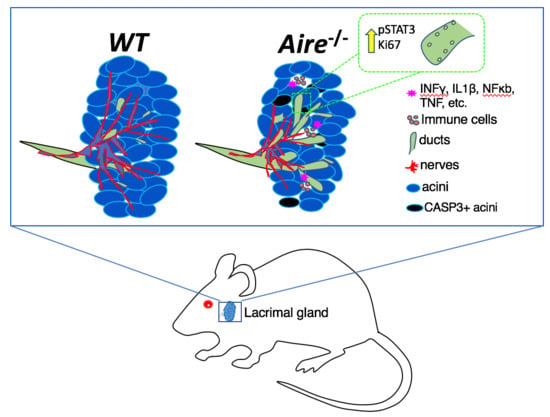
Deciphering Molecular and Phenotypic Changes Associated with Early Autoimmune Disease in the Aire-Deficient Mouse Model of Sjögren’s Syndrome
Abstract
1. Introduction
2. Results
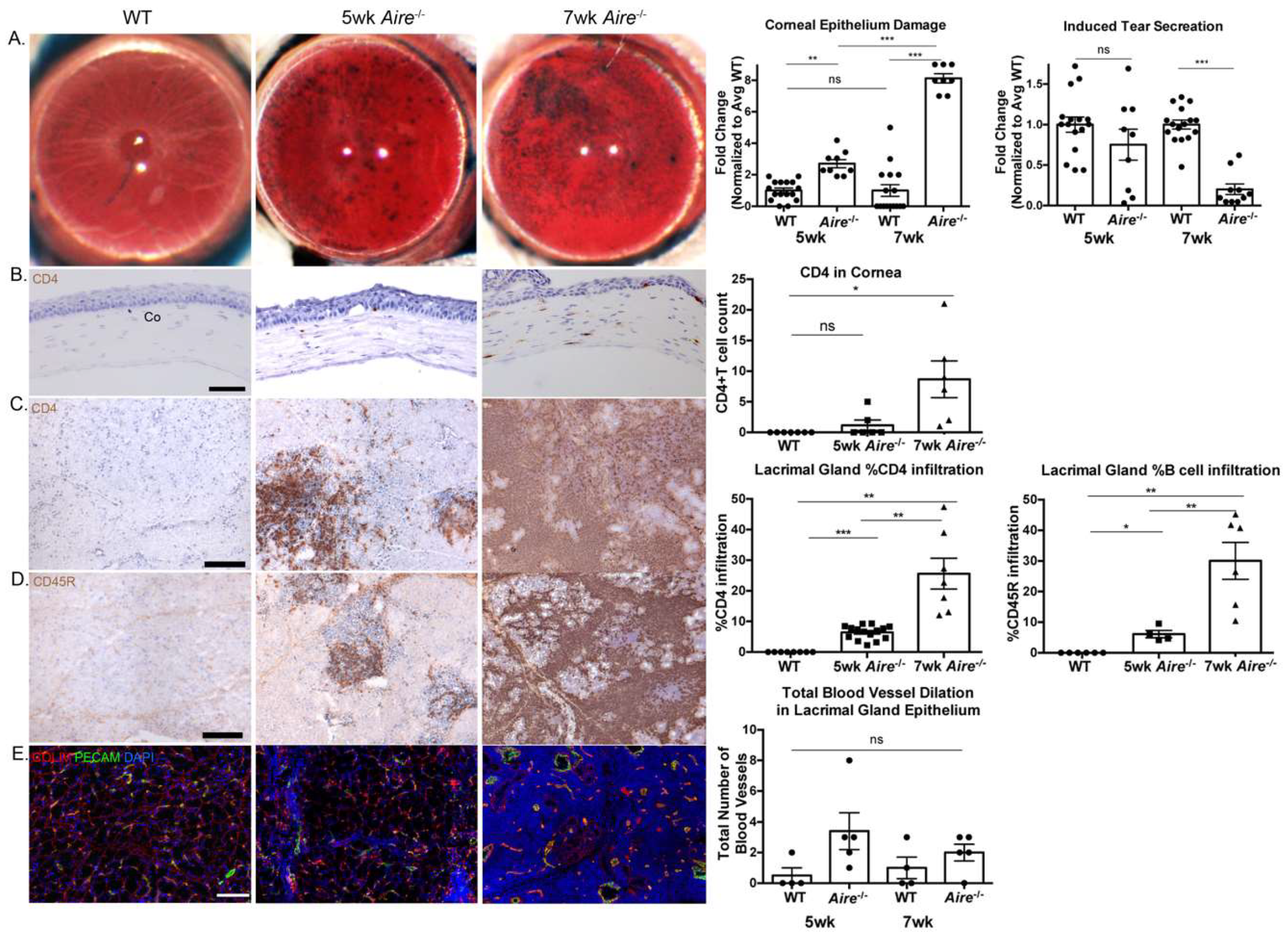
2.1. Aire-/- at 5 Weeks of Age Exhibit Mild Lacrimal Gland Disease Compared to Mice at 7 Weeks
2.2. Gene Expression Analysis Reveals Multiple Signaling Pathways Upregulated in the Lacrimal Glands of Mice Exhibiting Mild Disease
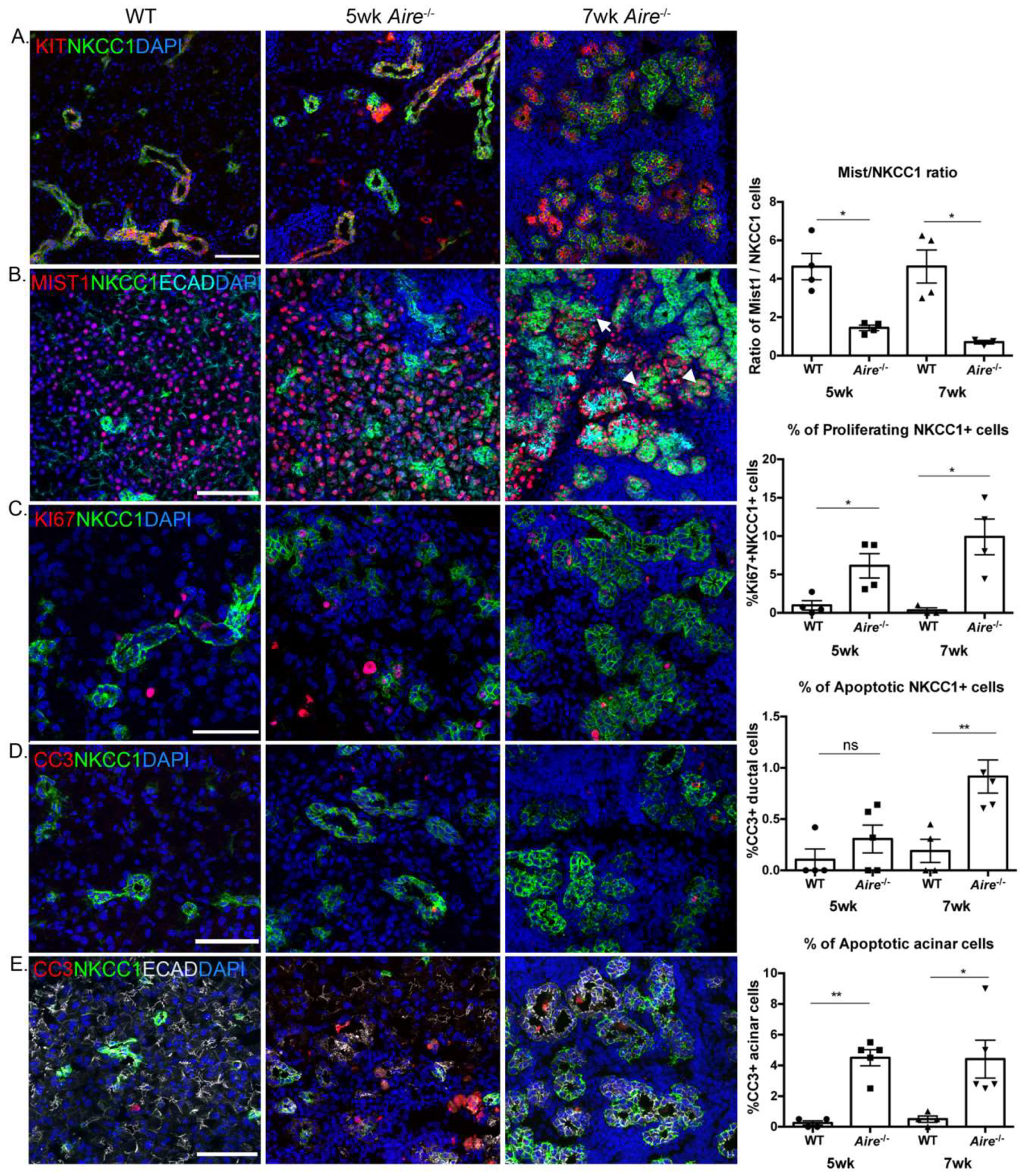
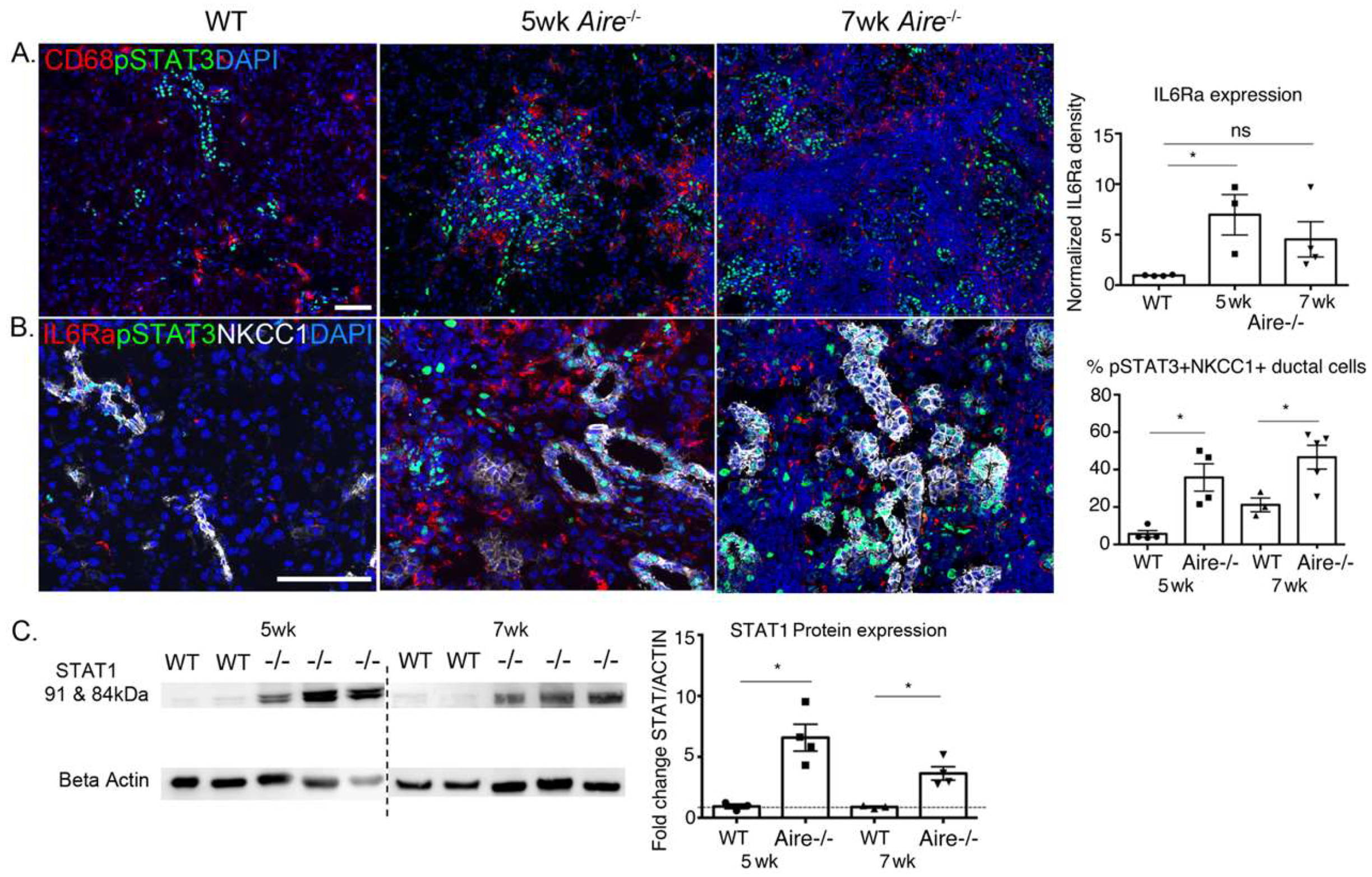
2.3. Validation of Gene Expression Data Confirms the Presence of Multiple Inflammatory Pathways Apparent at Early Stages of Disease Development
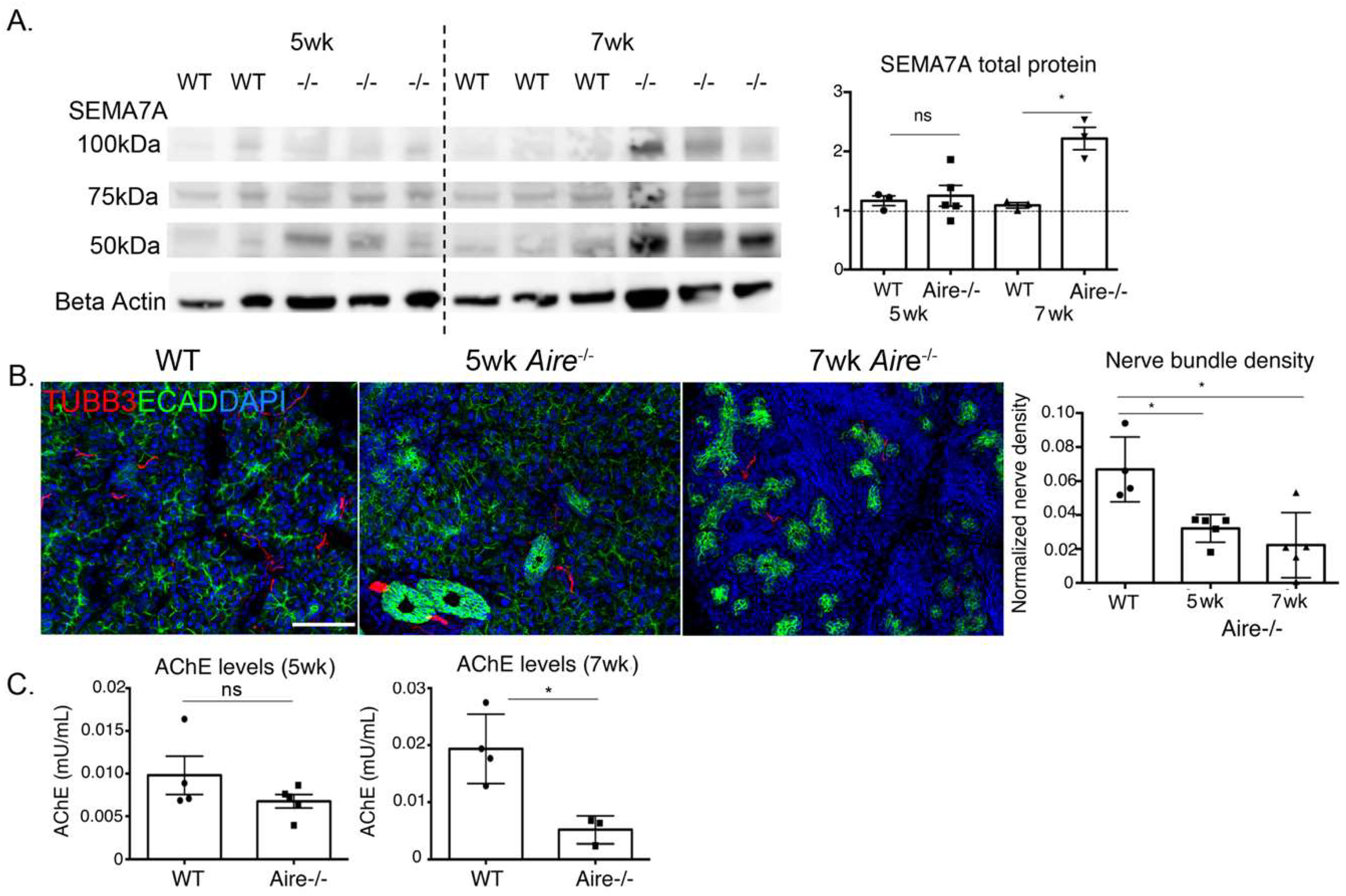
2.4. Denervation of the LG Begins at Early Disease Stages
3. Discussion
4. Materials and Methods
4.1. Animal Model
4.2. Immunohistological and Immunofluorescence Analysis
4.3. Western Blotting and Analysis
4.4. Lissamine Green Staining of the Ocular Surface
4.5. Tear Secretion Measurement
4.6. RNA Isolation and RNAseq Analysis
4.7. Acetylcholinesterase Assay
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Appendix A
| Gene Set | Description | Enrichment Score | p-Value |
|---|---|---|---|
| GO:0002376 | immune system process | 122.222 | 8.32 × 10−54 |
| GO:0045321 | leukocyte activation | 63.9325 | 1.72 × 10−28 |
| GO:0046649 | lymphocyte activation | 50.3351 | 1.38 × 10−22 |
| GO:0045087 | innate immune response | 38.6218 | 1.69 × 10−17 |
| GO:0042110 | T cell activation | 31.974 | 1.30 × 10−14 |
| GO:0002521 | leukocyte differentiation | 30.9403 | 3.65 × 10−14 |
| GO:0032946 | positive regulation of mononuclear cell proliferation | 29.0482 | 2.42 × 10−13 |
| GO:0006935 | chemotaxis | 28.0803 | 6.38 × 10−13 |
| GO:0030098 | lymphocyte differentiation | 26.614 | 2.76 × 10−12 |
| GO:0042113 | B cell activation | 25.1664 | 1.18 × 10−11 |
| GO:0010941 | regulation of cell death | 23.8593 | 4.35 × 10−11 |
| GO:1902107 | positive regulation of leukocyte differentiation | 21.3797 | 5.19 × 10−10 |
| GO:0032675 | regulation of interleukin-6 production | 18.6679 | 7.81 × 10−9 |
| GO:0030890 | positive regulation of B cell proliferation | 18.5907 | 8.44 × 10−9 |
| GO:0035458 | cellular response to interferon-β | 17.6558 | 2.15 × 10−8 |
| GO:0042102 | positive regulation of T cell proliferation | 16.1908 | 9.30 × 10−8 |
| GO:0002474 | antigen processing and presentation of peptide antigen via MHC class I | 15.9125 | 1.23 × 10−7 |
