Expression, Localization, and Activity of the Aryl Hydrocarbon Receptor in the Human Placenta
Abstract
1. Introduction
2. Results
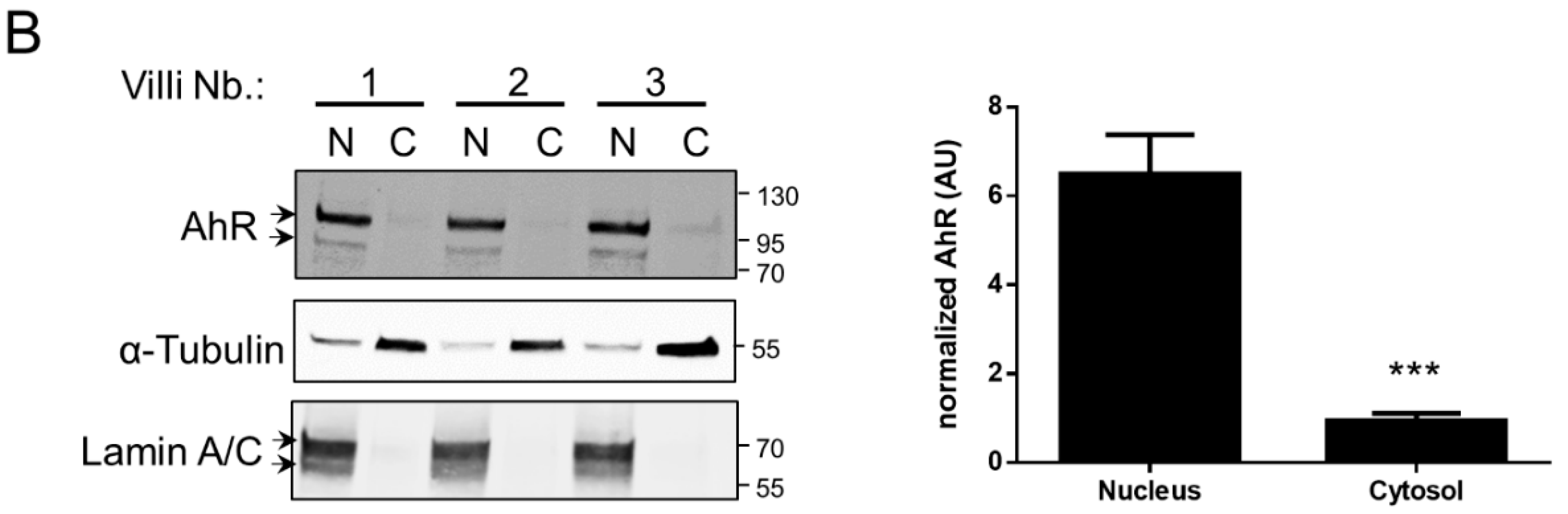
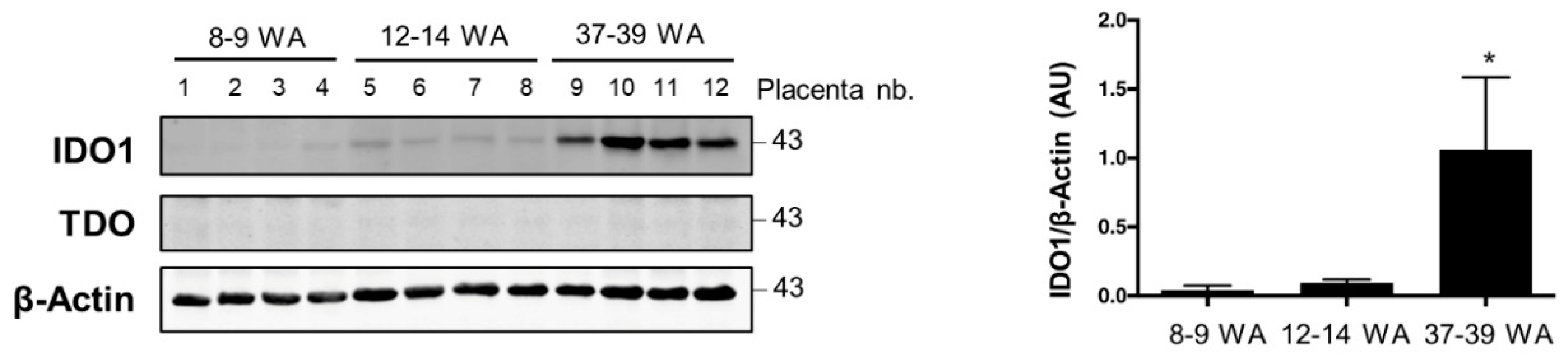
2.1. AhR Expression in Human Placenta During Ontogeny
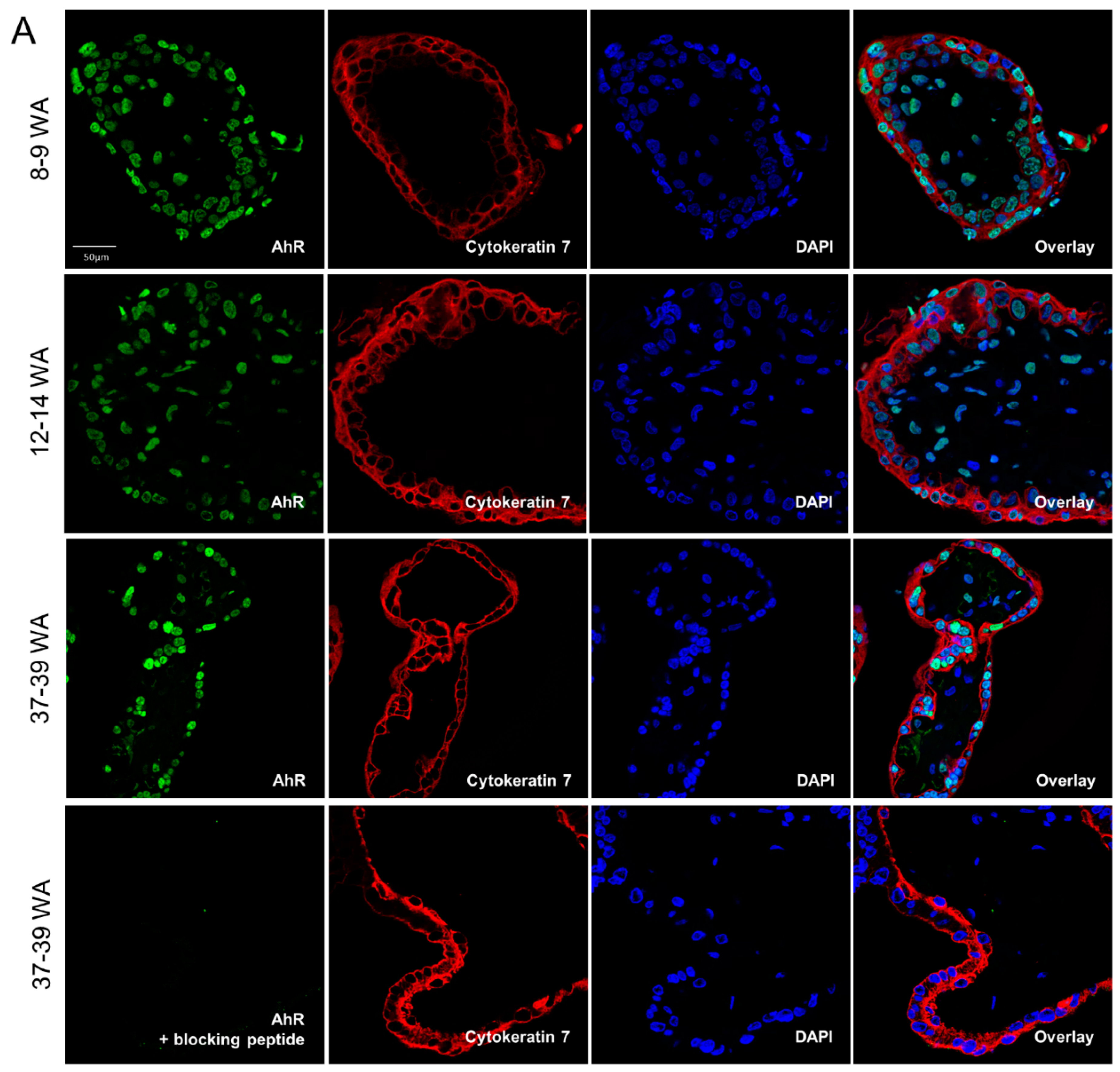
2.2. AhR Localization in Human Placental Chorionic Villi During Ontogeny
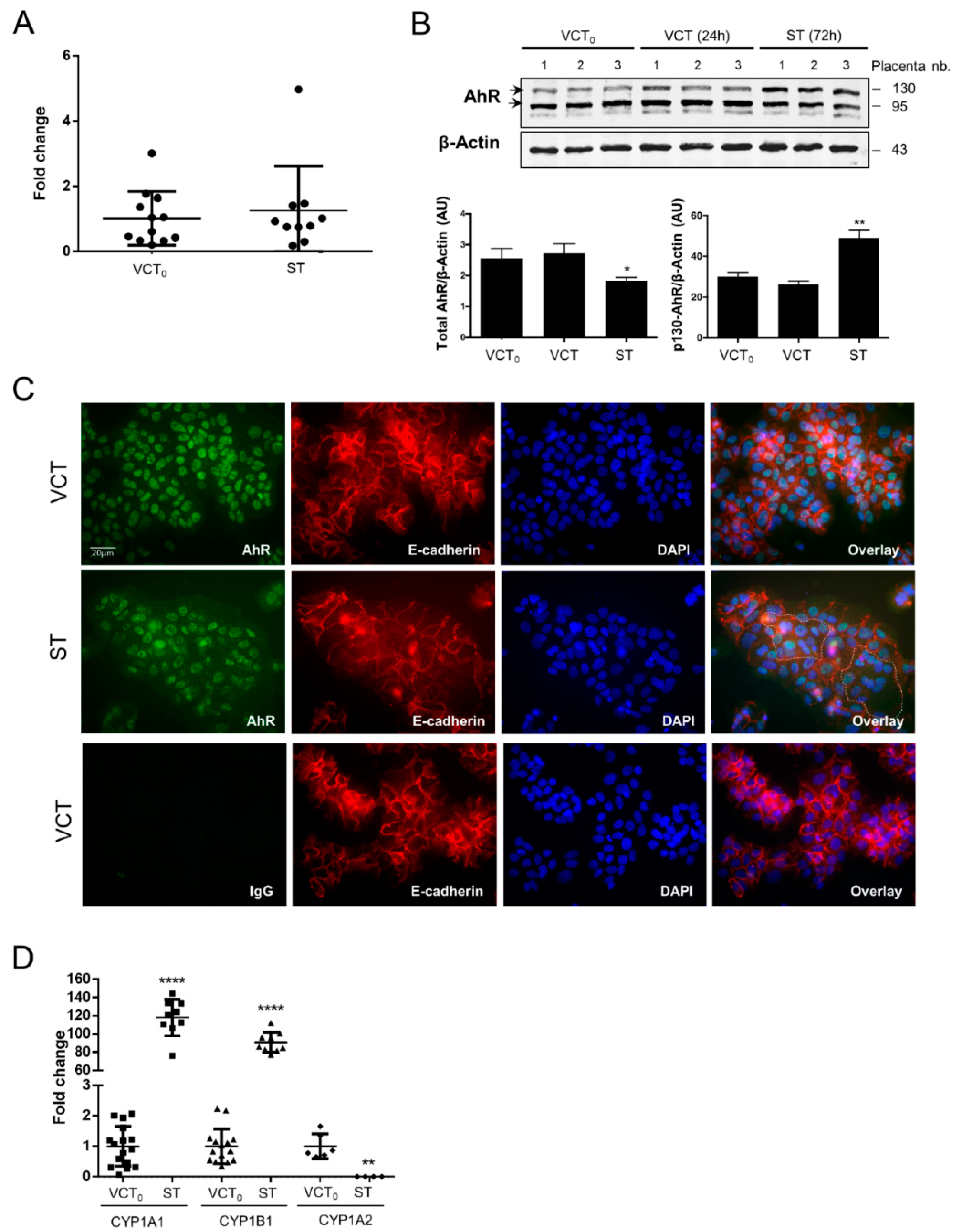
2.3. AhR Expression, Localization, and Activity in Human Purified Trophoblasts
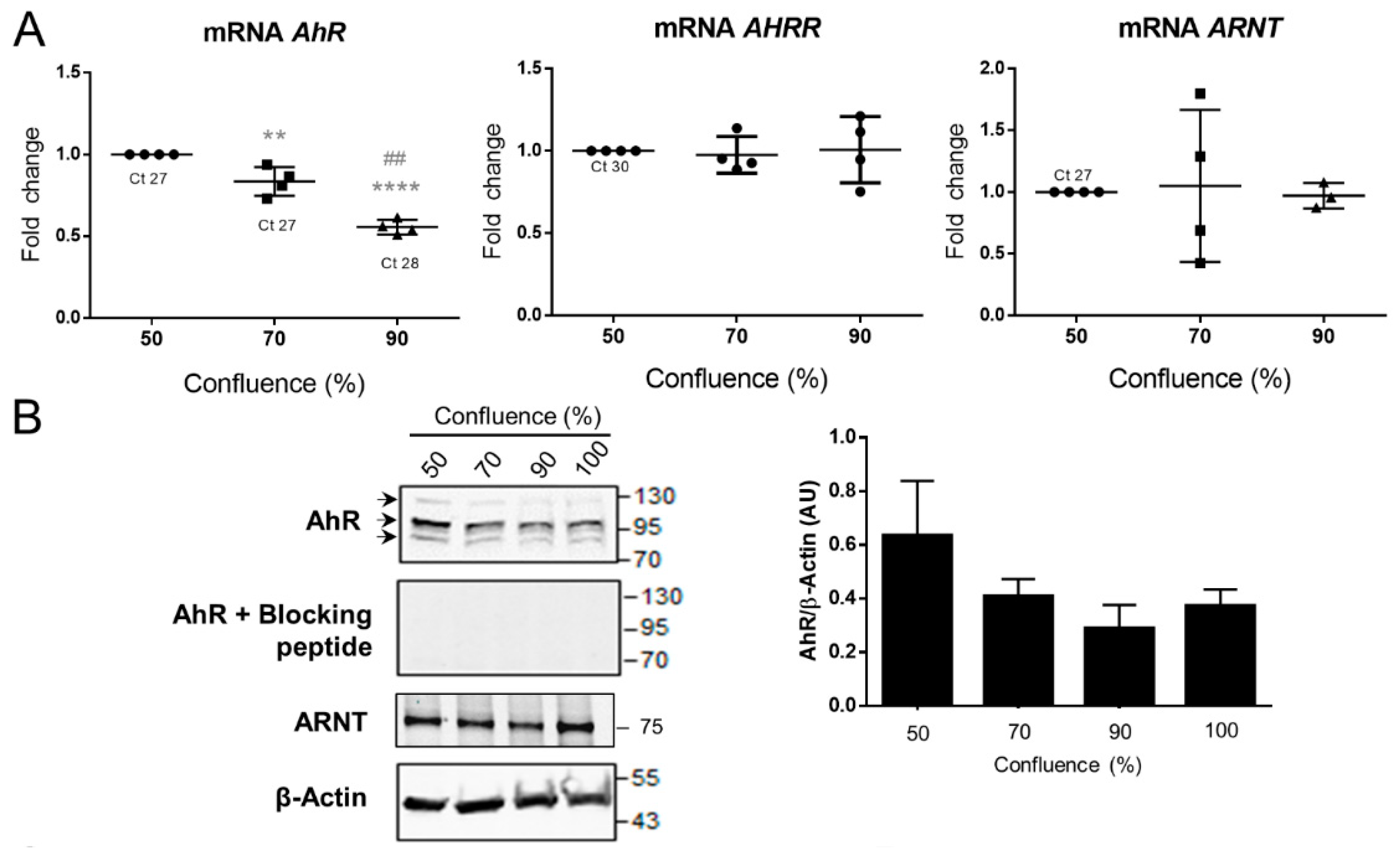
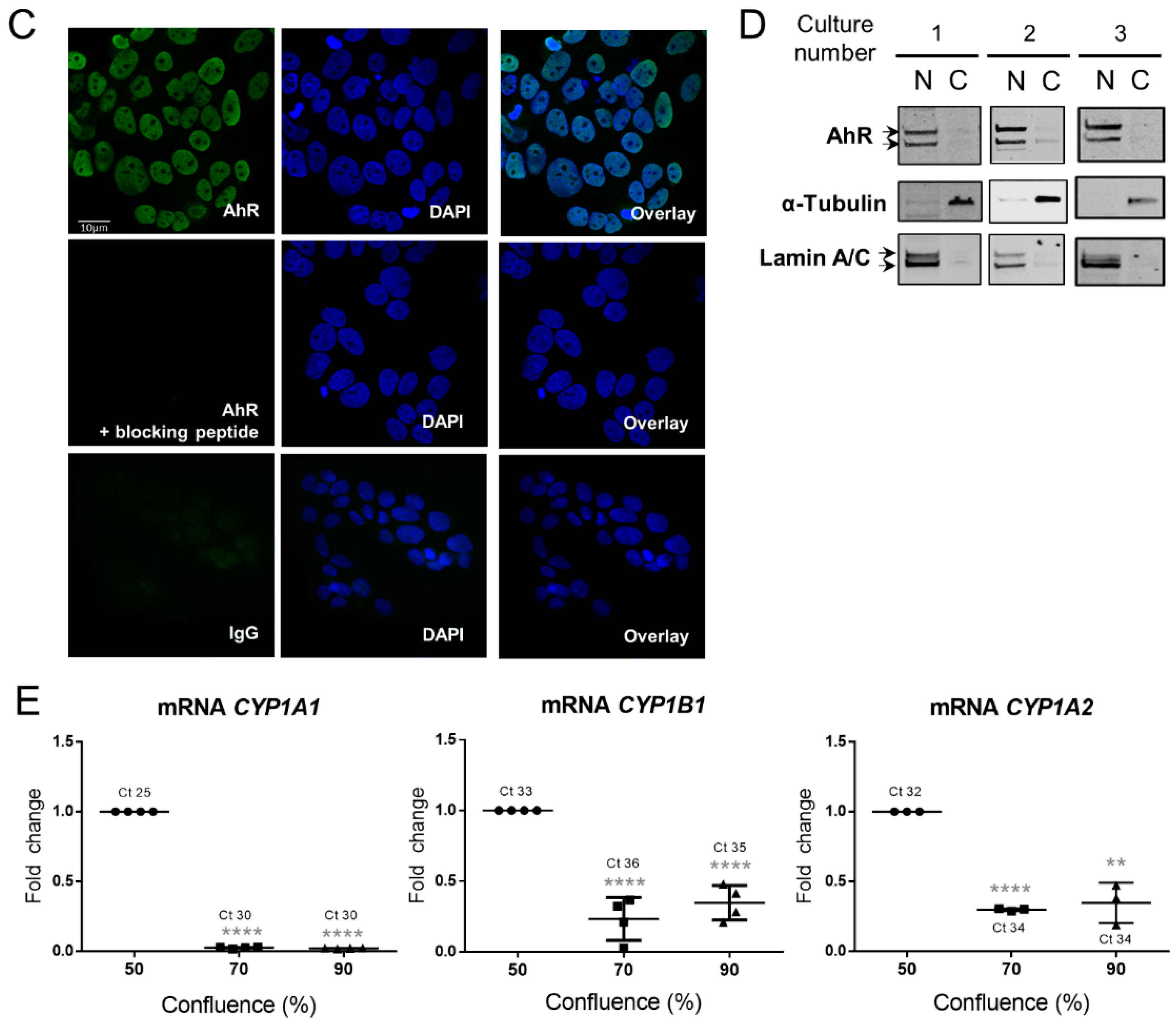
2.4. AhR Expression, Localization, and Activity in the BeWo Trophoblast Model
2.5. Effect of Benzo-(a)-Pyrene on AhR Activity in the BeWo Model
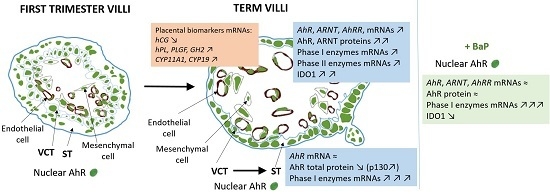
3. Discussion
4. Materials & Methods
4.1. Placentae Collection and Chorionic Villi Isolation
4.2. Explants
4.3. Cell Culture
4.4. Other Cell Types
4.5. Cell and Explant Incubation
4.6. RNA Extraction, Reverse Transcription, and Quantitative Real-Time PCR
4.7. RNA Extraction, Reverse Transcription and PCR
4.8. Cell Fractionation
4.9. Western Blot
4.10. Tissue Immunolabelling
4.11. Cell Immunolabelling
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Burton, G.J.; Jauniaux, E. What is the placenta? Am. J. Obstet. Gynecol. 2015, 213 (Suppl. 4), S6.e1–S6.e4. [Google Scholar] [CrossRef] [PubMed]
- Burton, G.J.; Fowden, A.L. The placenta: A multifaceted, transient organ. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2015, 370, 20140066. [Google Scholar] [CrossRef] [PubMed]
- Challier, J.C.; Vervelle, C.; Uzan, S. Ontogenesis of villi and fetal vessels in the human placenta. Fetal Diagn. Ther. 2001, 16, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Denison, M.S.; Nagy, S.R. Activation of the aryl hydrocarbon receptor by structurally diverse exogenous and endogenous chemicals. Ann. Rev. Pharmacol. Toxicol. 2003, 43, 309–334. [Google Scholar] [CrossRef] [PubMed]
- Juricek, L.; Bui, L.C.; Busi, F.; Pierre, S.; Guyot, E.; Lamouri, A.; Dupret, J.M.; Barouki, R.; Coumoul, X.; Rodrigues-Lima, F. Activation of the aryl hydrocarbon receptor by carcinogenic aromatic amines and modulatory effects of their N-acetylated metabolites. Arch. Toxicol. 2015, 89, 2403–2412. [Google Scholar] [CrossRef] [PubMed]
- Barouki, R.; Aggerbeck, M.; Aggerbeck, L.; Coumoul, X. The aryl hydrocarbon receptor system. Drug Metab. Drug Interact. 2012, 27, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Kawajiri, K.; Fujii-Kuriyama, Y. Cytochrome P450 gene regulation and physiological functions mediated by the aryl hydrocarbon receptor. Arch. Biochem. Biophys. 2007, 464, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Pollenz, R.S. Ligand-dependent and independent modulation of aryl hydrocarbon receptor localization, degradation, and gene regulation. Mol. Pharmacol. 2002, 62, 806–816. [Google Scholar] [CrossRef] [PubMed]
