Needle-Free Immunization with Chitosan-Based Systems
Abstract
1. Introduction
2. Routes of Needle-Free Immunization
2.1. Cutaneous Routes
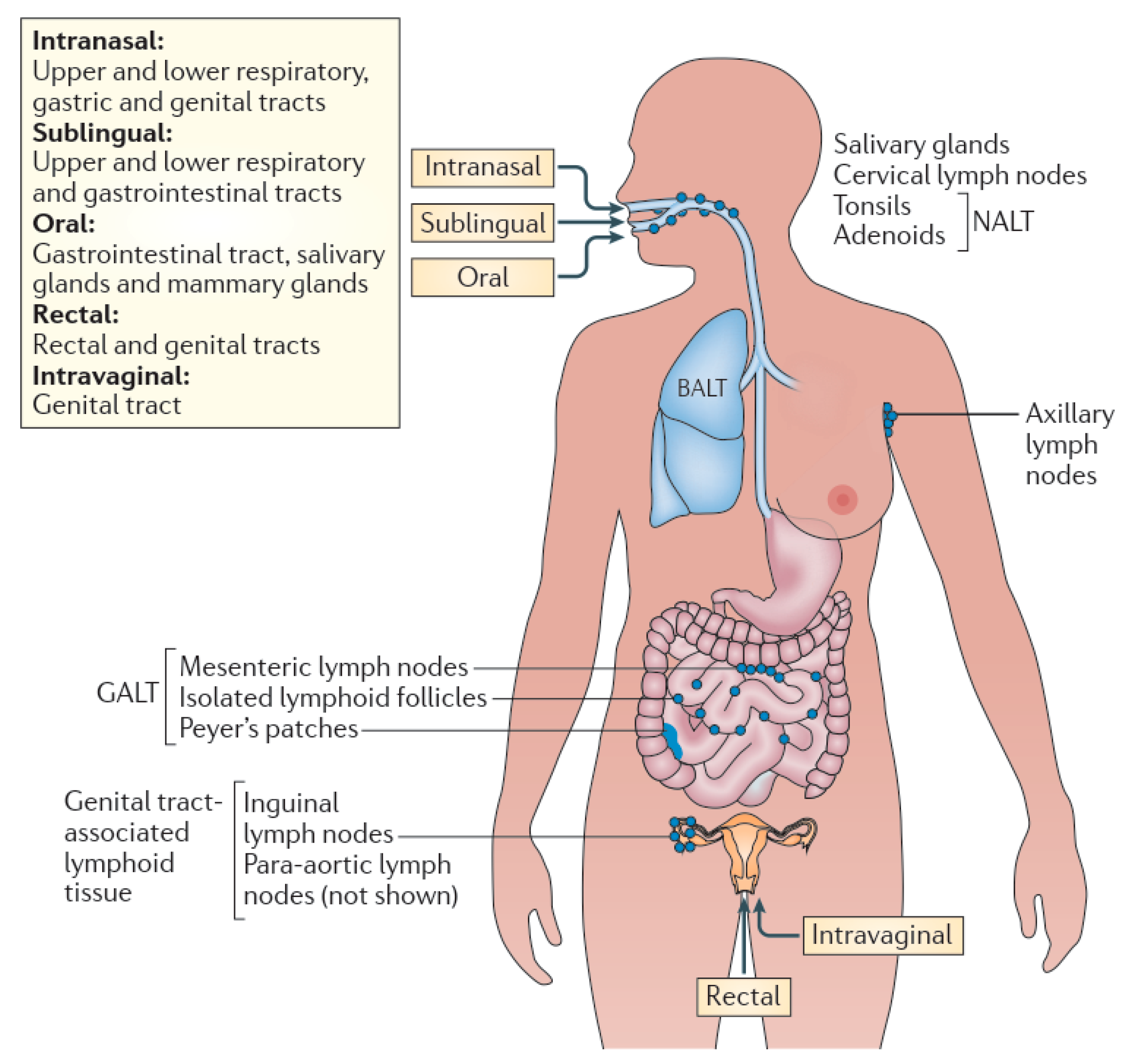
2.2. Mucosal Routes
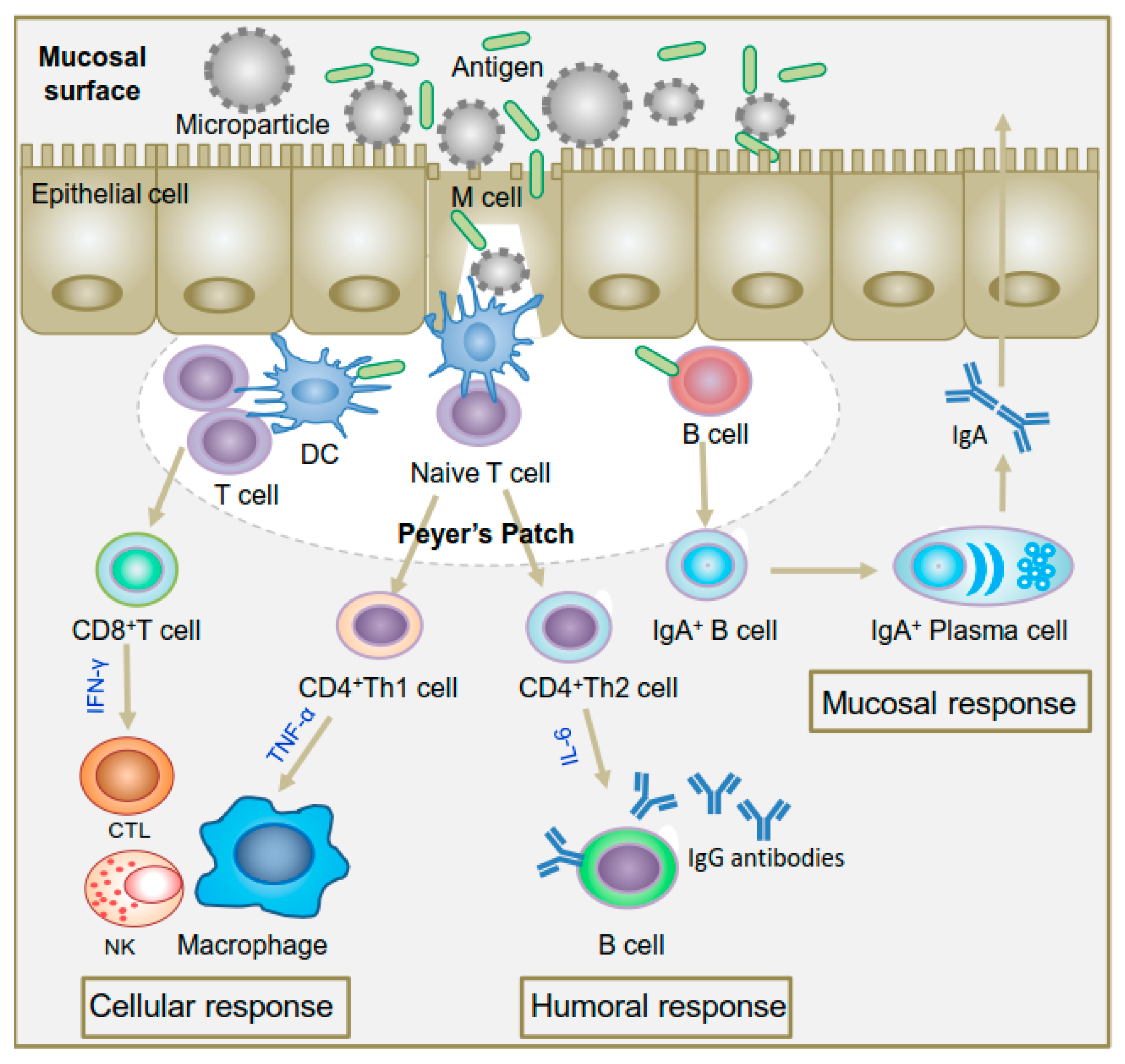
2.2.1. Oral Route
2.2.2. Nasal Route
2.2.3. Urinogenital Route
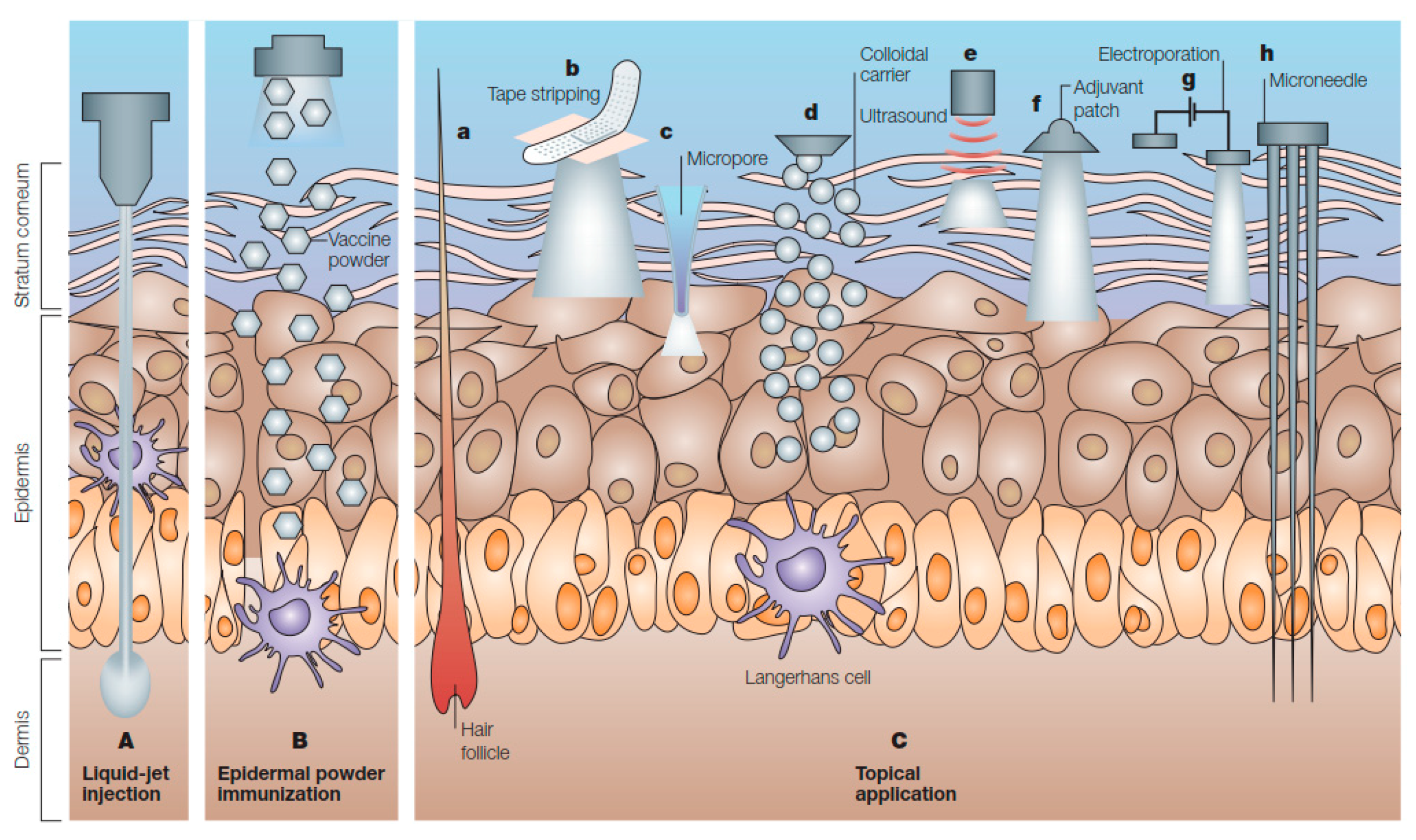
3. Needle-Free Cutaneous Immunization
3.1. Liquid-Jet Injections
3.2. Powder Injections
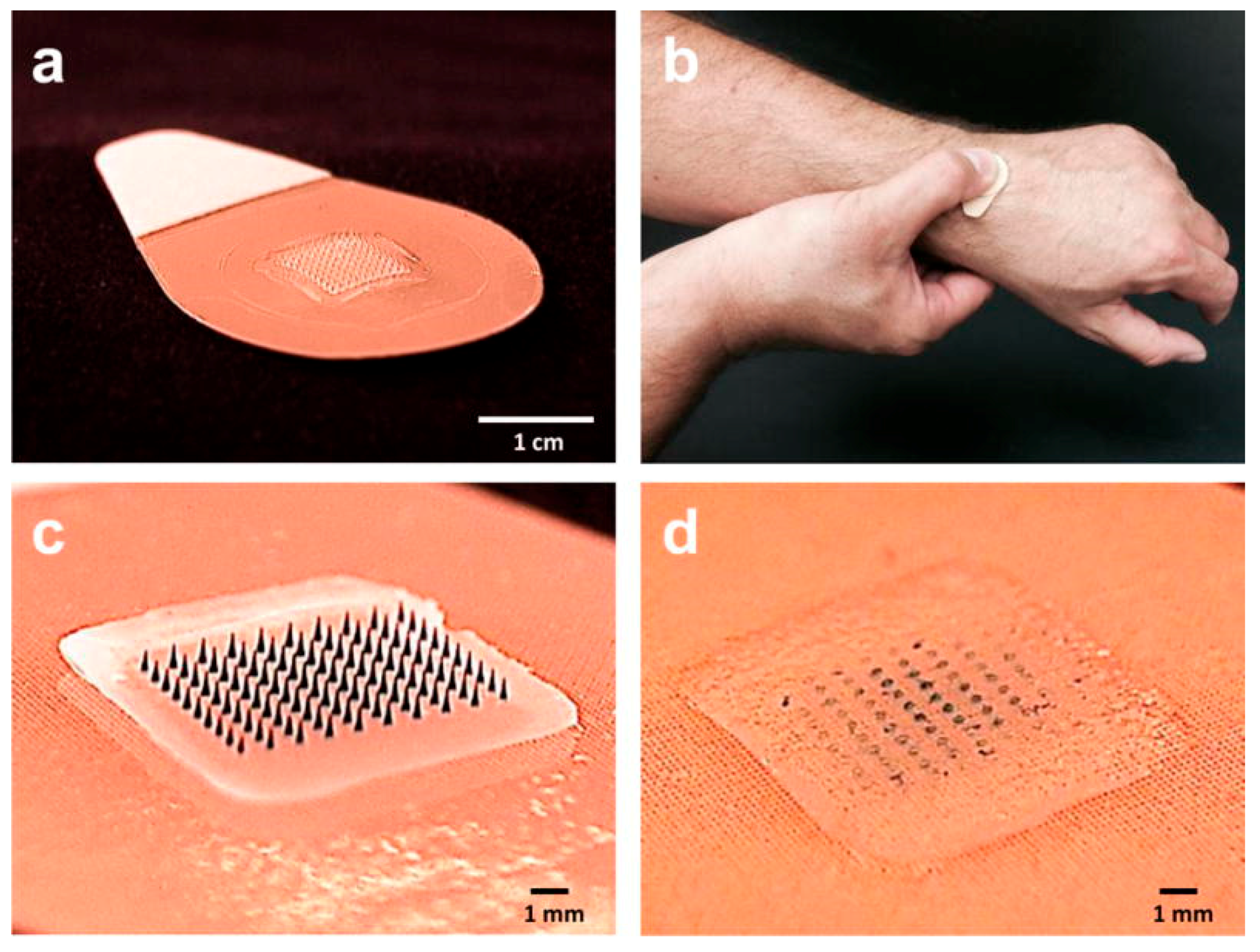
3.3. Microarray (Microneedle) Patches
4. Needle-Free Immunization with Chitosan Systems
4.1. Formulations of Chitosan Particles
4.2. Mucoadhesive Chitosan Particles
4.3. Targeted Chitosan Particles
4.4. Adjuvant Activity of Chitosan Particles
5. Conclusions and Prospectives
Funding
Conflicts of Interest
References
- Mitragotri, S. Immunization without needles. Nat. Rev. Immunol. 2005, 5, 905–916. [Google Scholar] [CrossRef] [PubMed]
- Holmgren, J.; Czerkinsky, C. Mucosal immunity and vaccines. Nat. Med. 2005, 11, S45–S53. [Google Scholar] [CrossRef] [PubMed]
- Lycke, N. Recent progress in mucosal vaccine development: Potential and limitations. Nat. Rev. Immunol. 2012, 12, 592. [Google Scholar] [CrossRef] [PubMed]
- Mestecky, J. The common mucosal immune system and current strategies for induction of immune responses in external secretions. J. Clin. Immunol. 1987, 7, 265–276. [Google Scholar] [CrossRef] [PubMed]
- Neutra, M.R.; Kozlowski, P.A. Mucosal vaccines: The promise and the challenge. Nat. Rev. Immunol. 2006, 6, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Maharjan, S.; Cho, K.H.; Cui, L.; Park, I.K.; Choi, Y.J.; Cho, C.S. Chitosan-based particulate systems for the delivery of mucosal vaccines against infectious diseases. Int. J. Biol. Macromol. 2018, 110, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Maharjan, S.; Jiang, T.; Kang, S.K.; Choi, Y.J.; Cho, C.S. Combinatorial approach of antigen delivery using m cell-homing peptide and mucoadhesive vehicle to enhance the efficacy of oral vaccine. Mol. Pharm. 2015, 12, 3816–3828. [Google Scholar] [CrossRef] [PubMed]
- Slütter, B.; Hagenaars, N.; Jiskoot, W. Rational design of nasal vaccines. J. Drug Target. 2008, 16, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Jabbal-Gill, I.; Watts, P.; Smith, A. Chitosan-based delivery systems for mucosal vaccines. Expert Opin. Drug Deliv. 2012, 9, 1051–1067. [Google Scholar] [CrossRef] [PubMed]
- Schlosser, E.; Mueller, M.; Fischer, S.; Basta, S.; Busch, D.H.; Gander, B.; Groettrup, M. Tlr ligands and antigen need to be coencapsulated into the same biodegradable microsphere for the generation of potent cytotoxic T lymphocyte responses. Vaccine 2008, 26, 1626–1637. [Google Scholar] [CrossRef] [PubMed]
- Fischer, S.; Schlosser, E.; Mueller, M.; Csaba, N.; Merkle, H.P.; Groettrup, M.; Gander, B. Concomitant delivery of a CTL-restricted peptide antigen and CpG ODN by PLGA microparticles induces cellular immune response. J. Drug Target. 2009, 17, 652–661. [Google Scholar] [CrossRef] [PubMed]
- Tafaghodi, M.; Jaafari, M.R.; Sajadi Tabassi, S.A. Nasal immunization studies using liposomes loaded with tetanus toxoid and CpG-ODN. Eur. J. Pharm. Biopharm. 2006, 64, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Bubiuk, S.; Baca-Estrada, M.; Babiuk, L.A.; Ewen, C.; Foldvari, M. Cutaneous vaccination: The skin as an immunologically active tissue and the challenge of antigen delivery (vol 66, pg 199, 2000). J. Control. Release 2000, 67, 415. [Google Scholar] [CrossRef]
- Bodey, B.; Bodey, B.; Kaiser, H.E. Dendritic type, accessory cells within the mammalian thymic microenvironment. Antigen presentation in the dendritic neuro-endocrine-immune cellular network. In Vivo 1997, 11, 351–370. [Google Scholar] [PubMed]
- Stoitzner, P.; Holzmann, S.; McLellan, A.D.; Ivarsson, L.; Stossel, H.; Kapp, M.; Kammerer, U.; Douillard, P.; Kampgen, E.; Koch, F.; et al. Visualization and characterization of migratory langerhans cells in murine skin and lymph nodes by antibodies against langerin/CD207. J. Invesig. Dermatol. 2003, 120, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Salmon, J.K.; Armstrong, C.A.; Ansel, J.C. The skin as an immune organ. West. J. Med. 1994, 160, 146–152. [Google Scholar] [PubMed]
- Czerkinsky, C.; Anjuere, F.; McGhee, J.R.; George-Chandy, A.; Holmgren, J.; Kieny, M.P.; Fujiyashi, K.; Mestecky, J.F.; Pierrefite-Carle, V.; Rask, C.; et al. Mucosal immunity and tolerance: Relevance to vaccine development. Immunol. Rev. 1999, 170, 197–222. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-B.; Yoon, S.-Y.; Singh, B.; Oh, S.-H.; Cui, L.; Yan, C.; Kang, S.-K.; Choi, Y.-J.; Cho, C.-S. Oral immunization of fmdv vaccine using ph-sensitive and mucoadhesive thiolated cellulose acetate phthalate microparticles. Tissue Eng. Regen. Med. 2018, 15, 1–11. [Google Scholar] [CrossRef]