| GO:0050766 | positive regulation of phagocytosis | 15.7837 | 1.40 × 10−7 |
| GO:0032755 | positive regulation of interleukin-6 production | 15.3477 | 2.16 × 10−7 |
| GO:0034121 | regulation of toll-like receptor signaling pathway | 14.6507 | 4.34 × 10−7 |
| GO:1901342 | regulation of vasculature development | 14.1787 | 6.95 × 10−7 |
| GO:0048534 | hematopoietic or lymphoid organ development | 12.8039 | 2.75 × 10−6 |
| GO:1904018 | positive regulation of vasculature development | 11.3609 | 1.16 × 10−5 |
| GO:1904894 | positive regulation of STAT cascade | 10.2205 | 3.64 × 10−5 |
| GO:0046427 | positive regulation of JAK-STAT cascade | 10.2205 | 3.64 × 10−5 |
| GO:0042116 | macrophage activation | 9.6339 | 6.55 × 10−5 |
| GO:0032663 | regulation of interleukin-2 production | 9.45996 | 7.79 × 10−5 |
| GO:0032649 | regulation of interferon-gamma production | 9.51548 | 7.37 × 10−5 |
| GO:0051092 | positive regulation of NF-kappaB transcription factor activity | 9.0688 | 0.00011521 |
| GO:0032956 | regulation of actin cytoskeleton organization | 8.20972 | 0.000272 |
| GO:0060333 | interferon-gamma-mediated signaling pathway | 8.15329 | 0.00028779 |
| GO:0030101 | natural killer cell activation | 7.79743 | 0.00041079 |
| GO:0071526 | semaphorin-plexin signaling pathway | 7.40996 | 0.00060519 |
| GO:0002825 | regulation of T-helper 1 type immune response | 6.67123 | 0.00126683 |
References
- Fox, R.I. Sjögren’s syndrome. Lancet 2029, 366, 321–331. [Google Scholar] [CrossRef]
- Taylor, K.E.; Wong, Q.; Levine, D.M.; McHugh, C.; Laurie, C.; Doheny, K.; Lam, M.Y.; Baer, A.N.; Challacombe, S.; Schiødt, M.; et al. Genome-wide association analysis reveals genetic heterogeneity of Sjögren’s syndrome according to ancestry. Arthritis Rheumatol. 2017, 69, 1294–1305. [Google Scholar] [CrossRef] [PubMed]
- Lessard, C.J.; Li, H.; Adrianto, I.; Ice, J.A.; Rasmussen, A.; Grundahl, K.M.; Kelly, J.A.; Dozmorov, M.G.; Miceli-Richard, C.; Lester, S.; et al. Variants at multiple loci implicated in both innate and adaptive immune responses are associated with Sjögren’s syndrome. Nat. Genet. 2013, 45, 1284–1292. [Google Scholar] [CrossRef] [PubMed]
- Hjelmervik, T.O.; Petersen, K.; Jonassen, I.; Jonsson, R.; Bolstad, A.I. Gene expression profiling of minor salivary glands clearly distinguishes primary Sjögren’s syndrome patients from healthy control subjects. Arthritis Rheum. 2005, 52, 1534–1544. [Google Scholar] [CrossRef] [PubMed]