- Mimura, J.; Ema, M.; Sogawa, K.; Fujii-Kuriyama, Y. Identification of a novel mechanism of regulation of Ah (dioxin) receptor function. Genes Dev. 1999, 13, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Cella, M.; Colonna, M. Aryl hydrocarbon receptor: Linking environment to immunity. Semin. Immunol. 2015, 27, 310–314. [Google Scholar] [CrossRef] [PubMed]
- Julliard, W.; Fechner, J.H.; Mezrich, J.D. The aryl hydrocarbon receptor meets immunology: Friend or foe? A little of both. Front. Immunol. 2014, 5, 458. [Google Scholar] [PubMed]
- Hao, N.; Whitelaw, M.L. The emerging roles of AhR in physiology and immunity. Biochem. Pharmacol. 2013, 86, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Guyot, E.; Chevallier, A.; Barouki, R.; Coumoul, X. The AhR twist: Ligand-dependent AhR signaling and pharmaco-toxicological implications. Drug Discov. Today 2013, 18, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Stejskalova, L.; Dvorak, Z.; Pavek, P. Endogenous and exogenous ligands of aryl hydrocarbon receptor: Current state of art. Curr. Drug Metab. 2011, 12, 198–212. [Google Scholar] [CrossRef] [PubMed]
- Abbott, B.D.; Schmid, J.E.; Pitt, J.A.; Buckalew, A.R.; Wood, C.R.; Held, G.A.; Diliberto, J.J. Adverse reproductive outcomes in the transgenic Ah receptor-deficient mouse. Toxicol. Appl. Pharmacol. 1999, 155, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Murray, I.A.; Patterson, A.D.; Perdew, G.H. Aryl hydrocarbon receptor ligands in cancer: Friend and foe. Nat. Rev. Cancer 2014, 14, 801–814. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Salguero, P.; Pineau, T.; Hilbert, D.M.; McPhail, T.; Lee, S.S.; Kimura, S.; Nebert, D.W.; Rudikoff, S.; Ward, J.M.; Gonzalez, F.J. Immune system impairment and hepatic fibrosis in mice lacking the dioxin-binding Ah receptor. Science 1995, 268, 722–726. [Google Scholar] [CrossRef] [PubMed]
- Barouki, R.; Coumoul, X.; Fernandez-Salguero, P.M. The aryl hydrocarbon receptor, more than a xenobiotic-interacting protein. FEBS Lett. 2007, 581, 3608–3615. [Google Scholar] [CrossRef] [PubMed]
- Juricek, L.; Carcaud, J.; Pelhaitre, A.; Riday, T.T.; Chevallier, A.; Lanzini, J.; Auzeil, N.; Laprevote, O.; Dumont, F.; Jacques, S.; et al. AhR-deficiency as a cause of demyelinating disease and inflammation. Sci. Rep. 2017, 7, 9794. [Google Scholar] [CrossRef] [PubMed]
- Baba, T.; Mimura, J.; Nakamura, N.; Harada, N.; Yamamoto, M.; Morohashi, K.; Fujii-Kuriyama, Y. Intrinsic function of the aryl hydrocarbon (dioxin) receptor as a key factor in female reproduction. Mol. Cell Biol. 2005, 25, 10040–10051. [Google Scholar] [CrossRef] [PubMed]
