DNA Vaccines—How Far From Clinical Use?
Abstract
1. Introduction
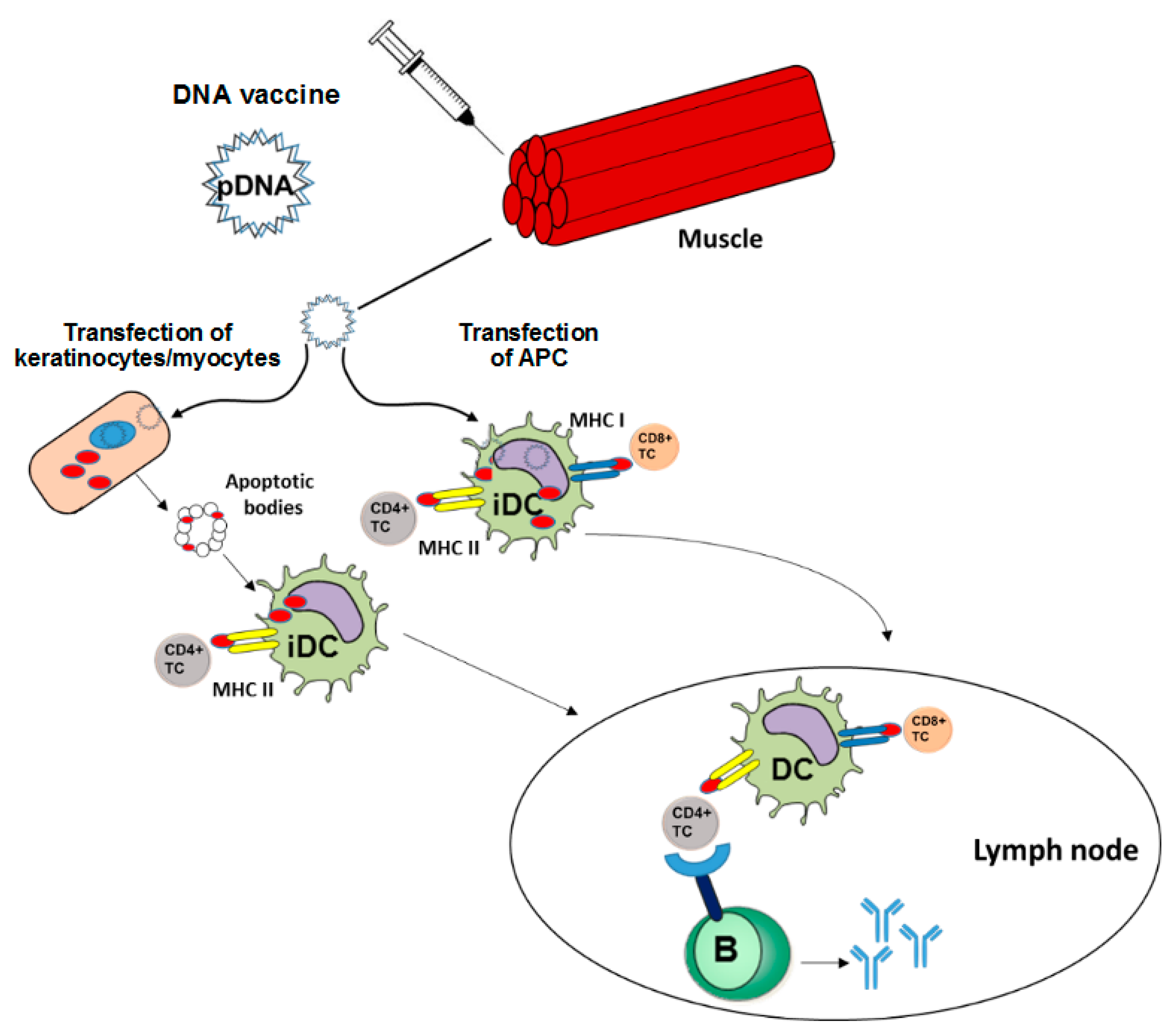
2. Course of Action of DNA Vaccines
3. DNA Vaccines in the Clinic
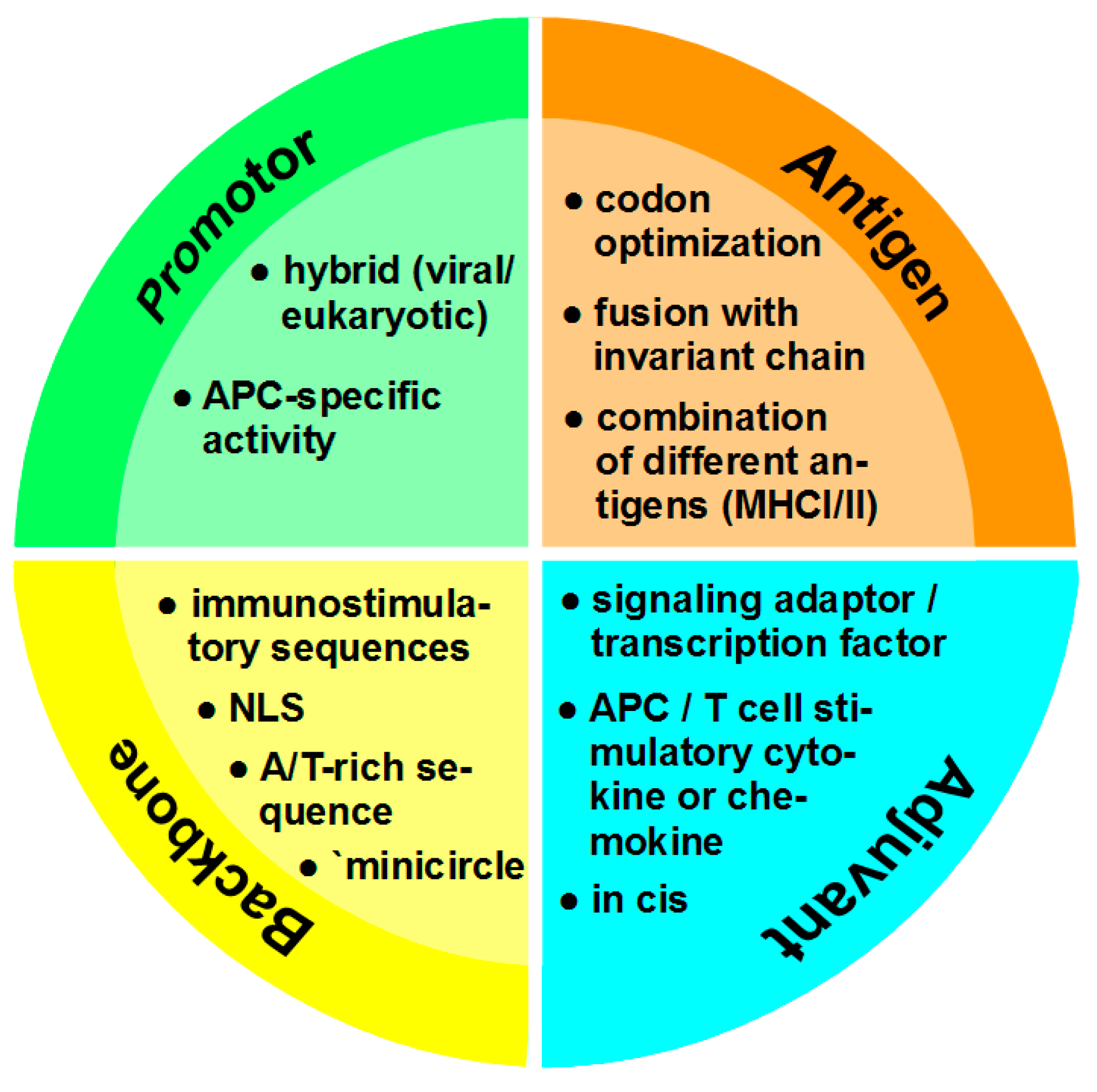
4. Optimization of DNA Vaccines
4.1. Promotor
4.2. Antigen
4.3. Adjuvant
4.4. Vector Backbone
5. Nano-Carriers for Transfer of DNA Vaccines
- (i)
- (ii)
- The delivery system has to show stability against serum proteins that may form a protein corona around the NC and thereby affect its targeting and uptake efficiency [134].
- (iii)
- After uptake by the cell, the NC cargo has to evade endo/lysosomal degradation and to enter the cytoplasm by endosomal escape [153]. While released mRNA is translated directly in the cytoplasm, DNA vaccines need to translocate into the nucleus for subsequent transcription which may be enhanced by NLS [154].
5.1. Nano-Carriers Composed of Inorganic Material
5.2. Lipid-Based Nano-Carriers
5.3. Protein-Based Nano-Carriers
5.4. Polymeric Nano-Carriers
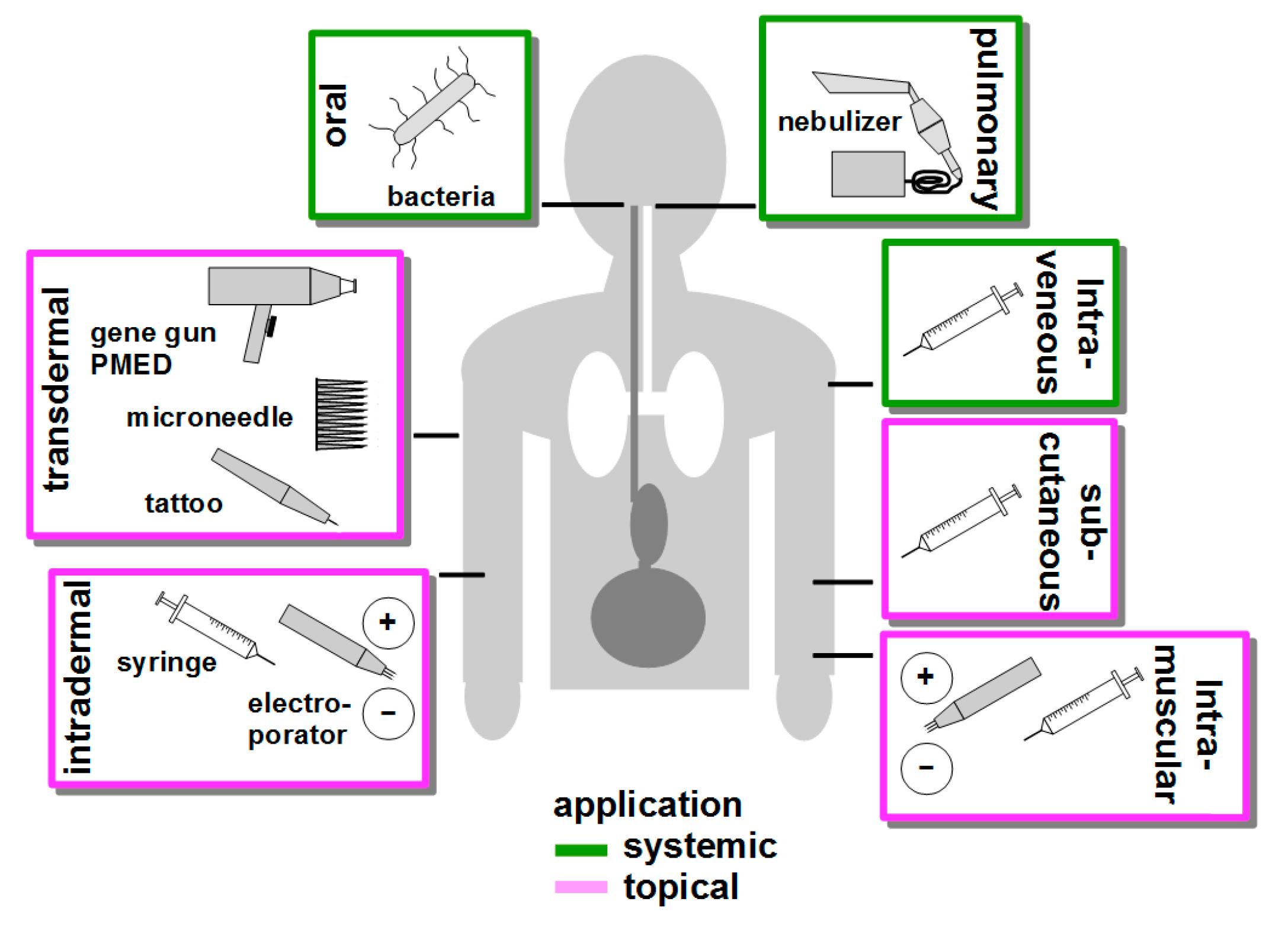
6. Route of Vaccination
7. Targeting of Antigen Presenting Cells
8. Concluding Remarks
Funding
Conflicts of Interest
Abbreviations
| APC | antigen presenting cell |
| AuNP | gold nanoparticle |
| CLR | C-type lectin receptor |
| CPP | cell penetrating peptide |
| CNT | carbon nanotube |
| CTL | cytotoxic T lymphocyte Cutaneous and Mucosal HIV Vaccination |
| CUTHIVAC | cutaneous and mucosal HIV vaccination |
| DC | dendritic cell |
| DC-SIGN | dendritic cell-specific intercellular adhesion molecule-3-grabbing non-integrin |
| DC-STAMP | dendrocyte expressed seven transmembrane protein |
| DOTAP | N-[1-(2,3-Dioleoyloxy)propyl]-N,N,N-trimethylammonium chloride |
| IRF | interferon-regulatory factor |
| HLA | human leukocyte antigen |
| LC | Langerhans cell |
| MDSC | myeloid-derived suppressor cell |
| MHC | major histocompatibility class |
| NC | nano-carrier |
| NK | natural killer cell |
| miRNA | micro-RNA |
| NF-κB | nuclear factor ‘kappa-light-chain-enhancer’ of activated B-cells |
| NLS | nuclear localization signal |
| PEG | polyethyleneglycol |
| PEI | polyethylenimine |
| PLGA | poly-d,l-lactic-coglycolic acid |
| scFv | single chain antibody fragment |
| SV40 | Simian virus 40 |
| TAA | tumor-associated antigen |
| TAM | tumor-associated macrophage |
| Th | T helper cell |
| TLR | toll-like receptor |
| Treg | regulatory T cell |
| VLP | virus-like particle |
References
- Prazeres, D.M.F.; Monteiro, G.A. Plasmid Biopharmaceuticals. Microbiol. Spectrum 2014, 2. [Google Scholar] [CrossRef] [PubMed]
- Fioretti, D.; Iurescia, S.; Rinaldi, M. Recent advances in design of immunogenic and effective naked DNA vaccines against cancer. Recent Pat. Anti-Canc. Drug Discov. 2014, 9, 66–82. [Google Scholar] [CrossRef]
- Scheiblhofer, S.; Thalhamer, J.; Weiss, R. DNA and mRNA vaccination against allergies. Pediat. Allergy Immu. 2018. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Nandakumar, K.S. Recent advances in the development of vaccines for chronic inflammatory autoimmune diseases. Vaccine 2018, 36, 3208–3220. [Google Scholar] [CrossRef] [PubMed]
- Maslow, J.N. Vaccines for emerging infectious diseases: Lessons from MERS coronavirus and Zika virus. Hum. Vacc. Immunother. 2017, 13, 2918–2930. [Google Scholar] [CrossRef] [PubMed]
- Weniger, B.G.; Anglin, I.E.; Tong, T.; Pensiero, M.; Pullen, J.K. Workshop report: Nucleic acid delivery devices for HIV vaccines: Workshop proceedings, National Institute of Allergy and Infectious Diseases, Bethesda, Maryland, USA, May 21, 2015. Vaccine 2018, 36, 427–437. [Google Scholar] [CrossRef] [PubMed]
- Tiptiri-Kourpeti, A.; Spyridopoulou, K.; Pappa, A.; Chlichlia, K. DNA vaccines to attack cancer: Strategies for improving immunogenicity and efficacy. Pharmacol. Therapeut. 2016, 165, 32–49. [Google Scholar] [CrossRef] [PubMed]
