.; Juusela, H.; Tammela, T.L. Differences between local and review urinary cytology in diagnosis of bladder cancer. An interobserver multicenter analysis. Eur. Urol. 2002, 41, 284–289. [Google Scholar] [CrossRef]
- Babjuk, M.; Bohle, A.; Burger, M.; Capoun, O.; Cohen, D.; Comperat, E.M.; Hernandez, V.; Kaasinen, E.; Palou, J.; Roupret, M.; et al. EAU Guidelines on Non-Muscle-invasive Urothelial Carcinoma of the Bladder: Update 2016. Eur. Urol. 2017, 71, 447–461. [Google Scholar] [CrossRef]
- Babjuk, M.; Oosterlinck, W.; Sylvester, R.; Kaasinen, E.; Bohle, A.; Palou-Redorta, J.; Roupret, M. EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder, the 2011 update. Eur. Urol. 2011, 59, 997–1008. [Google Scholar] [CrossRef]
- Sturgeon, C.M.; Duffy, M.J.; Hofmann, B.R.; Lamerz, R.; Fritsche, H.A.; Gaarenstroom, K.; Bonfrer, J.; Ecke, T.H.; Grossman, H.B.; Hayes, P.; et al. National Academy of Clinical Biochemistry Laboratory Medicine Practice Guidelines for use of tumor markers in liver, bladder, cervical, and gastric cancers. Clin. Chem. 2010, 56, e1–e48. [Google Scholar] [CrossRef]
- Hall, M.C.; Chang, S.S.; Dalbagni, G.; Pruthi, R.S.; Seigne, J.D.; Skinner, E.C.; Wolf, J.S., Jr.; Schellhammer, P.F. Guideline for the management of nonmuscle invasive bladder cancer (stages Ta, T1, and Tis): 2007 update. J. Urol. 2007, 178, 2314–2330. [Google Scholar] [CrossRef]
- Banek, S.; Schwentner, C.; Tager, D.; Pesch, B.; Nasterlack, M.; Leng, G.; Gawrych, K.; Bonberg, N.; Johnen, G.; Kluckert, M.; et al. Prospective evaluation of fluorescence-in situ-hybridization to detect bladder cancer: Results from the UroScreen-Study. Urol. Oncol. 2013, 31, 1656–1662. [Google Scholar] [CrossRef]
- Friedrich, M.G.; Hellstern, A.; Hautmann, S.H.; Graefen, M.; Conrad, S.; Huland, E.; Huland, H. Clinical use of urinary markers for the detection and prognosis of bladder carcinoma: A comparison of immunocytology with monoclonal antibodies against Lewis X and 486p3/12 with the BTA STAT and NMP22 tests. J. Urol. 2002, 168, 470–474. [Google Scholar] [CrossRef]
- Van Rhijn, B.W.; Catto, J.W.; Goebell, P.J.; Knuchel, R.; Shariat, S.F.; van der Poel, H.G.; Sanchez-Carbayo, M.; Thalmann, G.N.; Schmitz-Drager, B.J.; Kiemeney, L.A. Molecular markers for urothelial bladder cancer prognosis: Toward implementation in clinical practice. Urol. Oncol. 2014, 32, 1078–1087. [Google Scholar] [CrossRef] [PubMed]
- Ecke, T.H. Focus on urinary bladder cancer markers: A review. Ital. J. Urol. Nephrol. 2008, 60, 237–246. [Google Scholar]
- Yafi, F.A.; Brimo, F.; Steinberg, J.; Aprikian, A.G.; Tanguay, S.; Kassouf, W. Prospective analysis of sensitivity and specificity of urinary cytology and other urinary biomarkers for bladder cancer. Urol. Oncol. 2015, 33, 66.e25–66.e31. [Google Scholar] [CrossRef] [PubMed]