- Kollaritsch, H.; Que, J.U.; Kunz, C.; Wiedermann, G.; Herzog, C.; Cryz, S.J. Safety and immunogenicity of live oral cholera and typhoid vaccines administered alone or in combination with antimalarial drugs, oral polio vaccine, or yellow fever vaccine. J. Infect. Dis. 1997, 175, 871–875. [Google Scholar] [CrossRef] [PubMed]
- Lambkin, I.; Pinilla, C. Targeting approaches to oral drug delivery. Expert Opin. Biol. Ther. 2002, 2, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Maharjan, S.; Jiang, T.; Kang, S.K.; Choi, Y.J.; Cho, C.S. Attuning hydroxypropyl methylcellulose phthalate to oral delivery vehicle for effective and selective delivery of protein vaccine in ileum. Biomaterials 2015, 59, 144–159. [Google Scholar] [CrossRef] [PubMed]
- Nellore, R.V.; Pande, P.G.; Young, D.; Bhagat, H.R. Evaluation of biodegradable microspheres as vaccine adjuvant for hepatitis b surface antigen. J. Parent. Sci. Technol. Publ. Parenter. Drug Assoc. 1992, 46, 176–180. [Google Scholar]
- Esparza, I.; Kissel, T. Parameters affecting the immunogenicity of microencapsulated tetanus toxoid. Vaccine 1992, 10, 714–720. [Google Scholar] [CrossRef]
- Ahire, V.J.; Sawant, K.K.; Doshi, J.B.; Ravetkar, S.D. Chitosan microparticles as oral delivery system for tetanus toxoid. Drug Dev. Ind. Pharm. 2007, 33, 1112–1124. [Google Scholar] [CrossRef] [PubMed]
- Challacombe, S.J.; Rahman, D.; Jeffery, H.; Davis, S.S.; Ohagan, D.T. Enhanced secretory iga and systemic igg antibody-responses after oral immunization with biodegradable microparticles containing antigen. Immunology 1992, 76, 164–168. [Google Scholar] [PubMed]
- AllaouiAttarki, K.; Pecquet, S.; Fattal, E.; Trolle, S.; Chachaty, E.; Couvreur, P.; Andremont, A. Protective immunity against salmonella typhimurium elicited in mice by oral vaccination with phosphorylcholine encapsulated in poly(dl-lactide-co-glycolide) microspheres. Infect. Immun. 1997, 65, 853–857. [Google Scholar]
- Freytag, L.C.; Clements, J.D. Mucosal adjuvants. Vaccine 2005, 23, 1804–1813. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, K.; Holmgren, J. Recent advances in mucosal vaccines and adjuvants. Curr. Opin. Immunol. 2002, 14, 666–672. [Google Scholar] [CrossRef]
- Pizza, M.; Giuliani, M.M.; Fontana, M.R.; Monaci, E.; Douce, G.; Dougan, G.; Mills, K.H.G.; Rappuoli, R.; Del Giudice, G. Mucosal vaccines: Non toxic derivatives of lt and ct as mucosal adjuvants. Vaccine 2001, 19, 2534–2541. [Google Scholar] [CrossRef]
- Brandtzaeg, P. Potential of nasopharynx-associated lymphoid tissue for vaccine responses in the airways. Am. J. Respir. Crit. Care Med. 2011, 183, 1595–1604. [Google Scholar] [CrossRef] [PubMed]
- Jabbal-Gill, I. Nasal vaccine innovation. J. Drug Target. 2010, 18, 771–786. [Google Scholar] [CrossRef] [PubMed]
- Tribble, D.; Kaminski, R.; Cantrell, J.; Nelson, M.; Porter, C.; Baqar, S.; Williams, C.; Arora, R.; Saunders, J.; Ananthakrishnan, M.; et al. Safety and immunogenicity of a shigella flexneri 2a invaplex 50 intranasal vaccine in adult volunteers. Vaccine 2010, 28, 6076–6085. [Google Scholar] [CrossRef] [PubMed]
- Aggerbeck, H.; Gizurarson, S.; Wantzin, J.; Heron, I. Intranasal booster vaccination against diphtheria and tetanus in man. Vaccine 1997, 15, 307–316. [Google Scholar] [CrossRef]
- Betancourt, A.A.; Delgado, C.A.G.; Estévez, Z.C.; Martínez, J.C.; Ríos, G.V.; Aureoles-Roselló, S.R.M.; Zaldívar, R.A.; Guzmán, M.A.; Baile, N.F.; Reyes, P.A.D.; et al. Phase i clinical trial in healthy adults of a nasal vaccine candidate containing recombinant hepatitis b surface and core antigens. Int. J. Infect. Dis. 2007, 11, 394–401. [Google Scholar] [CrossRef] [PubMed]
- Mielcarek, N.; Debrie, A.S.; Raze, D.; Bertout, J.; Rouanet, C.; Younes, A.B.; Creusy, C.; Engle, J.; Goldman, W.E.; Locht, C. Live attenuated b. Pertussis as a single-dose nasal vaccine against whooping cough. PLoS Pathog. 2006, 2, e65. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Michalek, S.M.; Dasanayake, A.P.; Li, Y.; Kirk, K.; Childers, N.K. Intranasal immunization of humans with streptococcus mutans antigens. Oral Microbiol. Immunol. 2003, 18, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Davis, S.S. Nasal vaccines. Adv. Drug Deliv. Rev. 2001, 51, 21–42. [Google Scholar] [CrossRef]
- Roth, Y.; Chapnik, J.S.; Cole, P. Feasibility of aerosol vaccination in humans. Ann. Otol. Rhinol. Laryngol. 2003, 112, 264–270. [Google Scholar] [CrossRef] [PubMed]