- Gottenberg, J.E.; Cagnard, N.; Lucchesi, C.; Letourneur, F.; Mistou, S.; Lazure, T.; Jacques, S.; Ba, N.; Ittah, M.; Labetoulle, M.; et al. Activation of IFN pathways and plasmacytoid dendritic cell recruitment in target organs of primary Sjögren’s syndrome. Proc. Natl. Acad. Sci. USA 2006, 103, 2770–2775. [Google Scholar] [CrossRef] [PubMed]
- Katsifis, G.E.; Rekka, S.; Moutsopoulos, N.M.; Pillemer, S.; Wahl, S.M. Systemic and local interleukin-17 and linked cytokines associated with Sjögren’s syndrome immunopathogenesis. Am. J. Pathol. 2009, 175, 1167–1177. [Google Scholar] [CrossRef] [PubMed]
- Youinou, P.; Pers, J.O. Disturbance of cytokine networks in Sjögren’s syndrome. Arthritis Res. Ther. 2011, 13, 227. [Google Scholar] [CrossRef] [PubMed]
- Hansen, A.; Lipsky, P.E.; Dorner, T. B cells in Sjögren’s syndrome: Indications for disturbed selection and differentiation in ectopic lymphoid tissue. Arthritis Res. Ther. 2007, 9, 218. [Google Scholar] [CrossRef] [PubMed]
- Stott, D.I.; Hiepe, F.; Hummel, M.; Steinhauser, G.; Berek, C. Antigen-driven clonal proliferation of B cells within the target tissue of an autoimmune disease. the salivary glands of patients with Sjögren’s syndrome. J. Clin. Investig. 1998, 102, 938–946. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.T.; Lazarev, S.; Bahrami, A.F.; Noble, L.B.; Chen, F.Y.; Zhou, D.; Gallup, M.; Yadav, M.; McNamara, N.A. Interleukin-1 receptor mediates the interplay between CD4+ T cells and ocular resident cells to promote keratinizing squamous metaplasia in Sjögren’s syndrome. Lab. Investig. 2012, 92, 556–570. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Jin, J.O.; Patel, E.S.; Yu, Q. Interleukin-6 inhibits apoptosis of exocrine gland tissues under inflammatory conditions. Cytokine 2015, 76, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Spachidou, M.P.; Bourazopoulou, E.; Maratheftis, C.I.; Kapsogeorgou, E.K.; Moutsopoulos, H.M.; Tzioufas, A.G.; Manoussakis, M.N. Expression of functional toll-like receptors by salivary gland epithelial cells: Increased mRNA expression in cells derived from patients with primary Sjögren’s syndrome. Clin. Exp. Immunol. 2007, 147, 497–503. [Google Scholar] [CrossRef] [PubMed]
- Kwok, S.K.; Cho, M.L.; Her, Y.M.; Oh, H.J.; Park, M.K.; Lee, S.Y.; Woo, Y.J.; Ju, H.J.; Park, K.-S.; Park, S.H.; et al. TLR2 ligation induces the production of IL-23/IL-17 via IL-6, STAT3 and NF-κB pathway in patients with primary Sjögren’s syndrome. Arthritis Res. Ther. 2012, 14, R64. [Google Scholar] [CrossRef] [PubMed]
