Role of Endogenous Glucocorticoids in Cancer in the Elderly
Abstract
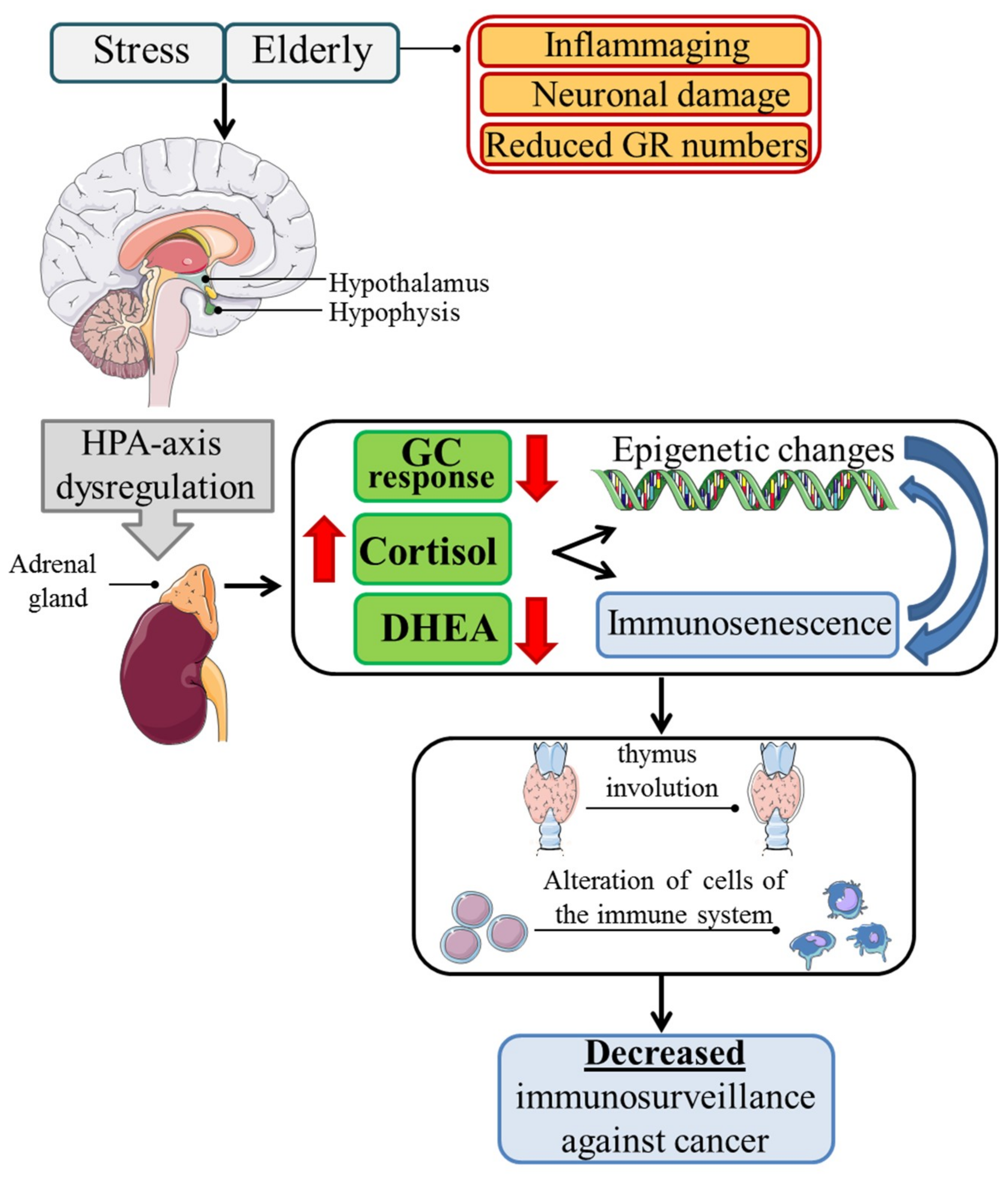
1. Introduction
2. Glucocorticoids in the Elderly
2.1. Immunosenescence
2.2. Immunosenescence and Cancer
2.3. Glucocorticoids and Cancer
3. Conclusions
Funding
Conflicts of Interest
References
- Lopez-Otin, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The hallmarks of aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef] [PubMed]
- McHugh, D.; Gil, J. Senescence and aging: Causes, consequences, and therapeutic avenues. J. Cell Biol. 2018, 217, 65–77. [Google Scholar] [CrossRef] [PubMed]
- Niccoli, T.; Partridge, L. Ageing as a risk factor for disease. Curr. Biol. CB 2012, 22, R741–R752. [Google Scholar] [CrossRef] [PubMed]
- Frenk, S.; Houseley, J. Gene expression hallmarks of cellular ageing. Biogerontology 2018, 19, 547–566. [Google Scholar] [CrossRef] [PubMed]
- Fraga, M.F.; Esteller, M. Epigenetics and aging: The targets and the marks. Trends Genet. TIG 2007, 23, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Garinis, G.A.; van der Horst, G.T.; Vijg, J.; Hoeijmakers, J.H. DNA damage and ageing: New-age ideas for an age-old problem. Nat. Cell Biol. 2008, 10, 1241–1247. [Google Scholar] [CrossRef] [PubMed]
- Armanios, M. Syndromes of telomere shortening. Annu. Rev. Genom. Hum. Genet. 2009, 10, 45–61. [Google Scholar] [CrossRef] [PubMed]
- Jenny, N.S. Inflammation in aging: Cause, effect, or both? Discov. Med. 2012, 13, 451–460. [Google Scholar] [PubMed]
- Fulop, T.; Larbi, A.; Dupuis, G.; Le Page, A.; Frost, E.H.; Cohen, A.A.; Witkowski, J.M.; Franceschi, C. Immunosenescence and Inflamm-Aging As Two Sides of the Same Coin: Friends or Foes? Front. Immunol. 2017, 8, 1960. [Google Scholar] [CrossRef] [PubMed]
- Leonardi, G.C.; Accardi, G.; Monastero, R.; Nicoletti, F.; Libra, M. Ageing: From inflammation to cancer. Immun. Ageing I A 2018, 15, 1. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, C.; Bonafè, M.; Valensin, S.; Olivieri, F.; De Luca, M.; Ottaviani, E.; De Benedictis, G. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann. N. Y. Acad. Sci. 2000, 908, 244–254. [Google Scholar] [CrossRef] [PubMed]
- Pawelec, G. Hallmarks of human “immunosenescence”: Adaptation or dysregulation? Immun. Ageing I A 2012, 9, 15. [Google Scholar] [CrossRef] [PubMed]
- D’Adda di Fagagna, F. Living on a break: Cellular senescence as a DNA-damage response. Nat. Rev. Cancer 2008, 8, 512–522. [Google Scholar] [CrossRef] [PubMed]
- Di Mitri, D.; Alimonti, A. Non-Cell-Autonomous Regulation of Cellular Senescence in Cancer. Trends Cell Biol. 2016, 26, 215–226. [Google Scholar] [CrossRef] [PubMed]
- Coppe, J.P.; Desprez, P.Y.; Krtolica, A.; Campisi, J. The senescence-associated secretory phenotype: The dark side of tumor suppression. Annu. Rev. Pathol. 2010, 5, 99–118. [Google Scholar] [CrossRef] [PubMed]
