Most Women with Previous Gestational Diabetes Mellitus Have Impaired Glucose Metabolism after a Decade
Abstract
1. Introduction
2. Results
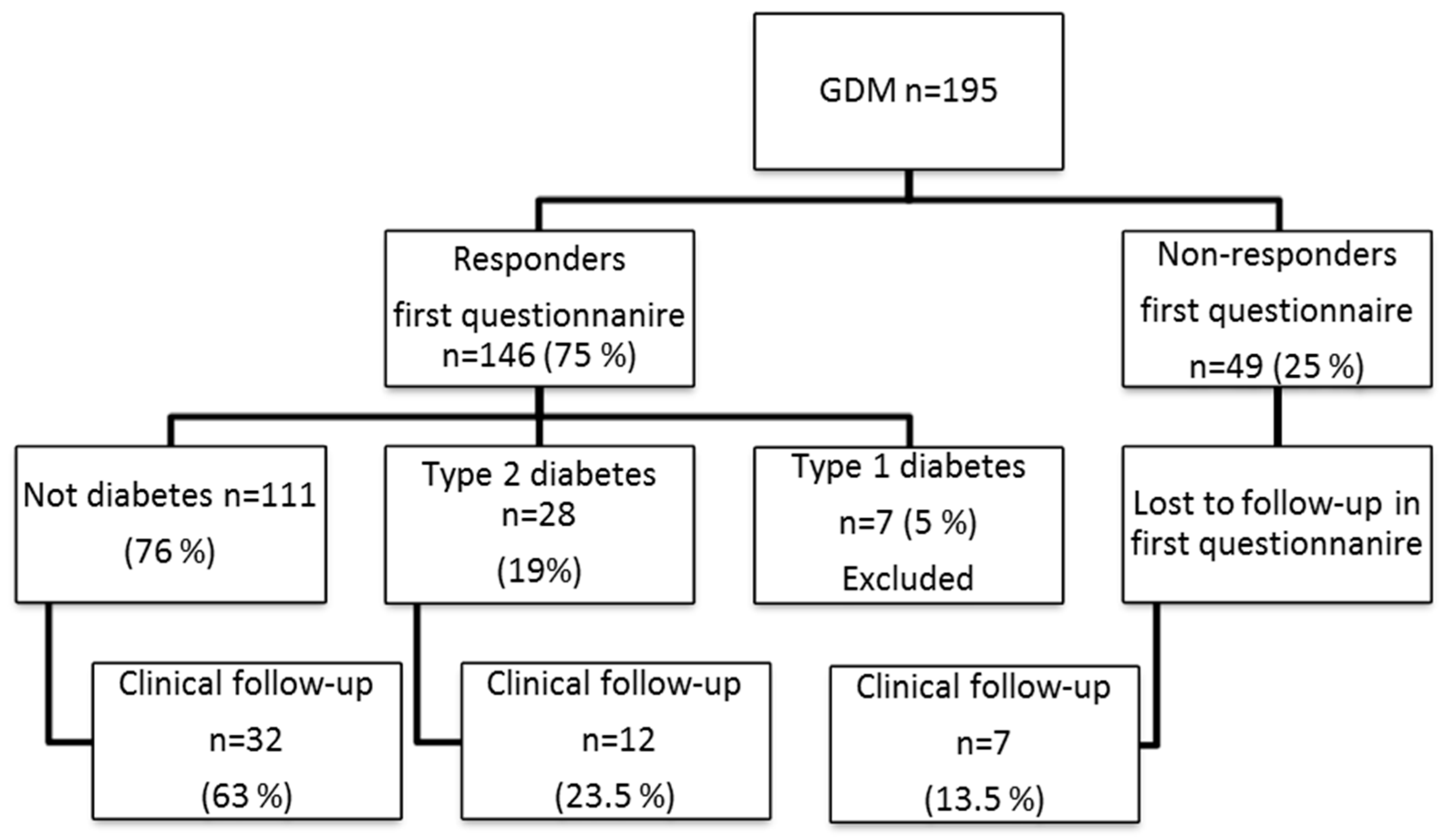
2.1. Characteristics of Women with Previous GDM at Clinical Follow-Up (n = 51)
2.2. Manifest Diabetes Mellitus
2.3. Normal Glucose Tolerance, IFG, and IGT
2.4. Heredity, Country of Origin, and Additional Pregnancies
2.5. Other Diseases and Other Medication
2.6. Characteristics of the Group with Manifest Diabetes Mellitus
2.7. GAD Antibodies, ln(Proinsulin), and C-Peptide
3. Discussion
4. Materials and Methods
4.1. Participants
4.2. Measurements
4.3. Ethics
4.4. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| OGTT | Oral Glucose Tolerance Test |
| GDM | Gestational Diabetes Mellitus |
| IFG | Impaired fasting plasma glucose |
| IGT | Impaired glucose tolerance |
| GAD | Glutamic acid decarboxylase |
| DPSG | The Diabetic Pregnancy Study Group |
| IADPSG | The International Association of the Diabetes and Pregnancy Study Groups |
| ADA | American Diabetes Association |
| EASD | European Association for the Study of Diabetes |
| HAPO | The Hyperglycemia and Adverse Pregnancy Outcome Study |
| NGSP | National Glycohemoglobin Standardization Program |
| IFCC | International Federation of Clinical Chemistry and Laboratory Medicine |
| WHO | World Health Organization |
References
- Coustan, D.R.; Carpenter, M.W.; O’Sullivan, P.S.; Carr, S.R. Gestational diabetes: predictors of subsequent disordered glucose metabolism. Am. J. Obstet. Gynecol. 1993, 168, 1139–1144. [Google Scholar] [CrossRef]
- Damm, P.; Kuhl, C.; Bertelsen, A.; Molsted-Pedersen, L. Predictive factors for the development of diabetes in women with previous gestational diabetes mellitus. Am. J. Obstet. Gynecol. 1992, 167, 607–616. [Google Scholar] [CrossRef]
- Ekelund, M.; Shaat, N.; Almgren, P.; Groop, L.; Berntorp, K. Prediction of postpartum diabetes in women with gestational diabetes mellitus. Diabetologia 2010, 53, 452–457. [Google Scholar] [CrossRef] [PubMed]
- Linne, Y.; Barkeling, B.; Rossner, S. Natural course of gestational diabetes mellitus: long term follow up of women in the SPAWN study. BJOG 2002, 109, 1227–1231. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, J.B.; Mahan, C.M. Criteria for the Oral Glucose Tolerance Test in Pregnancy. Diabetes 1964, 13, 278–285. [Google Scholar] [PubMed]
- Bellamy, L.; Casas, J.P.; Hingorani, A.D.; Williams, D. Type 2 diabetes mellitus after gestational diabetes: A systematic review and meta-analysis. Lancet 2009, 373, 1773–1779. [Google Scholar] [CrossRef]