- Marino, M.; Scuderi, F.; Provenzano, C.; Bartoccioni, E. Skeletal muscle cells: From local inflammatory response to active immunity. Gene Ther. 2011, 18, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Hengge, U.R.; Chan, E.F.; Foster, R.A.; Walker, P.S.; Vogel, J.C. Cytokine gene expression in epidermis with biological effects following injection of naked DNA. Nat. Genet. 1995, 10, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Porgador, A.; Irvine, K.R.; Iwasaki, A.; Barber, B.H.; Restifo, N.P.; Germain, R.N. Predominant role for directly transfected dendritic cells in antigen presentation to CD8+ T cells after gene gun immunization. J. Exp. Med. 1998, 188, 1075–1082. [Google Scholar] [CrossRef] [PubMed]
- Bai, H.; Lester, G.M.S.; Petishnok, L.C.; Dean, D.A. Cytoplasmic transport and nuclear import of plasmid DNA. Biosci. Rep. 2017, 37. [Google Scholar] [CrossRef] [PubMed]
- Coban, C.; Kobiyama, K.; Jounai, N.; Tozuka, M.; Ishii, K.J. DNA vaccines: A simple DNA sensing matter? Hum. Vacc. Immunother. 2013, 9, 2216–2221. [Google Scholar] [CrossRef] [PubMed]
- Joffre, O.; Nolte, M.A.; Sporri, R.; Reis e Sousa, C. Inflammatory signals in dendritic cell activation and the induction of adaptive immunity. Immunol. Rev. 2009, 227, 234–247. [Google Scholar] [CrossRef] [PubMed]
- Sudowe, S.; Dominitzki, S.; Montermann, E.; Bros, M.; Grabbe, S.; Reske-Kunz, A.B. Uptake and presentation of exogenous antigen and presentation of endogenously produced antigen by skin dendritic cells represent equivalent pathways for the priming of cellular immune responses following biolistic DNA immunization. Immunology 2009, 128, e193–e205. [Google Scholar] [CrossRef] [PubMed]
- Den Haan, J.M.; Arens, R.; van Zelm, M.C. The activation of the adaptive immune system: Cross-talk between antigen-presenting cells, T cells and B cells. Immunol. Lett. 2014, 162, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Macri, C.; Dumont, C.; Johnston, A.P.; Mintern, J.D. Targeting dendritic cells: A promising strategy to improve vaccine effectiveness. Clin. Transl. Immunol. 2016, 5, e66. [Google Scholar] [CrossRef] [PubMed]
- Lazzaro, S.; Giovani, C.; Mangiavacchi, S.; Magini, D.; Maione, D.; Baudner, B.; Geall, A.J.; De Gregorio, E.; D’Oro, U.; Buonsanti, C. CD8 T-cell priming upon mRNA vaccination is restricted to bone-marrow-derived antigen-presenting cells and may involve antigen transfer from myocytes. Immunology 2015, 146, 312–326. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, A.; Torres, C.A.; Ohashi, P.S.; Robinson, H.L.; Barber, B.H. The dominant role of bone marrow-derived cells in CTL induction following plasmid DNA immunization at different sites. J. Immunol. 1997, 159, 11–14. [Google Scholar] [PubMed]
- Maecker, H.T.; Umetsu, D.T.; DeKruyff, R.H.; Levy, S. Cytotoxic T cell responses to DNA vaccination: Dependence on antigen presentation via class II MHC. J. Immunol. 1998, 161, 6532–6536. [Google Scholar] [PubMed]
- Shimizu, K.; Iyoda, T.; Okada, M.; Yamasaki, S.; Fujii, S.I. Immune suppression and reversal of the suppressive tumor microenvironment. Int. Immunol. 2018, 30, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Concha-Benavente, F.; Srivastava, R.; Ferrone, S.; Ferris, R.L. Immunological and clinical significance of HLA class I antigen processing machinery component defects in malignant cells. Oral Oncol. 2016, 58, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Garrido, F.; Aptsiauri, N.; Doorduijn, E.M.; Garcia Lora, A.M.; van Hall, T. The urgent need to recover MHC class I in cancers for effective immunotherapy. Curr. Opin. Immunol. 2016, 39, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Wischhusen, J.; Waschbisch, A.; Wiendl, H. Immune-refractory cancers and their little helpers--an extended role for immunetolerogenic MHC molecules HLA-G and HLA-E? Semin. Cancer Biol. 2007, 17, 459–468. [Google Scholar] [CrossRef] [PubMed]