- Quintana, F.J.; Basso, A.S.; Iglesias, A.H.; Korn, T.; Farez, M.F.; Bettelli, E.; Caccamo, M.; Oukka, M.; Weiner, H.L. Control of T(reg) and T(H)17 cell differentiation by the aryl hydrocarbon receptor. Nature 2008, 453, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Stevens, E.A.; Mezrich, J.D.; Bradfield, C.A. The aryl hydrocarbon receptor: A perspective on potential roles in the immune system. Immunology 2009, 127, 299–311. [Google Scholar] [CrossRef] [PubMed]
- Somerset, D.A.; Zheng, Y.; Kilby, M.D.; Sansom, D.M.; Drayson, M.T. Normal human pregnancy is associated with an elevation in the immune suppressive CD25+ CD4+ regulatory T-cell subset. Immunology 2004, 112, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Hao, K.; Zhou, Q.; Chen, W.; Jia, W.; Zheng, J.; Kang, J.; Wang, K.; Duan, T. Possible role of the ‘IDO-AhR axis’ in maternal-foetal tolerance. Cell Biol. Int. 2013, 37, 105–108. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, Y.; Sakai, M.; Miyazaki, S.; Higuma, S.; Shiozaki, A.; Saito, S. Decidual and peripheral blood CD4+CD25+ regulatory T cells in early pregnancy subjects and spontaneous abortion cases. Mol. Hum. Reprod. 2004, 10, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Sedlmayr, P. Indoleamine 2,3-dioxygenase in materno-fetal interaction. Curr. Drug Metab. 2007, 8, 205–208. [Google Scholar] [CrossRef] [PubMed]
- Marshall, N.B.; Kerkvliet, N.I. Dioxin and immune regulation: Emerging role of aryl hydrocarbon receptor in the generation of regulatory T cells. Ann. N. Y. Acad. Sci. 2010, 1183, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Stejskalova, L.; Pavek, P. The function of cytochrome P450 1A1 enzyme (CYP1A1) and aryl hydrocarbon receptor (AhR) in the placenta. Curr. Pharm. Biotechnol. 2011, 12, 715–730. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.Z.; Wang, K.; Fang, R.; Zheng, J. Expression of aryl hydrocarbon receptor in human placentas and fetal tissues. J. Histochem. Cytochem. 2010, 58, 679–685. [Google Scholar] [CrossRef] [PubMed]
- Stejskalova, L.; Vecerova, L.; Perez, L.M.; Vrzal, R.; Dvorak, Z.; Nachtigal, P.; Pavek, P. Aryl hydrocarbon receptor and aryl hydrocarbon nuclear translocator expression in human and rat placentas and transcription activity in human trophoblast cultures. Toxicol. Sci. 2011, 123, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Cocquebert, M.; Berndt, S.; Segond, N.; Guibourdenche, J.; Murthi, P.; Aldaz-Carroll, L.; Evain-Brion, D.; Fournier, T. Comparative expression of hCG beta-genes in human trophoblast from early and late first-trimester placentas. Am. J. Physiol. Endocrinol. Metab. 2012, 303, E950–E958. [Google Scholar] [CrossRef] [PubMed]
- Ikuta, T.; Kobayashi, Y.; Kawajiri, K. Cell density regulates intracellular localization of aryl hydrocarbon receptor. J. Biol. Chem. 2004, 279, 19209–19216. [Google Scholar] [CrossRef] [PubMed]
