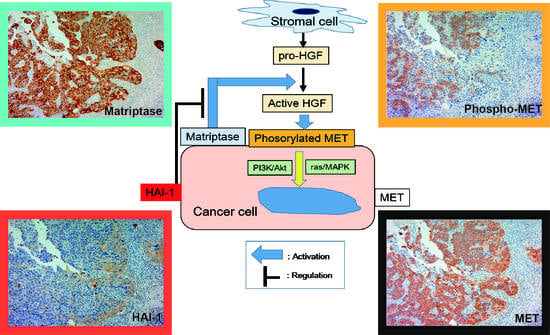
Matriptase-Induced Phosphorylation of MET is Significantly Associated with Poor Prognosis in Invasive Bladder Cancer; an Immunohistochemical Analysis
Abstract
1. Introduction
2. Results
2.1. Immunohistochemical Study
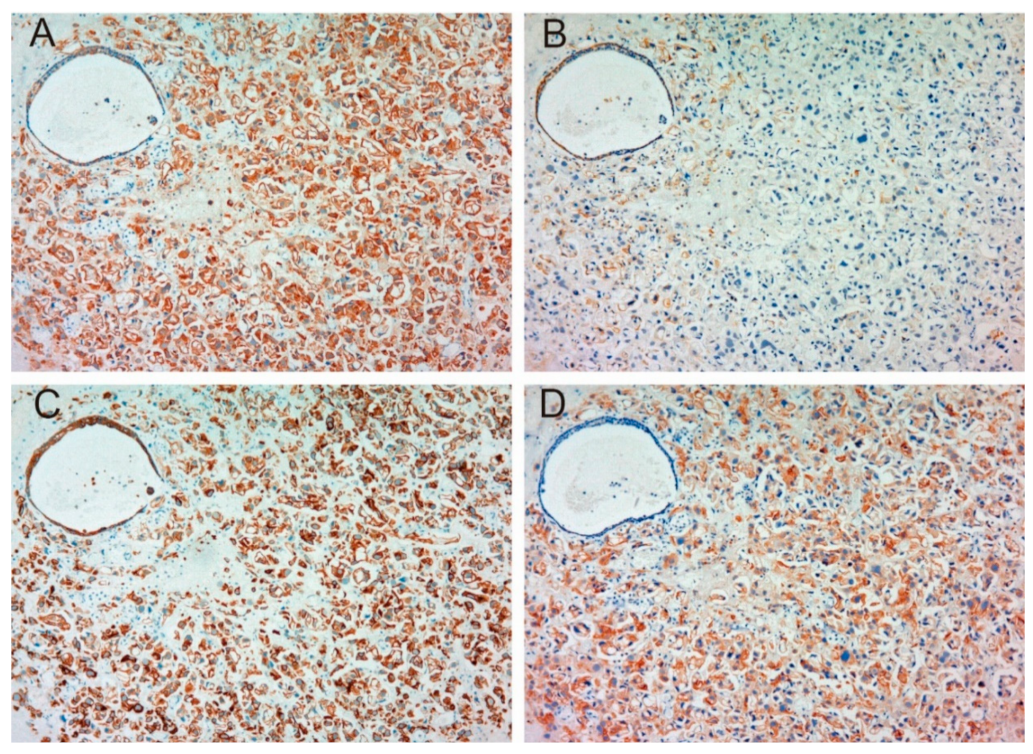
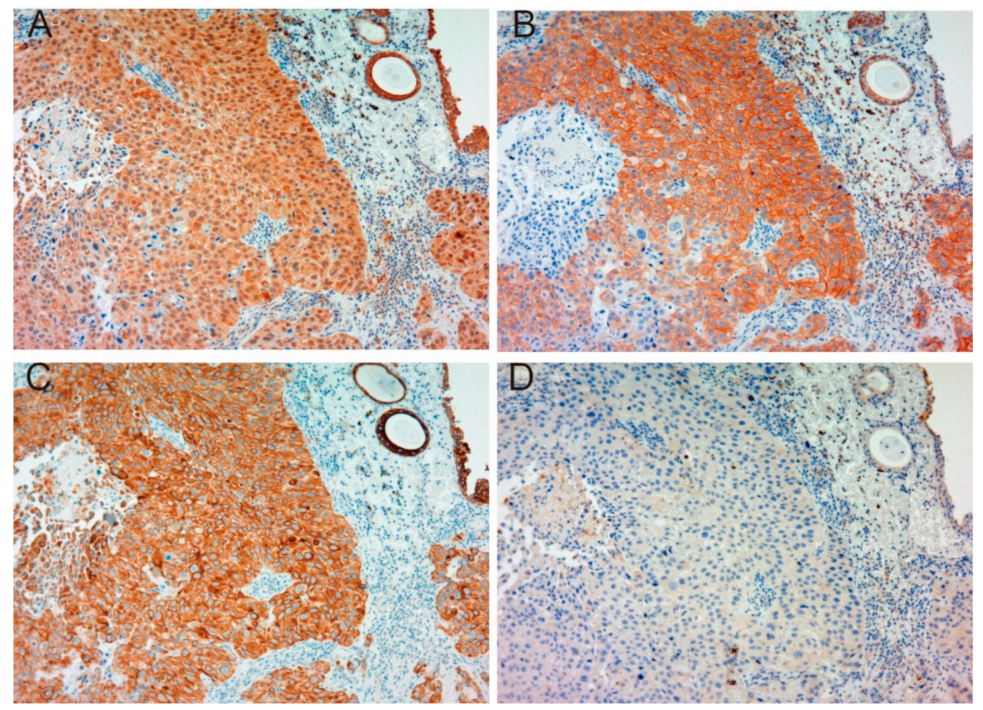
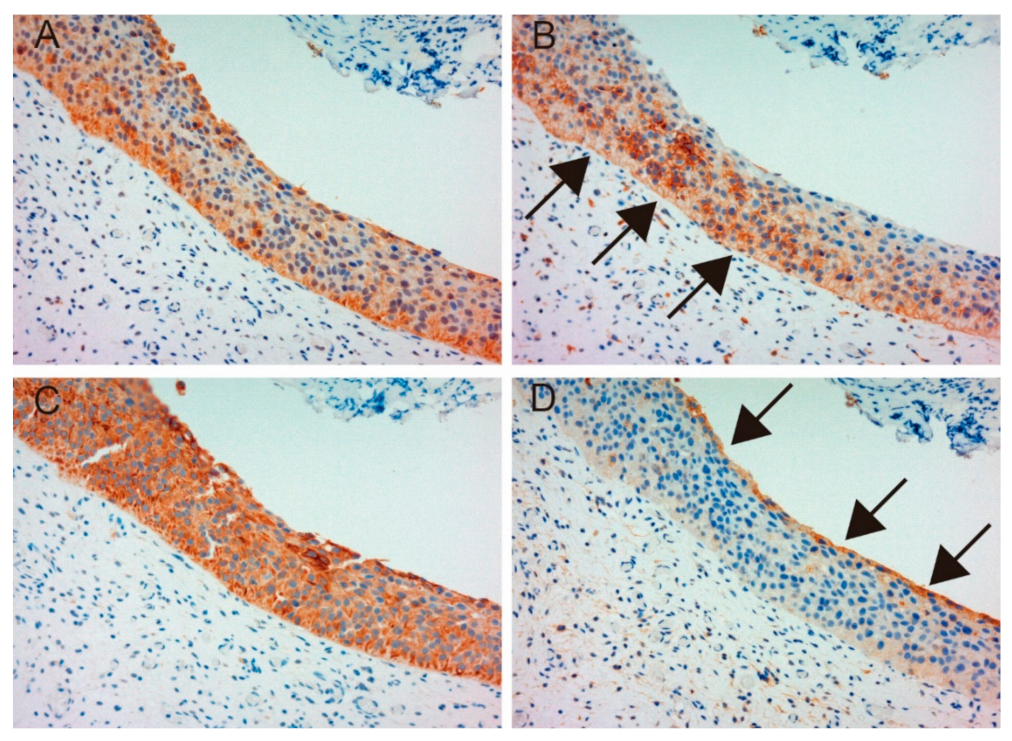
2.1.1. Expression of MET, Phospho-MET, Matriptase and HAI-1 in Cancer Tissue
2.1.2. Correlation between Pathological T Stage and Expression of Each Molecule
2.1.3. Correlation between Matriptase, HAI-1 and Phospho-MET
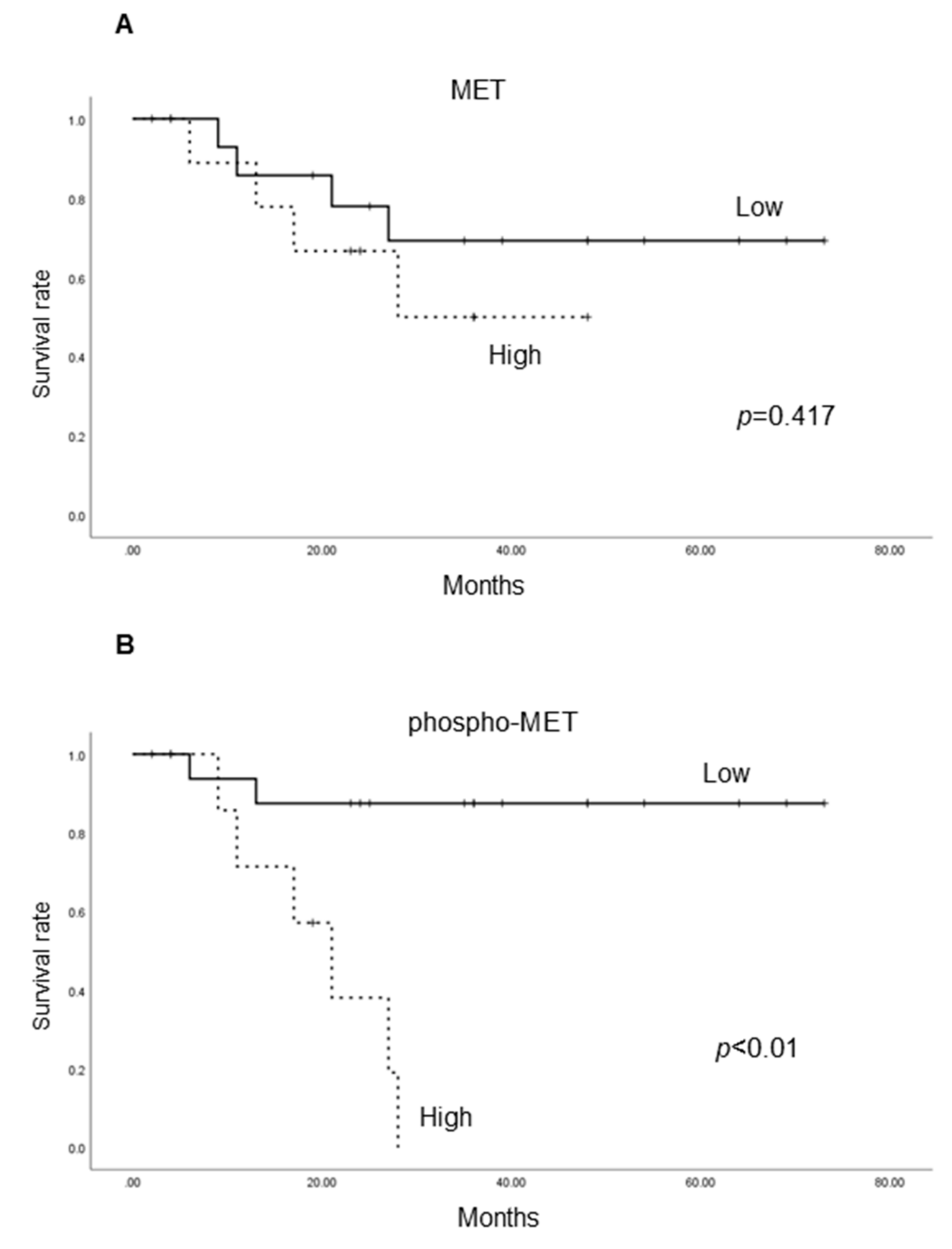
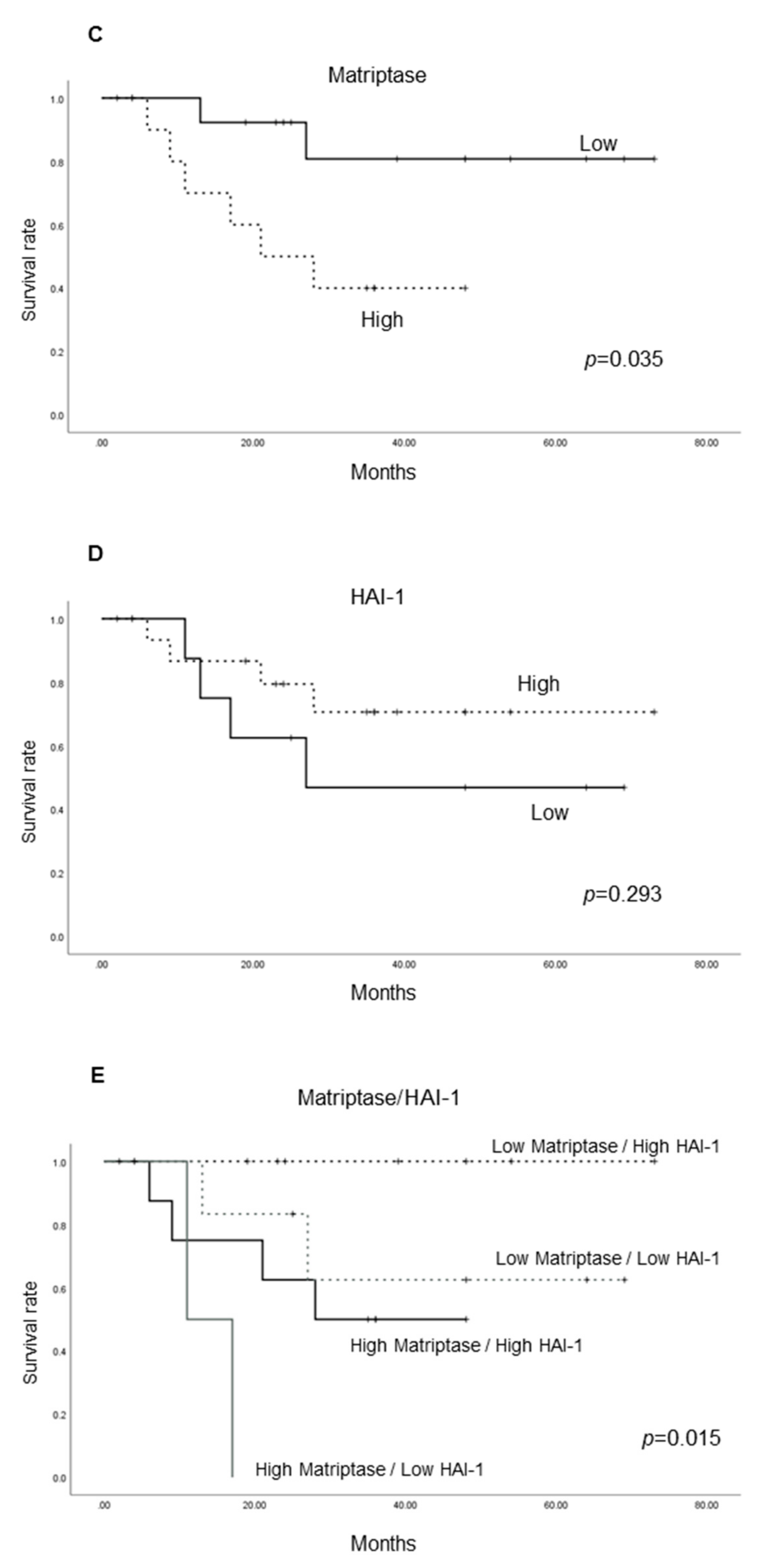
2.1.4. Cancer-Specific Survival According to the Expression of Each Molecule
3. Discussion
4. Materials and Methods
4.1. Immunohistochemistry and Analysis
4.2. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| HGF | Hepatocyto Growth Factor |
| HAI | Hepatocyto Growth Factor Activator Inhibitor |
| RNA | Rebonucleic Acid |
| NMIBC | Non-Muscle Invasive Bladder Cancer |
| MIBC | Muscle Invasive Bladder Cancer |
| TURBT | Transurethral Resection of Bladder Tumor |
| TTSP | Type II Transmembrane Serine Protease |
| EMT | Epithelial-Mesenchymal Transition |
| PAR | Protease Activated Receptor |
| PDGF | Platelet-Derived Growth Factor |
| MSP | Macrophage Stimulating Protein |
| NC | Negative Control |
| EGFR | Epidermal Growth Factor Receptor |
| TKI | Tyrosine Kinase Inhibitor |
| TMPRSS | Transmembrane Protease, Serine |
| KLK | Kallikrein 1-Related Peptidase |
| DNA | Deoxyribonucleic Acid |
References
- Parkin, D.M. The global burden of urinary bladder cancer. Scand. J. Urol. Nephrol. Suppl. 2008, 12–20. [Google Scholar] [CrossRef]
