The Protective and Restorative Effects of Growth Hormone and Insulin-Like Growth Factor-1 on Methadone-Induced Toxicity In Vitro
Abstract
1. Introduction
2. Results
2.1. High Concentrations of rhGH, but Not IGF-1, Stabilize Membrane Integrity in Untreated Cells
2.2. rhGH and IGF-1 Treatment Do Not Alter Mitochondrial Function in Untreated Cells
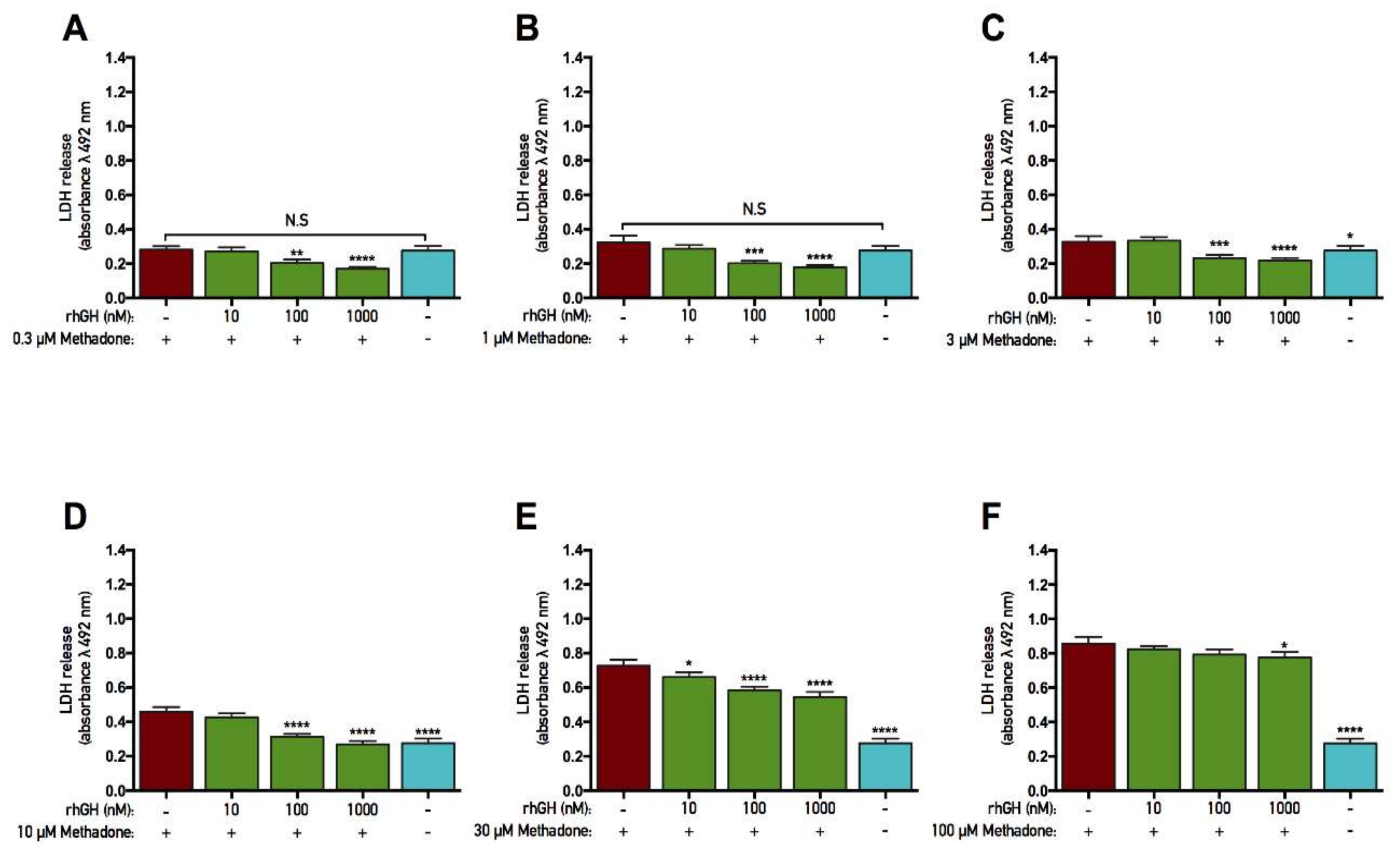
2.3. rhGH, unlike IGF-1, Is Protective Against Methadone-Induced Membrane Damage
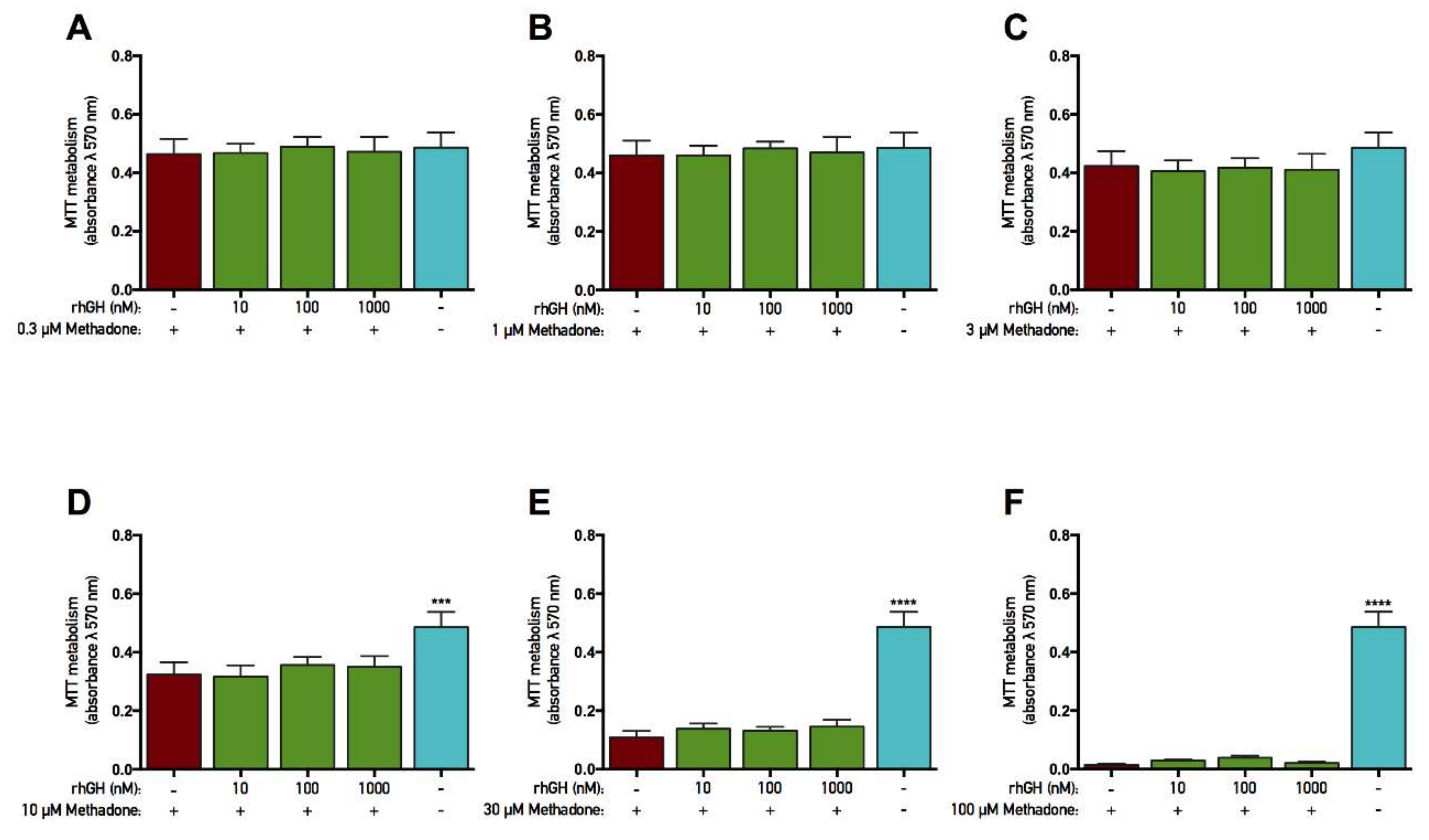
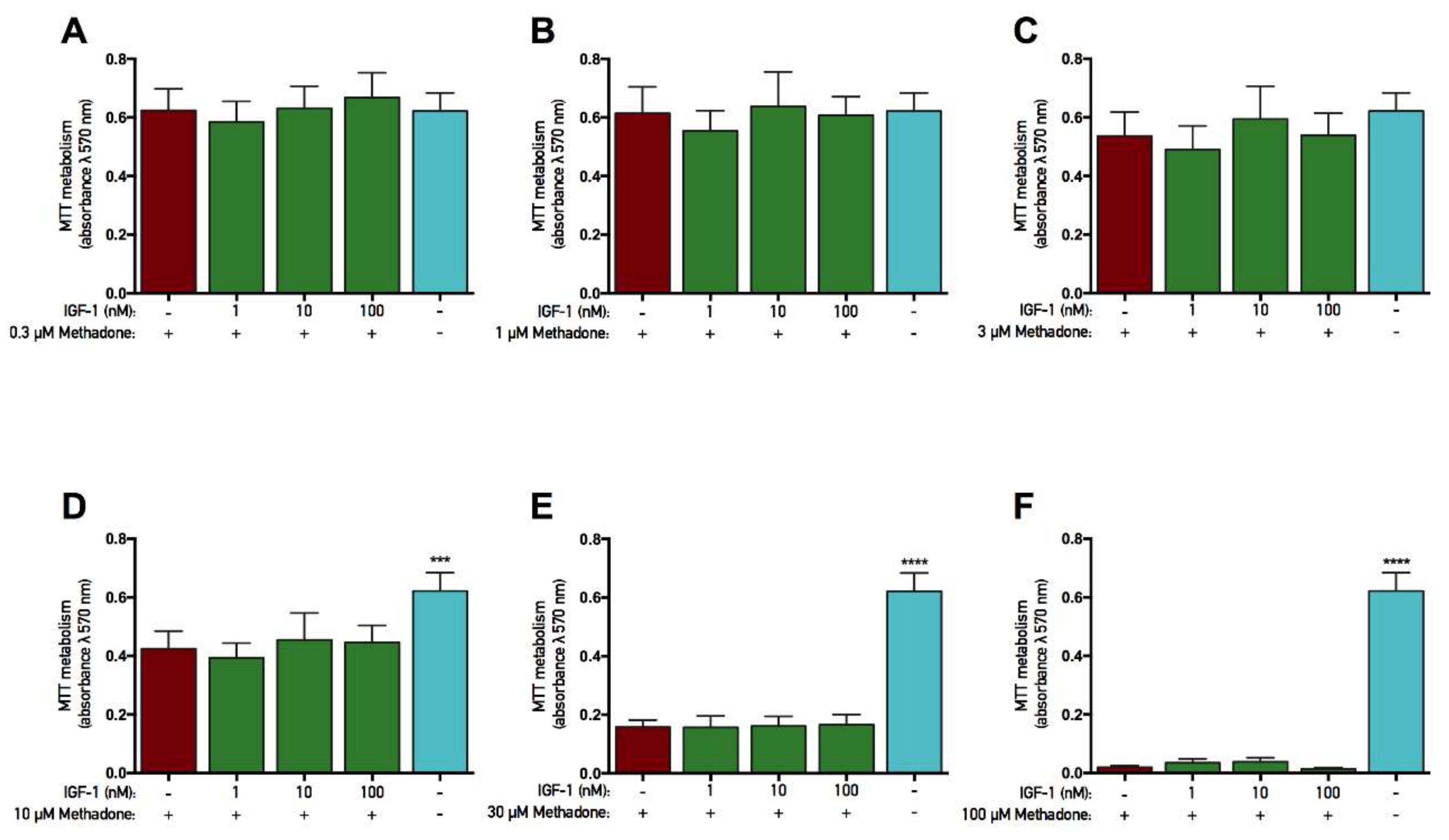
2.4. rhGH and IGF-1 Are Not Protective Against Methadone-Induced Mitochondrial Dysfunction
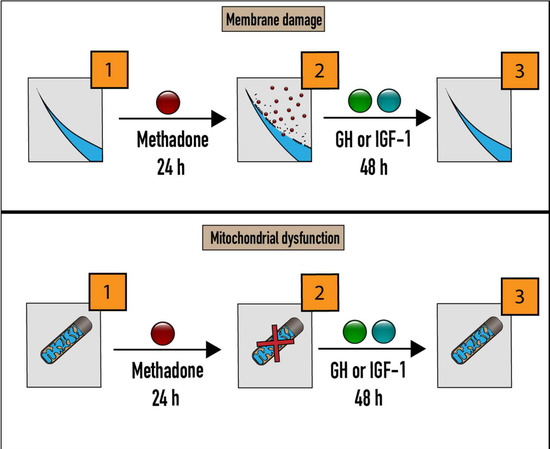
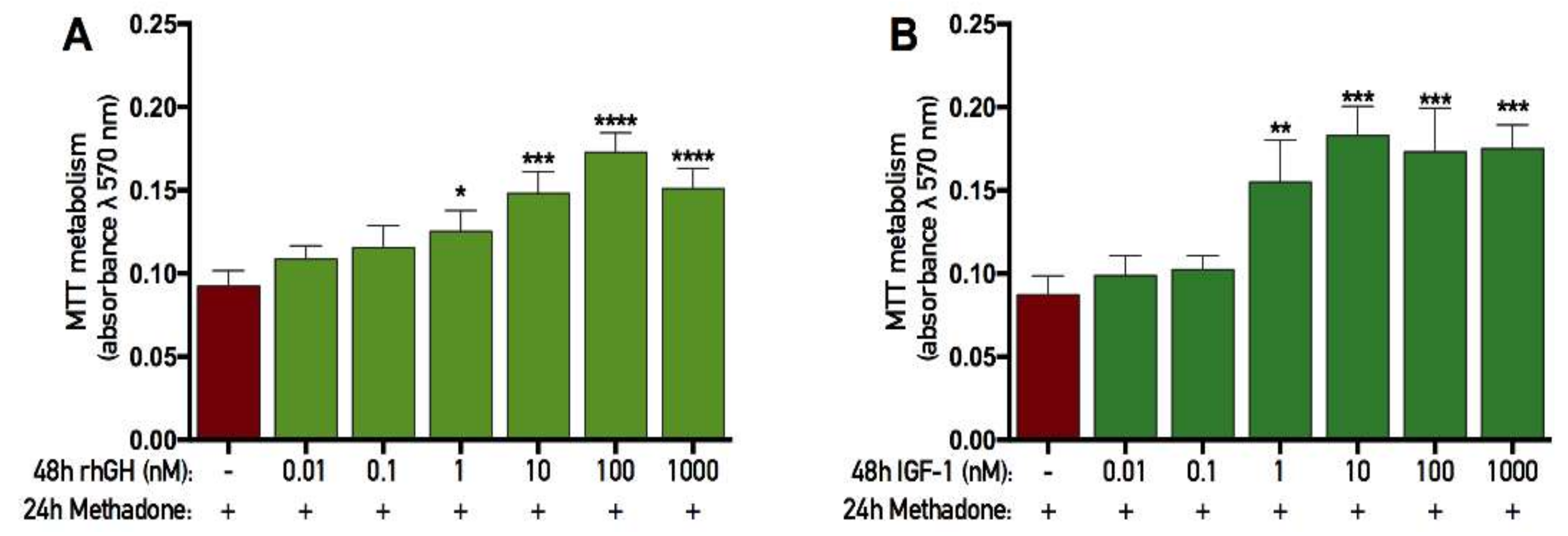
2.5. rhGH and IGF-1 Restore Mitochondria Function Following Acute Methadone Treatment
2.6. rhGH and IGF-1 Restore Membrane Integrity Following Acute Methadone Treatment
3. Discussion
4. Materials and Methods
4.1. Primary Cell Cultures
4.2. Treatments
4.2.1. GH and IGF-1 Treatment
4.2.2. GH/IGF-1 and Protection Experiments
4.2.3. GH/IGF-1 and Recovery Experiments
4.3. Mitochondrial Function
4.4. Lactate Dehydrogenase
4.5. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ANOVA | Analysis of variance |
| CNS | Central nervous system |
| DIV | Day(s) in vitro |
| E17 | Embryonic day 17 |
| GH | Growth hormone |
| IGF-1 | Insulin-like growth factor-1 |
| LDH | Lactate dehydrogenase |
| LTP | Long-term potentiation |
| MTT | Tetrazolium bromide |
| NBM | Neurobasal medium |
| NMDA | N-methyl-d-aspartate |
| rhGH | Recombinant human growth hormone |
| SEM | Standard error of the mean |
References
- Nyberg, F. Growth hormone in the brain: Characteristics of specific brain targets for the hormone and their functional significance. Front. Neuroendocrinol. 2000, 21, 330–348. [Google Scholar] [CrossRef] [PubMed]