- Gladen, B.C.; Zadorozhnaja, T.D.; Chislovska, N.; Hryhorczuk, D.O.; Kennicutt, M.C., 2nd; Little, R.E. Polycyclic aromatic hydrocarbons in placenta. Hum. Exp. Toxicol. 2000, 19, 597–603. [Google Scholar] [CrossRef] [PubMed]
- Topinka, J.; Milcova, A.; Libalova, H.; Novakova, Z.; Rossner, P., Jr.; Balascak, I.; Sram, R.J. Biomarkers of exposure to tobacco smoke and environmental pollutants in mothers and their transplacental transfer to the foetus. Part I: Bulky DNA adducts. Mutat. Res. 2009, 669, 13–19. [Google Scholar] [PubMed]
- Mulero-Navarro, S.; Fernandez-Salguero, P.M. New Trends in Aryl Hydrocarbon Receptor Biology. Front. Cell Dev. Biol. 2016, 4, 45. [Google Scholar] [CrossRef] [PubMed]
- Veldhoen, M.; Hirota, K.; Westendorf, A.M.; Buer, J.; Dumoutier, L.; Renauld, J.C.; Stockinger, B. The aryl hydrocarbon receptor links TH17-cell-mediated autoimmunity to environmental toxins. Nature 2008, 453, 106–109. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Chen, X.; Chang, X.; Huang, Y.J.; Bao, S.; He, Q.; Li, Y.; Zheng, J.; Duan, T.; Wang, K. Potential involvement of placental AhR in unexplained recurrent spontaneous abortion. Reprod. Toxicol. 2016, 59, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Su, X.; Yang, B.; Zhao, A. Aryl hydrocarbon receptor and unexplained miscarriage. J. Obstet. Gynaecol. Res. 2017, 43, 1029–1036. [Google Scholar] [CrossRef] [PubMed]
- Mori, M.; Ishikawa, G.; Luo, S.S.; Mishima, T.; Goto, T.; Robinson, J.M.; Matsubara, S.; Takeshita, T.; Kataoka, H.; Takizawa, T. The cytotrophoblast layer of human chorionic villi becomes thinner but maintains its structural integrity during gestation. Biol. Reprod. 2007, 76, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Nebert, D.W.; Jones, J.E. Regulation of the mammalian cytochrome P1-450 (CYP1A1) gene. Int. J. Biochem. 1989, 21, 243–252. [Google Scholar] [CrossRef]
- Eaton, D.L.; Gallagher, E.P.; Bammler, T.K.; Kunze, K.L. Role of cytochrome P4501A2 in chemical carcinogenesis: Implications for human variability in expression and enzyme activity. Pharmacogenetics 1995, 5, 259–274. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.M.; Wo, Y.Y.; Stewart, J.; Hawkins, A.L.; Griffin, C.A.; Sutter, T.R.; Greenlee, W.F. Isolation and characterization of the human cytochrome P450 CYP1B1 gene. J. Biol. Chem. 1996, 271, 28324–28330. [Google Scholar] [CrossRef] [PubMed]
- Hakkola, J.; Pasanen, M.; Hukkanen, J.; Pelkonen, O.; Maenpaa, J.; Edwards, R.J.; Boobis, A.R.; Raunio, H. Expression of xenobiotic-metabolizing cytochrome P450 forms in human full-term placenta. Biochem. Pharmacol. 1996, 51, 403–411. [Google Scholar] [CrossRef]