- Abol-Enein, H.; Kava, B.R.; Carmack, A.J. Nonurothelial cancer of the bladder. Urology 2007, 69, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Burger, M.; Catto, J.W.; Dalbagni, G.; Grossman, H.B.; Herr, H.; Karakiewicz, P.; Kassouf, W.; Kiemeney, L.A.; La Vecchia, C.; Shariat, S.; et al. Epidemiology and risk factors of urothelial bladder cancer. Eur. Urol. 2013, 63, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Vale, C.L. Neoadjuvant chemotherapy in invasive bladder cancer: Update of a systematic review and meta-analysis of individual patient data advanced bladder cancer (ABC) meta-analysis collaboration. Eur. Urol. 2005, 48, 202–205. [Google Scholar] [CrossRef] [PubMed]
- Pagano, F.; Bassi, P.; Galetti, T.P.; Meneghini, A.; Milani, C.; Artibani, W.; Garbeglio, A. Results of contemporary radical cystectomy for invasive bladder cancer: A clinicopathological study with an emphasis on the inadequacy of the tumor, nodes and metastases classification. J. Urol. 1991, 145, 45–50. [Google Scholar] [CrossRef]
- Stein, J.P.; Lieskovsky, G.; Cote, R.; Groshen, S.; Feng, A.C.; Boyd, S.; Skinner, E.; Bochner, B.; Thangathurai, D.; Mikhail, M.; et al. Radical cystectomy in the treatment of invasive bladder cancer: Long-term results in 1054 patients. J. Clin. Oncol. 2001, 19, 666–675. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K.; Umitsu, M.; De Silva, D.M.; Roy, A.; Bottaro, D.P. Hepatocyte growth factor/MET in cancer progression and biomarker discovery. Cancer Sci. 2017, 108, 296–307. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, H.; Kawaguchi, M.; Fukushima, T.; Shimomura, T. Hepatocyte growth factor activator inhibitors (HAI-1 and HAI-2): Emerging key players in epithelial integrity and cancer. Pathol. Int. 2018, 68, 145–158. [Google Scholar] [CrossRef] [PubMed]
- List, K.; Bugge, T.H.; Szabo, R. Matriptase: Potent proteolysis on the cell surface. Mol. Med. 2006, 12, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T. Hepatocyte growth factor as mitogen, motogen and morphogen, and its roles in organ regeneration. Princess Takamatsu Symp. 1994, 24, 195–213. [Google Scholar] [PubMed]