- Nyberg, F.; Hallberg, M. Growth hormone and cognitive function. Nat. Rev. Endocrinol. 2013, 9, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Le Greves, M.; Zhou, Q.; Berg, M.; Le Greves, P.; Fholenhag, K.; Meyerson, B.; Nyberg, F. Growth hormone replacement in hypophysectomized rats affects spatial performance and hippocampal levels of NMDA receptor subunit and PSD-95 gene transcript levels. Exp. Brain Res. 2006, 173, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Ramis, M.; Sarubbo, F.; Sola, J.; Aparicio, S.; Garau, C.; Miralles, A.; Esteban, S. Cognitive improvement by acute growth hormone is mediated by NMDA and AMPA receptors and MEK pathway. Prog. Neuro.-Psychoph. 2013, 45, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Gronbladh, A.; Johansson, J.; Nostl, A.; Nyberg, F.; Hallberg, M. GH improves spatial memory and reverses certain anabolic androgenic steroid-induced effects in intact rats. J. Endocrinol. 2013, 216, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Enhamre-Brolin, E.; Carlsson, A.; Hallberg, M.; Nyberg, F. Growth hormone reverses streptozotocin-induced cognitive impairments in male mice. Behav. Brain Res. 2013, 238, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Studzinski, A.L.; Barros, D.M.; Marins, L.F. Growth hormone (GH) increases cognition and expression of ionotropic glutamate receptors (AMPA and NMDA) in transgenic zebrafish (Danio rerio). Behav. Brain Res. 2015, 294, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Heredia, M.; Fuente, A.; Criado, J.; Yajeya, J.; Devesa, J.; Riolobos, A.S. Early growth hormone (GH) treatment promotes relevant motor functional improvement after severe frontal cortex lesion in adult rats. Behav. Brain Res. 2013, 247, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Aberg, D. Role of the growth hormone/insulin-like growth factor 1 axis in neurogenesis. Endocr. Dev. 2010, 17, 63–76. [Google Scholar] [PubMed]