- Takamatsu, H.; Okuno, T.; Kumanogoh, A. Regulation of immune cell responses by semaphorins and their receptors. Cell. Mol. Immunol. 2010, 7, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Chapoval, S.P.; Vadasz, Z.; Chapoval, A.I.; Toubi, E. Semaphorins 4A and 4D in chronic inflammatory diseases. Inflamm. Res. 2017, 66, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Kumanogoh, A.; Watanabe, C.; Shi, W.; Yoshida, K.; Kikutani, H. Functional soluble CD100/Sema4D released from activated lymphocytes: Possible role in normal and pathologic immune responses. Blood 2001, 97, 3498–3504. [Google Scholar] [CrossRef] [PubMed]
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, B.; Chaisson, M.; Gingeras, T.R. STAR: Ultrafast universal RNA-seq aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, N.; Ping, L.; Zhenjun, L.; Takada, Y.; Sugai, S. Involvement of the interferon-gamma-induced T cell-attracting chemokines, interferon-gamma-inducible 10-kd protein (CXCL10) and monokine induced by interferon-gamma (CXCL9), in the salivary gland lesions of patients with Sjögren’s syndrome. Arthritis Rheum. 2002, 46, 2730–2741. [Google Scholar] [CrossRef] [PubMed]
- Su, M.A.; Davini, D.; Cheng, P.; Giang, K.; Fan, U.; DeVoss, J.J.; Johannes, K.P.A.; Taylor, L.; Shum, A.K.; Meloni, A. Defective autoimmune regulator-dependent central tolerance to myelin protein zero is linked to autoimmune peripheral neuropathy. J. Immunol. 2012, 188, 4906–4912. [Google Scholar] [CrossRef] [PubMed]
- Khodarev, N.N.; Roizman, B.; Weichselbaum, R.R. Molecular pathways: Interferon/stat1 pathway: Role in the tumor resistance to genotoxic stress and aggressive growth. Clin. Cancer Res. 2012, 18, 3015–3021. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Nikulina, K.; DeVoss, J.; Wu, A.J.; Strauss, E.C.; Anderson, M.S.; McNamara, N.A. Small proline-rich protein 1B (SPRR1B) is a biomarker for squamous metaplasia in dry eye disease. Investig. Ophthalmol. Vis. Sci. 2008, 49, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Emmerson, E.; Knox, S.M. Salivary gland stem cells: A review of development, regeneration and cancer. Genesis 2018, 56, e23211. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.H.; Toshida, H.; Schurr, J.; Beuerman, R.W. Microarray analysis of the rat lacrimal gland following the loss of parasympathetic control of secretion. Physiol. Genom. 2004, 18, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.Y.; Lee, A.; Ge, S.; Nathan, S.; Knox, S.M.; McNamara, N.A. Aire-deficient mice provide a model of corneal and lacrimal gland neuropathy in Sjögren’s syndrome. PLoS ONE. 2017, 12, e0184916. [Google Scholar] [CrossRef] [PubMed]