- Comoglio, P.M.; Giordano, S.; Trusolino, L. Drug development of MET inhibitors: Targeting oncogene addiction and expedience. Nat. Rev. Drug Discov. 2008, 7, 504–516. [Google Scholar] [CrossRef] [PubMed]
- Shintani, T.; Kusuhara, Y.; Daizumoto, K.; Dondoo, T.O.; Yamamoto, H.; Mori, H.; Fukawa, T.; Nakatsuji, H.; Fukumori, T.; Takahashi, M.; et al. The involvement of hepatocyte growth factor-MET-Matrix metalloproteinase 1 signaling in bladder cancer invasiveness and proliferation. Effect of the MET inhibitor, Cabozantinib (XL184), on bladder cancer cells. Urology 2017, 101, 169.e7–169.e13. [Google Scholar] [CrossRef] [PubMed]
- Bergum, C.; Zoratti, G.; Boerner, J.; List, K. Strong expression association between matriptase and its substrate prostasin in breast cancer. J. Cell Physiol. 2012, 227, 1604–1609. [Google Scholar] [CrossRef] [PubMed]
- Oberst, M.D.; Johnson, M.D.; Dickson, R.B.; Lin, C.Y.; Singh, B.; Stewart, M.; Williams, A.; Al-Nafussi, A.; Smyth, J.F.; Gabra, H.; et al. Expression of the serine protease matriptase and its inhibitor HAI-1 in epithelial ovarian cancer: Correlation with clinical outcome and tumor clinicopathological parameters. Clin. Cancer Res. 2002, 8, 1101–1107. [Google Scholar] [PubMed]
- Lee, J.W.; Yong Song, S.; Choi, J.J.; Lee, S.J.; Kim, B.G.; Park, C.S.; Lee, J.H.; Lin, C.Y.; Dickson, R.B.; Bae, D.S. Increased expression of matriptase is associated with histopathologic grades of cervical neoplasia. Hum. Pathol. 2005, 36, 626–633. [Google Scholar] [CrossRef] [PubMed]
- Najy, A.J.; Won, J.J.; Movilla, L.S.; Kim, H.R. Differential tumorigenic potential and matriptase activation between PDGF B versus PDGF D in prostate cancer. Mol. Cancer Res. 2012, 10, 1087–1097. [Google Scholar] [CrossRef] [PubMed]
- Bocheva, G.; Rattenholl, A.; Kempkes, C.; Goerge, T.; Lin, C.Y.; D’Andrea, M.R.; Ständer, S.; Steinhoff, M. Role of matriptase and proteinase-activated receptor-2 in nonmelanoma skin cancer. J. Invest. Dermatol. 2009, 129, 1816–1823. [Google Scholar] [CrossRef] [PubMed]