- Petty, A.J.; Yang, Y. Tumor-associated macrophages: Implications in cancer immunotherapy. Immunotherapy 2017, 9, 289–302. [Google Scholar] [CrossRef] [PubMed]
- Ahrends, T.; Borst, J. The opposing roles of CD4(+) T cells in anti-tumour immunity. Immunology 2018. [Google Scholar] [CrossRef]
- Weber, R.; Fleming, V.; Hu, X.; Nagibin, V.; Groth, C.; Altevogt, P.; Utikal, J.; Umansky, V. Myeloid-Derived Suppressor Cells Hinder the Anti-Cancer Activity of Immune Checkpoint Inhibitors. Front. Immunol. 2018, 9, 1310. [Google Scholar] [CrossRef] [PubMed]
- Wolff, J.A.; Ludtke, J.J.; Acsadi, G.; Williams, P.; Jani, A. Long-term persistence of plasmid DNA and foreign gene expression in mouse muscle. Hum. Mol. Genet. 1992, 1, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Troilo, P.J.; Wang, X.; Griffiths, T.G.; Pacchione, S.J.; Barnum, A.B.; Harper, L.B.; Pauley, C.J.; Niu, Z.; Denisova, L.; et al. Detection of integration of plasmid DNA into host genomic DNA following intramuscular injection and electroporation. Gene Ther. 2004, 11, 711–721. [Google Scholar] [CrossRef] [PubMed]
- Manam, S.; Ledwith, B.J.; Barnum, A.B.; Troilo, P.J.; Pauley, C.J.; Harper, L.B.; Griffiths, T.G., 2nd; Niu, Z.; Denisova, L.; Follmer, T.T.; et al. Plasmid DNA vaccines: Tissue distribution and effects of DNA sequence, adjuvants and delivery method on integration into host DNA. Intervirology 2000, 43, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Jiao, S.; Williams, P.; Berg, R.K.; Hodgeman, B.A.; Liu, L.; Repetto, G.; Wolff, J.A. Direct gene transfer into nonhuman primate myofibers in vivo. Hum. Gene Ther. 1992, 3, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Mairhofer, J.; Lara, A.R. Advances in host and vector development for the production of plasmid DNA vaccines. Methods Mol. Biol. 2014, 1139, 505–541. [Google Scholar] [CrossRef] [PubMed]
- Myhr, A.I. DNA Vaccines: Regulatory Considerations and Safety Aspects. Curr. Issues Mol. Biol. 2017, 22, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Dauphin, G.; Zientara, S. West Nile virus: Recent trends in diagnosis and vaccine development. Vaccine 2007, 25, 5563–5576. [Google Scholar] [CrossRef] [PubMed]
- Atherton, M.J.; Morris, J.S.; McDermott, M.R.; Lichty, B.D. Cancer immunology and canine malignant melanoma: A comparative review. Vet. Immunol. Immunop. 2016, 169, 15–26. [Google Scholar] [CrossRef] [PubMed]
- MacGregor, R.R.; Boyer, J.D.; Ugen, K.E.; Lacy, K.E.; Gluckman, S.J.; Bagarazzi, M.L.; Chattergoon, M.A.; Baine, Y.; Higgins, T.J.; Ciccarelli, R.B.; et al. First human trial of a DNA-based vaccine for treatment of human immunodeficiency virus type 1 infection: Safety and host response. J. Infect. Dis. 1998, 178, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Doolan, D.L.; Le, T.P.; Hedstrom, R.C.; Coonan, K.M.; Charoenvit, Y.; Jones, T.R.; Hobart, P.; Margalith, M.; Ng, J.; et al. Induction of antigen-specific cytotoxic T lymphocytes in humans by a malaria DNA vaccine. Science 1998, 282, 476–480. [Google Scholar] [CrossRef] [PubMed]
- Rottinghaus, S.T.; Poland, G.A.; Jacobson, R.M.; Barr, L.J.; Roy, M.J. Hepatitis B DNA vaccine induces protective antibody responses in human non-responders to conventional vaccination. Vaccine 2003, 21, 4604–4608. [Google Scholar] [CrossRef]
- Mincheff, M.; Tchakarov, S.; Zoubak, S.; Loukinov, D.; Botev, C.; Altankova, I.; Georgiev, G.; Petrov, S.; Meryman, H.T. Naked DNA and adenoviral immunizations for immunotherapy of prostate cancer: A phase I/II clinical trial. Eur. Urol. 2000, 38, 208–217. [Google Scholar] [CrossRef] [PubMed]
- McNeel, D.G.; Becker, J.T.; Johnson, L.E.; Olson, B.M. DNA Vaccines for Prostate Cancer. Curr. Cancer Ther. Rev. 2012, 8, 254–263. [Google Scholar] [CrossRef] [PubMed]
- Tagawa, S.T.; Lee, P.; Snively, J.; Boswell, W.; Ounpraseuth, S.; Lee, S.; Hickingbottom, B.; Smith, J.; Johnson, D.; Weber, J.S. Phase I study of intranodal delivery of a plasmid DNA vaccine for patients with Stage IV melanoma. Cancer 2003, 98, 144–154. [Google Scholar] [CrossRef] [PubMed]