- Kim, C.; Newton, K.M.; Knopp, R.H. Gestational diabetes and the incidence of type 2 diabetes: A systematic review. Diabetes Care 2002, 25, 1862–1868. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, J.M.; Contreras, R.; Chen, W.; Sacks, D.A. Trends in the prevalence of preexisting diabetes and gestational diabetes mellitus among a racially/ethnically diverse population of pregnant women, 1999–2005. Diabetes Care 2008, 31, 899–904. [Google Scholar] [CrossRef] [PubMed]
- Ostlund, I.; Hanson, U.; Bjorklund, A.; Hjertberg, R.; Eva, N.; Nordlander, E.; Swahn, M.L.; Wager, J. Maternal and fetal outcomes if gestational impaired glucose tolerance is not treated. Diabetes Care 2003, 26, 2107–2111. [Google Scholar] [CrossRef] [PubMed]
- Kessous, R.; Shoham-Vardi, I.; Pariente, G.; Sherf, M.; Sheiner, E. An association between gestational diabetes mellitus and long-term maternal cardiovascular morbidity. Heart 2013, 99, 1118–1121. [Google Scholar] [CrossRef] [PubMed]
- Lauenborg, J.; Hansen, T.; Jensen, D.M.; Vestergaard, H.; Molsted-Pedersen, L.; Hornnes, P.; Locht, H.; Pedersen, O.; Damm, P. Increasing incidence of diabetes after gestational diabetes: A long-term follow-up in a Danish population. Diabetes Care 2004, 27, 1194–1199. [Google Scholar] [CrossRef] [PubMed]
- Wahlberg, J.; Ekman, B.; Nystrom, L.; Hanson, U.; Persson, B.; Arnqvist, H.J. Gestational diabetes: Glycaemic predictors for fetal macrosomia and maternal risk of future diabetes. Diabetes Res. Clin. Pract 2016, 114, 99–105. [Google Scholar] [CrossRef] [PubMed]
- McGovern, A.; Butler, L.; Jones, S.; van Vlymen, J.; Sadek, K.; Munro, N.; Carr, H.; de Lusignan, S. Diabetes screening after gestational diabetes in England: A quantitative retrospective cohort study. Br. J. Gen. Pract. 2014, 64, e17–e23. [Google Scholar] [CrossRef] [PubMed]
- Battarbee, A.N.; Yee, L.M. Barriers to Postpartum Follow-Up and Glucose Tolerance Testing in Women with Gestational Diabetes Mellitus. Am. J. Perinatol. 2018, 35, 354–360. [Google Scholar] [PubMed]
- Lind, T.; Phillips, P.R. Influence of pregnancy on the 75-g OGTT. A prospective multicenter study. The Diabetic Pregnancy Study Group of the European Association for the Study of Diabetes. Diabetes 1991, 40 Suppl. 2, 8–13. [Google Scholar] [CrossRef]
- Brown, C.J.; Dawson, A.; Dodds, R.; Gamsu, H.; Gillmer, M.; Hall, M.; Hounsome, B.; Knopfler, A.; Ostler, J.; Peacock, I.; et al. Report of the Pregnancy and Neonatal Care Group. Diabet. Med. 1996, 9 (Suppl. 4), S43–S53. [Google Scholar]
