Aberrant Epigenetic Regulation in Head and Neck Cancer Due to Distinct EZH2 Overexpression and DNA Hypermethylation
Abstract
1. Introduction
2. Results
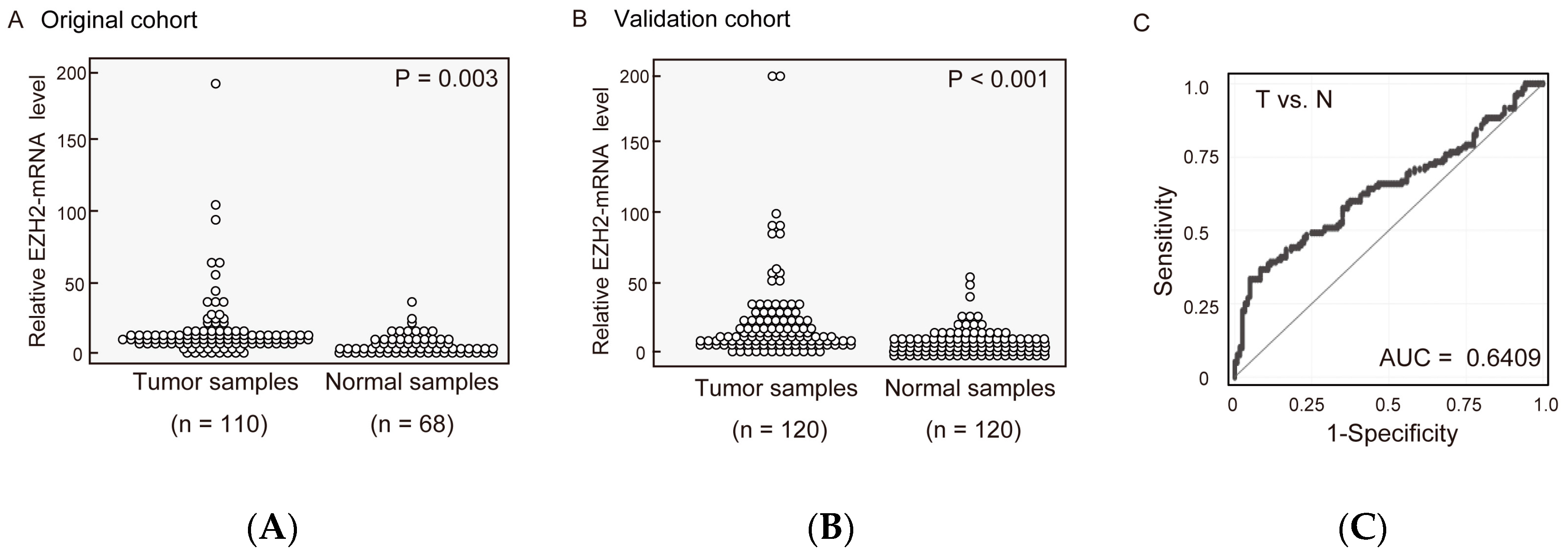
2.1. EZH2 mRNA Expression Levels in Pairs of HNSCC and Normal Mucosal Tissues
2.2. Correlation between EZH2 mRNA Expression and Clinicopathological Assessment
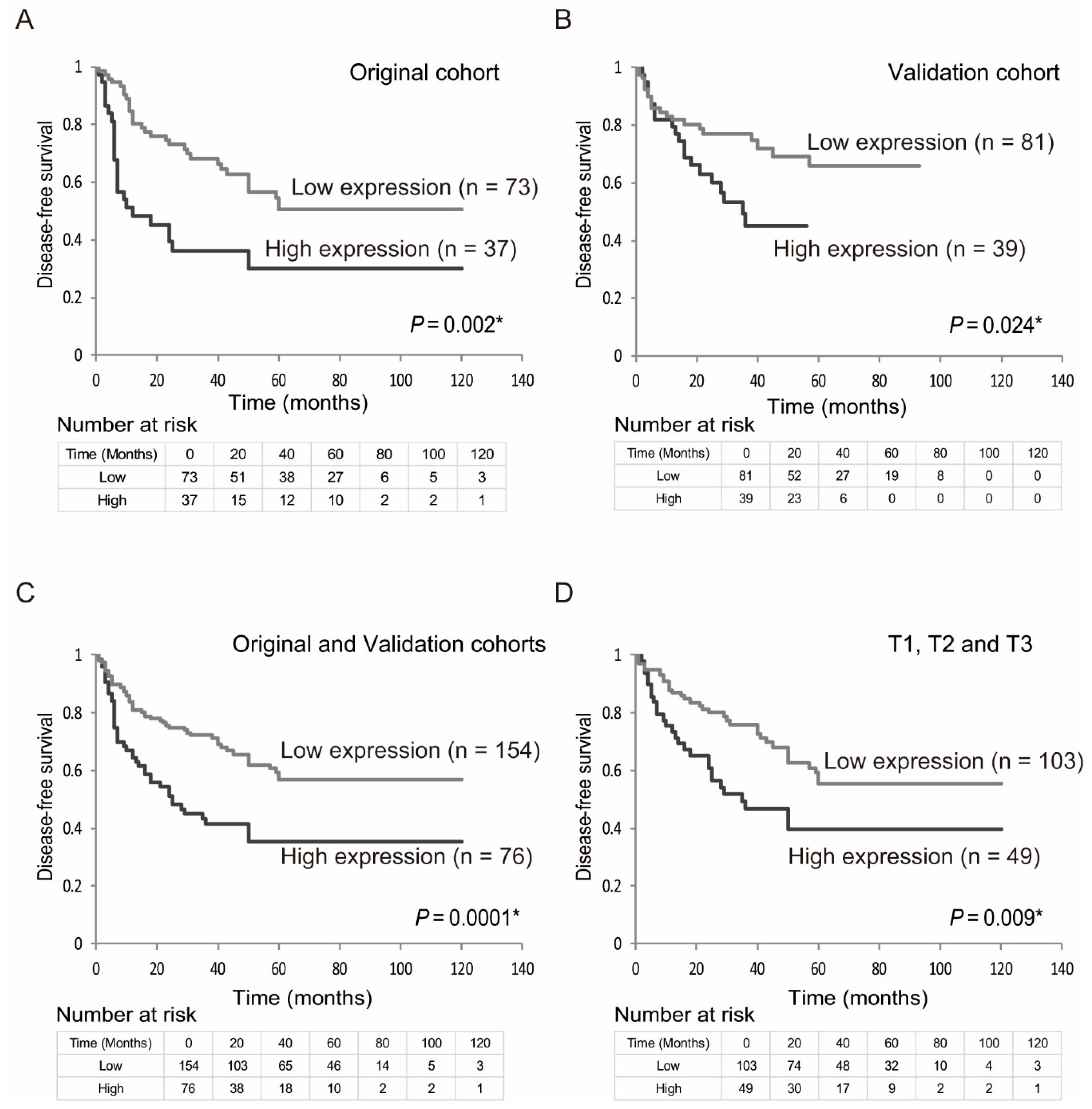
2.3. Prognostic Value of EZH2 Gene Expression
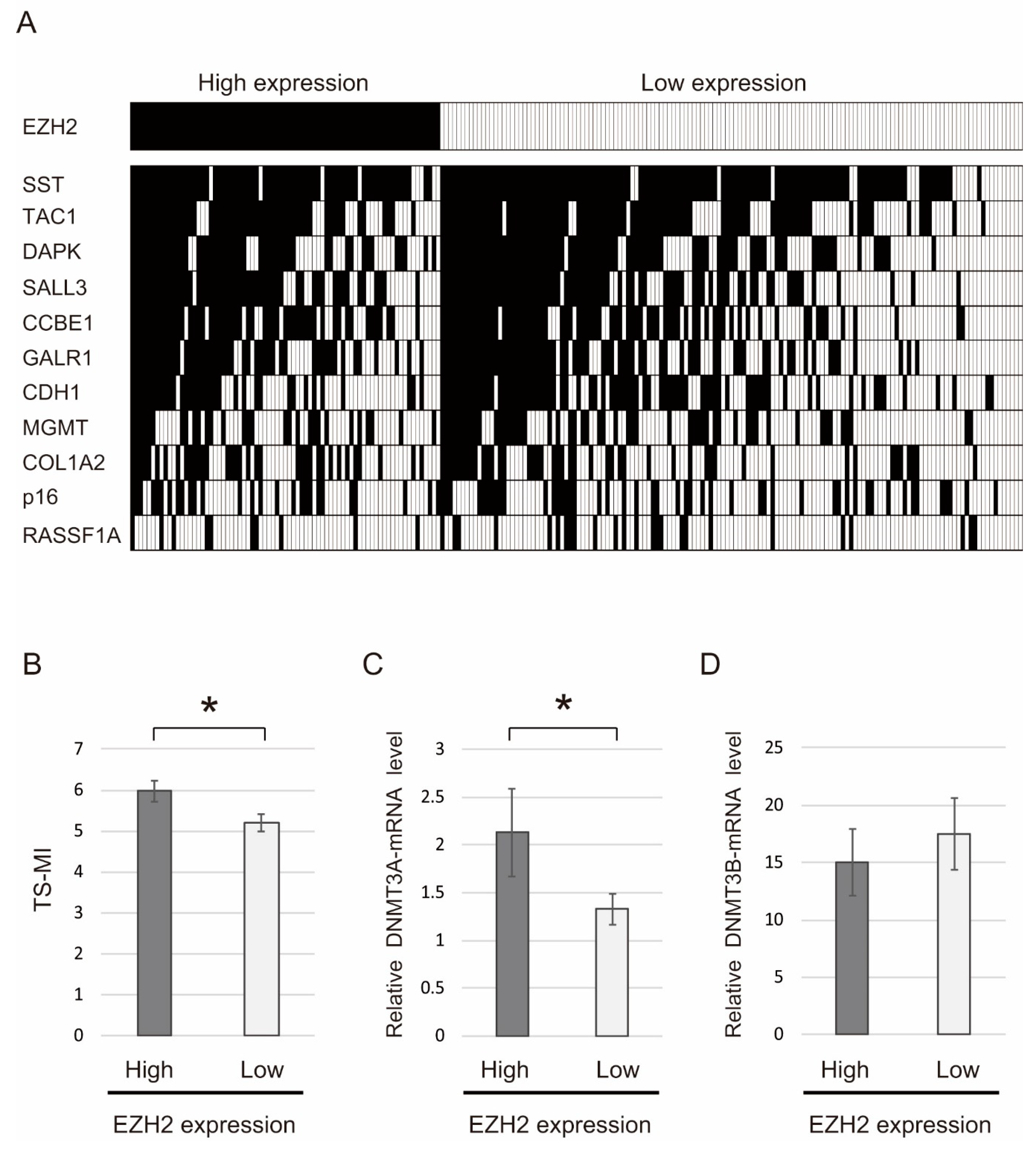
2.4. Correlation between Methylation Levels of 11 Tumor Suppressor Genes and EZH2 Expression
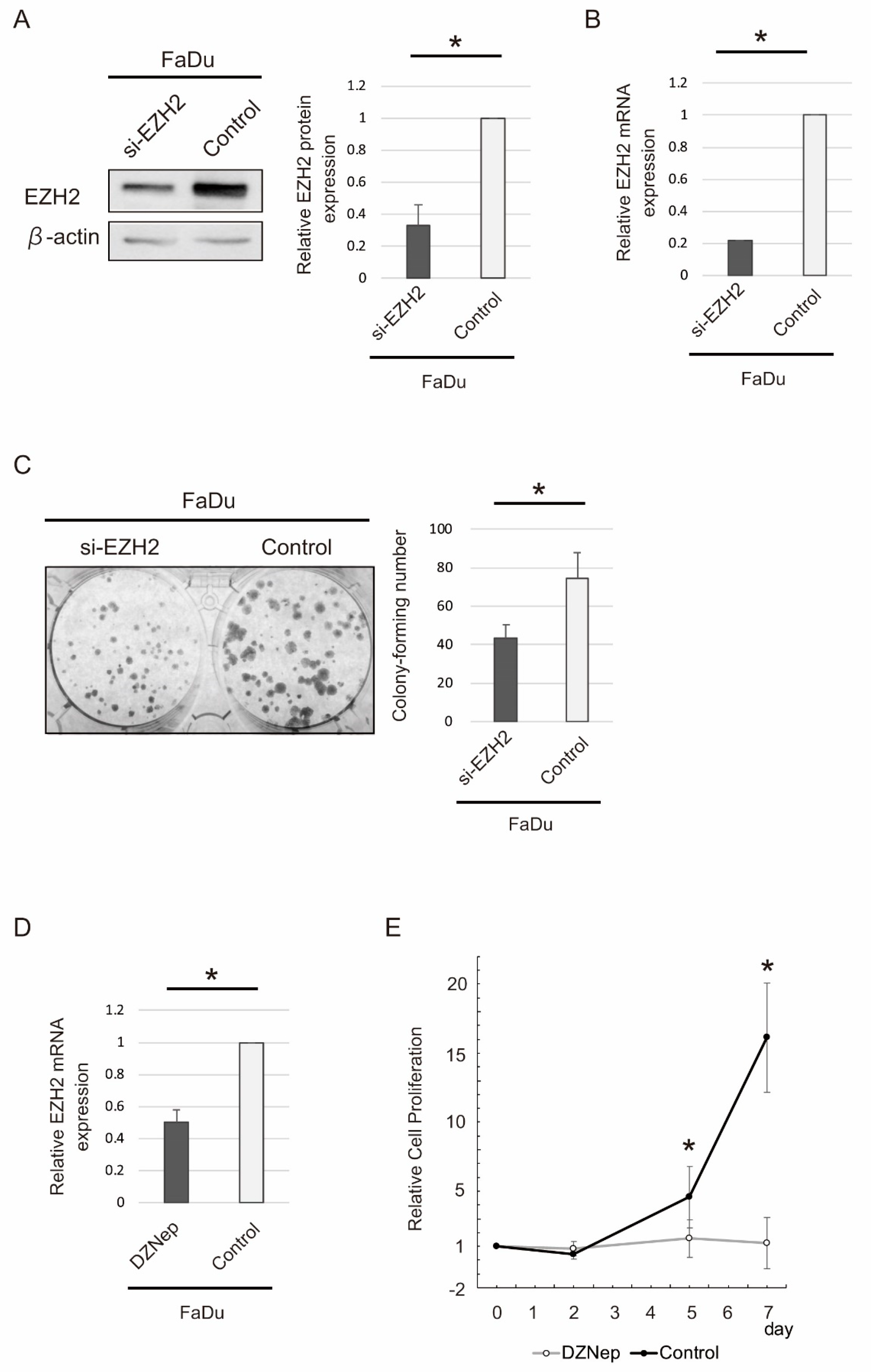
2.5. siRNA-Mediated EZH2 Knockdown and DZNep Administration
2.6. External Validation of Results from TCGA
3. Discussion
4. Materials and Methods
4.1. Tumor Samples
4.2. Cell Culture
4.3. RNA Extraction and Quantitative Reverse Transcription PCR (qRT-PCR)
4.4. EZH2 siRNA Transfection, Colony forming Assay, and Cell Proliferation Assay
4.5. Western Blot Analysis
4.6. DNA Extraction, Bisulfite Treatment, and Quantitative Methylation-Specific PCR (QMSP) Analysis
4.7. Collection of Publicly Available Data from TCGA
4.8. Data Analysis and Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Pai, S.I.; Westra, W.H. Molecular pathology of head and neck cancer: Implications for diagnosis, prognosis, and treatment. Ann. Rev. Pathol. 2009, 4, 49–70. [Google Scholar] [CrossRef] [PubMed]
- Bose, P.; Brockton, N.T.; Dort, J.C. Head and neck cancer: From anatomy to biology. Int. J. Cancer 2013, 133, 2013–2023. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Kiess, A.; Chung, C.H. Emerging biomarkers in head and neck cancer in the era of genomics. Nat. Rev. Clin. Oncol. 2015, 12, 11–26. [Google Scholar] [CrossRef] [PubMed]