- Zinger, A.; Cho, W.C.; Ben-Yehuda, A. Cancer and Aging—The Inflammatory Connection. Aging Dis. 2017, 8, 611–627. [Google Scholar] [CrossRef] [PubMed]
- Bauer, M.E.; Jeckel, C.M.; Luz, C. The role of stress factors during aging of the immune system. Ann. N. Y. Acad. Sci. 2009, 1153, 139–152. [Google Scholar] [CrossRef] [PubMed]
- Pawelec, G. Immunosenescence and cancer. Biogerontology 2017, 18, 717–721. [Google Scholar] [CrossRef] [PubMed]
- Biddie, S.C.; Conway-Campbell, B.L.; Lightman, S.L. Dynamic regulation of glucocorticoid signalling in health and disease. Rheumatology 2012, 51, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Nicolaides, N.C.; Charmandari, E.; Kino, T.; Chrousos, G.P. Stress-Related and Circadian Secretion and Target Tissue Actions of Glucocorticoids: Impact on Health. Front. Endocrinol. 2017, 8, 70. [Google Scholar] [CrossRef] [PubMed]
- Sapolsky, R.M.; Romero, L.M.; Munck, A.U. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr. Rev. 2000, 21, 55–89. [Google Scholar] [CrossRef] [PubMed]
- Bauer, M.E. Stress, glucocorticoids and ageing of the immune system. Stress 2005, 8, 69–83. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, E.; Cravello, L.; Muzzoni, B.; Casarotti, D.; Paltro, M.; Solerte, S.B.; Fioravanti, M.; Cuzzoni, G.; Pontiggia, B.; Magri, F. Age-related changes of the hypothalamic-pituitary-adrenal axis: Pathophysiological correlates. Eur. J. Endocrinol. 2001, 144, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Rhen, T.; Cidlowski, J.A. Antiinflammatory action of glucocorticoids—New mechanisms for old drugs. N. Engl. J. Med. 2005, 353, 1711–1723. [Google Scholar] [CrossRef] [PubMed]
- Pufall, M.A. Glucocorticoids and Cancer. Adv. Exp. Med. Biol. 2015, 872, 315–333. [Google Scholar] [CrossRef] [PubMed]
- Coleman, R.E. Glucocorticoids in cancer therapy. Biotherapy 1992, 4, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.T.; Wang, L.H. New dimension of glucocorticoids in cancer treatment. Steroids 2016, 111, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Vandevyver, S.; Dejager, L.; Libert, C. Comprehensive overview of the structure and regulation of the glucocorticoid receptor. Endocr. Rev. 2014, 35, 671–693. [Google Scholar] [CrossRef] [PubMed]
- De Bosscher, K.; Vanden Berghe, W.; Haegeman, G. The interplay between the glucocorticoid receptor and nuclear factor-kappaB or activator protein-1: Molecular mechanisms for gene repression. Endocr. Rev. 2003, 24, 488–522. [Google Scholar] [CrossRef] [PubMed]
- Cain, D.W.; Cidlowski, J.A. Immune regulation by glucocorticoids. Nat. Rev. Immunol. 2017, 17, 233–247. [Google Scholar] [CrossRef] [PubMed]
- Antonica, A.; Ayroldi, E.; Magni, F.; Paolocci, N. Lymphocyte traffic changes induced by monolateral vagal denervation in mouse thymus and peripheral lymphoid organs. J. Neuroimmunol. 1996, 64, 115–122. [Google Scholar] [CrossRef]