- Tuominen, I.S.; Konttinen, Y.T.; Vesaluoma, M.H.; Moilanen, J.A.; Helinto, M.; Tervo, T.M. Corneal innervation and morphology in primary Sjögren’s syndrome. Investig. Ophthalmol. Vis. Sci. 2003, 44, 2545–2549. [Google Scholar] [CrossRef]
- McNamara, N.A.; Ge, S.; Lee, S.M.; Enghauser, A.M.; Kuehl, L.; Chen, F.Y.T.; McKown, R.L. Reduced levels of tear lacritin are associated with corneal neuropathy in patients with the ocular component of Sjögren’s syndrome. Investig. Ophthalmol. Vis. Sci. 2016, 57, 5237–5243. [Google Scholar] [CrossRef] [PubMed]
- Widenfalk, J.; Nosrat, C.; Tomac, A.; Westphal, H.; Hoffer, B.; Olson, L. Neurturin and glial cell line-derived neurotrophic factor receptor-beta (GDNFR-beta), novel proteins related to GDNF and GDNFR-alpha with specific cellular patterns of expression suggesting roles in the developing and adult nervous system and in peripheral organs. J. Neurosci. 1997, 17, 8506–8519. [Google Scholar] [PubMed]
- Knox, S.M.; Lombaert, I.M.; Haddox, C.L.; Abrams, S.R.; Cotrim, A.; Wilson, A.J.; Hoffman, M.P. Parasympathetic stimulation improves epithelial organ regeneration. Nat. Commun. 2013, 4, 1494. [Google Scholar] [CrossRef] [PubMed]
- Xiao, N.; Lin, Y.; Cao, H.; Sirjani, D.; Giaccia, A.J.; Koong, A.C.; Kong, C.S.; Diehn, M.; Le, Q.T. Neurotrophic factor GDNF promotes survival of salivary stem cells. J. Clin Investig. 2014, 124, 3364–3377. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.T.; Nikulina, K.; Lazarev, S.; Bahrami, A.F.; Noble, L.B.; Gallup, M.; McNamara, N.A. Interleukin-1 as a phenotypic immunomodulator in keratinizing squamous metaplasia of the ocular surface in Sjögren’s syndrome. Am. J. Pathol. 2010, 177, 1333–1343. [Google Scholar] [CrossRef] [PubMed]
- Afkarian, M.; Sedy, J.R.; Yang, J.; Jacobson, N.G.; Cereb, N.; Yang, S.Y.; Murphy, T.L.; Murphy, K.M. T-bet is a STAT1-induced regulator of IL-12R expression in naive CD4+ T cells. Nat. Immunol. 2002, 3, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Wakamatsu, E.; Matsumoto, I.; Yasukochi, T.; Naito, Y.; Goto, D.; Mamura, M.; Ito, S.; Tsutsumi, A.; Sumida, T. Overexpression of phosphorylated STAT-1alpha in the labial salivary glands of patients with Sjögren’s syndrome. Arthritis Rheum. 2006, 54, 3476–3484. [Google Scholar] [CrossRef] [PubMed]
