Study on Thermal Decomposition Behaviors of Terpolymers of Carbon Dioxide, Propylene Oxide, and Cyclohexene Oxide
Abstract
1. Introduction
2. Results and Discussion
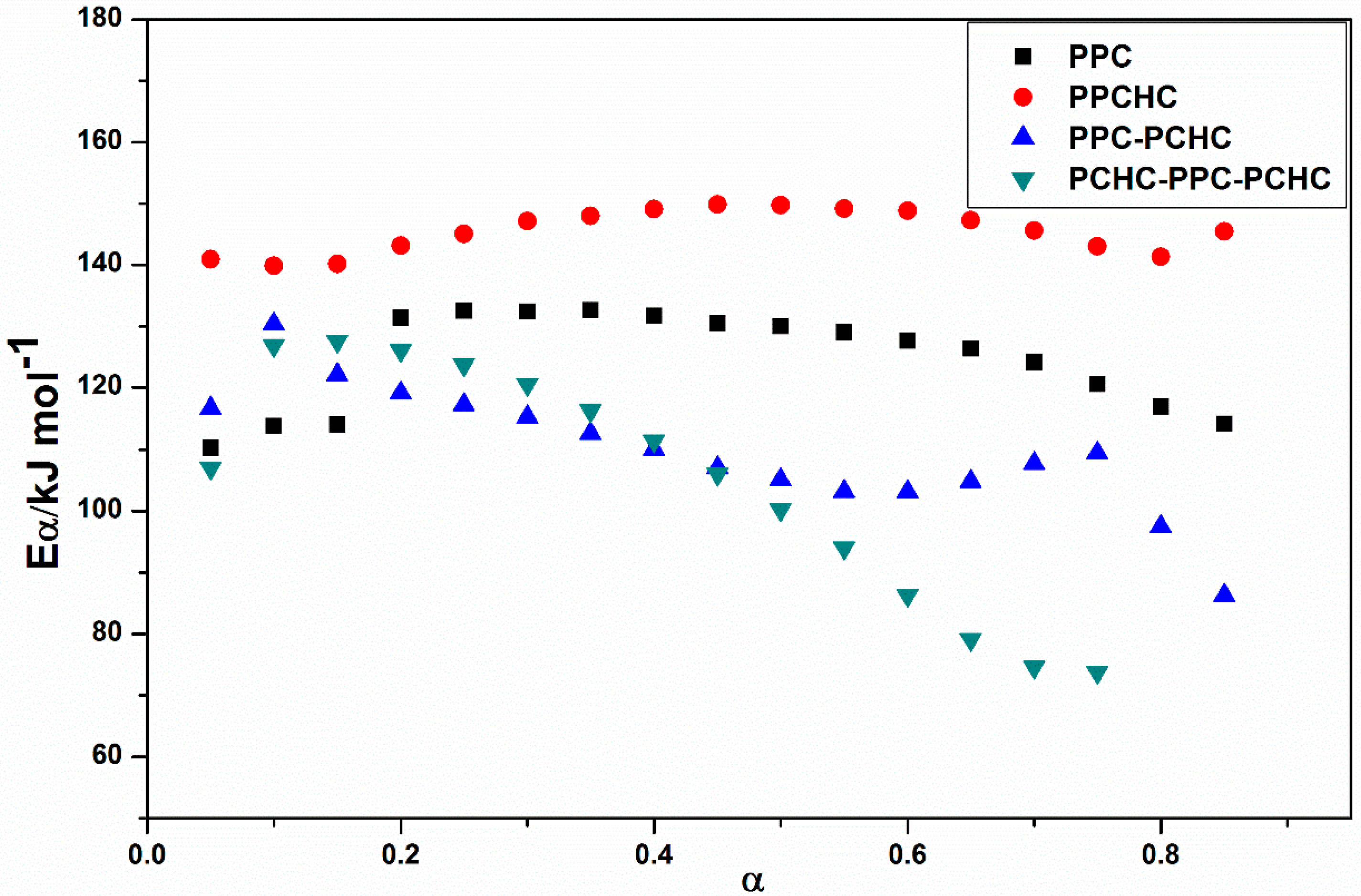
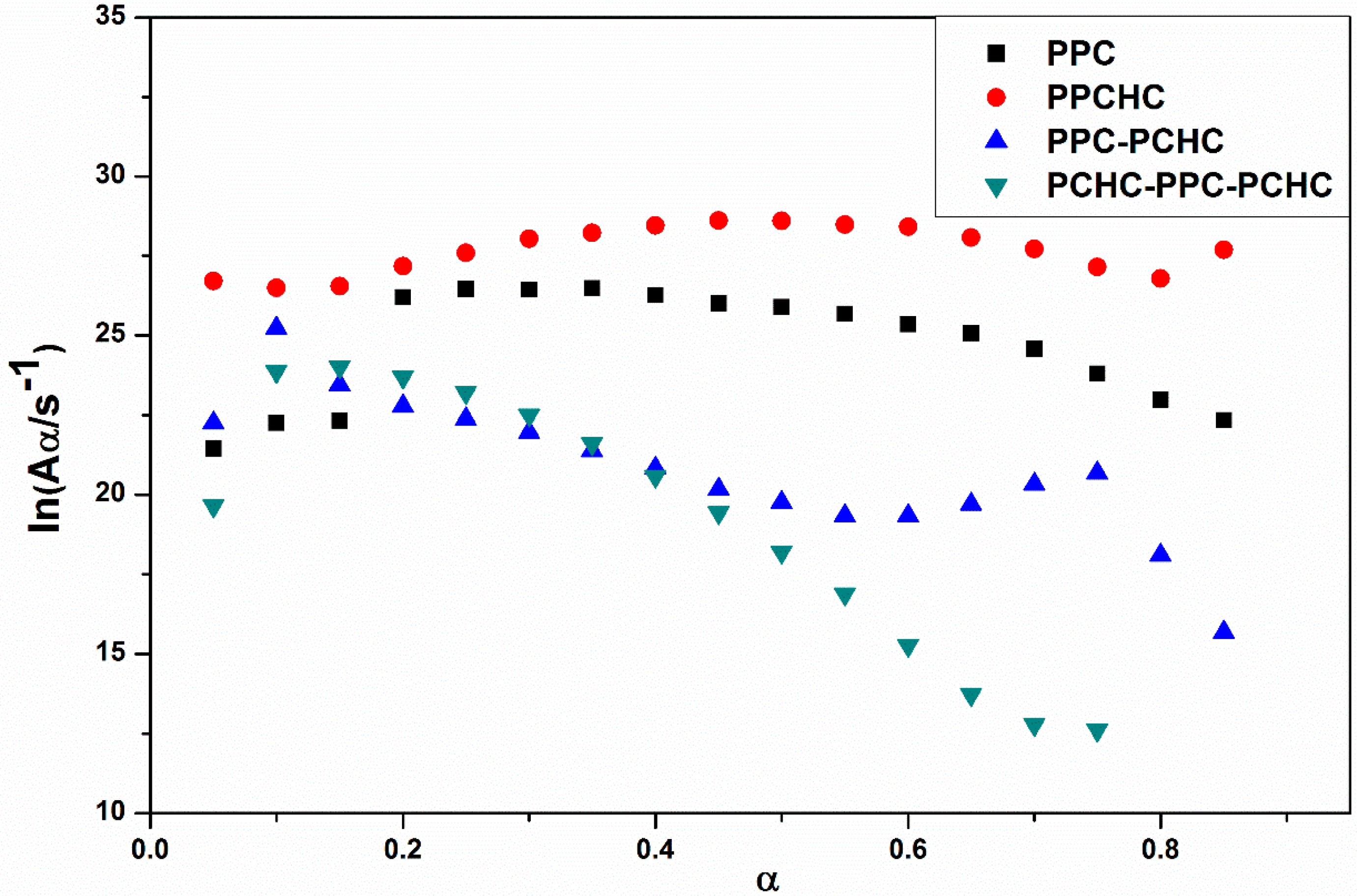
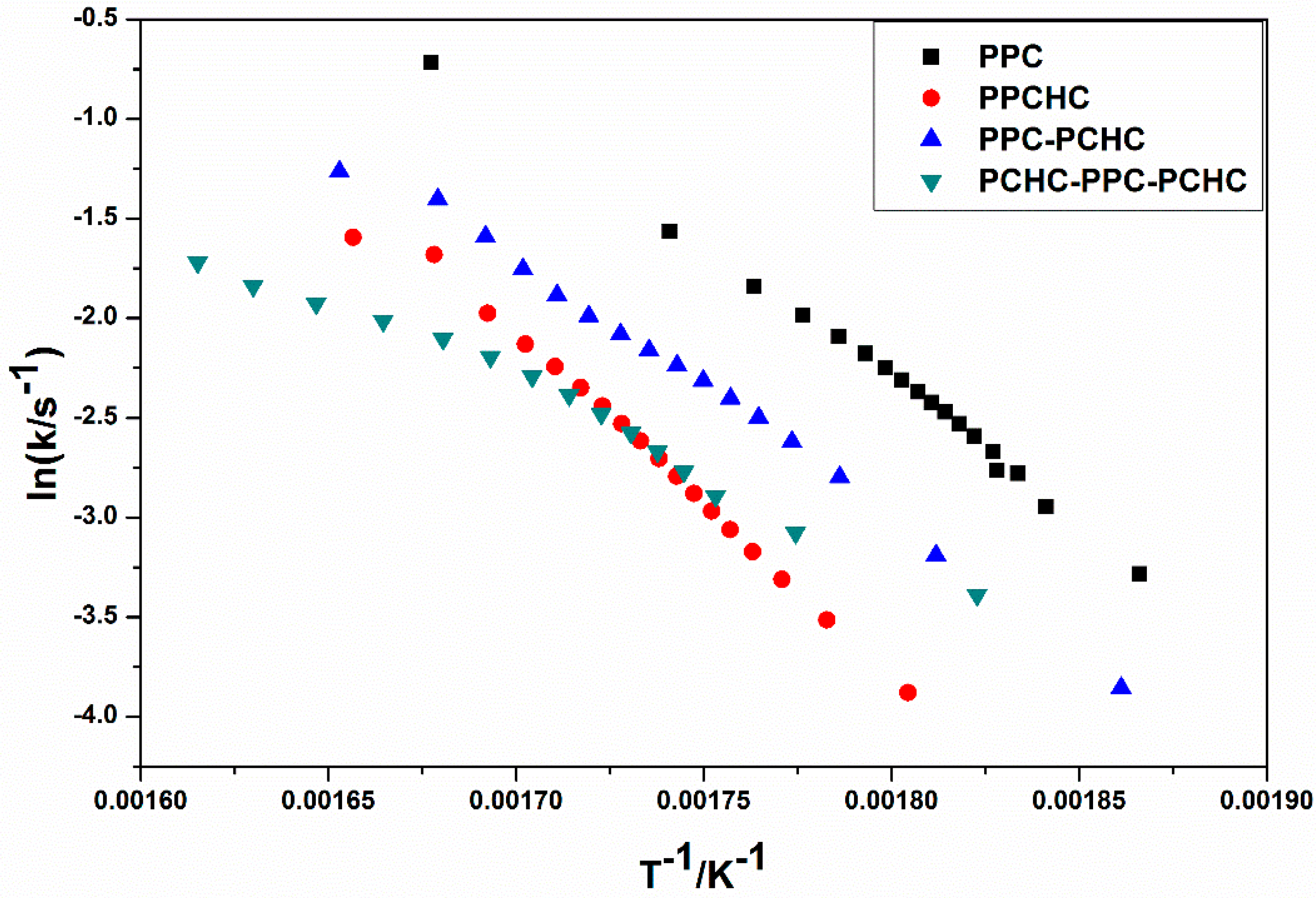
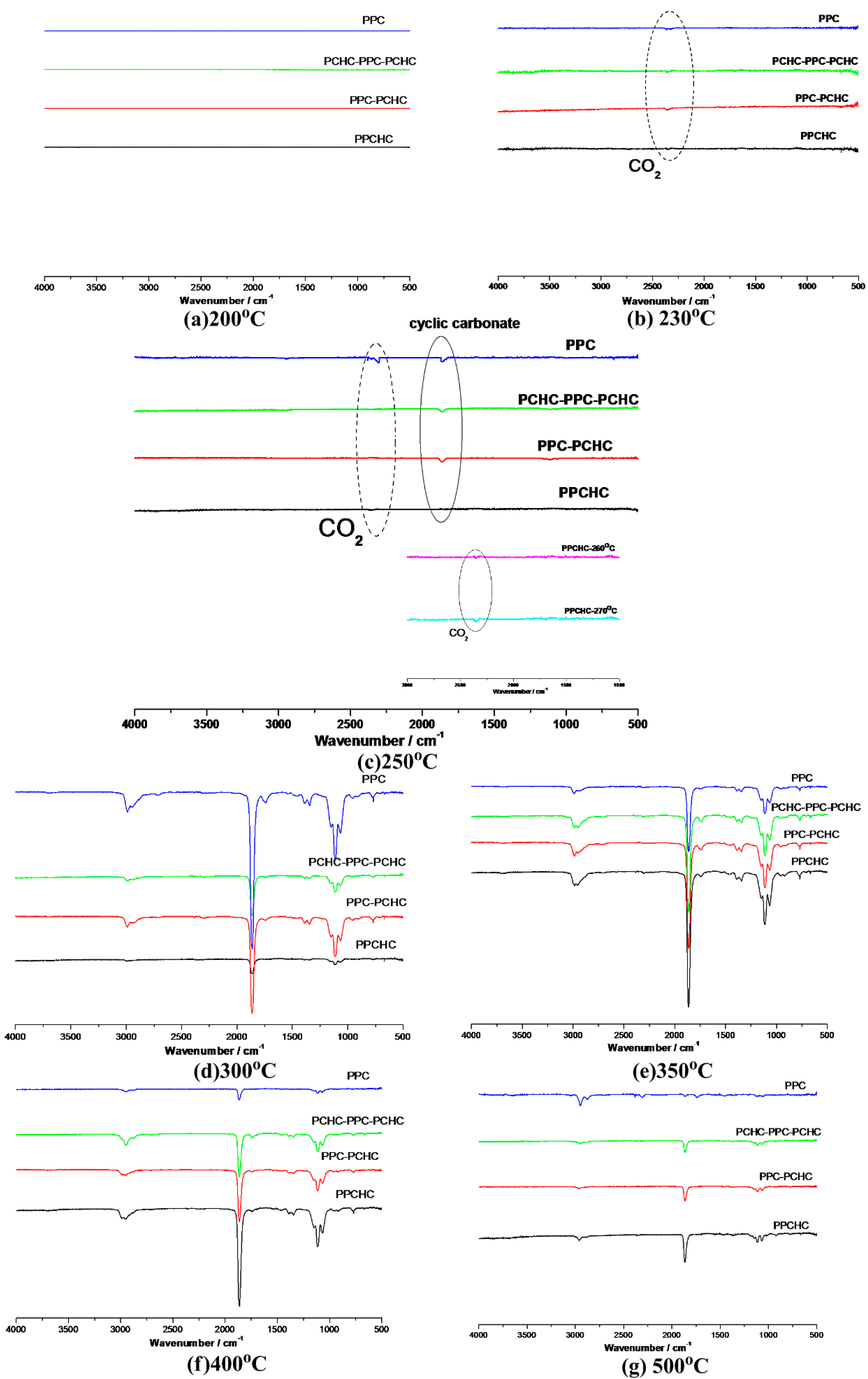
2.1. Thermal Decomposition Behavior
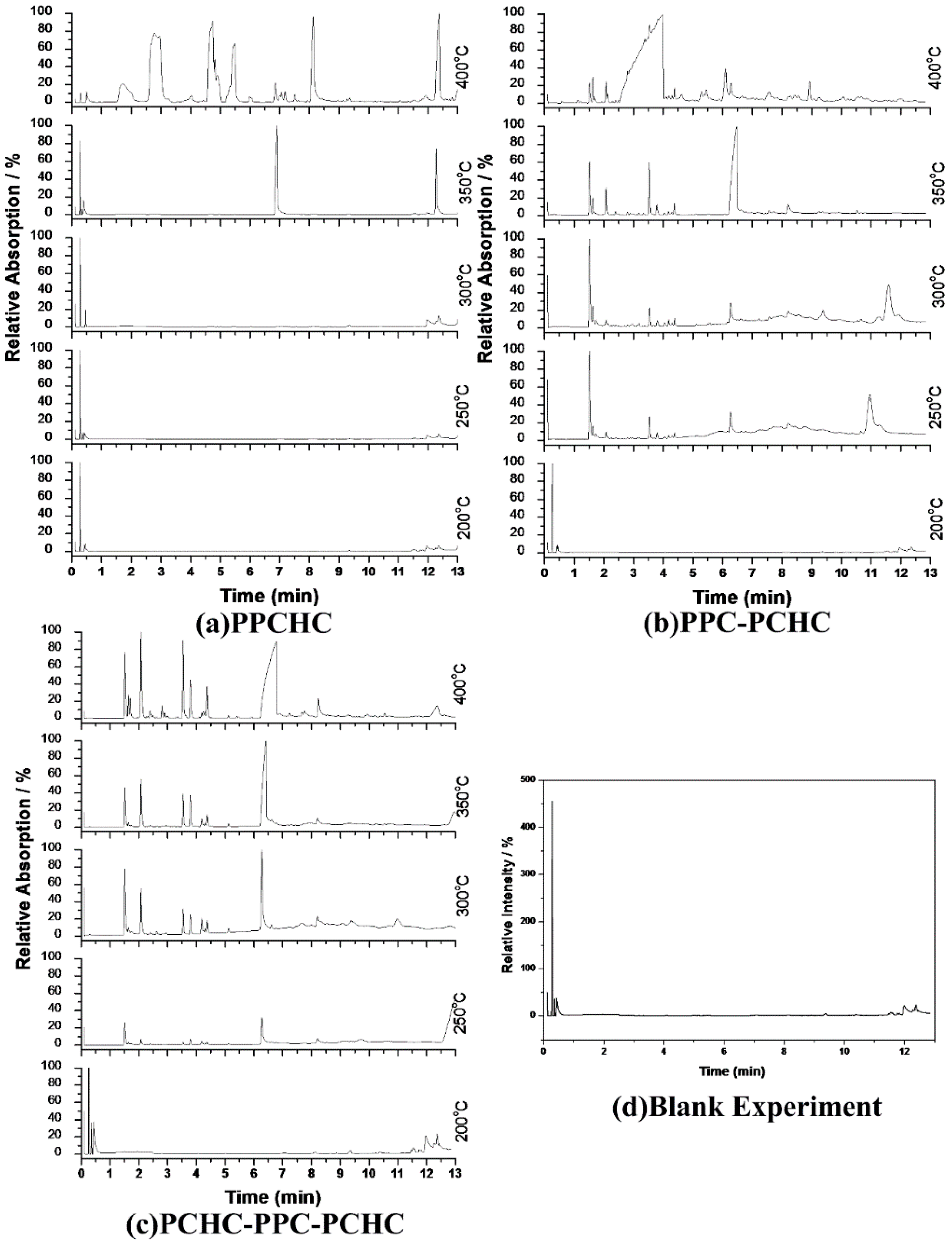
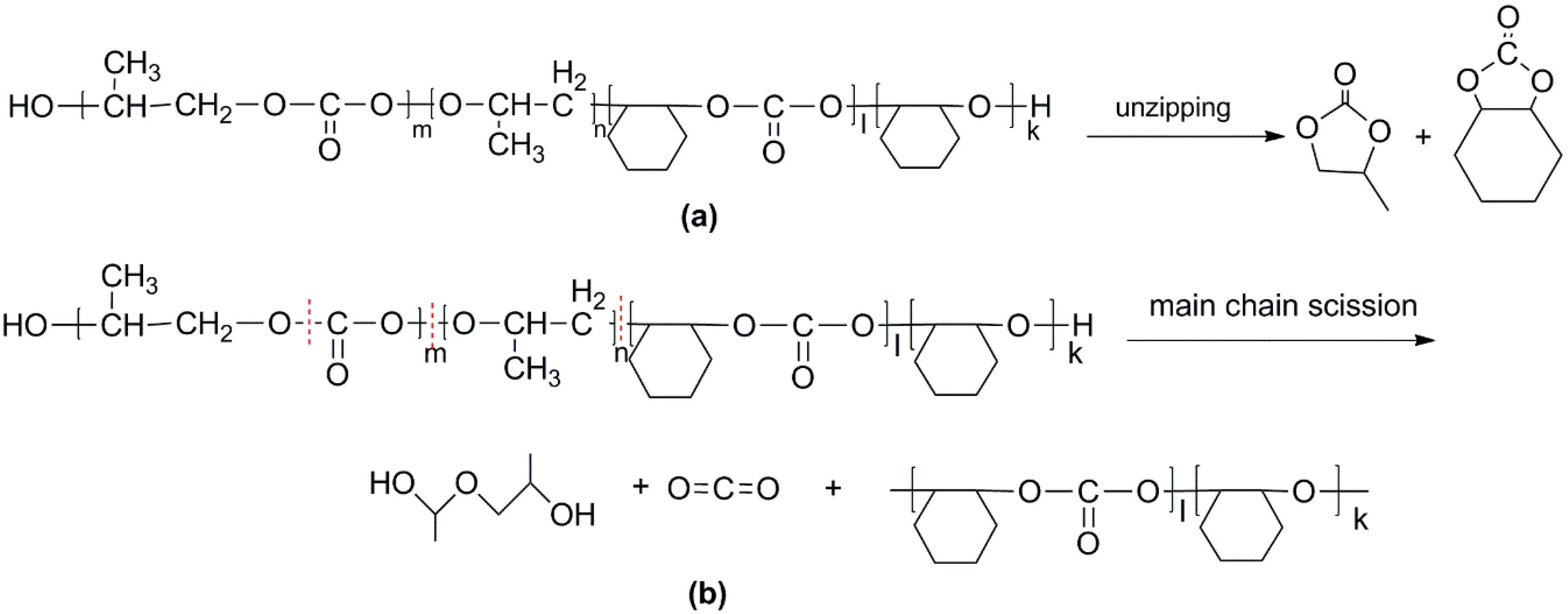
2.2. Study the Thermal Decomposition Behavior Using Py-GC/MS and TG/FTIR
3. Materials and Methods
3.1. Materials
3.2. Preparation of Random Terpolymers and Block Copolymers
3.3. Determination of the Composition and the Molecular Weight of Polymers
3.4. Py-GC/MS Measurement
3.5. TG/FTIR Measurement
3.6. Thermal Decomposition Kinetics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| TG/FTIR | Thermogravimetric analysis/ Fourier transform infrared spectrometry |
| Py-GC/MS | Pyrolysis-gas chromatography/mass spectrometry |
| PPC | poly (propylene carbonate) |
| PPCHC | Poly (propylene cyclohexene carbonate) |
| PPC-PCHC | Poly (propylene carbonate–cyclohexyl carbonate) |
| PCHC-PPC-PCHC | Poly (cyclohexyl carbonate–propylene carbonate–cyclohexyl carbonate) |
References
- Inoue, S.; Koinuma, H.; Tsuruta, T. Copolymerization of carbon dioxide and epoxide. J. Polym. Sci. Part B Polym. Lett. 1969, 7, 287–292. [Google Scholar] [CrossRef]