- Hasan, K.M.; Rahman, M.S.; Arif, K.M.; Sobhani, M.E. Psychological stress and aging: Role of glucocorticoids (GCs). Age 2012, 34, 1421–1433. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, C.; Monti, D.; Barbieri, D.; Grassilli, E.; Troiano, L.; Salvioli, S.; Negro, P.; Capri, M.; Guido, M.; Azzi, R.; et al. Immunosenescence in humans: Deterioration or remodelling? Int. Rev. Immunol. 1995, 12, 57–74. [Google Scholar] [CrossRef] [PubMed]
- Cambier, J. Immunosenescence: A problem of lymphopoiesis, homeostasis, microenvironment, and signaling. Immunol. Rev. 2005, 205, 5–6. [Google Scholar] [CrossRef] [PubMed]
- Gruver, A.L.; Hudson, L.L.; Sempowski, G.D. Immunosenescence of ageing. J. Pathol. 2007, 211, 144–156. [Google Scholar] [CrossRef] [PubMed]
- Bektas, A.; Schurman, S.H.; Sen, R.; Ferrucci, L. Human T cell immunosenescence and inflammation in aging. J. Leukoc. Biol. 2017, 102, 977–988. [Google Scholar] [CrossRef] [PubMed]
- McEwen, B.S.; Biron, C.A.; Brunson, K.W.; Bulloch, K.; Chambers, W.H.; Dhabhar, F.S.; Goldfarb, R.H.; Kitson, R.P.; Miller, A.H.; Spencer, R.L.; et al. The role of adrenocorticoids as modulators of immune function in health and disease: Neural, endocrine and immune interactions. Brain Res. Brain Res. Rev. 1997, 23, 79–133. [Google Scholar] [CrossRef]
- Elwenspoek, M.M.C.; Kuehn, A.; Muller, C.P.; Turner, J.D. The effects of early life adversity on the immune system. Psychoneuroendocrinology 2017, 82, 140–154. [Google Scholar] [CrossRef] [PubMed]
- Gassen, N.C.; Chrousos, G.P.; Binder, E.B.; Zannas, A.S. Life stress, glucocorticoid signaling, and the aging epigenome: Implications for aging-related diseases. Neurosci. Biobehav. Rev. 2017, 74, 356–365. [Google Scholar] [CrossRef] [PubMed]
- Roshan, S.; Nader, S.; Orlander, P. Review: Ageing and hormones. Eur. J. Clin. Investig. 1999, 29, 210–213. [Google Scholar] [CrossRef]
- Luz, C.; Dornelles, F.; Preissler, T.; Collaziol, D.; da Cruz, I.M.; Bauer, M.E. Impact of psychological and endocrine factors on cytokine production of healthy elderly people. Mech. Ageing Dev. 2003, 124, 887–895. [Google Scholar] [CrossRef]
- Jovanovic, I.; Ugrenović, S.; Ljubomirović, M.; Vasović, L.; Cukuranović, R.; Stefanović, V. Folliculo-stellate cells—Potential mediators of the inflammaging-induced hyperactivity of the hypothalamic-pituitary-adrenal axis in healthy elderly individuals. Med. Hypotheses 2014, 83, 501–505. [Google Scholar] [CrossRef] [PubMed]
- Hechter, O.; Grossman, A.; Chatterton, R.T., Jr. Relationship of dehydroepiandrosterone and cortisol in disease. Med. Hypotheses 1997, 49, 85–91. [Google Scholar] [CrossRef]
- Pinto, A.; Malacrida, B.; Oieni, J.; Serafini, M.M.; Davin, A.; Galbiati, V.; Corsini, E.; Racchi, M. DHEA modulates the effect of cortisol on RACK1 expression via interference with the splicing of the glucocorticoid receptor. Br. J. Pharmacol. 2015, 172, 2918–2927. [Google Scholar] [CrossRef] [PubMed]