- De Jonge, M.I.; Hamstra, H.J.; Jiskoot, W.; Roholl, P.; Williams, N.A.; Dankert, J.; van Alphen, L.; van der Ley, P. Intranasal immunisation of mice with liposomes containing recombinant meningococcal opab and opaj proteins. Vaccine 2004, 22, 4021–4028. [Google Scholar] [CrossRef] [PubMed]
- Alpar, H.O.; Somavarapu, S.; Atuah, K.N.; Bramwell, V. Biodegradable mucoadhesive particulates for nasal and pulmonary antigen and DNA delivery. Adv. Drug Deliv. Rev. 2005, 57, 411–430. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; O’Hagan, D.T. Recent advances in vaccine adjuvants. Pharm. Res. 2002, 19, 715–728. [Google Scholar] [CrossRef] [PubMed]
- Vajdy, M.; Srivastava, I.; Polo, J.; Donnelly, J.; O’Hagan, D.; Singh, M. Mucosal adjuvants and delivery systems for protein-, DNA- and RNA-based vaccines. Immunol. Cell. Biol. 2004, 82, 617–627. [Google Scholar] [CrossRef] [PubMed]
- Becker, P.D.; Bertot, G.M.; Souss, D.; Ebensen, T.; Guzman, C.A.; Grinstein, S. Intranasal vaccination with recombinant outer membrane protein cd and adamantylamide dipeptide as the mucosal adjuvant enhances pulmonary clearance of moraxella catarrhalis in an experimental murine model. Infect. Immun. 2007, 75, 1778–1784. [Google Scholar] [CrossRef] [PubMed]
- Rharbaoui, F.; Westendorf, A.; Link, C.; Felk, S.; Buer, J.; Gunzer, M.; Guzman, C.A. The mycoplasma-derived macrophage-activating 2-kilodalton lipopeptide triggers global immune activation on nasal mucosa-associated lymphoid tissues. Infect. Immun. 2004, 72, 6978–6986. [Google Scholar] [CrossRef] [PubMed]
- Becker, P.D.; Fiorentini, S.; Link, C.; Tosti, G.; Ebensen, T.; Caruso, A.; Guzman, C.A. The HIV-1 matrix protein p 17 can be efficiently delivered by intranasal route in mice using the TLR 2/6 agonist MALP-2 as mucosal adjuvant. Vaccine 2006, 24, 5269–5276. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M.; Briles, D.E.; Yamamoto, S.; Ohmura, M.; Kiyono, H.; McGhee, J.R. A nontoxic adjuvant for mucosal immunity to pneumococcal surface protein A. J. Immunol. 1998, 161, 4115–4121. [Google Scholar] [PubMed]
- Jakobsen, H.; Bjarnarson, S.; Del Giudice, G.; Moreau, M.; Siegrist, C.A.; Jonsdottir, I. Intranasal immunization with pneumococcal conjugate vaccines with lT-K63, a nontoxic mutant of heat-labile enterotoxin, as adjuvant rapidly induces protective immunity against lethal pneumococcal infections in neonatal mice. Infect. Immun. 2002, 70, 1443–1452. [Google Scholar] [CrossRef] [PubMed]
- Mutsch, M.; Zhou, W.G.; Rhodes, P.; Bopp, M.; Chen, R.T.; Linder, T.; Spyr, C.; Steffen, R. Use of the inactivated intranasal influenza vaccine and the risk of bell’s palsy in switzerland. N. Engl. J. Med. 2004, 350, 896–903. [Google Scholar] [CrossRef] [PubMed]
- Falkeborn, T.; Brave, A.; Larsson, M.; Akerlind, B.; Schroder, U.; Hinkula, J. Endocine, N3OA and N3OASq; three mucosal adjuvants that enhance the immune response to nasal influenza vaccination. PLoS ONE 2013, 8, e70527. [Google Scholar] [CrossRef] [PubMed]
- Maltais, A.K.; Stittelaar, K.J.; Veldhuis Kroeze, E.J.; van Amerongen, G.; Dijkshoorn, M.L.; Krestin, G.P.; Hinkula, J.; Arwidsson, H.; Lindberg, A.; Osterhaus, A.D. Intranasally administered endocine formulated 2009 pandemic influenza H1N1 vaccine induces broad specific antibody responses and confers protection in ferrets. Vaccine 2014, 32, 3307–3315. [Google Scholar] [CrossRef] [PubMed]
- Brekke, K.; Lind, A.; Holm-Hansen, C.; Haugen, I.L.; Sorensen, B.; Sommerfelt, M.; Kvale, D. Intranasal administration of a therapeutic hiv vaccine (VACC-4x) induces dose-dependent systemic and mucosal immune responses in a randomized controlled trial. PLoS ONE 2014, 9, e112556. [Google Scholar] [CrossRef] [PubMed]
- Illum, L.; Jabbal-Gill, I.; Hinchcliffe, M.; Fisher, A.N.; Davis, S.S. Chitosan as a novel nasal delivery system for vaccines. Adv. Drug Deliv. Rev. 2001, 51, 81–96. [Google Scholar] [CrossRef]
- Ryan, E.J.; Daly, L.M.; Mills, K.H.G. Immunomodulators and delivery systems for vaccination by mucosal routes. Trends Biotechnol. 2001, 19, 293–304. [Google Scholar] [CrossRef]
- Gherardi, M.M.; Najera, J.L.; Perez-Jimenez, E.; Guerra, S.; Garcia-Sastre, A.; Esteban, M. Prime-boost immunization schedules based on influenza virus and vaccinia virus vectors potentiate cellular immune responses against human immunodeficiency virus env protein systemically and in the genitorectal draining lymph nodes. J. Virol. 2003, 77, 7048–7057. [Google Scholar] [CrossRef] [PubMed]
- Fujkuyama, Y.; Tokuhara, D.; Kataoka, K.; Gilbert, R.S.; McGhee, J.R.; Yuki, Y.; Kiyono, H.; Fujihashi, K. Novel vaccine development strategies for inducing mucosal immunity. Expert Rev. Vaccines 2012, 11, 367–379. [Google Scholar] [CrossRef] [PubMed]