- Falleti, M.G.; Maruff, P.; Burman, P.; Harris, A. The effects of growth hormone (GH) deficiency and GH replacement on cognitive performance in adults: A meta-analysis of the current literature. Psychoneuroendocrinology 2006, 31, 681–691. [Google Scholar] [CrossRef] [PubMed]
- Elbornsson, M.; Horvath, A.; Gotherstrom, G.; Bengtsson, B.A.; Johannsson, G.; Svensson, J. Seven years of growth hormone (GH) replacement improves quality of life in hypopituitary patients with adult-onset GH deficiency. Eur. J. Endocrinol. 2017, 176, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Devesa, J.; Reimunde, P.; Devesa, P.; Barbera, M.; Arce, V. Growth hormone (GH) and brain trauma. Horm. Behav. 2013, 63, 331–344. [Google Scholar] [CrossRef] [PubMed]
- Devesa, J.; Lema, H.; Zas, E.; Munin, B.; Taboada, P.; Devesa, P. Learning and Memory Recoveries in a Young Girl Treated with Growth Hormone and Neurorehabilitation. J. Clin. Med. 2016, 5, 14. [Google Scholar] [CrossRef] [PubMed]
- Rhodin, A.; von Ehren, M.; Skottheim, B.; Gronbladh, A.; Ortiz-Nieto, F.; Raininko, R.; Gordh, T.; Nyberg, F. Recombinant human growth hormone improves cognitive capacity in a pain patient exposed to chronic opioids. Acta Anaesthesiol. Scand. 2014, 58, 759–765. [Google Scholar] [CrossRef] [PubMed]
- Scheepens, A.; Sirimanne, E.S.; Breier, B.H.; Clark, R.G.; Gluckman, P.D.; Williams, C.E. Growth hormone as a neuronal rescue factor during recovery from CNS injury. Neuroscience 2001, 104, 677–687. [Google Scholar] [CrossRef]
- Alba-Betancourt, C.; Luna-Acosta, J.L.; Ramirez-Martinez, C.E.; Avila-Gonzalez, D.; Granados-Avalos, E.; Carranza, M.; Martinez-Coria, H.; Aramburo, C.; Luna, M. Neuro-protective effects of growth hormone (GH) after hypoxia-ischemia injury in embryonic chicken cerebellum. Gen. Comp. Endocrinol. 2013, 183, 17–31. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Moreno, C.G.; Fleming, T.; Carranza, M.; Avila-Mendoza, J.; Luna, M.; Harvey, S.; Aramburo, C. Growth hormone protects against kainate excitotoxicity and induces BDNF and NT3 expression in chicken neuroretinal cells. Exp. Eye Res. 2018, 166, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Nylander, E.; Gronbladh, A.; Zelleroth, S.; Diwakarla, S.; Nyberg, F.; Hallberg, M. Growth hormone is protective against acute methadone-induced toxicity by modulating the NMDA receptor complex. Neuroscience 2016, 339, 538–547. [Google Scholar] [CrossRef] [PubMed]
- Svensson, A.L.; Bucht, N.; Hallberg, M.; Nyberg, F. Reversal of opiate-induced apoptosis by human recombinant growth hormone in murine foetus primary hippocampal neuronal cell cultures. Proc. Natl. Acad. Sci. USA 2008, 105, 7304–7308. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.; Yu, Y.; Cain, C.M.; Nyberg, F.; Couraud, P.O.; Kastin, A.J. Permeation of growth hormone across the blood-brain barrier. Endocrinology 2005, 146, 4898–4904. [Google Scholar] [CrossRef] [PubMed]
- Lai, Z.N.; Emtner, M.; Roos, P.; Nyberg, F. Characterization of putative growth hormone receptors in human choroid plexus. Brain Res. 1991, 546, 222–226. [Google Scholar] [CrossRef]
- Le Roith, D.; Bondy, C.; Yakar, S.; Liu, J.L.; Butler, A. The somatomedin hypothesis: 2001. Endocr. Rev. 2001, 22, 53–74. [Google Scholar] [CrossRef] [PubMed]
- Schneider, H.J.; Pagotto, U.; Stalla, G.K. Central effects of the somatotropic system. Eur. J. Endocrinol. 2003, 149, 377–392. [Google Scholar] [CrossRef] [PubMed]
- Bluthe, R.M.; Frenois, F.; Kelley, K.W.; Dantzer, R. Pentoxifylline and insulin-like growth factor-I (IGF-I) abrogate kainic acid-induced cognitive impairment in mice. J. Neuroimmunol. 2005, 169, 50–58. [Google Scholar] [CrossRef] [PubMed]
- D'Mello, S.R.; Galli, C.; Ciotti, T.; Calissano, P. Induction of apoptosis in cerebellar granule neurons by low potassium: Inhibition of death by insulin-like growth factor I and cAMP. Proc. Natl. Acad. Sci. USA 1993, 90, 10989–10993. [Google Scholar] [CrossRef] [PubMed]