- McEwen, B.S. Interacting mediators of allostasis and allostatic load: Towards an understanding of resilience in aging. Metab. Clin. Exp. 2003, 52, 10–16. [Google Scholar] [CrossRef]
- Volden, P.A.; Conzen, S.D. The influence of glucocorticoid signaling on tumor progression. Brain Behav. Immunity 2013, 30, S26–S31. [Google Scholar] [CrossRef] [PubMed]
- Shin, K.J.; Lee, Y.J.; Yang, Y.R.; Park, S.; Suh, P.G.; Follo, M.Y.; Cocco, L.; Ryu, S.H. Molecular Mechanisms Underlying Psychological Stress and Cancer. Curr. Pharm. Des. 2016, 22, 2389–2402. [Google Scholar] [CrossRef] [PubMed]
- Malaguarnera, L.; Cristaldi, E.; Malaguarnera, M. The role of immunity in elderly cancer. Crit. Rev. Oncol./Hematol. 2010, 74, 40–60. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Taboada, V.; Bartolomé, M.J.; Amado, J.A.; Blanco, R.; García-Unzueta, M.T.; Rodríguez-Valverde, V.; López-Hoyos, M. Changes in peripheral blood lymphocyte subsets in elderly subjects are associated with an impaired function of the hypothalamic-pituitary-adrenal axis. Mech. Ageing Dev. 2002, 123, 1477–1486. [Google Scholar] [CrossRef]
- Potestio, M.; Pawelec, G.; Di Lorenzo, G.; Candore, G.; D’Anna, C.; Gervasi, F.; Lio, D.; Tranchida, G.; Caruso, C.; Romano, G.C. Age-related changes in the expression of CD95 (APO1/FAS) on blood lymphocytes. Exp. Gerontol. 1999, 34, 659–673. [Google Scholar] [CrossRef]
- Schmidt, M.; Lügering, N.; Lügering, A.; Pauels, H.G.; Schulze-Osthoff, K.; Domschke, W.; Kucharzik, T. Role of the CD95/CD95 ligand system in glucocorticoid-induced monocyte apoptosis. J. Immunol. 2001, 166, 1344–1351. [Google Scholar] [CrossRef] [PubMed]
- Raynor, J.; Lages, C.S.; Shehata, H.; Hildeman, D.A.; Chougnet, C.A. Homeostasis and function of regulatory T cells in aging. Curr. Opin. Immunol. 2012, 24, 482–487. [Google Scholar] [CrossRef] [PubMed]
- Ugor, E.; Prenek, L.; Pap, R.; Berta, G.; Ernszt, D.; Najbauer, J.; Németh, P.; Boldizsár, F.; Berki, T. Glucocorticoid hormone treatment enhances the cytokine production of regulatory T cells by upregulation of Foxp3 expression. Immunobiology 2018, 223, 422–431. [Google Scholar] [CrossRef] [PubMed]
- Elenkov, I.J. Glucocorticoids and the Th1/Th2 balance. Ann. N. Y. Acad. Sci. 2004, 1024, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Do, T.T.H.; Marie, G.; Héloïse, D.; Guillaume, D.; Marthe, M.; Bruno, F.; Marion, B. Glucocorticoid-induced insulin resistance is related to macrophage visceral adipose tissue infiltration. J. Steroid Biochem. Mol. Biol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Li, W.P.; Meng, C.; Ivashkiv, L.B. Inhibition of IFN-gamma signaling by glucocorticoids. J. Immunol. 2003, 170, 4833–4839. [Google Scholar] [CrossRef] [PubMed]