- Barros, M.H.; Hauck, F.; Dreyer, J.H.; Kempkes, B.; Niedobitek, G. Macrophage polarisation: An immunohistochemical approach for identifying M1 and M2 macrophages. PLoS ONE 2013, 8, e80908. [Google Scholar] [CrossRef] [PubMed]
- Grisius, M.M.; Bermudez, D.K.; Fox, P.C. Salivary and serum interleukin 6 in primary Sjögren’s syndrome. J. Rheumatol. 1997, 24, 1089–1091. [Google Scholar] [PubMed]
- Tanaka, T.; Kishimoto, T. Targeting interleukin-6: All the way to treat autoimmune and inflammatory diseases. Int. J. Biol. Sci. 2012, 8, 1227–1236. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Chen, Y.T.; Chen, F.; Gallup, M.; Vijmasi, T.; Bahrami, A.F.; Noble, L.B.; Rooijen, N.; McNamara, N.A. Critical involvement of macrophage infiltration in the development of Sjögren’s syndrome-associated dry eye. Am. J. Pathol. 2012, 181, 753–760. [Google Scholar] [CrossRef] [PubMed]
- Kolodkin, A.L.; Matthes, D.J.; Goodman, C.S. The semaphorin genes encode a family of transmembrane and secreted growth cone guidance molecules. Cell 1993, 75, 1389–1399. [Google Scholar] [CrossRef]
- Wen-Hsiang, T. Document Image Analysis. In Moment-Preserving Thresholding: A New Approach; O’Gorman, L., Kasturi, R., Eds.; IEEE Computer Society Press: Los Alamitos, CA, USA, 1995; pp. 44–60. [Google Scholar]
- Andrews, S. FastQC: A Quality Control Tool for High Throughput Sequence Data. 2010. Available online: http://www.bioinformatics.babraham.ac.uk/projects/fastqc (accessed on 14 November 2018).

| Cell/Process | Gene Symbol | Gene Description | Fold Change (-/- vs. WT) | p-value (-/- vs. WT) |
|---|---|---|---|---|
| Immune cell | ||||
| Adaptive | Cd79b | B-cell antigen receptor complex-associated protein β chain | 200.00 | 5.35 × 10−3 |
| Cd19 | B-lymphocyte antigen CD19 | 172.92 | 3.30 × 10−7 | |
| Cd3d | T-cell surface glycoprotein CD3 δ chain | 106.92 | 8.57 × 10−3 | |
| Cd3g | T-cell surface glycoprotein CD3, gamma polypeptide | 100.81 | 9.69 × 10−3 | |
| Lat | linker for activation of T cells | 42.93 | 1.76 × 10−6 | |
| Innate | Srgn | Serglycin (mast cell) | 5.70 | 4.65 × 10−6 |
| Ncf1 | neutrophil cytosolic factor 1 | 12.36 | 1.67 × 10−5 | |
| Klrk1 | killer cell lectin-like receptor subfamily K, member 1 | 32.18 | 1.23 × 10−6 | |
| Cd68 | CD68 antigen | 3.65 | 1.95 × 10−5 | |
| Vascular | Vegfd | vascular endothelial growth factor D | 3.70 | 1.03 × 10−3 |