- Parrizas, M.; Saltiel, A.R.; LeRoith, D. Insulin-like growth factor 1 inhibits apoptosis using the phosphatidylinositol 3′-kinase and mitogen-activated protein kinase pathways. J. Biol. Chem. 1997, 272, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Johnston, B.M.; Mallard, E.C.; Williams, C.E.; Gluckman, P.D. Insulin-like growth factor-1 is a potent neuronal rescue agent after hypoxic-ischemic injury in fetal lambs. J. Clin. Invest. 1996, 97, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Beck, K.D.; Powell-Braxton, L.; Widmer, H.R.; Valverde, J.; Hefti, F. Igf1 gene disruption results in reduced brain size, CNS hypomyelination, and loss of hippocampal granule and striatal parvalbumin-containing neurons. Neuron 1995, 14, 717–730. [Google Scholar] [CrossRef]
- Sonntag, W.E.; Ramsey, M.; Carter, C.S. Growth hormone and insulin-like growth factor-1 (IGF-1) and their influence on cognitive aging. Ageing Res. Rev. 2005, 4, 195–212. [Google Scholar] [CrossRef] [PubMed]
- van Dam, P.S.; Aleman, A. Insulin-like growth factor-I, cognition and brain aging. Eur. J. Pharmacol. 2004, 490, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Andersen, J.M.; Olaussen, C.F.; Ripe, A.; Morland, J. Long-term methadone treatment impairs novelty preference in rats both when present and absent in brain tissue. Pharmacol. Biochem. Be. 2011, 98, 412–416. [Google Scholar] [CrossRef] [PubMed]
- Hepner, I.J.; Homewood, J.; Taylor, A.J. Methadone disrupts performance on the working memory version of the Morris water task. Physiol. Behav. 2002, 76, 41–49. [Google Scholar] [CrossRef]
- Cummins, E.; Allen, C.P.; Ricchetti, A.; Boughner, E.; Christenson, K.; Haines, M.; Limebeer, C.L.; Parker, L.A.; Leri, F. The effects of acute and chronic steady state methadone on memory retrieval in rats. Psychopharmacology 2012, 222, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Tramullas, M.; Martinez-Cue, C.; Hurle, M.A. Chronic methadone treatment and repeated withdrawal impair cognition and increase the expression of apoptosis-related proteins in mouse brain. Psychopharmacology 2007, 193, 107–120. [Google Scholar] [CrossRef] [PubMed]