- Prall, S.P.; Larson, E.E.; Muehlenbein, M.P. The role of dehydroepiandrosterone on functional innate immune responses to acute stress. Stress Health 2017, 33, 656–664. [Google Scholar] [CrossRef] [PubMed]
- Revskoy, S.; Redei, E. Decreased in vitro sensitivity to dexamethasone in corticotropes from middle-age rats. Exp. Gerontol. 2000, 35, 237–242. [Google Scholar] [CrossRef]
- Juruena, M.F.; Cleare, A.J.; Bauer, M.E.; Pariante, C.M. Molecular mechanisms of glucocorticoid receptor sensitivity and relevance to affective disorders. Acta Neuropsychiatr. 2003, 15, 354–367. [Google Scholar] [CrossRef] [PubMed]
- Ramamoorthy, S.; Cidlowski, J.A. Exploring the molecular mechanisms of glucocorticoid receptor action from sensitivity to resistance. Endocr. Dev. 2013, 24, 41–56. [Google Scholar] [CrossRef] [PubMed]
- Adcock, I.M.; Barnes, P.J. Molecular mechanisms of corticosteroid resistance. Chest 2008, 134, 394–401. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.S.; Mellman, I. Oncology meets immunology: The cancer-immunity cycle. Immunity 2013, 39, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Nikolich-Zugich, J.; Li, G.; Uhrlaub, J.L.; Renkema, K.R.; Smithey, M.J. Age-related changes in CD8 T cell homeostasis and immunity to infection. Semin. Immunol. 2012, 24, 356–364. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.; Janicki-Deverts, D.; Doyle, W.J.; Miller, G.E.; Frank, E.; Rabin, B.S.; Turner, R.B. Chronic stress, glucocorticoid receptor resistance, inflammation, and disease risk. Proc. Nat. Acad. Sci. USA 2012, 109, 5995–5999. [Google Scholar] [CrossRef] [PubMed]
- Lang, K.; Entschladen, F.; Weidt, C.; Zaenker, K.S. Tumor immune escape mechanisms: Impact of the neuroendocrine system. Cancer Immunol. Immunother. CII 2006, 55, 749–760. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Kim, C.; Yel, L.; Gollapudi, S. A role of fas-associated death domain (FADD) in increased apoptosis in aged humans. J. Clin. Immunol. 2004, 24, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Warner, H.R.; Hodes, R.J.; Pocinki, K. What does cell death have to do with aging? J. Am. Geriatr. Soc. 1997, 45, 1140–1146. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, S.; Gupta, S. Increased apoptosis of T cell subsets in aging humans: Altered expression of Fas (CD95), Fas ligand, Bcl-2, and Bax. J. Immunol. 1998, 160, 1627–1637. [Google Scholar] [PubMed]
- Azher, S.; Azami, O.; Amato, C.; McCullough, M.; Celentano, A.; Cirillo, N. The Non-Conventional Effects of Glucocorticoids in Cancer. J. Cell. Physiol. 2016, 231, 2368–2373. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Liu, L.; Zhang, C.; Zheng, T.; Wang, J.; Lin, M.; Zhao, Y.; Wang, X.; Levine, A.J.; Hu, W. Chronic restraint stress attenuates p53 function and promotes tumorigenesis. Proc. Nat. Acad. Sci. USA 2012, 109, 7013–7018. [Google Scholar] [CrossRef] [PubMed]