- Qiao, Q.; Lindstrom, J.; Valle, T.T.; Tuomilehto, J. Progression to clinically diagnosed and treated diabetes from impaired glucose tolerance and impaired fasting glycaemia. Diabet. Med. 2003, 20, 1027–1033. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes, A. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2018. Diabetes Care 2018, 41 (Suppl. 1), S13–S27. [Google Scholar] [CrossRef] [PubMed]
- International Association of, D.; Pregnancy Study Groups Consensus, P.; Metzger, B.E.; Gabbe, S.G.; Persson, B.; Buchanan, T.A.; Catalano, P.A.; Damm, P.; Dyer, A.R.; Leiva, A.; et al. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care 2010, 33, 676–682. [Google Scholar]
- American Diabetes, A. Standards of medical care in diabetes--2013. Diabetes Care 2013, (Suppl. 1), S11–S66. [Google Scholar] [CrossRef] [PubMed]
- Diagnostic Criteria and Classification of Hyperglycaemia First Detected in Pregnancy; World Health Organization: Geneva, 2013. Available online: https://www.ncbi.nlm.nih.gov/books/NBK169024/ (accessed on 13 October 2018).
- Vandorsten, J.P.; Dodson, W.C.; Espeland, M.A.; Grobman, W.A.; Guise, J.M.; Mercer, B.M.; Minkoff, H.L.; Poindexter, B.; Prosser, L.A.; Sawaya, G.F.; et al. NIH consensus development conference: Diagnosing gestational diabetes mellitus. NIH Consens. State. Sci. Statements 2013, 29, 1–31. [Google Scholar] [PubMed]
- Blackwell, S.C. Counterpoint: enough evidence to treat? The American College of Obstetricians and Gynecologists guidelines. Clin. Chem. 2012, 58, 1098–1100. [Google Scholar] [CrossRef] [PubMed]
- Long, H. Diagnosing gestational diabetes: can expert opinions replace scientific evidence? Diabetologia 2011, 54, 2211–2213. [Google Scholar] [CrossRef] [PubMed]
- Ryan, E.A. Diagnosing gestational diabetes. Diabetologia 2011, 54, 480–486. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, H.D. Diagnosing gestational diabetes mellitus: rationed or rationally related to risk? Diabetes Care 2013, 36, 2879–2880. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, H.D.; Colagiuri, S.; Roglic, G.; Hod, M. Diagnosis of GDM: A suggested consensus. Best Pract. Res. Clin. Obstet. Gynaecol. 2015, 29, 194–205. [Google Scholar] [CrossRef] [PubMed]
- Boney, C.M.; Verma, A.; Tucker, R.; Vohr, B.R. Metabolic syndrome in childhood: Association with birth weight, maternal obesity, and gestational diabetes mellitus. Pediatrics 2005, 115, e290–e296. [Google Scholar] [CrossRef] [PubMed]
- Lauenborg, J.; Mathiesen, E.; Hansen, T.; Glumer, C.; Jorgensen, T.; Borch-Johnsen, K.; Hornnes, P.; Pedersen, O.; Damm, P. The prevalence of the metabolic syndrome in a danish population of women with previous gestational diabetes mellitus is three-fold higher than in the general population. J. Clin. Endocrinol. Metab. 2005, 90, 4004–4010. [Google Scholar] [CrossRef] [PubMed]