- Schiltenwolf, M.; Akbar, M.; Hug, A.; Pfuller, U.; Gantz, S.; Neubauer, E.; Flor, H.; Wang, H.L. Evidence of Specific Cognitive Deficits in Patients with Chronic Low Back Pain under Long-Term Substitution Treatment of Opioids. Pain Physician 2014, 17, 9–19. [Google Scholar] [PubMed]
- Zeng, H.; Su, D.Q.; Jiang, X.; Zhu, L.; Ye, H.S. The similarities and differences in impulsivity and cognitive ability among ketamine, methadone, and non-drug users. Psychiat. Res. 2016, 243, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Konijnenberg, C.; Melinder, A. Prenatal exposure to methadone and buprenorphine: A review of the potential effects on cognitive development. Child Neuropsychol. 2011, 17, 495–519. [Google Scholar] [CrossRef] [PubMed]
- Perez-Alvarez, S.; Cuenca-Lopez, M.D.; de Mera, R.M.; Puerta, E.; Karachitos, A.; Bednarczyk, P.; Kmita, H.; Aguirre, N.; Galindo, M.F.; Jordan, J. Methadone induces necrotic-like cell death in SH-SY5Y cells by an impairment of mitochondrial ATP synthesis. Biochim. Biophys. Acta 2010, 1802, 1036–1047. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.; Sung, B.; Ji, R.R.; Lim, G. Neuronal apoptosis associated with morphine tolerance: Evidence for an opioid-induced neurotoxic mechanism. J. Neurosci. 2002, 22, 7650–7661. [Google Scholar] [CrossRef] [PubMed]
- Eisch, A.J.; Barrot, M.; Schad, C.A.; Self, D.W.; Nestler, E.J. Opiates inhibit neurogenesis in the adult rat hippocampus. Proc. Natl. Acad. Sci. USA 2000, 97, 7579–7584. [Google Scholar] [CrossRef] [PubMed]
- Younger, J.W.; Chu, L.F.; D’Arcy, N.T.; Trott, K.E.; Jastrzab, L.E.; Mackey, S.C. Prescription opioid analgesics rapidly change the human brain. Pain 2011, 152, 1803–1810. [Google Scholar] [CrossRef] [PubMed]