- Mochizuki, D.; Adams, A.; Warner, K.A.; Zhang, Z.; Pearson, A.T.; Misawa, K.; McLean, S.A.; Wolf, G.T.; Nor, J.E. Anti-tumor effect of inhibition of il-6 signaling in mucoepidermoid carcinoma. Oncotarget 2015, 6, 22822–22835. [Google Scholar] [CrossRef] [PubMed]
- Stransky, N.; Egloff, A.M.; Tward, A.D.; Kostic, A.D.; Cibulskis, K.; Sivachenko, A.; Kryukov, G.V.; Lawrence, M.S.; Sougnez, C.; McKenna, A.; et al. The mutational landscape of head and neck squamous cell carcinoma. Science 2011, 333, 1157–1160. [Google Scholar] [CrossRef] [PubMed]
- Laytragoon-Lewin, N.; Rutqvist, L.E.; Lewin, F. DNA methylation in tumour and normal mucosal tissue of head and neck squamous cell carcinoma (HNSCC) patients: New diagnostic approaches and treatment. Med. Oncol. 2013, 30, 654. [Google Scholar] [CrossRef] [PubMed]
- Koffler, J.; Sharma, S.; Hess, J. Predictive value of epigenetic alterations in head and neck squamous cell carcinoma. Mol. Cell. Oncol. 2014, 1, e954827. [Google Scholar] [CrossRef] [PubMed]
- Simon, J.A.; Lange, C.A. Roles of the EZH2 histone methyltransferase in cancer epigenetics. Mutat. Res. 2008, 647, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Li, Y.; Huang, X.; Wei, W.; Qu, Y. Expression of EZH2 in uveal melanomas patients and associations with prognosis. Oncotarget 2017, 8, 76423–76431. [Google Scholar] [CrossRef] [PubMed]
- Labbe, D.P.; Sweeney, C.J.; Brown, M.; Galbo, P.; Rosario, S.; Wadosky, K.M.; Ku, S.Y.; Sjostrom, M.; Alshalalfa, M.; Erho, N.; et al. TOP2A and EZH2 provide early detection of an aggressive prostate cancer subgroup. Clin. Cancer Res. 2017, 23, 7072–7083. [Google Scholar] [CrossRef] [PubMed]