- Shariat, S.F.; Karam, J.A.; Lotan, Y.; Karakiewizc, P.I. Critical evaluation of urinary markers for bladder cancer detection and monitoring. Rev. Urol. 2008, 10, 120–135. [Google Scholar] [PubMed]
- Lotan, Y.; Capitanio, U.; Shariat, S.F.; Hutterer, G.C.; Karakiewicz, P.I. Impact of clinical factors, including a point-of-care nuclear matrix protein-22 assay and cytology, on bladder cancer detection. BJU Int. 2009, 103, 1368–1374. [Google Scholar] [CrossRef] [PubMed]
- Youden, W.J. Index for rating diagnostic tests. Cancer 1950, 3, 32–35. [Google Scholar] [CrossRef]

| NMI-LG | NMI-HG | MI-HG | NED | Control | p-Value | |
|---|---|---|---|---|---|---|
| n | 134 | 48 | 60 | 62 | 226 | |
| Age Mean (SD) Median Range | 71.0 (11.9) 73.5 26–92 | 73.8 (10.6) 75 51–94 | 73.6 (10.0) 74.5 52–98 | 70.8 (11.4) 72 46–88 | 68.9 (12.2) 71 31–93 | 0.036 |
| Sex Female (%) Male (%) | 34 (25.4) 100 (74.6) | 6 (12.5) 42 (87.5) | 19 (31.7) 41 (68.3) | 10 (16.1) 52 (83.9) | 70 (31.0) 156 (69.0) | 0.021 |
| Diabetes | 25 (18.7%) | 8 (16.7%) | 12 (20%) | 10(16.1%) | 34 (15%) | 0.849 |
| Erythrocyte (urine dipstick) | 83 (61.9%) | 44 (91.7%) | 58 (96.7%) | 38 (61.3%) | 73 (32.3%) | <0.001 |
| Leucocytes (urine dipstick) Mean (SD) Median Range | 84.6 (172.6) 0 0–500 | 109.9 (167.6) 25 0–500 | 229.7 (228.1) 100 0–500 | 127.8 (206.6) 20 0–500 | 64.3 (150.3) 0 0–500 | <0.001 |
| Nitrite pos. | 5 (3.7%) | 5 (10.4%) | 8 (13.3%) | 3 (4.8%) | 12 (5.3%) | 0.085 |
| Cystoscopy | 100 (74.6%) | 33 (68.8%) | 42 (70%) | 49 (79%) | 36 (15.9%) | <0.001 |
| UBC (µg/L) Mean (SD) Median Range | 30.9 (63.4) 6.4 5–300 | 95.5 (104.6) 46 5–300 | 66.9 (90.1) 24.85 5–300 | 10 (9.79) 5 5–56.5 | 7.7 (13.92) 5 5–166 | <0.001 |
| Sensitivity Specificity PPV NPV | 38.8% 93.8% 78.8% 72.1% | 75.0% 93.8% 72.0% 94.6% | 68.3% 93.8% 74.6% 94.6% | 22.6% 93.8% 50% 81.5% |
| NMI-LG | NMI-HG | MI-HG | NED | Control | p-Value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Center 1 | Center 2 | Center 1 | Center 2 | Center 1 | Center 2 | Center 1 | Center 2 | Center 1 | Center 2 | Center 1 | Center 2 | |
| n | 78 | 56 | 26 | 22 | 25 | 35 | 30 | 32 | 78 | 148 | ||
| Age Mean (SD) Median Range | 71.1 (11.6) 74 33–90 | 70.8 (12.4) 72 26–92 | 74.9 (11.6) 78.5 52–94 | 72.5 (9.4) 75 51–92 | 74.2 (11.0) 75 52–98 | 73.2 (9.3) 74 53–88 | 73.0 (8.8) 73.5 46–88 | 68.8 (13.1) 70.5 46–88 | 67.6 (12.64) 69.5 31–86 | 69.6 (11.94) 71.5 33–93 | 0.042 | 0.554 |
| Gender Female (%) Male (%) | 20 (25.6) 58 (74.4) | 14 (25.0) 42 (75.0) | 4 (15.4) 22 (84.6) | 2 (9.1) 20 (90.9) | 6 (24.0) 19 (76.0) | 13 (37.1) 22 (62.9) | 3 (10.0) 27 (90.0) | 7 (21.9) 25 (78) | 23 (29.5) 55 (70.5) | 47 (31.8) 101 (68.2) | 0.220 | 0.127 |
| Diabetes | 22 (28.2%) | 3 (5.4%) | 6 (23.1) | 2 (9.1%) | 6 (24%) | 6 (17.1%) | 5 (16.7%) | 5 (15.6%) | 13 (16.7%) | 21 (14.2%) | 0.469 | 0.337 |