- Mahic, M.; Fredheim, O.M.; Borchgrevink, P.C.; Skurtveit, S. Use of prescribed opioids by children and adolescents: Differences between Denmark, Norway and Sweden. Eur. J. Pain 2015, 19, 1095–1100. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Compton, W.M.; Jones, C.M.; Cai, R. Nonmedical Prescription Opioid Use and Use Disorders Among Adults Aged 18 Through 64 Years in the United States, 2003–2013. JAMA 2015, 314, 1468–1478. [Google Scholar] [CrossRef] [PubMed]
- Ong, L.K.; Chow, W.Z.; TeBay, C.; Kluge, M.; Pietrogrande, G.; Zalewska, K.; Crock, P.; Aberg, N.D.; Bivard, A.; Johnson, S.J.; et al. Growth Hormone Improves Cognitive Function After Experimental Stroke. Stroke 2018, 49, 1257–1266. [Google Scholar] [CrossRef] [PubMed]
- Torres-Aleman, I. Toward a comprehensive neurobiology of IGF-I. Dev. Neurobiol. 2010, 70, 384–396. [Google Scholar] [CrossRef] [PubMed]
- Le Greves, M.; Steensland, P.; Le Greves, P.; Nyberg, F. Growth hormone induces age-dependent alteration in the expression of hippocampal growth hormone receptor and N-methyl-d-aspartate receptor subunits gene transcripts in male rats. Proc. Natl. Acad. Sci. USA 2002, 99, 7119–7123. [Google Scholar] [CrossRef] [PubMed]
- Tsien, J.Z.; Huerta, P.T.; Tonegawa, S. The essential role of hippocampal CA1 NMDA receptor-dependent synaptic plasticity in spatial memory. Cell 1996, 87, 1327–1338. [Google Scholar] [CrossRef]
- Ebert, B.; Andersen, S.; Krogsgaard-Larsen, P. Ketobemidone, methadone and pethidine are non-competitive N-methyl-d-aspartate (NMDA) antagonists in the rat cortex and spinal cord. Neurosci. Lett. 1995, 187, 165–168. [Google Scholar] [CrossRef]
- Callahan, R.J.; Au, J.D.; Paul, M.; Liu, C.; Yost, C.S. Functional inhibition by methadone of N-methyl-d-aspartate receptors expressed in Xenopus oocytes: Stereospecific and subunit effects. Anesth. Analg. 2004, 98, 653–659. [Google Scholar] [CrossRef] [PubMed]