- Mukai, S.; Yorita, K.; Kawagoe, Y.; Katayama, Y.; Nakahara, K.; Kamibeppu, T.; Sugie, S.; Tukino, H.; Kamoto, T.; Kataoka, H. Matriptase and MET are prominently expressed at the site of bone metastasis in renal cell carcinoma: Immunohistochemical analysis. Hum. Cell 2015, 28, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, H.; Miyata, S.; Uchinokura, S.; Itoh, H. Roles of hepatocyte growth factor (HGF) activator and HGF activator inhibitor in the pericellular activation of HGF/scatter factor. Cancer Metastasis Rev. 2003, 22, 223–236. [Google Scholar] [CrossRef] [PubMed]
- Ha, S.Y.; Kim, K.Y.; Lee, N.K.; Kim, M.G.; Kim, S.H. Overexpression of matriptase correlates with poor prognosis in esophageal squamous cell carcinoma. Virchows Arch. 2014, 464, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Kanemaru, A.; Yamamoto, K.; Kawaguchi, M.; Fukushima, T.; Lin, C.Y.; Johnson, M.D.; Camerer, E.; Kataoka, H. Deregulated matriptase activity in oral squamous cell carcinoma promotes the infiltration of cancer-associated fibroblasts by paracrine activation of protease-activated receptor 2. Int. J. Cancer 2017, 140, 130–141. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Jiang, Z.; Chen, X.; Xue, L.; Mao, X.; Ruan, G.; Song, Y.; Mustea, A. Decreasing the ratio of matriptase/HAI-1 by downregulation of matriptase as a potential adjuvant therapy in ovarian cancer. Mol. Med. Rep. 2016, 14, 1465–1474. [Google Scholar] [CrossRef] [PubMed]
- Trusolino, L.; Bertotti, A.; Comoglio, P.M. MET signalling: Principles and functions in development, organ regeneration and cancer. Nat. Rev. Mol. Cell Biol. 2010, 11, 834–848. [Google Scholar] [CrossRef] [PubMed]
- Benvenuti, S.; Comoglio, P.M. The MET receptor tyrosine kinase in invasion and metastasis. J. Cell Physiol. 2007, 213, 316–325. [Google Scholar] [CrossRef] [PubMed]