- Gundisch, S.; Boeckeler, E.; Behrends, U.; Amtmann, E.; Ehrhardt, H.; Jeremias, I. Glucocorticoids augment survival and proliferation of tumor cells. Anticancer Res. 2012, 32, 4251–4261. [Google Scholar] [PubMed]
- Zannas, A.S. Editorial Perspective: Psychological stress and epigenetic aging—What can we learn and how can we prevent? J. Child Psychol. Psychiatry Allied Discip. 2016, 57, 674–675. [Google Scholar] [CrossRef] [PubMed]
- Thomassin, H.; Flavin, M.; Espinas, M.L.; Grange, T. Glucocorticoid-induced DNA demethylation and gene memory during development. EMBO J. 2001, 20, 1974–1983. [Google Scholar] [CrossRef] [PubMed]
- Teschendorff, A.E.; Menon, U.; Gentry-Maharaj, A.; Ramus, S.J.; Weisenberger, D.J.; Shen, H.; Campan, M.; Noushmehr, H.; Bell, C.G.; Maxwell, A.P.; et al. Age-dependent DNA methylation of genes that are suppressed in stem cells is a hallmark of cancer. Genome Res. 2010, 20, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Bird, A. Genetic determinants of the epigenome in development and cancer. Swiss Med. Wkly. 2017, 147, w14523. [Google Scholar] [CrossRef] [PubMed]
- Smetana, K., Jr.; Lacina, L.; Szabo, P.; Dvořánková, B.; Brož, P.; Šedo, A. Ageing as an Important Risk Factor for Cancer. Anticancer Res. 2016, 36, 5009–5017. [Google Scholar] [CrossRef] [PubMed]
- Finkel, T.; Serrano, M.; Blasco, M.A. The common biology of cancer and ageing. Nature 2007, 448, 767–774. [Google Scholar] [CrossRef] [PubMed]
- Tserel, L.; Kolde, R.; Limbach, M.; Tretyakov, K.; Kasela, S.; Kisand, K.; Saare, M.; Vilo, J.; Metspalu, A.; Milani, L.; et al. Age-related profiling of DNA methylation in CD8+ T cells reveals changes in immune response and transcriptional regulator genes. Sci. Rep. 2015, 5, 13107. [Google Scholar] [CrossRef] [PubMed]
- DuPage, M.; Bluestone, J.A. Harnessing the plasticity of CD4(+) T cells to treat immune-mediated disease. Nat. Rev. Immunol. 2016, 16, 149–163. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ayroldi, E.; Cannarile, L.; Adorisio, S.; Delfino, D.V.; Riccardi, C. Role of Endogenous Glucocorticoids in Cancer in the Elderly. Int. J. Mol. Sci. 2018, 19, 3774. https://doi.org/10.3390/ijms19123774
Ayroldi E, Cannarile L, Adorisio S, Delfino DV, Riccardi C. Role of Endogenous Glucocorticoids in Cancer in the Elderly. International Journal of Molecular Sciences. 2018; 19(12):3774. https://doi.org/10.3390/ijms19123774
Chicago/Turabian StyleAyroldi, Emira, Lorenza Cannarile, Sabrina Adorisio, Domenico V. Delfino, and Carlo Riccardi. 2018. "Role of Endogenous Glucocorticoids in Cancer in the Elderly" International Journal of Molecular Sciences 19, no. 12: 3774. https://doi.org/10.3390/ijms19123774
APA StyleAyroldi, E., Cannarile, L., Adorisio, S., Delfino, D. V., & Riccardi, C. (2018). Role of Endogenous Glucocorticoids in Cancer in the Elderly. International Journal of Molecular Sciences, 19(12), 3774. https://doi.org/10.3390/ijms19123774