- Seavey, M.M.; Mosmann, T.R. Estradiol-induced vaginal mucus inhibits antigen penetration and CD8(+) t cell priming in response to intravaginal immunization. Vaccine 2009, 27, 2342–2349. [Google Scholar] [CrossRef] [PubMed]
- Wira, C.R.; Fahey, J.V.; Ghosh, M.; Patel, M.V.; Hickey, D.K.; Ochiel, D.O. Sex hormone regulation of innate immunity in the female reproductive tract: The role of epithelial cells in balancing reproductive potential with protection against sexually transmitted pathogens. Am. J. Reprod. Immunol. 2010, 63, 544–565. [Google Scholar] [CrossRef] [PubMed]
- Marks, E.; Helgeby, A.; Andersson, J.O.; Schön, K.; Lycke, N.Y. Cd4+ T-cell immunity in the female genital tract is critically dependent on local mucosal immunization. Eur. J. Immunol. 2011, 41, 2642–2653. [Google Scholar] [CrossRef] [PubMed]
- Pettini, E.; Prota, G.; Ciabattini, A.; Boianelli, A.; Fiorino, F.; Pozzi, G.; Vicino, A.; Medaglini, D. Vaginal immunization to elicit primary t-cell activation and dissemination. PLoS ONE 2013, 8, e80545. [Google Scholar] [CrossRef] [PubMed]
- Uehling, D.T.; Hopkins, W.J.; James, L.J.; Balish, E. Vaginal immunization of monkeys against urinary tract infection with a multi-strain vaccine. J. Urol. 1994, 151, 214–216. [Google Scholar] [CrossRef]
- Hopkins, W.J.; Elkahwaji, J.; Beierle, L.M.; Leverson, G.E.; Uehling, D.T. Vaginal mucosal vaccine for recurrent urinary tract infections in women: Results of a phase 2 clinical trial. J. Urol. 2007, 177, 1349–1353. [Google Scholar] [CrossRef] [PubMed]
- McKay, P.F.; Mann, J.F.S.; Pattani, A.; Kett, V.; Aldon, Y.; King, D.; Malcolm, R.K.; Shattock, R.J. Intravaginal immunisation using a novel antigen-releasing ring device elicits robust vaccine antigen-specific systemic and mucosal humoral immune responses. J. Control. Release 2017, 249, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Lewis, D.J.; Wang, Y.; Huo, Z.; Giemza, R.; Babaahmady, K.; Rahman, D.; Shattock, R.J.; Singh, M.; Lehner, T. Effect of vaginal immunization with HIVGP140 and HSP70 on HIV-1 replication and innate and t cell adaptive immunity in women. J. Virol. 2014, 88, 11648–11657. [Google Scholar] [CrossRef] [PubMed]
- Parez, N.; Fourgeux, C.; Mohamed, A.; Dubuquoy, C.; Pillot, M.; Dehee, A.; Charpilienne, A.; Poncet, D.; Schwartz-Cornil, I.; Garbarg-Chenon, A. Rectal immunization with rotavirus virus-like particles induces systemic and mucosal humoral immune responses and protects mice against rotavirus infection. J. Virol. 2006, 80, 1752–1761. [Google Scholar] [CrossRef] [PubMed]
- Kozlowski, P.A.; Cu-Uvin, S.; Neutra, M.R.; Flanigan, T.P. Comparison of the oral, rectal, and vaginal immunization routes for induction of antibodies in rectal and genital tract secretions of women. Infect. Immun. 1997, 65, 1387–1394. [Google Scholar] [PubMed]
- Williams, J.; Fox-Leyva, L.; Christensen, C.; Fisher, D.; Schlicting, E.; Snowball, M.; Negus, S.; Mayers, J.; Koller, R.; Stout, R. Hepatitis a vaccine administration: Comparison between jet-injector and needle injection. Vaccine 2000, 18, 1939–1943. [Google Scholar] [CrossRef]
- Chen, D.; Maa, Y.F.; Haynes, J.R. Needle-free epidermal powder immunization. Expert Rev. Vaccines 2002, 1, 265–276. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Erickson, C.A.; Endres, R.L.; Periwal, S.B.; Chu, Q.; Shu, C.; Maa, Y.F.; Payne, L.G. Adjuvantation of epidermal powder immunization. Vaccine 2001, 19, 2908–2917. [Google Scholar] [CrossRef]
- Dean, H.J.; Chen, D. Epidermal powder immunization against influenza. Vaccine 2004, 23, 681–686. [Google Scholar] [CrossRef] [PubMed]
- Roy, M.J.; Wu, M.S.; Barr, L.J.; Fuller, J.T.; Tussey, L.G.; Speller, S.; Culp, J.; Burkholder, J.K.; Swain, W.F.; Dixon, R.M.; et al. Induction of antigen-specific CD8+ t cells, t helper cells, and protective levels of antibody in humans by particle-mediated administration of a hepatitis B virus DNA vaccine. Vaccine 2000, 19, 764–778. [Google Scholar] [CrossRef]
- Roberts, L.K.; Barr, L.J.; Fuller, D.H.; McMahon, C.W.; Leese, P.T.; Jones, S. Clinical safety and efficacy of a powdered hepatitis B nucleic acid vaccine delivered to the epidermis by a commercial prototype device. Vaccine 2005, 23, 4867–4878. [Google Scholar] [CrossRef] [PubMed]
- Drape, R.J.; Macklin, M.D.; Barr, L.J.; Jones, S.; Haynes, J.R.; Dean, H.J. Epidermal DNA vaccine for influenza is immunogenic in humans. Vaccine 2006, 24, 4475–4481. [Google Scholar] [CrossRef] [PubMed]
- DeMuth, P.C.; Li, A.V.; Abbink, P.; Liu, J.; Li, H.; Stanley, K.A.; Smith, K.M.; Lavine, C.L.; Seaman, M.S.; Kramer, J.A.; et al. Vaccine delivery with microneedle skin patches in nonhuman primates. Nat. Biotechnol. 2013, 31, 1082–1085. [Google Scholar] [CrossRef] [PubMed]