- Mukai, S.; Yorita, K.; Yamasaki, K.; Nagai, T.; Kamibeppu, T.; Sugie, S.; Kida, K.; Onizuka, C.; Tsukino, H.; Kamimura, T.; et al. Expression of human kallikrein 1-related peptidase 4 (KLK4) and MET phosphorylation in prostate cancer tissue: Immunohistochemical analysis. Hum. Cell 2015, 28, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Betsunoh, H.; Mukai, S.; Akiyama, Y.; Fukushima, T.; Minamiguchi, N.; Hasui, Y.; Osada, Y.; Kataoka, H. Clinical relevance of hepsin and hepatocyte growth factor activator inhibitor type 2 expression in renal cell carcinoma. Cancer Sci. 2007, 98, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Suda, K.; Mizuuchi, H.; Maehara, Y.; Mitsudomi, T. Acquired resistance mechanisms to tyrosine kinase inhibitors in lung cancer with activating epidermal growth factor receptor mutation-diversity, ductility, and destiny. Cancer Metastasis Rev. 2012, 31, 807–814. [Google Scholar] [CrossRef] [PubMed]
- Pennacchietti, S.; Cazzanti, M.; Bertotti, A.; Rideout, W.M.; Han, M.; Gyuris, J.; Perera, T.; Comoglio, P.M.; Trusolino, L.; Michieli, P. Microenvironment-derived HGF overcomes genetically determined sensitivity to anti-MET drugs. Cancer Res. 2014, 74, 6598–6609. [Google Scholar] [CrossRef] [PubMed]
- Joffre, C.; Barrow, R.; Ménard, L.; Calleja, V.; Hart, I.R.; Kermorgant, S. A direct role for Met endocytosis in tumorigenesis. Nat. Cell Biol. 2011, 13, 827–837. [Google Scholar] [CrossRef] [PubMed]
- Szabo, R.; Bugge, T.H. Type II transmembrane serine proteases in development and disease. Int. J. Biochem. Cell Biol. 2008, 40, 1297–1316. [Google Scholar] [CrossRef] [PubMed]
- Kojima, K.; Tsuzuki, S.; Fushiki, T.; Inouye, K. Roles of functional and structural domains of hepatocyte growth factor activator inhibitor type 1 in the inhibition of matriptase. J. Biol. Chem. 2008, 283, 2478–2487. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.Y.; Wang, J.K.; Torri, J.; Dou, L.; Sang, Q.A.; Dickson, R.B. Characterization of a novel, membrane-bound, 80-kDa matrix-degrading protease from human breast cancer cells. Monoclonal antibody production, isolation, and localization. J. Biol. Chem. 1997, 272, 9147–9152. [Google Scholar] [CrossRef] [PubMed]