- Mertens, I.; Van Gaal, L.F. New International Diabetes Federation (IDF) and National Cholesterol Education Program Adult Treatment panel III (NCEP-ATPIII) criteria and the involvement of hemostasis and fibrinolysis in the metabolic syndrome. J. Thromb. Haemost. 2006, 4, 1164–1166. [Google Scholar] [CrossRef] [PubMed]
- Lappas, M.; Jinks, D.; Shub, A.; Willcox, J.C.; Georgiou, H.M.; Permezel, M. Postpartum IGF-I and IGFBP-2 levels are prospectively associated with the development of type 2 diabetes in women with previous gestational diabetes mellitus. Diabetes Metab. 2016, 42, 442–447. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, C.; Ursing, D.; Torn, C.; Aberg, A.; Landin-Olsson, M. Presence of GAD antibodies during gestational diabetes mellitus predicts type 1 diabetes. Diabetes Care 2007, 30, 1968–1971. [Google Scholar] [CrossRef] [PubMed]
- Rono, K.; Grotenfelt, N.E.; Klemetti, M.M.; Stach-Lempinen, B.; Huvinen, E.; Meinila, J.; Valkama, A.; Tiitinen, A.; Roine, R.P.; Poyhonen-Alho, M.; et al. Effect of a lifestyle intervention during pregnancy-findings from the Finnish gestational diabetes prevention trial (RADIEL). J. Perinatol. 2018, 38, 1157–1164. [Google Scholar] [CrossRef] [PubMed]
- Ignell, C.; Berntorp, K. Evaluation of the relationship between capillary and venous plasma glucose concentrations obtained by the HemoCue Glucose 201+ system during an oral glucose tolerance test. Scand. J. Clin. Lab. Invest. 2011, 71, 670–675. [Google Scholar] [CrossRef] [PubMed]
- Stahl, M.; Brandslund, I.; Jorgensen, L.G.; Hyltoft Petersen, P.; Borch-Johnsen, K.; de Fine Olivarius, N. Can capillary whole blood glucose and venous plasma glucose measurements be used interchangeably in diagnosis of diabetes mellitus? Scand. J. Clin. Lab. Invest. 2002, 62, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Daly, N.; Carroll, C.; Flynn, I.; Harley, R.; Maguire, P.J.; Turner, M.J. Evaluation of point-of-care maternal glucose measurements for the diagnosis of gestational diabetes mellitus. BJOG 2017, 124, 1746–1752. [Google Scholar] [CrossRef] [PubMed]
- Nord, E.; Hanson, U.; Persson, B. Blood glucose limits in the diagnosis of impaired glucose tolerance during pregnancy. Relation to morbidity. Acta Obstet. Gynecol. Scand. 1995, 74, 589–593. [Google Scholar] [CrossRef] [PubMed]
- Voss, E.M.; Cembrowski, G.S. Performance characteristics of the HemoCue B-Glucose analyzer using whole-blood samples. Arch. Pathol. Lab. Med. 1993, 117, 711–713. [Google Scholar] [PubMed]
- Report of a WHO/IDF Consultation: Definition and Diagnosis of Diabetes Mellitus and Intermediate Hyperglycemia; WHO Document Production Services: Geneva, Switzerland, 2006.