- Weber, J.; Boswell, W.; Smith, J.; Hersh, E.; Snively, J.; Diaz, M.; Miles, S.; Liu, X.; Obrocea, M.; Qiu, Z.; et al. Phase 1 trial of intranodal injection of a Melan-A/MART-1 DNA plasmid vaccine in patients with stage IV melanoma. J. Immunother. 2008, 31, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Ottensmeier, C.; Bowers, M.; Hamid, D.; Maishman, T.; Regan, S.; Wood, W.; Cazaly, A.; Stanton, L. Efficacy and Mechanism Evaluation. In Wilms’ Tumour Antigen 1 Immunity via DNA Fusion Gene Vaccination in Haematological Malignancies by Intramuscular Injection Followed by Intramuscular Electroporation: A Phase II Non-Randomised Clinical Trial (WIN); NIHR Journals Library.: Southampton, UK, 2016. [Google Scholar] [CrossRef]
- Haidari, G.; Cope, A.; Miller, A.; Venables, S.; Yan, C.; Ridgers, H.; Reijonen, K.; Hannaman, D.; Spentzou, A.; Hayes, P.; et al. Combined skin and muscle vaccination differentially impact the quality of effector T cell functions: The CUTHIVAC-001 randomized trial. Sci. Rep. 2017, 7, 13011. [Google Scholar] [CrossRef] [PubMed]
- Pierini, S.; Perales-Linares, R.; Uribe-Herranz, M.; Pol, J.G.; Zitvogel, L.; Kroemer, G.; Facciabene, A.; Galluzzi, L. Trial watch: DNA-based vaccines for oncological indications. Oncoimmunology 2017, 6, e1398878. [Google Scholar] [CrossRef] [PubMed]
- Suschak, J.J.; Williams, J.A.; Schmaljohn, C.S. Advancements in DNA vaccine vectors, non-mechanical delivery methods, and molecular adjuvants to increase immunogenicity. Hum. Vacc. Immunother. 2017, 13, 2837–2848. [Google Scholar] [CrossRef] [PubMed]
- Dupuis, M.; Denis-Mize, K.; Woo, C.; Goldbeck, C.; Selby, M.J.; Chen, M.; Otten, G.R.; Ulmer, J.B.; Donnelly, J.J.; Ott, G.; et al. Distribution of DNA vaccines determines their immunogenicity after intramuscular injection in mice. J. Immunol. 2000, 165, 2850–2858. [Google Scholar] [CrossRef] [PubMed]
- Lechardeur, D.; Verkman, A.S.; Lukacs, G.L. Intracellular routing of plasmid DNA during non-viral gene transfer. Adv. Drug Deliv. Rev. 2005, 57, 755–767. [Google Scholar] [CrossRef] [PubMed]
- Osborn, M.J.; McElmurry, R.T.; Lees, C.J.; DeFeo, A.P.; Chen, Z.Y.; Kay, M.A.; Naldini, L.; Freeman, G.; Tolar, J.; Blazar, B.R. Minicircle DNA-based gene therapy coupled with immune modulation permits long-term expression of alpha-L-iduronidase in mice with mucopolysaccharidosis type I. Mol. Ther. 2011, 19, 450–460. [Google Scholar] [CrossRef] [PubMed]
- Grubor-Bauk, B.; Yu, W.; Wijesundara, D.; Gummow, J.; Garrod, T.; Brennan, A.J.; Voskoboinik, I.; Gowans, E.J. Intradermal delivery of DNA encoding HCV NS3 and perforin elicits robust cell-mediated immunity in mice and pigs. Gene Ther. 2016, 23, 26–37. [Google Scholar] [CrossRef] [PubMed]
- Krinner, S.; Heitzer, A.; Asbach, B.; Wagner, R. Interplay of Promoter Usage and Intragenic CpG Content: Impact on GFP Reporter Gene Expression. Hum. Gene Ther. 2015, 26, 826–840. [Google Scholar] [CrossRef] [PubMed]
- Vanniasinkam, T.; Reddy, S.T.; Ertl, H.C. DNA immunization using a non-viral promoter. Virology 2006, 344, 412–420. [Google Scholar] [CrossRef] [PubMed]
- Metlay, J.P.; Witmer-Pack, M.D.; Agger, R.; Crowley, M.T.; Lawless, D.; Steinman, R.M. The distinct leukocyte integrins of mouse spleen dendritic cells as identified with new hamster monoclonal antibodies. J. Exp. Med. 1990, 171, 1753–1771. [Google Scholar] [CrossRef] [PubMed]
- Erdei, A.; Lukacsi, S.; Macsik-Valent, B.; Nagy-Balo, Z.; Kurucz, I.; Bajtay, Z. Non-identical twins: Different faces of CR3 and CR4 in myeloid and lymphoid cells of mice and men. Semin. Cell Dev. Biol. 2017. [Google Scholar] [CrossRef]
- Brocker, T.; Riedinger, M.; Karjalainen, K. Driving gene expression specifically in dendritic cells. Adv. Exp. Med. Biol. 1997, 417, 55–57. [Google Scholar] [PubMed]