| Pecam1 | platelet/endothelial cell adhesion molecule 1 | 2.67 | 4.3 × 10-4 | |
| Vcam1 | vascular cell adhesion molecule 1 | 23.10 | 5.68 × 10−8 | |
| Proliferation | Aurkb | aurora kinase B | 20.12 | 1.26 × 10−5 |
| Top2a | topoisomerase (DNA) II α | 3.69 | 3.17 × 10−4 | |
| Tpx2 | Microtubule Nucleation Factor, microtubule-associated | 11.59 | 2.28 × 10−5 | |
| Cell death | Bid | BH3 interacting domain death agonist | 9.69 | 6.13 × 10−6 |
| Casp3 | caspase 3 | 3.73 | 7.69 × 10−5 | |
| Trp53 | transformation related protein 53 | 2.30 | 2.60 × 10−3 | |
| Cell migration | Cxcl9 | chemokine (C–X–C motif) ligand 9 | 167.93 | 6.30 × 10−3 |
| Cxcr3 | chemokine (C–X–C motif) receptor 3 | 43.23 | 1.34 × 10−7 | |
| Csf1r | colony stimulating factor 1 receptor | 3.25 | 1.30 × 10−4 | |
| Sell | selectin, lymphocyte | 223.19 | 4.79 × 10−3 | |
| Axon guidance/ immunoregulation | Sema4a | sema domain, immunoglobulin domain (Ig), transmembrane domain (TM) and short cytoplasmic domain, (semaphorin) 4A(Sema4a) | 2.42 | 9.17 × 10−4 |
| Sema4c | sema domain, immunoglobulin domain (Ig), transmembrane domain (TM) and short cytoplasmic domain, (semaphorin) 4C(Sema4c) | 4.12 | 3.72 × 10−4 | |
| Sema4d | sema domain, immunoglobulin domain (Ig), transmembrane domain (TM) and short cytoplasmic domain, (semaphorin) 4D(Sema4d) | 2.35 | 2.03 × 10−4 | |
| Sema6d | sema domain, transmembrane domain (TM), and cytoplasmic domain, (semaphorin) 6D | 3.56 | 9.57 × 10−4 | |
| Sema7a | sema domain, immunoglobulin domain (Ig), and GPI membrane anchor, (semaphorin) 7A | 3.02 | 2.62 × 10−4 | |
| Plxnb1 | plexin B1 | 4.78 | 2.33 × 10−6 | |
| Plxnd1 | plexin D1 | 3.12 | 2.72 × 10−4 | |
| Secretion | Scgb1b19 | secretoglobin, family 1B, member 19 | −4.61 | 3.33 × 10−3 |
| Pnliprp1 | pancreatic lipase related protein 1 | −4.08 | 8.44 × 10−3 | |
| Scgb1b3 | secretoglobin, family 1B, member 3 | −3.57 | 2.24 × 10−4 | |
| Scgb1b20 | secretoglobin, family 1B, member 20 | −3.09 | 7.62 × 10−5 | |
| Pip | prolactin induced protein | −2.31 | 3.22 × 10−4 | |
| Bhlha15 | basic helix–loop–helix family, member a15(Bhlha15) | −2.04 | 7.58 × 10−3 | |
| Esp6 | exocrine gland secreted peptide 6 | −2.39 | 5.81 × 10−3 | |
| Signaling pathways | ||||
| TNF | Tnfrsf18 | tumor necrosis factor receptor superfamily, member 18 | 33.63 | 3.64 × 10−6 |
| Tnfrsf13b | tumor necrosis factor receptor superfamily, member 13b | 27.71 | 1.09 × 10−6 | |
| NF-κB | Nfkbie | nuclear factor of kappa light polypeptide gene enhancer in B cells inhibitor, epsilon | 28.23 | 4.08 × 10−6 |