- Darensbourg, D.J.; Holtcamp, M.W. Catalysts for the reactions of epoxides and carbon dioxide. Coord. Chem. Rev. 1996, 153, 155–174. [Google Scholar] [CrossRef]
- Meng, Y.Z.; Du, L.C.; Tiong, S.C. Effects of the structure and morphology of zinc glutarate on the fixation of carbon dioxide into polymer. J. Polym. Sci. Part A Polym. Chem. 2002, 40, 3579–3591. [Google Scholar] [CrossRef]
- Coates, G.W.; Moore, D.R. Discrete metal-based catalysts for the copolymerization of CO2 and epoxides: Discovery, reactivity, optimization, and mechanism. Angew. Chem. Int. Ed. 2004, 43, 6618–6639. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.B.; Ren, W.M.; Wu, G.P. CO2 copolymers from epoxides: Catalyst activity, product selectivity, and stereochemistry control. Acc. Chem. Res. 2012, 45, 1721–1735. [Google Scholar] [CrossRef] [PubMed]
- Czaplewski, D.A.; Kameoka, J.; Mathers, R.; Coats, G.W.; Craighead, H.G. Nanofluidic channels with elliptical cross sections formed using a nonlithographic process. Appl. Phys. Lett. 2003, 83, 4836–4838. [Google Scholar] [CrossRef]
- Cao, M.; Xiao, M.; Lu, Y.; Meng, Y. Novel in situ preparation of crosslinked ethylene-vinyl alcohol copolymer foams with propylene carbonate. Mater. Lett. 2006, 60, 3286–3291. [Google Scholar]
- Zeng, S.; Wang, S.; Xiao, M.; Meng, Y. Preparation and properties of biodegradable blend containing poly (propylene carbonate) and starch acetate with different degrees of substitution. Carbohydr. Polym. 2011, 86, 1260–1265. [Google Scholar] [CrossRef]