- Lauterbach, H.; Gruber, A.; Ried, C.; Cheminay, C.; Brocker, T. Insufficient APC capacities of dendritic cells in gene gun-mediated DNA vaccination. J. Immunol. 2006, 176, 4600–4607. [Google Scholar] [CrossRef] [PubMed]
- Eleveld-Trancikova, D.; Triantis, V.; Moulin, V.; Looman, M.W.; Wijers, M.; Fransen, J.A.; Lemckert, A.A.; Havenga, M.J.; Figdor, C.G.; Janssen, R.A.; et al. The dendritic cell-derived protein DC-STAMP is highly conserved and localizes to the endoplasmic reticulum. J. Leukocyte Biol. 2005, 77, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Yagi, M.; Miyamoto, T.; Toyama, Y.; Suda, T. Role of DC-STAMP in cellular fusion of osteoclasts and macrophage giant cells. J. Bone Miner. Metab. 2006, 24, 355–358. [Google Scholar] [CrossRef] [PubMed]
- Dresch, C.; Edelmann, S.L.; Marconi, P.; Brocker, T. Lentiviral-mediated transcriptional targeting of dendritic cells for induction of T cell tolerance in vivo. J. Immunol. 2008, 181, 4495–4506. [Google Scholar] [CrossRef] [PubMed]
- Goyal, S.; Castrillon-Betancur, J.C.; Klaile, E.; Slevogt, H. The Interaction of Human Pathogenic Fungi With C-Type Lectin Receptors. Front. Immunol. 2018, 9, 1261. [Google Scholar] [CrossRef] [PubMed]
- Ariizumi, K.; Shen, G.L.; Shikano, S.; Ritter, R., 3rd; Zukas, P.; Edelbaum, D.; Morita, A.; Takashima, A. Cloning of a second dendritic cell-associated C-type lectin (dectin-2) and its alternatively spliced isoforms. J. Biol. Chem. 2000, 275, 11957–11963. [Google Scholar] [CrossRef] [PubMed]
- Bonkobara, M.; Zukas, P.K.; Shikano, S.; Nakamura, S.; Cruz, P.D., Jr.; Ariizumi, K. Epidermal Langerhans cell-targeted gene expression by a dectin-2 promoter. J. Immunol. 2001, 167, 6893–6900. [Google Scholar] [CrossRef] [PubMed]
- Lopes, A.; Vanvarenberg, K.; Preat, V.; Vandermeulen, G. Codon-Optimized P1A-Encoding DNA Vaccine: Toward a Therapeutic Vaccination against P815 Mastocytoma. Mol. Ther.-Nucl. Acids 2017, 8, 404–415. [Google Scholar] [CrossRef] [PubMed]
- Moulin, V.; Morgan, M.E.; Eleveld-Trancikova, D.; Haanen, J.B.; Wielders, E.; Looman, M.W.; Janssen, R.A.; Figdor, C.G.; Jansen, B.J.; Adema, G.J. Targeting dendritic cells with antigen via dendritic cell-associated promoters. Cancer Gene Ther. 2012, 19, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Yamashiro, S. Functions of fascin in dendritic cells. Crit. Rev. Immunol. 2012, 32, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Ross, R.; Jonuleit, H.; Bros, M.; Ross, X.L.; Yamashiro, S.; Matsumura, F.; Enk, A.H.; Knop, J.; Reske-Kunz, A.B. Expression of the actin-bundling protein fascin in cultured human dendritic cells correlates with dendritic morphology and cell differentiation. J. Investig. Dermatol. 2000, 115, 658–663. [Google Scholar] [CrossRef] [PubMed]
- Ross, R.; Sudowe, S.; Beisner, J.; Ross, X.L.; Ludwig-Portugall, I.; Steitz, J.; Tuting, T.; Knop, J.; Reske-Kunz, A.B. Transcriptional targeting of dendritic cells for gene therapy using the promoter of the cytoskeletal protein fascin. Gene Ther. 2003, 10, 1035–1040. [Google Scholar] [CrossRef] [PubMed]
- Bros, M.; Ross, X.L.; Pautz, A.; Reske-Kunz, A.B.; Ross, R. The human fascin gene promoter is highly active in mature dendritic cells due to a stage-specific enhancer. J. Immunol. 2003, 171, 1825–1834. [Google Scholar] [CrossRef] [PubMed]
- Sudowe, S.; Ludwig-Portugall, I.; Montermann, E.; Ross, R.; Reske-Kunz, A.B. Transcriptional targeting of dendritic cells in gene gun-mediated DNA immunization favors the induction of type 1 immune responses. Mol. Ther. 2003, 8, 567–575. [Google Scholar] [CrossRef]
- Raker, V.; Maxeiner, J.; Reske-Kunz, A.B.; Sudowe, S. Efficiency of biolistic DNA vaccination in experimental type I allergy. Methods Mol. Biol. 2013, 940, 357–370. [Google Scholar] [CrossRef] [PubMed]
- Castor, T.; Yogev, N.; Blank, T.; Barwig, C.; Prinz, M.; Waisman, A.; Bros, M.; Reske-Kunz, A.B. Inhibition of experimental autoimmune encephalomyelitis by tolerance-promoting DNA vaccination focused to dendritic cells. PLoS ONE 2018, 13, e0191927. [Google Scholar] [CrossRef] [PubMed]