- Clermont, P.L.; Fornaro, L.; Crea, F. Elevated expression of a pharmacologic polycomb signature predicts poor prognosis in gastric and breast cancer. Epigenomics 2017, 9, 1329–1335. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhao, H.; Lv, L.; Bao, L.; Han, S. Prognostic significance of EZH2 expression in non-small cell lung cancer: A meta-analysis. Sci. Rep. 2016, 6, 19239. [Google Scholar] [CrossRef] [PubMed]
- He, L.R.; Liu, M.Z.; Li, B.K.; Jia, W.H.; Zhang, Y.; Liao, Y.J.; Chen, Y.C.; Zhang, L.J.; Guan, X.Y.; Zeng, Y.X.; et al. High expression of EZH2 is associated with tumor aggressiveness and poor prognosis in patients with esophageal squamous cell carcinoma treated with definitive chemoradiotherapy. Int. J. Cancer 2010, 127, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Kidani, K.; Osaki, M.; Tamura, T.; Yamaga, K.; Shomori, K.; Ryoke, K.; Ito, H. High expression of EZH2 is associated with tumor proliferation and prognosis in human oral squamous cell carcinomas. Oral Oncol. 2009, 45, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Oki, S.; Sone, K.; Oda, K.; Hamamoto, R.; Ikemura, M.; Maeda, D.; Takeuchi, M.; Tanikawa, M.; Mori-Uchino, M.; Nagasaka, K.; et al. Oncogenic histone methyltransferase EZH2: A novel prognostic marker with therapeutic potential in endometrial cancer. Oncotarget 2017, 8, 40402–40411. [Google Scholar] [CrossRef] [PubMed]
- Hayden, A.; Johnson, P.W.; Packham, G.; Crabb, S.J. S-adenosylhomocysteine hydrolase inhibition by 3-deazaneplanocin a analogues induces anti-cancer effects in breast cancer cell lines and synergy with both histone deacetylase and HER2 inhibition. Breast Cancer Res. Treat. 2011, 127, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Girard, N.; Bazille, C.; Lhuissier, E.; Benateau, H.; Llombart-Bosch, A.; Boumediene, K.; Bauge, C. 3-deazaneplanocin a (dznep), an inhibitor of the histone methyltransferase EZH2, induces apoptosis and reduces cell migration in chondrosarcoma cells. PLoS ONE 2014, 9, e98176. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.K.; Kim, K.C. Dznep, inhibitor of s-adenosylhomocysteine hydrolase, down-regulates expression of SETDB1 H3K9me3 hmtase in human lung cancer cells. Biochem. Biophys. Res. Commun. 2013, 438, 647–652. [Google Scholar] [CrossRef] [PubMed]
- Sha, M.; Mao, G.; Wang, G.; Chen, Y.; Wu, X.; Wang, Z. Dznep inhibits the proliferation of colon cancer HCT116 cells by inducing senescence and apoptosis. Acta Pharm. Sin. B 2015, 5, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, C.D.; Kostiuk, M.A.; Harris, J.; O’Connell, D.A.; Seikaly, H.; Biron, V.L. Efficacy of EZH2 inhibitory drugs in human papillomavirus-positive and human papillomavirus-negative oropharyngeal squamous cell carcinomas. Clin. Epigenet. 2017, 9, 95. [Google Scholar] [CrossRef] [PubMed]
- Vire, E.; Brenner, C.; Deplus, R.; Blanchon, L.; Fraga, M.; Didelot, C.; Morey, L.; Van Eynde, A.; Bernard, D.; Vanderwinden, J.M.; et al. The polycomb group protein EZH2 directly controls DNA methylation. Nature 2006, 439, 871–874. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, J.; Valind, A.; Jansson, C.; O’Sullivan, M.J.; Holmquist Mengelbier, L.; Gisselsson, D. Aberrant epigenetic regulation in clear cell sarcoma of the kidney featuring distinct DNA hypermethylation and EZH2 overexpression. Oncotarget 2016, 7, 11127–11136. [Google Scholar] [CrossRef] [PubMed]