- Benaud, C.M.; Oberst, M.; Dickson, R.B.; Lin, C.Y. Deregulated activation of matriptase in breast cancer cells. Clin. Exp. Metastasis 2002, 19, 639–649. [Google Scholar] [CrossRef] [PubMed]
- Zoratti, G.L.; Tanabe, L.M.; Hyland, T.E.; Duhaime, M.J.; Colombo, É.; Leduc, R.; Marsault, E.; Johnson, M.D.; Lin, C.Y.; Boerner, J.; et al. Matriptase regulates c-Met mediated proliferation and invasion in inflammatory breast cancer. Oncotarget 2016, 7, 58162–58173. [Google Scholar] [CrossRef] [PubMed]
- Szabo, R.; Rasmussen, A.L.; Moyer, A.B.; Kosa, P.; Schafer, J.M.; Molinolo, A.A.; Gutkind, J.S.; Bugge, T.H. c-Met-induced epithelial carcinogenesis is initiated by the serine protease matriptase. Oncogene 2011, 30, 2003–2016. [Google Scholar] [CrossRef] [PubMed]
- Miyata, Y.; Sagara, Y.; Kanda, S.; Hayashi, T.; Kanetake, H. Phosphorylated hepatocyte growth factor receptor/c-Met is associated with tumor growth and prognosis in patients with bladder cancer: Correlation with matrix metalloproteinase-2 and -7 and E-cadherin. Hum. Pathol. 2009, 40, 496–504. [Google Scholar] [CrossRef] [PubMed]
- Shimomura, T.; Denda, K.; Kitamura, A.; Kawaguchi, T.; Kito, M.; Kondo, J.; Kagaya, S.; Qin, L.; Takata, H.; Miyazawa, K.; et al. Hepatocyte growth factor activator inhibitor, a novel Kunitz-type serine protease inhibitor. J. Biol. Chem. 1997, 272, 6370–6376. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Jiang, N.; Wang, G.; Zheng, J.; Yang, W.; Yang, J. Expression of hepatocyte growth factor activator inhibitor-1 (HAI-1) gene in prostate cancer: Clinical and biological significance. J. BUON. 2014, 19, 215–220. [Google Scholar] [PubMed]