- Nazarkina Zh, K.; Khar’kova, M.V.; Antonets, D.V.; Morozkin, E.S.; Bazhan, S.I.; Karpenko, L.I.; Vlasov, V.V.; Ilyichev, A.A.; Laktionov, P.P. Design of Polyepitope DNA Vaccine against Breast Carcinoma Cells and Analysis of Its Expression in Dendritic Cells. Bull. Exp. Biol. Med. 2016, 160, 486–490. [Google Scholar] [CrossRef] [PubMed]
- Hirayama, M.; Nishimura, Y. The present status and future prospects of peptide-based cancer vaccines. Int. Immunol. 2016, 28, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Brennick, C.A.; George, M.M.; Corwin, W.L.; Srivastava, P.K.; Ebrahimi-Nik, H. Neoepitopes as cancer immunotherapy targets: Key challenges and opportunities. Immunotherapy 2017, 9, 361–371. [Google Scholar] [CrossRef] [PubMed]
- Kiyotani, K.; Chan, H.T.; Nakamura, Y. Immunopharmacogenomics towards personalized cancer immunotherapy targeting neoantigens. Cancer Sci. 2018, 109, 542–549. [Google Scholar] [CrossRef] [PubMed]
- Sultan, H.; Trillo-Tinoco, J.; Rodriguez, P.; Celis, E. Effective antitumor peptide vaccines can induce severe autoimmune pathology. Oncotarget 2017, 8, 70317–70331. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.C.; Yao, X.; Crystal, J.S.; Li, Y.F.; El-Gamil, M.; Gross, C.; Davis, L.; Dudley, M.E.; Yang, J.C.; Samuels, Y.; et al. Efficient identification of mutated cancer antigens recognized by T cells associated with durable tumor regressions. Clin. Cancer Res. 2014, 20, 3401–3410. [Google Scholar] [CrossRef] [PubMed]
- Bins, A.D.; Wolkers, M.C.; van den Boom, M.D.; Haanen, J.B.; Schumacher, T.N. In vivo antigen stability affects DNA vaccine immunogenicity. J. Immunol. 2007, 179, 2126–2133. [Google Scholar] [CrossRef] [PubMed]
- Rosa, D.S.; Ribeiro, S.P.; Fonseca, S.G.; Almeida, R.R.; Santana, V.C.; Apostolico Jde, S.; Kalil, J.; Cunha-Neto, E. Multiple Approaches for Increasing the Immunogenicity of an Epitope-Based Anti-HIV Vaccine. AIDS Res. Hum. Retrov. 2015, 31, 1077–1088. [Google Scholar] [CrossRef] [PubMed]
- Hoppes, R.; Oostvogels, R.; Luimstra, J.J.; Wals, K.; Toebes, M.; Bies, L.; Ekkebus, R.; Rijal, P.; Celie, P.H.; Huang, J.H.; et al. Altered peptide ligands revisited: Vaccine design through chemically modified HLA-A2-restricted T cell epitopes. J. Immunol. 2014, 193, 4803–4813. [Google Scholar] [CrossRef] [PubMed]
- Pielak, R.M.; O’Donoghue, G.P.; Lin, J.J.; Alfieri, K.N.; Fay, N.C.; Low-Nam, S.T.; Groves, J.T. Early T cell receptor signals globally modulate ligand:receptor affinities during antigen discrimination. Proc. Natl. Acad. Sci. USA 2017, 114, 12190–12195. [Google Scholar] [CrossRef] [PubMed]
- Seledtsova, G.V.; Shishkov, A.A.; Kaschenko, E.A.; Goncharov, A.G.; Gazatova, N.D.; Seledtsov, V.I. Xenogeneic cell-based vaccine therapy for stage III melanoma: Safety, immune-mediated responses and survival benefits. Eur. J. Dermatol. 2016, 26, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Garg, R.; Kaur, M.; Saxena, A.; Prasad, R.; Bhatnagar, R. Alum adjuvanted rabies DNA vaccine confers 80% protection against lethal 50 LD50 rabies challenge virus standard strain. Mol. Immunol. 2017, 85, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Hemmi, H.; Takeuchi, O.; Kawai, T.; Kaisho, T.; Sato, S.; Sanjo, H.; Matsumoto, M.; Hoshino, K.; Wagner, H.; Takeda, K.; et al. A Toll-like receptor recognizes bacterial DNA. Nature 2000, 408, 740–745. [Google Scholar] [CrossRef] [PubMed]
- Tudor, D.; Dubuquoy, C.; Gaboriau, V.; Lefevre, F.; Charley, B.; Riffault, S. TLR9 pathway is involved in adjuvant effects of plasmid DNA-based vaccines. Vaccine 2005, 23, 1258–1264. [Google Scholar] [CrossRef] [PubMed]