- Bartke, A.; Kopchick, J.J. The forgotten lactogenic activity of growth hormone: Important implications for rodent studies. Endocrinology 2015, 156, 1620–1622. [Google Scholar] [CrossRef] [PubMed]
- Diwakarla, S.; Nylander, E.; Gronbladh, A.; Vanga, S.R.; Khan, Y.S.; Gutierrez-de-Teran, H.; Ng, L.; Pham, V.; Savmarker, J.; Lundback, T.; et al. Binding to and Inhibition of Insulin-Regulated Aminopeptidase by Macrocyclic Disulfides Enhances Spine Density. Mol. Pharmacol. 2016, 89, 413–424. [Google Scholar] [CrossRef] [PubMed]
- Lew, M. Good statistical practice in pharmacology. Problem 2. Br. J. Pharmacol. 2007, 152, 299–303. [Google Scholar] [CrossRef] [PubMed]



© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nylander, E.; Zelleroth, S.; Nyberg, F.; Grönbladh, A.; Hallberg, M. The Protective and Restorative Effects of Growth Hormone and Insulin-Like Growth Factor-1 on Methadone-Induced Toxicity In Vitro. Int. J. Mol. Sci. 2018, 19, 3627. https://doi.org/10.3390/ijms19113627
Nylander E, Zelleroth S, Nyberg F, Grönbladh A, Hallberg M. The Protective and Restorative Effects of Growth Hormone and Insulin-Like Growth Factor-1 on Methadone-Induced Toxicity In Vitro. International Journal of Molecular Sciences. 2018; 19(11):3627. https://doi.org/10.3390/ijms19113627
Chicago/Turabian StyleNylander, Erik, Sofia Zelleroth, Fred Nyberg, Alfhild Grönbladh, and Mathias Hallberg. 2018. "The Protective and Restorative Effects of Growth Hormone and Insulin-Like Growth Factor-1 on Methadone-Induced Toxicity In Vitro" International Journal of Molecular Sciences 19, no. 11: 3627. https://doi.org/10.3390/ijms19113627
APA StyleNylander, E., Zelleroth, S., Nyberg, F., Grönbladh, A., & Hallberg, M. (2018). The Protective and Restorative Effects of Growth Hormone and Insulin-Like Growth Factor-1 on Methadone-Induced Toxicity In Vitro. International Journal of Molecular Sciences, 19(11), 3627. https://doi.org/10.3390/ijms19113627