- Parr, C.; Jiang, W.G. Hepatocyte growth factor activation inhibitors (HAI-1 and HAI-2) regulate HGF-induced invasion of human breast cancer cells. Int. J. Cancer 2006, 119, 1176–1183. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Abarzua, F.; Kodama, J.; Hongo, A.; Nasu, Y.; Kumon, H.; Hiramatsu, Y. Expression of hepatocyte growth factor activator inhibitors (HAI-1 and HAI-2) in ovarian cancer. Int. J. Oncol. 2009, 34, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Xue, L.; Song, Y.; Mao, X.; Chen, L.; Dong, B.; Braicu, E.L.; Sehouli, J. Regulation of matriptase and HAI-1 system, a novel therapeutic target in human endometrial cancer cells. Oncotarget 2018, 9, 12682–12694. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Wu, H.; Cao, J.; Ye, J. Hepatocyte growth factor activator inhibitor type-1 in cancer: Advances and perspectives (Review). Mol. Med. Rep. 2014, 10, 2779–2785. [Google Scholar] [CrossRef] [PubMed]

| Age (median) | 73 | |
|---|---|---|
| Gender | Male | 14 |
| Female | 12 | |
| Neoadjuvant chemotherapy | + | 12 |
| - | 14 | |
| pT stage | ≤ T1 | 6 |
| T2 | 15 | |
| ≥ T3 | 5 | |
| Follow-up period (months, median) | 26 | |
| Patient | MET | p-MET | HAI-1 | Matriptase |
|---|---|---|---|---|
| 1 | H | L | L | L |
| 2 | L | L | L | L |
| 3 | L | L | L | L |
| 4 | L | L | L | L |
| 5 | L | L | L | L |
| 6 | L | L | H | L |
| 7 | L | L | H | L |
| 8 | L | L | H | H |
| 9 | L | L | H | L |
| 10 | H | L | H | H |
| 11 | H | L | H | L |
| 12 | H | L | H | L |
| 13 | H | L | H | L |
| 14 | H | L | H | H |
| 15 | H | L | H | H |
| 16 | H | L | H | L |
| 17 | H | L | H | H |
| 18 | H | L | H | H |
| 19 | L | L | H | L |
| 20 | L | H | H | L |
| 21 | L | H | H | H |
| 22 | L | H | H | H |
| 23 | H | H | H | H |
| 24 | L | H | L | L |
| 25 | L | H | L | H |
| 26 | H | H | L | H |
| Molecules | pT Stage | ||||
|---|---|---|---|---|---|
| 1 | 2 | ≥3 | p Value | ||
| MET | Low | 4 | 10 | 0 | 0.027 |
| High | 2 | 5 | 5 | ||
| Phospho-MET | Low | 6 | 8 | 5 | 0.030 |