- Kordonouri, O.; Hartmann, R.; Gruters-Kieslich, A.; Knip, M.; Danne, T. Age-specific levels of diabetes-related GAD and IA-2 antibodies in healthy children and adults. J. Pediatr. Endocrinol. Metab. 2002, 15, 47–52. [Google Scholar] [CrossRef] [PubMed]
| Variable | a Normal N = 12 | b Diabetes N = 16 | c IFG N = 13 | d IGT N = 10 |
|---|---|---|---|---|
| Weight before pregnancy (kg) | 73.3 ± 14.4 | 79.1 ± 21.8 | 79.5 ± 20.9 | 73.9 ± 14.4 |
| Weight at follow-up (kg) | 74.4 ± 10 | 80.8 ± 18.5 | 83.4 ± 21.7 | 81.2 ± 17.9 |
| Age (years) | 45 ± 7 | 42 ± 6 | 41 ± 4 | 45 ± 6 |
| Height (cm) | 165 ± 5.3 | 166 ± 8.7 | 164 ± 6.6 | 164 ± 5.8 |
| BMI (kg/m2) | 26.9 ± 3.4 | 29.2 ± 5.8 | 31.1 ± 8.1 | 30.1 ± 5.6 |
| Waist | 91.9 ± 11.9 | 96.9 ± 14.4 | 99.7 ± 17.1 | 104.7 ± 17.7 |
| Sagittal diameter (cm) | 21.0 ± 2.9 | 22.4 ± 2.9 | 23.3 ± 4.7 | 24.3 ± 4.6 |
| Systolic BP supine (mmHg) | 116 ± 11 | 128 ± 15 * | 124 ± 7 | 134 ± 20 * |
| Diastolic BP supine (mmHg) | 73 ± 7 | 81 ± 11 | 79 ± 6 | 84 ± 13.2 |
| Heart rate supine (beat/min) | 66 ± 8 | 68 ± 11 | 64 ± 6 | 70 ± 8 |
| P-Cholesterol (mmol/L) | 5.2 ± 1.2 | 4.6 ± 0.99 | 5.0 ± 0.81 | 5.2 ± 0.89 |
| P-LDL-cholesterol (mmol/L) | 3.4 ± 1.1 | 2.8 ± 0.86 | 3.0 ± 0.82 | 3.3 ± 0.75 |
| P-HDL-cholesterol (mmol/L) | 1.4 ± 0.31 | 1.2 ± 0.35 | 1.4 ± 0.36 | 1.3 ± 0.20 |
| P-Triglycerides (mmol/L) | 1.1 ± 0.80 | 1.3 ± 0.63 | 1.17 ± 0.48 | 1.32 ± 0.60 |
| APOB/APOA1 | 0.71 ± 0.24 | 0.67 ± 0.16 | 0.74 ± 0.21 | 0.80 ± 0.17 |
| Breast feeding (%) | 75% (n = 9) | 56% (n = 9) | 85% (n = 11) | 80% (n = 8) |
| Pregnancies (n) | 2.5 ± 0.5 | 2.2 ± 0.8 | 2.2 ± 0.8 | 3.5 ± 2.4 |
| Birth weight ¥ | 3053 ± 342 | 3462 ± 755 | 3162 ± 414 | 3381 ± 538 |
| Heredity for diabetes † | 67% (n = 8) | 100% (n = 16) * | 77% (n = 10) | 80% (n = 8) |
| Variable | a Normal N = 12 | b Diabetes N = 16 | c IFG N = 13 | d IGT N = 10 |
|---|---|---|---|---|
| At diagnosis of gestational diabetes mellitus (GDM) (capillary blood glucose) | ||||
| Fasting B-Glucose (mmol/L) | 5.7 ± 0.8 | 5.6 ± 1.1 | 5.3 ± 0.9 | 5.3 ± 0.7 |
| 2 h 75 g OGTT before (mmol/L) | 11.1 ± 0.9 | 11.5 ± 1.3 | 10.9 ± 0.7 | 10.6 ± 0.5 |
| At clinical follow-up (capillary plasma glucose) | ||||
| Fasting P-Glucose (mmol/L) | 5.6 ± 0.4 | 7.2 ± 1.5 ** | 6.5 ± 0.2 | 6.1 ± 0.7 |
| 2 h OGTT after (mmol/L) | 6.4 ± 1.9 | 12.7 ± 3.5 *** | 7.7 ± 1.0 | 10.0 ± 0.9 * |
| HbA1c, NGSP (%) | 5.5 ± 0.3 | 6.3 ± 1 * | 5.5 ± 0.2 | 5.6 ± 0.5 |
| HbA1c, IFCC (mmol/mol) | 37 ± 3 | 45 ± 12 * | 37 ± 2 | 38 ± 5 |
| C-peptide (nmol/L) | 0.58 ± 0.29 | 0.54 ± 0.32 | 0.74 ± 0.32 | 0.73 ± 0.36 |