- Chen, W.; Pang, M.; Xiao, M.; Wen, L.; Meng, Y. Mechanical, thermal, and morphological properties of glass fiber-reinforced biodegradable poly (propylene carbonate) composites. J. Reinf. Plastics Compos. 2010, 29, 1545–1550. [Google Scholar] [CrossRef]
- Thorat, S.D.; Phillips, P.J.; Semenov, V.; Gakh, A. Physical properties of aliphatic polycarbonates made from CO2 and epoxides. J. Appl. Polym. Sci. 2003, 89, 1163–1176. [Google Scholar] [CrossRef]
- Kember, M.R.; Buchard, A.; Williams, C.K. Catalysts for CO2/epoxide copolymerization. Chem. Commun. 2011, 47, 141–163. [Google Scholar] [CrossRef] [PubMed]
- Li, X.H.; Meng, Y.Z.; Zhu, Q.; Tjong, S.C. Thermal decomposition characteristics of poly (propylene carbonate) using TG/IR and Py-GC/MS techniques. Polym. Degrad. STable 2003, 81, 157–165. [Google Scholar] [CrossRef]
- Lu, X.L.; Zhu, Q.; Meng, Y.Z. Kinetic analysis of thermal decomposition of poly (propylene carbonate). Polym. Degrad. STable 2005, 89, 282–288. [Google Scholar] [CrossRef]
- Wu, J.S.; Xiao, M.; He, H.; Wang, S.; Han, D.M.; Meng, Y.Z. Synthesis and characterization of high molecular weight poly (1, 2-propylene carbonate-co-1, 2-cyclohexylene carbonate) using zinc complex catalyst. Chin. J. Polym. Sci. 2011, 29, 552–559. [Google Scholar] [CrossRef]