| High | 0 | 7 | 0 | ||
| Matriptase | Low | 2 | 5 | 1 | 0.845 |
| High | 4 | 10 | 4 | ||
| HAI-1 | Low | 4 | 9 | 2 | 0.647 |
| High | 2 | 6 | 3 | ||
| Matriptase | HAI-1 | ||||||
|---|---|---|---|---|---|---|---|
| Low | High | p Value | Low | High | p Value | ||
| Phospho-MET | Low | 13 | 2 | 0.068 | 5 | 14 | 0.418 |
| High | 6 | 5 | 3 | 4 | |||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamasaki, K.; Mukai, S.; Nagai, T.; Nakahara, K.; Fujii, M.; Terada, N.; Ohno, A.; Sato, Y.; Toda, Y.; Kataoka, H.; et al. Matriptase-Induced Phosphorylation of MET is Significantly Associated with Poor Prognosis in Invasive Bladder Cancer; an Immunohistochemical Analysis. Int. J. Mol. Sci. 2018, 19, 3708. https://doi.org/10.3390/ijms19123708
Yamasaki K, Mukai S, Nagai T, Nakahara K, Fujii M, Terada N, Ohno A, Sato Y, Toda Y, Kataoka H, et al. Matriptase-Induced Phosphorylation of MET is Significantly Associated with Poor Prognosis in Invasive Bladder Cancer; an Immunohistochemical Analysis. International Journal of Molecular Sciences. 2018; 19(12):3708. https://doi.org/10.3390/ijms19123708
Chicago/Turabian StyleYamasaki, Koji, Shoichiro Mukai, Takahiro Nagai, Kozue Nakahara, Masato Fujii, Naoki Terada, Akinobu Ohno, Yuichiro Sato, Yoshinobu Toda, Hiroaki Kataoka, and et al. 2018. "Matriptase-Induced Phosphorylation of MET is Significantly Associated with Poor Prognosis in Invasive Bladder Cancer; an Immunohistochemical Analysis" International Journal of Molecular Sciences 19, no. 12: 3708. https://doi.org/10.3390/ijms19123708
APA StyleYamasaki, K., Mukai, S., Nagai, T., Nakahara, K., Fujii, M., Terada, N., Ohno, A., Sato, Y., Toda, Y., Kataoka, H., & Kamoto, T. (2018). Matriptase-Induced Phosphorylation of MET is Significantly Associated with Poor Prognosis in Invasive Bladder Cancer; an Immunohistochemical Analysis. International Journal of Molecular Sciences, 19(12), 3708. https://doi.org/10.3390/ijms19123708