- Li, Y.; Wang, K.; Zou, Q.Y.; Magness, R.R.; Zheng, J. 2,3,7,8-Tetrachlorodibenzo-p-dioxin differentially suppresses angiogenic responses in human placental vein and artery endothelial cells. Toxicology 2015, 336, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Le Vee, M.; Kolasa, E.; Jouan, E.; Collet, N.; Fardel, O. Differentiation of human placental BeWo cells by the environmental contaminant benzo(a)pyrene. Chem. Biol. Interact. 2014, 210, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi-Bardbori, A.; Bengtsson, J.; Rannug, U.; Rannug, A.; Wincent, E. Quercetin, resveratrol, and curcumin are indirect activators of the aryl hydrocarbon receptor (AHR). Chem. Res. Toxicol. 2012, 25, 1878–1884. [Google Scholar] [CrossRef] [PubMed]
- Ikuta, T.; Tachibana, T.; Watanabe, J.; Yoshida, M.; Yoneda, Y.; Kawajiri, K. Nucleocytoplasmic shuttling of the aryl hydrocarbon receptor. J. Biochem. 2000, 127, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Mezrich, J.D.; Fechner, J.H.; Zhang, X.; Johnson, B.P.; Burlingham, W.J.; Bradfield, C.A. An interaction between kynurenine and the aryl hydrocarbon receptor can generate regulatory T cells. J. Immunol. 2010, 185, 3190–3198. [Google Scholar] [CrossRef] [PubMed]
- Murthi, P.; Wallace, E.M.; Walker, D.W. Altered placental tryptophan metabolic pathway in human fetal growth restriction. Placenta 2017, 52, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Revel, A.; Raanani, H.; Younglai, E.; Xu, J.; Rogers, I.; Han, R.; Savouret, J.F.; Casper, R.F. Resveratrol, a natural aryl hydrocarbon receptor antagonist, protects lung from DNA damage and apoptosis caused by benzo[a]pyrene. J. Appl. Toxicol. 2003, 23, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Cvitic, S.; Longtine, M.S.; Hackl, H.; Wagner, K.; Nelson, M.D.; Desoye, G.; Hiden, U. The human placental sexome differs between trophoblast epithelium and villous vessel endothelium. PLoS ONE 2013, 8, e79233. [Google Scholar] [CrossRef] [PubMed]
- Nishizawa, H.; Imanishi, S.; Manabe, N. Effects of exposure in utero to bisphenol a on the expression of aryl hydrocarbon receptor, related factors, and xenobiotic metabolizing enzymes in murine embryos. J. Reprod. Dev. 2005, 51, 593–605. [Google Scholar] [CrossRef] [PubMed]
- Pollenz, R.S.; Sattler, C.A.; Poland, A. The aryl hydrocarbon receptor and aryl hydrocarbon receptor nuclear translocator protein show distinct subcellular localizations in Hepa 1c1c7 cells by immunofluorescence microscopy. Mol. Pharmacol. 1994, 45, 428–438. [Google Scholar] [PubMed]
- Richter, C.A.; Tillitt, D.E.; Hannink, M. Regulation of subcellular localization of the aryl hydrocarbon receptor (AhR). Arch. Biochem. Biophys. 2001, 389, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Detmar, J.; Rennie, M.Y.; Whiteley, K.J.; Qu, D.; Taniuchi, Y.; Shang, X.; Casper, R.F.; Adamson, S.L.; Sled, J.G.; Jurisicova, A. Fetal growth restriction triggered by polycyclic aromatic hydrocarbons is associated with altered placental vasculature and AhR-dependent changes in cell death. Am. J. Physiol. Endocrinol. Metab. 2008, 295, E519–E530. [Google Scholar] [CrossRef] [PubMed]