- Suh, H.; Shin, J.; Kim, Y.C. Microneedle patches for vaccine delivery. Clin. Exp. Vaccine Res. 2014, 3, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.C.; Quan, F.S.; Compans, R.W.; Kang, S.M.; Prausnitz, M.R. Formulation and coating of microneedles with inactivated influenza virus to improve vaccine stability and immunogenicity. J. Control. Release 2010, 142, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Matriano, J.A.; Cormier, M.; Johnson, J.; Young, W.A.; Buttery, M.; Nyam, K.; Daddona, P.E. Macroflux microprojection array patch technology: A new and efficient approach for intracutaneous immunization. Pharm. Res. 2002, 19, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Widera, G.; Johnson, J.; Kim, L.; Libiran, L.; Nyam, K.; Daddona, P.E.; Cormier, M. Effect of delivery parameters on immunization to ovalbumin following intracutaneous administration by a coated microneedle array patch system. Vaccine 2006, 24, 1653–1664. [Google Scholar] [CrossRef] [PubMed]
- Hiraishi, Y.; Nandakumar, S.; Choi, S.O.; Lee, J.W.; Kim, Y.C.; Posey, J.E.; Sable, S.B.; Prausnitz, M.R. Bacillus calmette-guerin vaccination using a microneedle patch. Vaccine 2011, 29, 2626–2636. [Google Scholar] [CrossRef] [PubMed]
- Gill, H.S.; Soderholm, J.; Prausnitz, M.R.; Sallberg, M. Cutaneous vaccination using microneedles coated with hepatitis c DNA vaccine. Gene Ther. 2010, 17, 811–814. [Google Scholar] [CrossRef] [PubMed]
- Moon, S.; Wang, Y.H.; Edens, C.; Gentsch, J.R.; Prausnitz, M.R.; Jiang, B.M. Dose sparing and enhanced immunogenicity of inactivated rotavirus vaccine administered by skin vaccination using a microneedle patch. Vaccine 2013, 31, 3396–3402. [Google Scholar] [CrossRef] [PubMed]
- Rouphael, N.G.; Paine, M.; Mosley, R.; Henry, S.; McAllister, D.V.; Kalluri, H.; Pewin, W.; Frew, P.M.; Yu, T.; Thornburg, N.J.; et al. The safety, immunogenicity, and acceptability of inactivated influenza vaccine delivered by microneedle patch (TIV-MNP 2015): A randomised, partly blinded, placebo-controlled, phase 1 trial. Lancet 2017, 390, 649–658. [Google Scholar] [CrossRef]
- Vassilieva, E.V.; Kalluri, H.; McAllister, D.; Taherbhai, M.T.; Esser, E.S.; Pewin, W.P.; Pulit-Penaloza, J.A.; Prausnitz, M.R.; Compans, R.W.; Skountzou, I. Improved immunogenicity of individual influenza vaccine components delivered with a novel dissolving microneedle patch stable at room temperature. Drug Deliv. Transl. Res. 2015, 5, 360–371. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.C.; Huang, S.F.; Lai, K.Y.; Ling, M.H. Fully embeddable chitosan microneedles as a sustained release depot for intradermal vaccination. Biomaterials 2013, 34, 3077–3086. [Google Scholar] [CrossRef] [PubMed]
- Van der Maaden, K.; Sekerdag, E.; Schipper, P.; Kersten, G.; Jiskoot, W.; Bouwstra, J. Layer-by-layer assembly of inactivated poliovirus and n-trimethyl chitosan on ph-sensitive microneedles for dermal vaccination. Langmuir ACS J. Surf. Colloids 2015, 31, 8654–8660. [Google Scholar] [CrossRef] [PubMed]
- Chiu, Y.-H.; Chen, M.-C.; Wan, S.-W. Sodium hyaluronate/chitosan composite microneedles as a single-dose intradermal immunization system. Biomacromolecules 2018, 19, 2278–2285. [Google Scholar] [CrossRef] [PubMed]
- Singla, A.K.; Chawla, M. Chitosan: Some pharmaceutical and biological aspects—An update. J. Pharm. Pharmacol. 2001, 53, 1047–1067. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, K.; Nishimura, S.; Nishi, N.; Saiki, I.; Tokura, S.; Azuma, I. Immunological activity of chitin and its derivatives. Vaccine 1984, 2, 93–99. [Google Scholar] [CrossRef]
- Nishimura, K.; Nishimura, S.; Nishi, N.; Numata, F.; Tone, Y.; Tokura, S.; Azuma, I. Adjuvant activity of chitin derivatives in mice and guinea-pigs. Vaccine 1985, 3, 379–384. [Google Scholar] [CrossRef]
- Bernkop-Schnurch, A.; Guggi, D.; Pinter, Y. Thiolated chitosans: Development and in vitro evaluation of a mucoadhesive, permeation enhancing oral drug delivery system. J. Control. Release 2004, 94, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Roldo, M.; Hornof, M.; Caliceti, P.; Bernkop-Schnurch, A. Mucoadhesive thiolated chitosans as platforms for oral controlled drug delivery: Synthesis and in vitro evaluation. Eur. J. Pharm. Biopharm. 2004, 57, 115–121. [Google Scholar] [CrossRef]
- Holme, K.R.; Perlin, A.S. Chitosan N-sulfate. A water-soluble polyelectrolyte. Carbohydr. Res. 1997, 302, 7–12. [Google Scholar] [CrossRef]
- Kurita, K. Chitin and chitosan: Functional biopolymers from marine crustaceans. Mar. Biotechnol. 2006, 8, 203–226. [Google Scholar] [CrossRef] [PubMed]