- Chen, S.Y.; Xiao, M.; Wang, S.J.; Han, D.M.; Meng, Y.Z. Novel Ternary Block Copolymerization of Carbon Dioxide with Cyclohexene Oxide and Propylene Oxide Using Zinc Complex Catalyst. J. Poly. Res. 2012, 19. [Google Scholar] [CrossRef]
- Bassilakis, R.; Carangelo, R.M.; Wojtowicz, M.A. TG-FTIR analysis of biomass pyrolysis. Fuel 2001, 80, 1765–1786. [Google Scholar] [CrossRef]
- Jiao, L.; Xiao, H.; Wang, Q.; Sun, J. Thermal degradation characteristics of rigid polyurethane foam and the volatile products analysis with TG-FTIR-MS. Polym. Degrad. STable 2013, 98, 2687–2696. [Google Scholar] [CrossRef]
- Bruno, S.S.; Ana Paula, D.M.; Ana Maria, R.F.T. TG-FTIR coupling to monitor the pyrolysis products from agricultural residues. J. Therm. Anal. Calorim. 2009, 97, 637–642. [Google Scholar]
- Rio, J.C.D.; Gutierrez, A.; Hernando, M.; Landin, P.; Romero, J.; Martinez, A.T. Determining the influence of eucalypt lignin composition in paper pulp yield using Py-GC/MS. J. Anal. Appl. Pyrolysis. 2005, 74, 110–115. [Google Scholar]
- Zhu, P.; Sui, S.; Wang, B.; Sun, K.; Sun, G. A study of pyrolysis and pyrolysis products of flame-retardant cotton fabrics by DSC, TGA, and PY-GC/MS. J. Anal. Appl. Pyrolysis. 2004, 71, 645–655. [Google Scholar] [CrossRef]
- Lu, X.Q.; Hanna, J.V.; Johnson, W.D. Source indicators of humic substances: An elemental composition, solid state 13 C CP/MAS NMR and Py-GC/MS study. Appl. Geochem. 2000, 15, 1019–1033. [Google Scholar] [CrossRef]