- Conway, D.E.; Sakurai, Y.; Weiss, D.; Vega, J.D.; Taylor, W.R.; Jo, H.; Eskin, S.G.; Marcus, C.B.; McIntire, L.V. Expression of CYP1A1 and CYP1B1 in human endothelial cells: Regulation by fluid shear stress. Cardiovasc. Res. 2009, 81, 669–677. [Google Scholar] [CrossRef] [PubMed]
- Perdew, G.H.; Hollenback, C.E. Evidence for two functionally distinct forms of the human Ah receptor. J. Biochem. Toxicol. 1995, 10, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Jans, D.A.; Hubner, S. Regulation of protein transport to the nucleus: Central role of phosphorylation. Physiol. Rev. 1996, 76, 651–685. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Baldwin, K.T. 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced degradation of aryl hydrocarbon receptor (AhR) by the ubiquitin-proteasome pathway. Role of the transcription activaton and DNA binding of AhR. J. Biol. Chem. 2000, 275, 8432–8438. [Google Scholar] [CrossRef] [PubMed]
- Xing, X.; Bi, H.; Chang, A.K.; Zang, M.X.; Wang, M.; Ao, X.; Li, S.; Pan, H.; Guo, Q.; Wu, H. SUMOylation of AhR modulates its activity and stability through inhibiting its ubiquitination. J. Cell Physiol. 2012, 227, 3812–3819. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Wang, Q.; Peng, J.; Wu, M.; Pan, B.; Xing, B. Transfer of polycyclic aromatic hydrocarbons from mother to fetus in relation to pregnancy complications. Sci. Total Environ. 2018, 636, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Yeung, A.W.; Terentis, A.C.; King, N.J.; Thomas, S.R. Role of indoleamine 2,3-dioxygenase in health and disease. Clin. Sci. 2015, 129, 601–672. [Google Scholar] [CrossRef] [PubMed]
- Wierzba, W.; Radowicki, S.; Bojar, I.; Pinkas, J. Effects of environmental pollution with aromatic hydrocarbons on endocrine and metabolic functions of the human placenta. Ann. Agric. Environ. Med. 2018, 25, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Valero, L.; Alhareth, K.; Gil, S.; Simasotchi, C.; Roques, C.; Scherman, D.; Mignet, N.; Fournier, T.; Andrieux, K. Assessment of dually labelled PEGylated liposomes transplacental passage and placental penetration using a combination of two ex-vivo human models: The dually perfused placenta and the suspended villous explants. Int. J. Pharm. 2017, 532, 729–737. [Google Scholar] [CrossRef] [PubMed]
- Kliman, H.J.; Nestler, J.E.; Sermasi, E.; Sanger, J.M.; Strauss, J.F., 3rd. Purification, characterization, and in vitro differentiation of cytotrophoblasts from human term placentae. Endocrinology 1986, 118, 1567–1582. [Google Scholar] [CrossRef] [PubMed]
- Alsat, E.; Mirlesse, V.; Fondacci, C.; Dodeur, M.; Evain-Brion, D. Parathyroid hormone increases epidermal growth factor receptors in cultured human trophoblastic cells from early and term placenta. J. Clin. Endocrinol. Metab. 1991, 73, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Wice, B.; Menton, D.; Geuze, H.; Schwartz, A.L. Modulators of cyclic AMP metabolism induce syncytiotrophoblast formation in vitro. Exp. Cell Res. 1990, 186, 306–316. [Google Scholar] [CrossRef]