| Proinsulin (pmol/L) | 7.3 ± 8.1 | 10.9 ± 14.6 | 8.6 ± 6.7 | 9.7 ± 8.7 |
| ln(Proinsulin) (pmol/L) | 1.6 ± 0.8 | 1.9 ± 0.9 | 1.9 ± 0.8 | 1.9 ± 0.9 |
| Ratio ln(Proinsulin)/C-peptide | 2.9 ± 1.0 | 3.9 ± 1.7 | 2.6 ± 0.8 | 2.7 ± 1.0 |
| Variable | C-Peptide nmol/L | ln(Proinsulin) pmol/L | ||
|---|---|---|---|---|
| r | p | r | p | |
| Age (yrs.) | 0.02 | n.s | 0.13 | n.s |
| Weight (kg) | 0.48 | <0.001 *** | 0.50 | <0.001 *** |
| BMI (kg/m2) | 0.52 | <0.001 *** | 0.54 | <0.001 *** |
| Waist circumference (cm) | 0.60 | <0.001 *** | 0.60 | 0.001 *** |
| Sagittal diameter (cm) | 0.51 | 0.001 *** | 0.55 | 0.001 *** |
| Systolic BP mmHg) | 0.40 | 0.003 ** | 0.44 | <0.001 *** |
| Diastolic BP (mmHg) | 0.36 | 0.009 ** | 0.35 | 0.013 * |
| HbA1c (mmol/mol) | 0.024 | n.s | 0.31 | 0.028 * |
| Fasting P-glucose (mmol/L) | 0.11 | n.s | 0.29 | 0.045 * |
| 2 h OGTT P-glucose (mmol/L) | 0.14 | n.s | 0.37 | <0.01 ** |
| P-Cholesterol (mmol/L) | 0.19 | n.s | 0.23 | n.s |
| P-HDL-Cholesterol (mmol/L) | −0.21 | n.s | −0.40 | 0.004 * |
| P-LDL-Cholesterol (mmol/L) | 0.16 | n.s | 0.20 | n.s |
| P-Triglycerides (mmol/L) | 0.39 | 0.005 ** | 0.48 | <0.001 *** |
| Parameter | Clinical Evaluation N = 51 | Not Investigated N = 144 | p-Value |
|---|---|---|---|
| Born in Sweden (%) | 88 | 72 | n.s. |
| Born Outside EU (%) | 10 | 22 | n.s. |
| Height (m) | 1.65 ± 0.07 | 1.65 ± 0.07 | n.s. |
| Weight (kg) | 77 ± 19 | 74 ± 17 | n.s. |
| BMI (kg/m2) | 28 ± 6 | 28 ± 6 | n.s. |
| OGTT 2 h Glucose (mmol/L) | 9.9 ± 0.9 | 10.5 ± 1.7 | 0.032 |
| Birthweight of the Child (g) | 3664 ± 611 | 3624 ± 5696 | n.s. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeanette, W.; Bertil, E.; Hans, A.J. Most Women with Previous Gestational Diabetes Mellitus Have Impaired Glucose Metabolism after a Decade. Int. J. Mol. Sci. 2018, 19, 3724. https://doi.org/10.3390/ijms19123724
Jeanette W, Bertil E, Hans AJ. Most Women with Previous Gestational Diabetes Mellitus Have Impaired Glucose Metabolism after a Decade. International Journal of Molecular Sciences. 2018; 19(12):3724. https://doi.org/10.3390/ijms19123724
Chicago/Turabian StyleJeanette, Wahlberg, Ekman Bertil, and Arnqvist J. Hans. 2018. "Most Women with Previous Gestational Diabetes Mellitus Have Impaired Glucose Metabolism after a Decade" International Journal of Molecular Sciences 19, no. 12: 3724. https://doi.org/10.3390/ijms19123724
APA StyleJeanette, W., Bertil, E., & Hans, A. J. (2018). Most Women with Previous Gestational Diabetes Mellitus Have Impaired Glucose Metabolism after a Decade. International Journal of Molecular Sciences, 19(12), 3724. https://doi.org/10.3390/ijms19123724