- Tsuge, S.; Ohtani, H. Structural characterization of polymeric materials by PyrolysisdGC/MS. Polym. Degrad. STable 1997, 58, 109–130. [Google Scholar] [CrossRef]
- Gu, X.L.; Ma, X.; Li, L.X.; Liu, C.; Cheng, K.H.; Li, Z.Z. Pyrolysis of poplar wood sawdust by TG-FTIR and Py-GC/MS. J. Anal. Appl. Pyrolysis. 2013, 102, 16–23. [Google Scholar] [CrossRef]
- Huang, G.; Zou, Y.; Xiao, M.; Wang, S.; Luo, W.; Han, D.; Meng, Y. Thermal degradation of poly (lactide-copropylene carbonate) measured by TG/FTIR and Py-GC/MS. Polym. Degrad. STable 2015, 117, 16–21. [Google Scholar] [CrossRef]
- Luo, W.; Xiao, M.; Wang, S.; Ren, S.; Meng, Y. Thermal degradation behavior of Copoly (propylene carbonateε-caprolactone) investigated using TG/FTIR and Py-GC/MS methodologies. Polymer Testing. 2017, 58, 13–20. [Google Scholar] [CrossRef]
- Kissinger, H.E. Variation of peak temperature with heating rate in differential thermal analysis. J. Res. Natl. Bur. Stand. 1956, 57, 217–221. [Google Scholar] [CrossRef]
- Kissinger, H.E. Reaction kinetics in differential thermal analysis. Anal. Chem. 1957, 29, 1702–1706. [Google Scholar] [CrossRef]
- Akahira, T.; Sunose, T. Method of determining activation deterioration constant of electrical insulating materials. Res. Rep. Chiba Inst. Technol. 1971, 16, 22–31. [Google Scholar]