- Sashiwa, H.; Kawasaki, N.; Nakayama, A.; Muraki, E.; Yajima, H.; Yamamori, N.; Ichinose, Y.; Sunamoto, J.; Aiba, S. Chemical modification of chitosan. Part 15: Synthesis of novel chitosan derivatives by substitution of hydrophilic amine using N-carboxyethylchitosan ethyl ester as an intermediate. Carbohydr. Res. 2003, 338, 557–561. [Google Scholar] [CrossRef]
- Abreu, F.R.D.; Campana-Filho, S.P. Preparation and characterization of carboxymethylchitosan. Polímeros 2005, 15, 79–83. [Google Scholar] [CrossRef]
- Kato, Y.; Onishi, H.; Machida, Y. N-succinyl-chitosan as a drug carrier: Water-insoluble and water-soluble conjugates. Biomaterials 2004, 25, 907–915. [Google Scholar] [CrossRef]
- Kang, M.L.; Jiang, H.L.; Kang, S.G.; Guo, D.D.; Lee, D.Y.; Cho, C.S.; Yoo, H.S. Pluronic f127 enhances the effect as an adjuvant of chitosan microspheres in the intranasal delivery of bordetella bronchiseptica antigens containing dermonecrotoxin. Vaccine 2007, 25, 4602–4610. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.-L.; Park, I.-K.; Kang, M.-L.; Yoo, H.-S.; Choi, Y.-J.; Akaike, T.; Cho, C.-S. Immune stimulating activity of an atrophic rhinitis vaccine associated to pegylated chitosan microspheres in vitro. Polym. Adv. Technol. 2007, 18, 220–225. [Google Scholar] [CrossRef]
- Kim, C.H.; Choi, J.W.; Chun, H.J.; Choi, K.S. Synthesis of chitosan derivatives with quaternary ammonium salt and their antibacterial activity. Polym. Bull. 1997, 38, 387–393. [Google Scholar] [CrossRef]
- Andrianov, A.K.; Payne, L.G. Polymeric carriers for oral uptake of microparticulates. Adv. Drug Deliv. Rev. 1998, 34, 155–170. [Google Scholar] [CrossRef]
- Des Rieux, A.; Fievez, V.; Garinot, M.; Schneider, Y.J.; Preat, V. Nanoparticles as potential oral delivery systems of proteins and vaccines: A mechanistic approach. J. Control. Release 2006, 116, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Mao, S.; Bakowsky, U.; Jintapattanakit, A.; Kissel, T. Self-assembled polyelectrolyte nanocomplexes between chitosan derivatives and insulin. J. Pharm. Sci. 2006, 95, 1035–1048. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Du, Y.; Huang, R.; Gao, L. Preparation and modification of N-(2-hydroxyl)propyl-3-trimethyl ammonium chitosan chloride nanoparticle as a protein carrier. Biomaterials 2003, 24, 5015–5022. [Google Scholar] [CrossRef]
- Calvo, P.; Remunan-Lopez, C.; Vila-Jato, J.L.; Alonso, M. Novel hydrophilic chitosan-polyethylene oxide nanoparticles as protein carriers. J. Appl. Polym. Sci. 1997, 63, 125–132. [Google Scholar] [CrossRef]
- Vila, A.; Sanchez, A.; Janes, K.; Behrens, I.; Kissel, T.; Vila Jato, J.L.; Alonso, M.J. Low molecular weight chitosan nanoparticles as new carriers for nasal vaccine delivery in mice. Eur. J. Pharm. Biopharm. 2004, 57, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Amidi, M.; Romeijn, S.G.; Verhoef, J.C.; Junginger, H.E.; Bungener, L.; Huckriede, A.; Crommelin, D.J.A.; Jiskoot, W. N-trimethyl chitosan (TMC) nanoparticles loaded with influenza subunit antigen for intranasal vaccination: Biological properties and immunogenicity in a mouse model. Vaccine 2007, 25, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Van der Lubben, I.M.; Verhoef, J.C.; van Aelst, A.C.; Borchard, G.; Junginger, H.E. Chitosan microparticles for oral vaccination: Preparation, characterization and preliminary in vivo uptake studies in murine peyer’s patches. Biomaterials 2001, 22, 687–694. [Google Scholar] [CrossRef]
- Slutter, B.; Soema, P.C.; Ding, Z.; Verheul, R.; Hennink, W.; Jiskoot, W. Conjugation of ovalbumin to trimethyl chitosan improves immunogenicity of the antigen. J. Control. Release 2010, 143, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Slutter, B.; Bal, S.M.; Que, I.; Kaijzel, E.; Lowik, C.; Bouwstra, J.; Jiskoot, W. Antigen-adjuvant nanoconjugates for nasal vaccination: An improvement over the use of nanoparticles? Mol. Pharm. 2010, 7, 2207–2215. [Google Scholar] [CrossRef] [PubMed]
- Westerink, M.A.; Smithson, S.L.; Srivastava, N.; Blonder, J.; Coeshott, C.; Rosenthal, G.J. Projuvant (pluronic f127/chitosan) enhances the immune response to intranasally administered tetanus toxoid. Vaccine 2001, 20, 711–723. [Google Scholar] [CrossRef]
- Hori, M.; Onishi, H.; Machida, Y. Evaluation of eudragit-coated chitosan microparticles as an oral immune delivery system. Int. J. Pharm. 2005, 297, 223–234. [Google Scholar] [CrossRef] [PubMed]
- Quan, J.S.; Jiang, H.L.; Kim, E.M.; Jeong, H.J.; Choi, Y.J.; Guo, D.D.; Yoo, M.K.; Lee, H.G.; Cho, C.S. Ph-sensitive and mucoadhesive thiolated eudragit-coated chitosan microspheres. Int. J. Pharm. 2008, 359, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Farhadian, A.; Dounighi, N.M.; Avadi, M. Enteric trimethyl chitosan nanoparticles containing hepatitis B surface antigen for oral delivery. Hum. Vaccines Immunother. 2015, 11, 2811–2818. [Google Scholar] [CrossRef] [PubMed]