- Tiffen, J.; Wilson, S.; Gallagher, S.J.; Hersey, P.; Filipp, F.V. Somatic copy number amplification and hyperactivating somatic mutations of EZH2 correlate with DNA methylation and drive epigenetic silencing of genes involved in tumor suppression and immune responses in melanoma. Neoplasia 2016, 18, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Poirier, J.T.; Gardner, E.E.; Connis, N.; Moreira, A.L.; de Stanchina, E.; Hann, C.L.; Rudin, C.M. DNA methylation in small cell lung cancer defines distinct disease subtypes and correlates with high expression of EZH2. Oncogene 2015, 34, 5869–5878. [Google Scholar] [CrossRef] [PubMed]
- Bachmann, I.M.; Halvorsen, O.J.; Collett, K.; Stefansson, I.M.; Straume, O.; Haukaas, S.A.; Salvesen, H.B.; Otte, A.P.; Akslen, L.A. EZH2 expression is associated with high proliferation rate and aggressive tumor subgroups in cutaneous melanoma and cancers of the endometrium, prostate, and breast. J. Clin. Oncol. 2006, 24, 268–273. [Google Scholar] [CrossRef] [PubMed]
- Vilorio-Marques, L.; Martin, V.; Diez-Tascon, C.; Gonzalez-Sevilla, M.F.; Fernandez-Villa, T.; Honrado, E.; Davila-Batista, V.; Molina, A.J. The role of EZH2 in overall survival of colorectal cancer: A meta-analysis. Sci. Rep. 2017, 7, 13806. [Google Scholar] [CrossRef] [PubMed]
- Castro, R.; Rivera, I.; Martins, C.; Struys, E.A.; Jansen, E.E.; Clode, N.; Graca, L.M.; Blom, H.J.; Jakobs, C.; de Almeida, I.T. Intracellular S-adenosylhomocysteine increased levels are associated with DNA hypomethylation in huvec. J. Mol. Med. (Berl.) 2005, 83, 831–836. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Yang, X.; Zhuang, L.; Jiang, X.; Chen, W.; Lee, P.L.; Karuturi, R.K.; Tan, P.B.; Liu, E.T.; Yu, Q. Pharmacologic disruption of polycomb-repressive complex 2-mediated gene repression selectively induces apoptosis in cancer cells. Genes Dev. 2007, 21, 1050–1063. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Ren, Y.; Kong, L.; Cai, G.; Sun, S.; Song, W.; Wang, Y.; Jin, R.; Qi, L.; Mei, M.; et al. Targeting EZH2 regulates tumor growth and apoptosis through modulating mitochondria dependent cell-death pathway in hnscc. Oncotarget 2015, 6, 33720–33732. [Google Scholar] [CrossRef] [PubMed]