- Suschak, J.J.; Wang, S.; Fitzgerald, K.A.; Lu, S. A cGAS-Independent STING/IRF7 Pathway Mediates the Immunogenicity of DNA Vaccines. J. Immunol. 2016, 196, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Van Delft, M.A.; Huitema, L.F.; Tas, S.W. The contribution of NF-kappaB signalling to immune regulation and tolerance. Eur. J. Clin. Investig. 2015, 45, 529–539. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, I.; Kaisho, T. Transcriptional control of dendritic cell differentiation. Curr. Topics Microbiol. 2014, 381, 257–278. [Google Scholar] [CrossRef]
- Shedlock, D.J.; Tingey, C.; Mahadevan, L.; Hutnick, N.; Reuschel, E.L.; Kudchodkar, S.; Flingai, S.; Yan, J.; Kim, J.J.; Ugen, K.E.; et al. Co-Administration of Molecular Adjuvants Expressing NF-Kappa B Subunit p65/RelA or Type-1 Transactivator T-bet Enhance Antigen Specific DNA Vaccine-Induced Immunity. Vaccines 2014, 2, 196–215. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, S.; Amara, R.R.; Yeow, W.S.; Pitha, P.M.; Robinson, H.L. Regulation of DNA-raised immune responses by cotransfected interferon regulatory factors. J. Virol. 2002, 76, 6652–6659. [Google Scholar] [CrossRef] [PubMed]
- Castaldello, A.; Sgarbanti, M.; Marsili, G.; Brocca-Cofano, E.; Remoli, A.L.; Caputo, A.; Battistini, A. Interferon regulatory factor-1 acts as a powerful adjuvant in tat DNA based vaccination. J. Cell. Physiol. 2010, 224, 702–709. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Qu, X.; Pan, R.; Zhu, D.; Zhang, Y.; Wu, J.; Pan, Z. The virus-induced signaling adaptor molecule enhances DNA-raised immune protection against H5N1 influenza virus infection in mice. Vaccine 2011, 29, 2561–2567. [Google Scholar] [CrossRef] [PubMed]
- Larsen, K.C.; Spencer, A.J.; Goodman, A.L.; Gilchrist, A.; Furze, J.; Rollier, C.S.; Kiss-Toth, E.; Gilbert, S.C.; Bregu, M.; Soilleux, E.J.; et al. Expression of tak1 and tram induces synergistic pro-inflammatory signalling and adjuvants DNA vaccines. Vaccine 2009, 27, 5589–5598. [Google Scholar] [CrossRef] [PubMed]
- De Andres, X.; Reina, R.; Ciriza, J.; Crespo, H.; Glaria, I.; Ramirez, H.; Grillo, M.J.; Perez, M.M.; Andresdottir, V.; Rosati, S.; et al. Use of B7 costimulatory molecules as adjuvants in a prime-boost vaccination against Visna/Maedi ovine lentivirus. Vaccine 2009, 27, 4591–4600. [Google Scholar] [CrossRef] [PubMed]
- Langrish, C.L.; McKenzie, B.S.; Wilson, N.J.; de Waal Malefyt, R.; Kastelein, R.A.; Cua, D.J. IL-12 and IL-23: Master regulators of innate and adaptive immunity. Immunol. Rev. 2004, 202, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Li, S.S.; Kochar, N.K.; Elizaga, M.; Hay, C.M.; Wilson, G.J.; Cohen, K.W.; De Rosa, S.C.; Xu, R.; Ota-Setlik, A.; Morris, D.; et al. DNA Priming Increases Frequency of T-Cell Responses to a Vesicular Stomatitis Virus HIV Vaccine with Specific Enhancement of CD8(+) T-Cell Responses by Interleukin-12 Plasmid DNA. Clin. Vaccine Immunol. 2017, 24. [Google Scholar] [CrossRef] [PubMed]
- Steel, J.C.; Waldmann, T.A.; Morris, J.C. Interleukin-15 biology and its therapeutic implications in cancer. Trends Pharmacol. Sci. 2012, 33, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Yuan, Q.; Xu, T.; Yao, L.; Feng, J.; Ma, J.; Wang, L.; Lv, C.; Wang, D. Novel adjuvant for immunization against tuberculosis: DNA vaccine expressing Mycobacterium tuberculosis antigen 85A and interleukin-15 fusion product elicits strong immune responses in mice. Biotechnol. Lett. 2017, 39, 1159–1166. [Google Scholar] [CrossRef] [PubMed]
- Spolski, R.; Li, P.; Leonard, W.J. Biology and regulation of IL-2: From molecular mechanisms to human therapy. Nat. Rev. Immunol. 2018, 18, 648–659. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liang, S.; Li, X.; Wang, L.; Zhang, J.; Xu, J.; Huo, S.; Cao, X.; Zhong, Z.; Zhong, F. Mutual enhancement of IL-2 and IL-7 on DNA vaccine immunogenicity mainly involves regulations on their receptor expression and receptor-expressing lymphocyte generation. Vaccine 2015, 33, 3480–3487. [Google Scholar] [CrossRef] [PubMed]