| Nfkbiz | nuclear factor of kappa light polypeptide gene enhancer in B cells inhibitor, zeta | 2.90 | 1.29 × 10−4 | |
| Eomes | eomesodermin | 16.56 | 4.90 × 10−6 | |
| Pim1 | proviral integration site 1 | 6.25 | 1.17 × 10−5 | |
| Toll-like | Tlr2 | toll-like receptor 2 | 10.24 | 3.30 × 10−6 |
| Tlr6 | toll-like receptor 6 | 5.63 | 1.17 × 10−3 | |
| Tlr7 | toll-like receptor 7 | 14.87 | 5.74 × 10−6 | |
| Tlr13 | toll-like receptor 13 | 13.19 | 2.39 × 10−4 | |
| JAK/STAT | Jak2 | Janus kinase 2 | 4.31 | 2.75 × 10−5 |
| Jak3 | Janus kinase 3 | 25.17 | 9.18 × 10−6 | |
| Stat1 | signal transducer and activator of transcription 1 | 12.07 | 2.42 × 10−8 | |
| Stat2 | signal transducer and activator of transcription 2 | 3.65 | 1.92 × 10−5 | |
| Interleukin | Il1b | interleukin 1β | 12.19 | 3.16 × 10−5 |
| Il2ra | interleukin 2 receptor, α chain | 9.35 | 2.49 × 10−4 | |
| Il2rg | interleukin 2 receptor, γ chain | 21.03 | 3.65 × 10−7 | |
| Il6ra | interleukin 6 receptor, α | 7.40 | 9.40 × 10−5 | |
| Interferon | Igtp | interferon gamma induced GTPase | 23.07 | 2.11 ×10−8 |
| Irf1 | interferon regulatory factor 1 | 8.23 | 2.06 × 10−6 | |
| Irf5 | interferon regulatory factor 5 | 11.99 | 4.43 × 10−6 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, F.Y.; Gaylord, E.; McNamara, N.; Knox, S. Deciphering Molecular and Phenotypic Changes Associated with Early Autoimmune Disease in the Aire-Deficient Mouse Model of Sjögren’s Syndrome. Int. J. Mol. Sci. 2018, 19, 3628. https://doi.org/10.3390/ijms19113628
Chen FY, Gaylord E, McNamara N, Knox S. Deciphering Molecular and Phenotypic Changes Associated with Early Autoimmune Disease in the Aire-Deficient Mouse Model of Sjögren’s Syndrome. International Journal of Molecular Sciences. 2018; 19(11):3628. https://doi.org/10.3390/ijms19113628
Chicago/Turabian StyleChen, Feeling YuTing, Eliza Gaylord, Nancy McNamara, and Sarah Knox. 2018. "Deciphering Molecular and Phenotypic Changes Associated with Early Autoimmune Disease in the Aire-Deficient Mouse Model of Sjögren’s Syndrome" International Journal of Molecular Sciences 19, no. 11: 3628. https://doi.org/10.3390/ijms19113628
APA StyleChen, F. Y., Gaylord, E., McNamara, N., & Knox, S. (2018). Deciphering Molecular and Phenotypic Changes Associated with Early Autoimmune Disease in the Aire-Deficient Mouse Model of Sjögren’s Syndrome. International Journal of Molecular Sciences, 19(11), 3628. https://doi.org/10.3390/ijms19113628