- Gannon, O.M.; Merida de Long, L.; Endo-Munoz, L.; Hazar-Rethinam, M.; Saunders, N.A. Dysregulation of the repressive H3K27 trimethylation mark in head and neck squamous cell carcinoma contributes to dysregulated squamous differentiation. Clin. Cancer Res. 2013, 19, 428–441. [Google Scholar] [CrossRef] [PubMed]
- Jiao, L.; Liu, X. Structural basis of histone H3K27 trimethylation by an active polycomb repressive complex 2. Science 2015, 350, aac4383. [Google Scholar] [CrossRef] [PubMed]
- Yan, K.S.; Lin, C.Y.; Liao, T.W.; Peng, C.M.; Lee, S.C.; Liu, Y.J.; Chan, W.P.; Chou, R.H. EZH2 in cancer progression and potential application in cancer therapy: A friend or foe? Int. J. Mol. Sci. 2017, 18, 1172. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.J.; Engers, R.; Florl, A.R.; Otte, A.P.; Muller, M.; Schulz, W.A. Expression changes in EZH2, but not in BMI-1, SIRT1, DNMT1 or DNMT3B are associated with DNA methylation changes in prostate cancer. Cancer Biol. Ther. 2007, 6, 1403–1412. [Google Scholar] [CrossRef] [PubMed]
- Misawa, K.; Mochizuki, D.; Imai, A.; Mima, M.; Misawa, Y.; Mineta, H. Analysis of site-specific methylation of tumor-related genes in head and neck cancer: Potential utility as biomarkers for prognosis. Cancers 2018, 10, 27. [Google Scholar] [CrossRef] [PubMed]
- Misawa, K.; Imai, A.; Mochizuki, D.; Mima, M.; Endo, S.; Misawa, Y.; Kanazawa, T.; Mineta, H. Association of tet3 epigenetic inactivation with head and neck cancer. Oncotarget 2018, 9, 24480–24493. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.Y.; Hsu, S.D.; Huang, H.Y.; Sun, Y.M.; Chou, C.H.; Weng, S.L.; Huang, H.D. Methhc: A database of DNA methylation and gene expression in human cancer. Nucleic Acids Res. 2015, 43, D856–D861. [Google Scholar] [CrossRef] [PubMed]