- Gerbaud, P.; Pidoux, G.; Guibourdenche, J.; Pathirage, N.; Costa, J.M.; Badet, J.; Frendo, J.L.; Murthi, P.; Evain-Brion, D. Mesenchymal activin-A overcomes defective human trisomy 21 trophoblast fusion. Endocrinology 2011, 152, 5017–5028. [Google Scholar] [CrossRef] [PubMed]
- Bompais, H.; Chagraoui, J.; Canron, X.; Crisan, M.; Liu, X.H.; Anjo, A.; Tolla-Le Port, C.; Leboeuf, M.; Charbord, P.; Bikfalvi, A.; et al. Human endothelial cells derived from circulating progenitors display specific functional properties compared with mature vessel wall endothelial cells. Blood 2004, 103, 2577–2584. [Google Scholar] [CrossRef] [PubMed]




| mRNA | Pregnancy Period | |
|---|---|---|
| First Trimester (n = 16) | Term (n = 8) | |
| Fold Change Average ± Standard Deviation | ||
| HO1 | 1.04 ± 0.33 | 2.24 ± 0.48 *** |
| SOD1 | 1.01 ± 0.16 | 3.99 ± 1.33 *** |
| SOD2 | 1.15 ± 0.64 | 3.09 ± 0.85 **** |
| NQO1 | 1.08 ± 0.51 | 3.53 ± 1.75 ** |
| GSTA2 | 1.05 ± 0.39 | 2.42 ± 0.84 ** |
| Catalase | 1.06 ± 0.37 | 6.52 ± 2.62 **** |
| mRNA | Confluence | |||
|---|---|---|---|---|
| 70% | 90% | |||
| HO1 | 0.55 ± 0.06 | **** | 0.37 ± 0.05 | ****, ## |
| SOD1 | 0.93 ± 0.05 | * | 0.64 ± 0.07 | ****, ### |
| SOD2 | 0.99 ± 0.13 | - | 0.56 ± 0.05 | ****, ### |
| NQO1 | 0.78 ± 0.11 | ** | 0.40 ± 0.07 | ****, ## |
| Catalase | 1.01 ± 0.12 | - | 0.66 ± 0.07 | ****, # |
| GSTA2 | 0.92 ± 0.14 | **** | 0.66 ± 0.10 | **, # |
| GSS | 1.06 ± 0.14 | - | 0.77 ± 0.12 | **, # |
| NRF2 | 1.02 ± 0.42 | - | 0.54 ± 0.15 | *** |
| AhR | forward 5′-TAACCCAGACCAGATTCCTC-3′ |
| reverse 5′-GCAAACAAAGCCAACTGAG-3′ | |
| AhRR | forward 5′-CGCCTCAGTGTCAGTTACC-3′ |
| reverse 5′-ACTCACGACCAGAGCAAAG-3′ | |
| ARNT | forward 5′-CCCCTCCTGTAACCATTC-3′ |
| reverse 5′-CTGCCCACACCAAACTG-3′ | |
| β-actin | forward 5′-CTCCTTAATGTCACGCACGATTTC-3′ |
| reverse 5′-ACAATGAGCTGCGTGTGGCT-3′ | |
| βhCG | forward 5′-GCTACTGCCCCACCATGACC-3′ |
| reverse 5′-ATGGACTCGAAGCGCACATC-3′ | |
| Catalase | forward 5′-TTCATCCAGAAGAAAGCGGTCAA-3′ |
| reverse 5′-TGGATGTGGCTCCCGTAGTCA-3′ | |
| CYP11A1 | forward 5′-TTTTTGCCCCTGTTGGATGCA-3′ |
| reverse 5′-CCCTGGCGCTCCCCAAAAAT-3′ | |
| CYP19 | forward 5′-CCTGAAGCCATGCCTGCTGC-3′ |
| reverse 5′-CCGATCCCCATCCACAGGAATCT-3′ | |
| CYP1A1 | forward 5′-CCACAGCACAACAAGAGAC-3′ |
| reverse 5′-CCATCAGGGGTGAGAAAC-3′ | |
| CYP1A2 | forward 5′-TGCCAAACAGCATCATCTTG-3′ |
| reverse 5′-ACAGCACAACAAGGGACACA-3′ | |
| CYP1B1 | forward 5′-AACGTACCGGCCACTATCAC-3′ |
| reverse 5′-CAGTGGTGGCATGAGGAATA-3′ | |
| GH2 | forward 5′-CACCTTCCAACAGGGTGAAAA-3′ |
| reverse 5′-GGTGGCGATAGACGTTGCT-3′ | |
| GSS | forward 5′-GGAACATCCATGTGATCCGAC-3′ |