- Coats, A.W.; Redfern, J.P. Kinetic parameters from thermogravimetric data. Nature 1964, 201, 68–69. [Google Scholar] [CrossRef]
- Yuan, J.J.; Tu, J.L.; Xu, Y.J.; Qin, F.G.F.; Li, B.; Wang, C.Z. Thermal stability and products chemical analysis of olive leaf extract after enzymolysis based on TG-FTIR and Py-GC-MS. J. Therm. Anal. Calor. 2018, 132, 1729–1740. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Burnham, A.K.; Criado, J.M.; Pέrez-Maqueda, L.A.; Popescu, C.; Sbirrazzuoli, N. ICTAC Kinetics Committee recommendations for performing kinetic computations on thermal analysis data. Thermochim. Acta. 2011, 520, 1–19. [Google Scholar] [CrossRef]
- Liavitskaya, T.; Birx, L.; Vyazovkin, S. Thermal stability of Malonic Acid Dissolved in Pomy(vinylpyrrolidone) and Other Polymeric Matrices. Ind. Eng. Chem. Res. 2018, 57, 5228–5233. [Google Scholar] [CrossRef]
- Osman, Y.B.; Liavitslaya, T.; Vyazovkin, S. Polyvinylpyrrolidone affects thermal stability of drugs in solid dispersions. Int. J. Pharm. 2018, 551, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Teng, C.T.; Win, K.Y.; Chen, Y.; Zhang, X.; Yang, D.; Li, Z.; Ye, E. Polymeric Encapsulation of Turmeric Extract for Bioimaging and Antimicrobial Applications. Macromol. Rapid Commun. 2018. [Google Scholar] [CrossRef] [PubMed]
- Luinstra, G. Poly (propylene carbonate), old copolymers of propylene oxideand carbon dioxide with new interests: Catalysis and material properties. Polym. Rev. 2008, 48, 192–219. [Google Scholar] [CrossRef]