| Patient and Tumor Characteristics | Original Cohort (n = 110) | Validation Cohort (n = 120) | Original and Validation Cohorts (n = 230) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| High (37) | Low (73) | p-Value § | High (39) | Low (81) | p-Value § | High (76) | Low (154) | p-Value § | |
| Age | |||||||||
| Under 70 | 25 | 57 | 26 | 49 | 51 | 106 | |||
| 70 and Older | 12 | 16 | 1 | 13 | 32 | 0.552 | 25 | 48 | 1 |
| Gender | |||||||||
| Male | 31 | 56 | 33 | 70 | 64 | 126 | |||
| Female | 6 | 17 | 0.463 | 6 | 11 | 1 | 12 | 28 | 0.715 |
| Alcohol Exposure | |||||||||
| Ever | 21 | 39 | 29 | 70 | 50 | 109 | |||
| Never | 16 | 34 | 0.84 | 10 | 11 | 1 | 26 | 45 | 1 |
| Smoking Status | |||||||||
| Smoker | 27 | 45 | 28 | 65 | 55 | 110 | |||
| Non-Smoker | 10 | 28 | 0.291 | 11 | 16 | 1 | 21 | 44 | 1 |
| Tumor Size | |||||||||
| T1–3 | 22 | 56 | 27 | 48 | 49 | 104 | |||
| T4 | 15 | 17 | 1 | 12 | 33 | 0.321 | 27 | 50 | 1 |
| Lympho-Node Status | |||||||||
| N0 | 15 | 36 | 18 | 37 | 33 | 73 | |||
| N+ | 22 | 37 | 0.423 | 21 | 44 | 1 | 43 | 81 | 0.578 |
| Stage | |||||||||
| I, II, III | 12 | 40 | 20 | 32 | 32 | 72 | |||
| IV | 25 | 33 | 0.043 * | 19 | 49 | 0.243 | 44 | 82 | 0.574 |
| Recurrence Events | |||||||||
| Positive | 25 | 31 | 19 | 21 | 44 | 52 | |||
| Negative | 12 | 42 | 0.016 * | 20 | 60 | 0.012 * | 32 | 102 | <0.001 * |
| Variables | Disease-Free Survival | |
|---|---|---|
| HR (95% CI) | p | |
| Age | ||
| 70 and older vs. <70 | 1.326 (0.860–2.044) | 0.202 |
| Gender | ||
| Male vs. Female | 1.065 (0.615–1.846) | 0.821 |