- Pawar, D.; Jaganathan, K.S. Mucoadhesive glycol chitosan nanoparticles for intranasal delivery of hepatitis B vaccine: Enhancement of mucosal and systemic immune response. Drug Deliv. 2016, 23, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Pawar, D.; Mangal, S.; Goswami, R.; Jaganathan, K.S. Development and characterization of surface modified plga nanoparticles for nasal vaccine delivery: Effect of mucoadhesive coating on antigen uptake and immune adjuvant activity. Eur. J. Pharm. Biopharm. 2013, 85, 550–559. [Google Scholar] [CrossRef] [PubMed]
- Phanse, Y.; Carrillo-Conde, B.R.; Ramer-Tait, A.E.; Roychoudhury, R.; Pohl, N.L.; Narasimhan, B.; Wannemuehler, M.J.; Bellaire, B.H. Functionalization of polyanhydride microparticles with di-mannose influences uptake by and intracellular fate within dendritic cells. Acta Biomater. 2013, 9, 8902–8909. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.L.; Kang, M.L.; Quan, J.S.; Kang, S.G.; Akaike, T.; Yoo, H.S.; Cho, C.S. The potential of mannosylated chitosan microspheres to target macrophage mannose receptors in an adjuvant-delivery system for intranasal immunization. Biomaterials 2008, 29, 1931–1939. [Google Scholar] [CrossRef] [PubMed]
- Cruz, L.J.; Tacken, P.J.; Pots, J.M.; Torensma, R.; Buschow, S.I.; Figdor, C.G. Comparison of antibodies and carbohydrates to target vaccines to human dendritic cells via dc-sign. Biomaterials 2012, 33, 4229–4239. [Google Scholar] [CrossRef] [PubMed]
- Cruz, L.J.; Tacken, P.J.; Fokkink, R.; Figdor, C.G. The influence of peg chain length and targeting moiety on antibody-mediated delivery of nanoparticle vaccines to human dendritic cells. Biomaterials 2011, 32, 6791–6803. [Google Scholar] [CrossRef] [PubMed]
- Apostolopoulos, V.; Thalhammer, T.; Tzakos, A.G.; Stojanovska, L. Targeting antigens to dendritic cell receptors for vaccine development. J. Drug Deliv. 2013, 2013, 869718. [Google Scholar] [CrossRef] [PubMed]
- Macri, C.; Dumont, C.; Johnston, A.P.; Mintern, J.D. Targeting dendritic cells: A promising strategy to improve vaccine effectiveness. Clin. Transl. Immunol. 2016, 5, e66. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.N.; Kang, S.K.; Yeo, G.H.; Li, H.Y.; Jiang, T.; Nah, J.W.; Bok, J.D.; Cho, C.S.; Choi, Y.J. Targeted delivery of vaccine to dendritic cells by chitosan nanoparticles conjugated with a targeting peptide ligand selected by phage display technique. Macromol. Biosci. 2015, 15, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Park, T.-E.; Singh, B.; Maharjan, S.; Jiang, T.; Yoon, S.-Y.; Kang, S.-K.; Bok, J.-D.; Choi, Y.-J.; Cho, C.-S. Mucosal delivery of vaccine by m cell targeting strategies. Curr. Drug Ther. 2014, 9, 9–20. [Google Scholar] [CrossRef]
- Lambkin, I.; Pinilla, C.; Hamashin, C.; Spindler, L.; Russell, S.; Schink, A.; Moya-Castro, R.; Allicotti, G.; Higgins, L.; Smith, M.; et al. Toward targeted oral vaccine delivery systems: Selection of lectin mimetics from combinatorial libraries. Pharm. Res. 2003, 20, 1258–1266. [Google Scholar] [CrossRef] [PubMed]
- Clark, M.A.; Jepson, M.A.; Simmons, N.L.; Booth, T.A.; Hirst, B.H. Differential expression of lectin-binding sites defines mouse intestinal m-cells. J. Histochem. Cytochem. 1993, 41, 1679–1687. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.N.; Khatri, K.; Goyal, A.K.; Mishra, N.; Vyas, S.P. M-cell targeted biodegradable plga nanoparticles for oral immunization against hepatitis B. J. Drug Target. 2007, 15, 701–713. [Google Scholar] [CrossRef] [PubMed]
- Malik, B.; Goyal, A.K.; Markandeywar, T.S.; Rath, G.; Zakir, F.; Vyas, S.P. Microfold-cell targeted surface engineered polymeric nanoparticles for oral immunization. J. Drug Target. 2012, 20, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Higgins, L.M.; Lambkin, I.; Donnelly, G.; Byrne, D.; Wilson, C.; Dee, J.; Smith, M.; O’Mahony, D.J. In vivo phage display to identify m cell-targeting ligands. Pharm. Res. 2004, 21, 695–705. [Google Scholar] [CrossRef] [PubMed]
- Fievez, V.; Plapied, L.; Plaideau, C.; Legendre, D.; des Rieux, A.; Pourcelle, V.; Freichels, H.; Jerome, C.; Marchand, J.; Preat, V.; et al. In vitro identification of targeting ligands of human m cells by phage display. Int. J. Pharm. 2010, 394, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Seo, K.W.; Kim, J.; Lee, K.Y.; Jang, Y.S. The m cell-targeting ligand promotes antigen delivery and induces antigen-specific immune responses in mucosal vaccination. J. Immunol. 2010, 185, 5787–5795. [Google Scholar] [CrossRef] [PubMed]