- Chisholm, M.H.; Navarro-Llobet, D.; Zhou, Z. Poly (propylene carbonate). 1. More about poly (propylene carbonate) formed from the copolymerization of propylene oxide and carbon dioxide employing a zinc glutarate catalyst. Macromolecules 2002, 35, 6494–6504. [Google Scholar] [CrossRef]
- Barreto, C.; Cannon, W.R.; Shanefield, D.J. Thermal decomposition behavior of poly (propylene carbonate): tailoring the composition and thermal properties of PPC. Polym. Degrad. Stab. 2012, 97, 893–904. [Google Scholar] [CrossRef]
- Zhu, Q.; Meng, Y.; Tjong, S.; Zhao, X.; Chen, Y. Thermally stable and high molecular weight poly(propylene carbonate)s from carbon dioxide and propylene oxide. Polym. Int. 2002, 51, 1079–1085. [Google Scholar] [CrossRef]
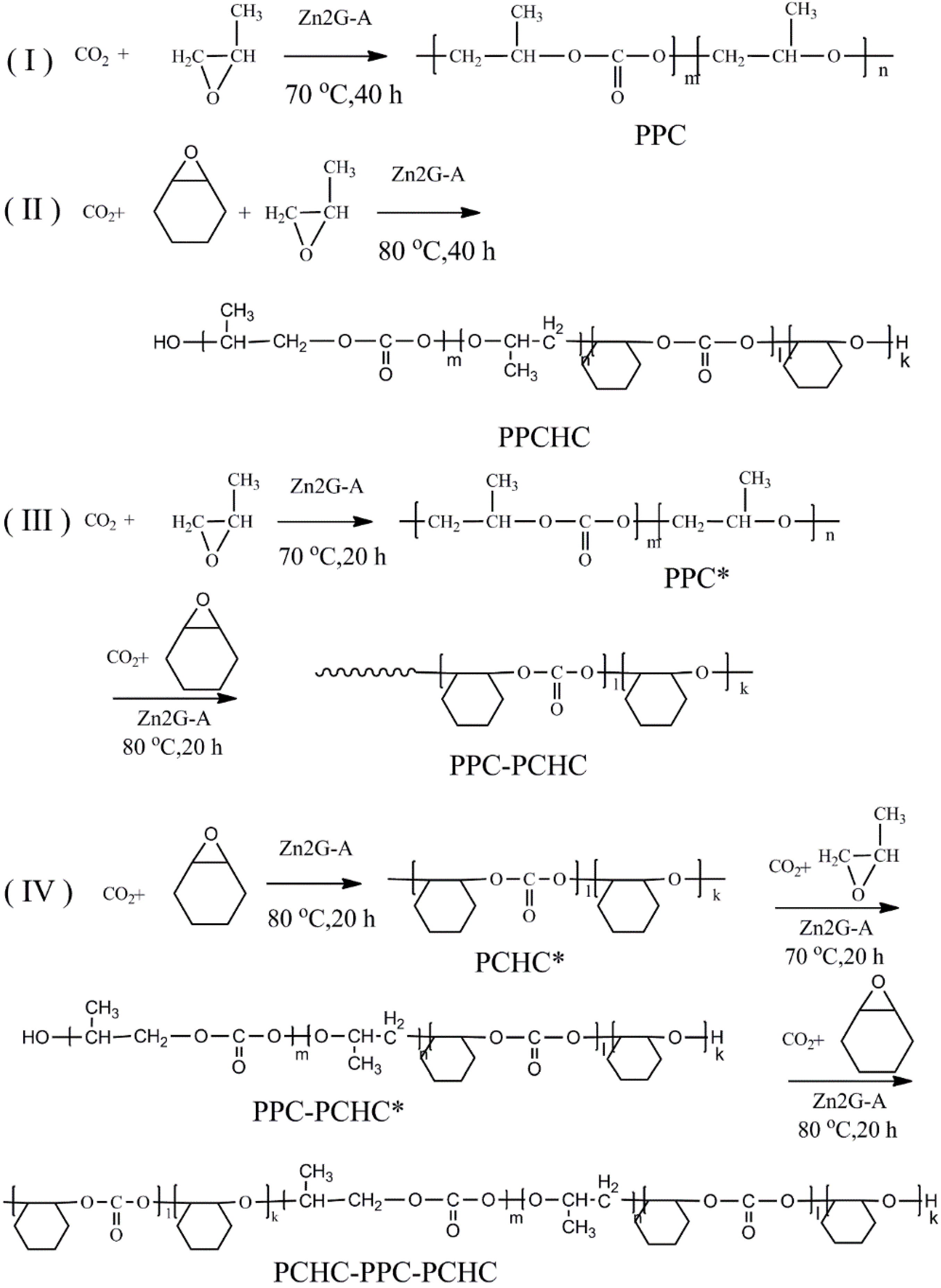

| Copolymer | Mn/Mw/PI a | T−5%/Tmax (°C) c | Composition (Molar Fraction %) b | |||
|---|---|---|---|---|---|---|
| PPC | 2.17 × 105/3.78 × 105/1.74 | 255.7/278.2 | 48.9 | 48.9 | - | 2.2 |
| PPCHC | 2.02 × 105/7.13 × 105/3.50 | 281.0/313.4 | 47.9 | 36.2 | 11.7 | 4.2 |
| PPC-PCHC | 2.97 × 105/7.35 × 105/2.47 | 261.2/304.2, 342.7 | 48.0 | 35.1 | 12.9 | 4.0 |
| PCHC-PPC-PCHC | 2.74 × 105/7.88 × 105/2.87 | 275.2/305.8, 345.3 | 47.7 | 34.1 | 13.6 | 4.6 |
| Compound | Retention Time (min) | Compound | Retention Time (min) |