| reverse 5′-GGAACATCCATGTGATCCGAC-3′ | |
| GSTA2 | forward 5′-TTGGGCTCTATGGGAAGGAC-3′ |
| reverse 5′-GGGAGATGTATTTGCAGCGGA-3′ | |
| hPL | forward 5′-GCATGACTCCCAGACCTCCTT-3′ |
| reverse 5′-TGCGGAGCAGCTCTAGATTGG-3′ | |
| HO1 | forward 5′-CGTTCCTGGTCAACATCC-3′ |
| reverse 5′-CTGTCGCCACCAGAAAG-3′ | |
| HPRT | forward 5′-GGCGTCGTGATTAGTGATG-3′ |
| reverse 5′-CAGAGGGCTACAATGTGATG-3′ | |
| KRT7 | forward 5′-GGACATCGAGATCGCCACCT-3′ |
| reverse 5′-ACCGCCACTGCTACTGCCA-3′ | |
| NQO1 | forward 5′-GCAGACCTTGTGATATTCCAG-3′ |
| reverse 5′-CCTATGAACACTCGCTCAAAC-3′ | |
| NRF2 | forward 5′-TGACATACTTTGGAGGCAAG-3′ |
| reverse 5′-CTAAATCAACAGGGGCTACC-3′ | |
| PLGF | forward 5′-GCTCGTCAGAGGTGGAAGTGGT-3′ |
| reverse 5′-CTCGCTGGGGTACTCGGACA-3 | |
| RPL0 | forward 5′- AACATCTCCCCCTTCTCCT-3′ |
| reverse 5′- ACTCGTTTGTACCCGTTGAT-3′ | |
| RPL13 | forward 5′- AAGGTCGTGCGTCTGAAG-3′ |
| reverse 5′-GAGTCCGTGGGTCTTGAG-3′ | |
| SDHA | forward 5′- CCACCACTGCATCAAATTCATG-3′ |
| reverse 5′-TGGGAACAAGAGGGCATCTG-3′ | |
| SOD1 | forward 5′-CTGAAGGCCTGCATGGATTC-3′ |
| reverse 5′-CCAAGTCTCCAACATGCCTCTC-3′ | |
| SOD2 | forward 5′-GTTCAATGGTGGTGGTCATA-3′ |
| reverse 5′-GTAAGTGTCCCCGTTCCTT-3′ | |
| Ubiquitin C | forward 5′-CACTTGGTCCTGCGCTTGA-3′ |
| reverse 5′-TTTTTTGGGAATGCAACAACTTT-3′ | |
| 18S | forward 5′- TCCCCCAACTTCTTAGAGG-3′ |
| reverse 5′-CTTATGACCCGCACTTACTG-3′ |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wakx, A.; Nedder, M.; Tomkiewicz-Raulet, C.; Dalmasso, J.; Chissey, A.; Boland, S.; Vibert, F.; Degrelle, S.A.; Fournier, T.; Coumoul, X.; et al. Expression, Localization, and Activity of the Aryl Hydrocarbon Receptor in the Human Placenta. Int. J. Mol. Sci. 2018, 19, 3762. https://doi.org/10.3390/ijms19123762
Wakx A, Nedder M, Tomkiewicz-Raulet C, Dalmasso J, Chissey A, Boland S, Vibert F, Degrelle SA, Fournier T, Coumoul X, et al. Expression, Localization, and Activity of the Aryl Hydrocarbon Receptor in the Human Placenta. International Journal of Molecular Sciences. 2018; 19(12):3762. https://doi.org/10.3390/ijms19123762
Chicago/Turabian StyleWakx, Anaïs, Margaux Nedder, Céline Tomkiewicz-Raulet, Jessica Dalmasso, Audrey Chissey, Sonja Boland, Françoise Vibert, Séverine A. Degrelle, Thierry Fournier, Xavier Coumoul, and et al. 2018. "Expression, Localization, and Activity of the Aryl Hydrocarbon Receptor in the Human Placenta" International Journal of Molecular Sciences 19, no. 12: 3762. https://doi.org/10.3390/ijms19123762
APA StyleWakx, A., Nedder, M., Tomkiewicz-Raulet, C., Dalmasso, J., Chissey, A., Boland, S., Vibert, F., Degrelle, S. A., Fournier, T., Coumoul, X., Gil, S., & Ferecatu, I. (2018). Expression, Localization, and Activity of the Aryl Hydrocarbon Receptor in the Human Placenta. International Journal of Molecular Sciences, 19(12), 3762. https://doi.org/10.3390/ijms19123762