| Erythrocyte pos. | 45 (57.7%) | 38 (67.9%) | 24 (92.3%) | 20 (91.0%) | 24 (96.0%) | 34 (97.1%) | 17 (56.7%) | 21 (65.6%) | 34 (43.6%) | 39 (26.4%) | <0.001 | <0.001 |
| Leucocytes Mean (SD) Median Range | 79.17 (165.2) 0 0–500 | 92.39 (183.8) 0 0–500 | 142.3 (182.04) 100 0–500 | 71.6 (143.4) 25 0–500 | 249.0 (229.41) 100 0–500 | 215.4 (229.5) 100 0–500 | 142.50 (220.1) 25 0–500 | 113.55 (195.2) 0 0–500 | 76.6 (165.60) 0 0–500 | 57.7 (141.68) 0 0–500 | <0.001 | <0.001 |
| Nitrite pos. | 3 (3.8%) | 2 (3.6%) | 4 (5.1%) | 1 (1.8%) | 2 (8%) | 6 (17.1%) | 1 (3.3%) | 2 (6.3%) | 3 (3.8%) | 9 (6.1%) | 0.199 | 0.185 |
| Cystoscopy | 47 (60.3%) | 53 (94.6%) | 11 (42.3%) | 22 (100%) | 11 (44%) | 31 (88.6%) | 20 (66.7%) | 29 (90.6%) | 16 (20.5%) | 20 (13.5%) | <0.001 | <0.001 |
| UBC [µg/L] Mean (SD) Median Range | 21.1 (43.7) 6.5 5–300 | 44.6 (81.9) 6.2 5–300 | 83.9 (94.8) 41.4 5–300 | 109.3 (115.8) 59.4 5–300 | 61.4 (80.9) 28.9 5–300 | 70.9 (97.1) 20.7 5–300 | 7.48 (7.06) 5 5–39.1 | 12.37 (11.4) 6.5 5–56.5 | 7.8 (13.9) 5 5–121 | 7.6 (14.0) 5 5–166 | <0.001 | <0.001 |
| Sensitivity Specificity PPV NPV | 38.5% 92.3% 83.3% 60.0% | 39.3% 94.6% 73.3% 80.5% | 73.1% 92.3% 76.0% 91.1% | 77.3% 94.6% 68.0% 96.6% | 68.0% 92.3% 73.9% 90.0% | 68.6% 94.6% 75.0% 92.7% | 6.7% 92.3% 25% 72% | 37.5% 94.6% 60% 87.5% | ||||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ecke, T.H.; Weiß, S.; Stephan, C.; Hallmann, S.; Arndt, C.; Barski, D.; Otto, T.; Gerullis, H. UBC® Rapid Test—A Urinary Point-of-Care (POC) Assay for Diagnosis of Bladder Cancer with a focus on Non-Muscle Invasive High-Grade Tumors: Results of a Multicenter-Study. Int. J. Mol. Sci. 2018, 19, 3841. https://doi.org/10.3390/ijms19123841
Ecke TH, Weiß S, Stephan C, Hallmann S, Arndt C, Barski D, Otto T, Gerullis H. UBC® Rapid Test—A Urinary Point-of-Care (POC) Assay for Diagnosis of Bladder Cancer with a focus on Non-Muscle Invasive High-Grade Tumors: Results of a Multicenter-Study. International Journal of Molecular Sciences. 2018; 19(12):3841. https://doi.org/10.3390/ijms19123841
Chicago/Turabian StyleEcke, Thorsten H., Sarah Weiß, Carsten Stephan, Steffen Hallmann, Christian Arndt, Dimitri Barski, Thomas Otto, and Holger Gerullis. 2018. "UBC® Rapid Test—A Urinary Point-of-Care (POC) Assay for Diagnosis of Bladder Cancer with a focus on Non-Muscle Invasive High-Grade Tumors: Results of a Multicenter-Study" International Journal of Molecular Sciences 19, no. 12: 3841. https://doi.org/10.3390/ijms19123841
APA StyleEcke, T. H., Weiß, S., Stephan, C., Hallmann, S., Arndt, C., Barski, D., Otto, T., & Gerullis, H. (2018). UBC® Rapid Test—A Urinary Point-of-Care (POC) Assay for Diagnosis of Bladder Cancer with a focus on Non-Muscle Invasive High-Grade Tumors: Results of a Multicenter-Study. International Journal of Molecular Sciences, 19(12), 3841. https://doi.org/10.3390/ijms19123841