| Alcohol exposure | ||
| Ever vs. Never | 0.516 (0.308–0.865) | 0.012 * |
| Smoking status | ||
| Smoker vs. Non Smoker | 1.988 (1.127–3.505) | 0.018 * |
| Stage | ||
| I, II, III vs. IV | 1.672 (1.088–2.568) | 0.019 * |
| EZH2 Expression | ||
| High vs. Low | 2.085 (1.390–3.127) | <0.001 * |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mochizuki, D.; Misawa, Y.; Kawasaki, H.; Imai, A.; Endo, S.; Mima, M.; Yamada, S.; Nakagawa, T.; Kanazawa, T.; Misawa, K. Aberrant Epigenetic Regulation in Head and Neck Cancer Due to Distinct EZH2 Overexpression and DNA Hypermethylation. Int. J. Mol. Sci. 2018, 19, 3707. https://doi.org/10.3390/ijms19123707
Mochizuki D, Misawa Y, Kawasaki H, Imai A, Endo S, Mima M, Yamada S, Nakagawa T, Kanazawa T, Misawa K. Aberrant Epigenetic Regulation in Head and Neck Cancer Due to Distinct EZH2 Overexpression and DNA Hypermethylation. International Journal of Molecular Sciences. 2018; 19(12):3707. https://doi.org/10.3390/ijms19123707
Chicago/Turabian StyleMochizuki, Daiki, Yuki Misawa, Hideya Kawasaki, Atsushi Imai, Shiori Endo, Masato Mima, Satoshi Yamada, Takuya Nakagawa, Takeharu Kanazawa, and Kiyoshi Misawa. 2018. "Aberrant Epigenetic Regulation in Head and Neck Cancer Due to Distinct EZH2 Overexpression and DNA Hypermethylation" International Journal of Molecular Sciences 19, no. 12: 3707. https://doi.org/10.3390/ijms19123707
APA StyleMochizuki, D., Misawa, Y., Kawasaki, H., Imai, A., Endo, S., Mima, M., Yamada, S., Nakagawa, T., Kanazawa, T., & Misawa, K. (2018). Aberrant Epigenetic Regulation in Head and Neck Cancer Due to Distinct EZH2 Overexpression and DNA Hypermethylation. International Journal of Molecular Sciences, 19(12), 3707. https://doi.org/10.3390/ijms19123707