- Yoo, M.K.; Kang, S.K.; Choi, J.H.; Park, I.K.; Na, H.S.; Lee, H.C.; Kim, E.B.; Lee, N.K.; Nah, J.W.; Choi, Y.J.; et al. Targeted delivery of chitosan nanoparticles to peyer’s patch using m cell-homing peptide selected by phage display technique. Biomaterials 2010, 31, 7738–7747. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Singh, B.; Li, H.S.; Kim, Y.K.; Kang, S.K.; Nah, J.W.; Choi, Y.J.; Cho, C.S. Targeted oral delivery of bmpb vaccine using porous plga microparticles coated with m cell homing peptide-coupled chitosan. Biomaterials 2014, 35, 2365–2373. [Google Scholar] [CrossRef] [PubMed]
- Vogel, F.R. Immunologic adjuvants for modern vaccine formulations. Ann. N. Y. Acad. Sci. 1995, 754, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Van der Lubben, I.M.; Verhoef, J.C.; Borchard, G.; Junginger, H.E. Chitosan and its derivatives in mucosal drug and vaccine delivery. Eur. J. Pharm. Sci. 2001, 14, 201–207. [Google Scholar] [CrossRef]
- Sivakumar, S.M.; Sukumaran, N.; Nirmala, L.; Swarnalakshmi, R.; Anilbabu, B.; Siva, L.; Anbu, J.; Shanmugarajan, T.S.; Ravichandran, V. Immunopotentiation of hepatitis B vaccine using biodegradable polymers as an adjuvant. J. Microbiol. Immunol. 2010, 43, 265–270. [Google Scholar] [CrossRef]
- Wang, Z.-B.; Shan, P.; Li, S.-Z.; Zhou, Y.; Deng, X.; Li, J.-L.; Zhang, Y.; Gao, J.-S.; Xu, J. The mechanism of action of acid-soluble chitosan as an adjuvant in the formulation of nasally administered vaccine against hbv. RSC Adv. 2016, 6, 96785–96797. [Google Scholar] [CrossRef]
- Chen, F.; Zhang, Z.R.; Yuan, F.; Qin, X.; Wang, M.; Huang, Y. In vitro and in vivo study of N-trimethyl chitosan nanoparticles for oral protein delivery. Int. J. Pharm. 2008, 349, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Qu, D.; Wang, H.; Sun, Z.; Liu, X.; Chen, J.; Li, C.; Li, X.; Chen, Z. Intranasal administration of chitosan against influenza a (H7N9) virus infection in a mouse model. Sci. Rep. 2016, 6, 28729. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, K.; Ishihara, C.; Ukei, S.; Tokura, S.; Azuma, I. Stimulation of cytokine production in mice using deacetylated chitin. Vaccine 1986, 4, 151–156. [Google Scholar] [CrossRef]
- Choi, B.; Jo, D.H.; Anower, A.K.; Islam, S.M.; Sohn, S. Chitosan as an immunomodulating adjuvant on t-cells and antigen-presenting cells in herpes simplex virus type 1 infection. Mediat. Inflamm. 2016, 2016, 4374375. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Tao, L.; Wang, F.; Liu, W.; Jing, L.; Liu, D.; Hu, S.; Xie, Y.; Zhou, N. Chitosan as an adjuvant for a helicobacter pylori therapeutic vaccine. Mol. Med. Rep. 2015, 12, 4123–4132. [Google Scholar] [CrossRef] [PubMed]
- Sui, Z.; Chen, Q.; Fang, F.; Zheng, M.; Chen, Z. Cross-protection against influenza virus infection by intranasal administration of m1-based vaccine with chitosan as an adjuvant. Vaccine 2010, 28, 7690–7698. [Google Scholar] [CrossRef] [PubMed]
- Bal, S.M.; Ding, Z.; Kersten, G.F.A.; Jiskoot, W.; Bouwstra, J.A. Microneedle-based transcutaneous immunisation in mice with n-trimethyl chitosan adjuvanted diphtheria toxoid formulations. Pharm. Res. 2010, 27, 1837–1847. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.-C.; Lai, K.-Y.; Ling, M.-H.; Lin, C.-W. Enhancing immunogenicity of antigens through sustained intradermal delivery using chitosan microneedles with a patch-dissolvable design. Acta Biomater. 2018, 65, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Arca, H.Ç.; Günbeyaz, M.; Şenel, S. Chitosan-based systems for the delivery of vaccine antigens. Expert Rev. Vaccines 2009, 8, 937–953. [Google Scholar] [CrossRef] [PubMed]
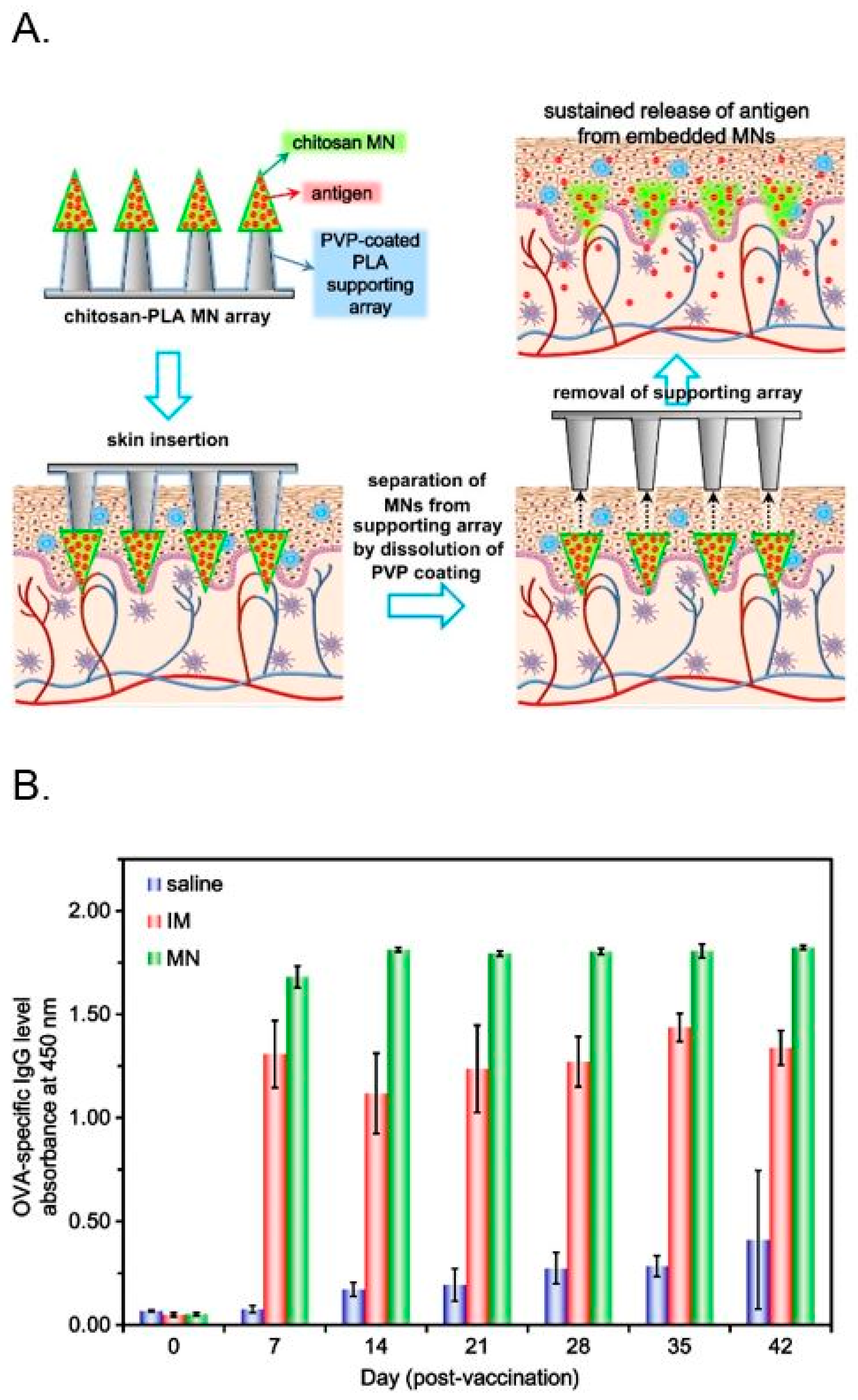
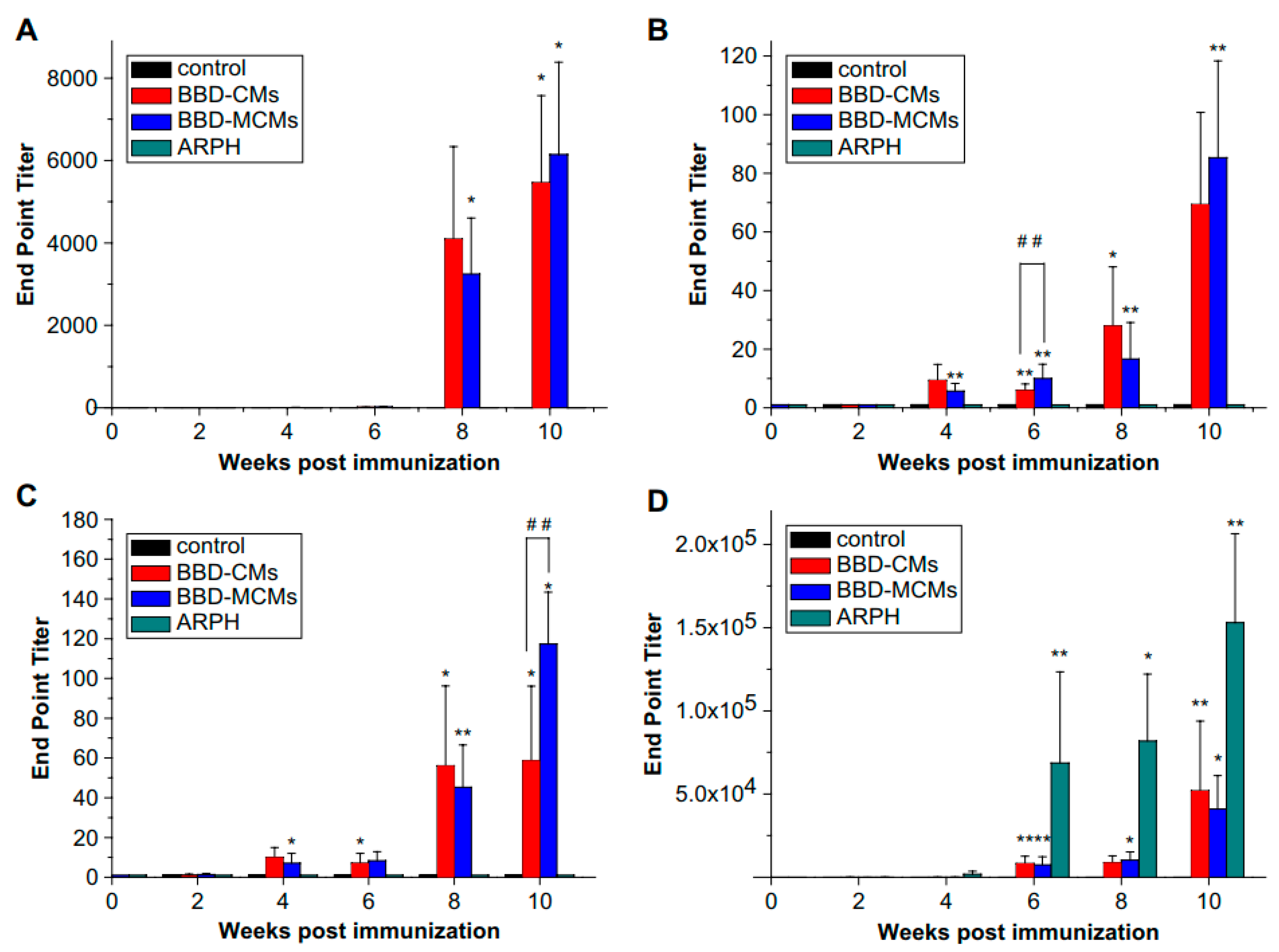
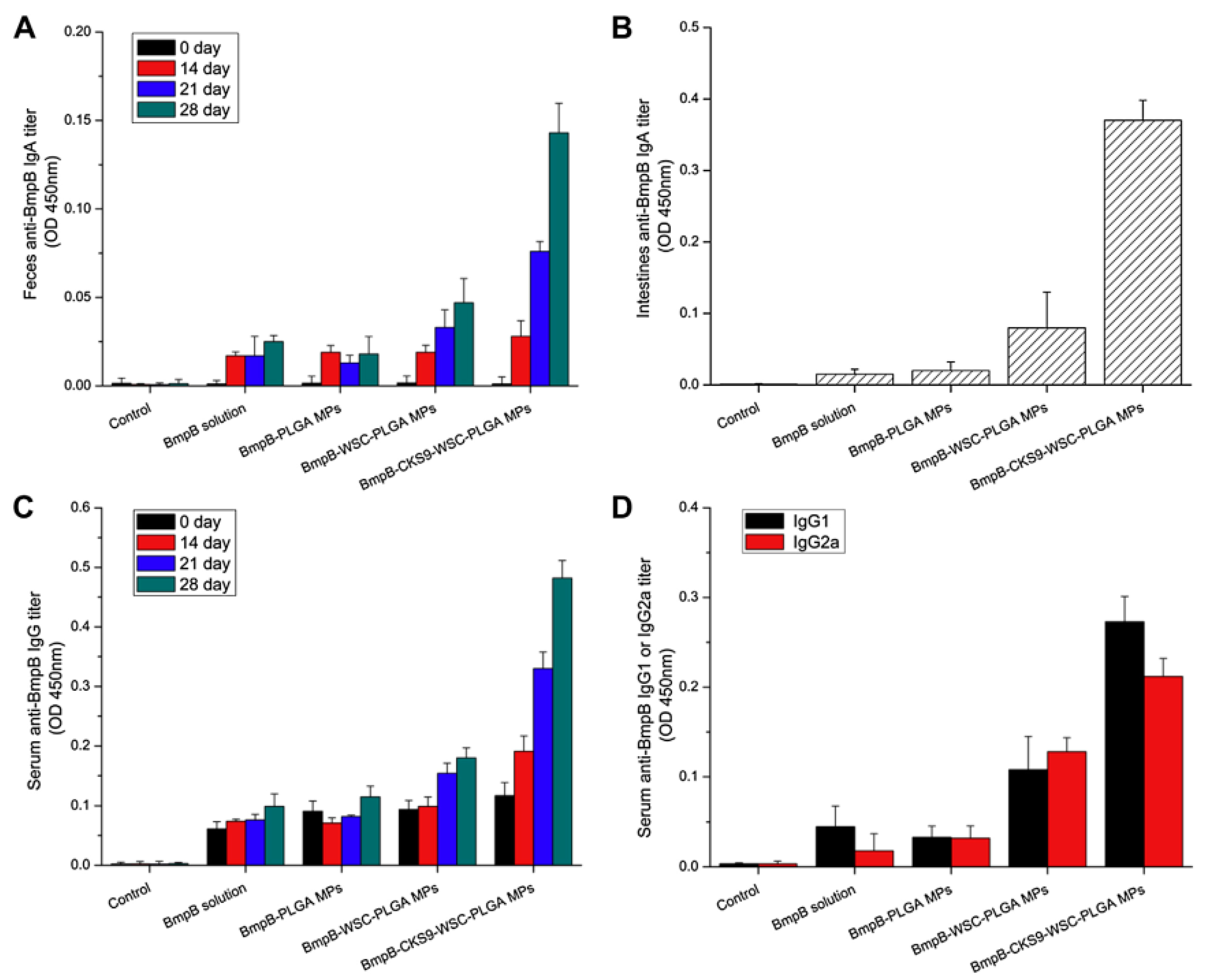
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, B.; Maharjan, S.; Sindurakar, P.; Cho, K.-H.; Choi, Y.-J.; Cho, C.-S. Needle-Free Immunization with Chitosan-Based Systems. Int. J. Mol. Sci. 2018, 19, 3639. https://doi.org/10.3390/ijms19113639
Singh B, Maharjan S, Sindurakar P, Cho K-H, Choi Y-J, Cho C-S. Needle-Free Immunization with Chitosan-Based Systems. International Journal of Molecular Sciences. 2018; 19(11):3639. https://doi.org/10.3390/ijms19113639
Chicago/Turabian StyleSingh, Bijay, Sushila Maharjan, Princy Sindurakar, Ki-Hyun Cho, Yun-Jaie Choi, and Chong-Su Cho. 2018. "Needle-Free Immunization with Chitosan-Based Systems" International Journal of Molecular Sciences 19, no. 11: 3639. https://doi.org/10.3390/ijms19113639
APA StyleSingh, B., Maharjan, S., Sindurakar, P., Cho, K.-H., Choi, Y.-J., & Cho, C.-S. (2018). Needle-Free Immunization with Chitosan-Based Systems. International Journal of Molecular Sciences, 19(11), 3639. https://doi.org/10.3390/ijms19113639