|---|---|---|---|
| CO2 | 1.5–2 min | ||
 | 4.0–4.5 min |  | 4.5–5.0 min |
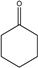 | 5.0–5.5 min | 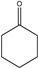 | 6.0–6.75 min |
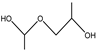 | 7.5–8.0 min | 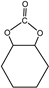 | 12.25–12.5 min |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, S.; Xiao, M.; Sun, L.; Meng, Y. Study on Thermal Decomposition Behaviors of Terpolymers of Carbon Dioxide, Propylene Oxide, and Cyclohexene Oxide. Int. J. Mol. Sci. 2018, 19, 3723. https://doi.org/10.3390/ijms19123723
Chen S, Xiao M, Sun L, Meng Y. Study on Thermal Decomposition Behaviors of Terpolymers of Carbon Dioxide, Propylene Oxide, and Cyclohexene Oxide. International Journal of Molecular Sciences. 2018; 19(12):3723. https://doi.org/10.3390/ijms19123723
Chicago/Turabian StyleChen, Shaoyun, Min Xiao, Luyi Sun, and Yuezhong Meng. 2018. "Study on Thermal Decomposition Behaviors of Terpolymers of Carbon Dioxide, Propylene Oxide, and Cyclohexene Oxide" International Journal of Molecular Sciences 19, no. 12: 3723. https://doi.org/10.3390/ijms19123723
APA StyleChen, S., Xiao, M., Sun, L., & Meng, Y. (2018). Study on Thermal Decomposition Behaviors of Terpolymers of Carbon Dioxide, Propylene Oxide, and Cyclohexene Oxide. International Journal of Molecular Sciences, 19(12), 3723. https://doi.org/10.3390/ijms19123723