- Abedini, F.; Ebrahimi, M.; Hosseinkhani, H. Technology of RNA Interference in Advanced Medicine. MicroRNA 2018, 7, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Wang, C.; Yi, H.; Li, P.; Pan, H.; Liu, L.; Cai, L.; Ma, Y. Nanovaccine loaded with poly I:C and STAT3 siRNA robustly elicits anti-tumor immune responses through modulating tumor-associated dendritic cells in vivo. Biomaterials 2015, 38, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Peng, X.; Hou, J.; Wu, S.; Shen, J.; Wang, L. Folic acid-functionalized polyethylenimine superparamagnetic iron oxide nanoparticles as theranostic agents for magnetic resonance imaging and PD-L1 siRNA delivery for gastric cancer. Int. J. Nanomed. 2017, 12, 5331–5343. [Google Scholar] [CrossRef] [PubMed]
- Self-Fordham, J.B.; Naqvi, A.R.; Uttamani, J.R.; Kulkarni, V.; Nares, S. MicroRNA: Dynamic Regulators of Macrophage Polarization and Plasticity. Front. Immunol. 2017, 8, 1062. [Google Scholar] [CrossRef] [PubMed]
- Migault, M.; Donnou-Fournet, E.; Galibert, M.D.; Gilot, D. Definition and identification of small RNA sponges: Focus on miRNA sequestration. Methods 2017, 117, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Scheiermann, J.; Klinman, D.M. Clinical evaluation of CpG oligonucleotides as adjuvants for vaccines targeting infectious diseases and cancer. Vaccine 2014, 32, 6377–6389. [Google Scholar] [CrossRef] [PubMed]
- Elizaga, M.L.; Li, S.S.; Kochar, N.K.; Wilson, G.J.; Allen, M.A.; Tieu, H.V.N.; Frank, I.; Sobieszczyk, M.E.; Cohen, K.W.; Sanchez, B.; et al. Safety and tolerability of HIV-1 multiantigen pDNA vaccine given with IL-12 plasmid DNA via electroporation, boosted with a recombinant vesicular stomatitis virus HIV Gag vaccine in healthy volunteers in a randomized, controlled clinical trial. PLoS ONE 2018, 13, e0202753. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.K.; Cha, S.C.; Smith, D.L.; Kim, K.H.; Parshottam, S.R.; Rao, S.; Popescu, M.; Lee, V.Y.; Neelapu, S.S.; Kwak, L.W. Phase I study of an active immunotherapy for asymptomatic phase Lymphoplasmacytic lymphoma with DNA vaccines encoding antigen-chemokine fusion: Study protocol. BMC Cancer 2018, 18, 187. [Google Scholar] [CrossRef] [PubMed]
- Kreiss, P.; Cameron, B.; Rangara, R.; Mailhe, P.; Aguerre-Charriol, O.; Airiau, M.; Scherman, D.; Crouzet, J.; Pitard, B. Plasmid DNA size does not affect the physicochemical properties of lipoplexes but modulates gene transfer efficiency. Nucleic Acids Res. 1999, 27, 3792–3798. [Google Scholar] [CrossRef] [PubMed]
- Gracey Maniar, L.E.; Maniar, J.M.; Chen, Z.Y.; Lu, J.; Fire, A.Z.; Kay, M.A. Minicircle DNA vectors achieve sustained expression reflected by active chromatin and transcriptional level. Mol. Ther. 2013, 21, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Hardee, C.L.; Arevalo-Soliz, L.M.; Hornstein, B.D.; Zechiedrich, L. Advances in Non-Viral DNA Vectors for Gene Therapy. Genes 2017, 8, 65. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Zhang, F.; Fire, A.Z.; Kay, M.A. Sequence-Modified Antibiotic Resistance Genes Provide Sustained Plasmid-Mediated Transgene Expression in Mammals. Mol. Ther. 2017, 25, 1187–1198. [Google Scholar] [CrossRef] [PubMed]
- Stenler, S.; Blomberg, P.; Smith, C.I. Safety and efficacy of DNA vaccines: Plasmids vs. minicircles. Hum. Vacc. Immunother. 2014, 10, 1306–1308. [Google Scholar] [CrossRef] [PubMed]
- Vargas, J.E.; Salton, G.; Sodre de Castro Laino, A.; Pires, T.D.; Bonamino, M.; Lenz, G.; Delgado-Canedo, A. pLR: A lentiviral backbone series to stable transduction of bicistronic genes and exchange of promoters. Plasmid 2012, 68, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Terenin, I.M.; Smirnova, V.V.; Andreev, D.E.; Dmitriev, S.E.; Shatsky, I.N. A researcher’s guide to the galaxy of IRESs. Cell. Mol. Life Sci. 2017, 74, 1431–1455. [Google Scholar] [CrossRef] [PubMed]
- Chng, J.; Wang, T.; Nian, R.; Lau, A.; Hoi, K.M.; Ho, S.C.; Gagnon, P.; Bi, X.; Yang, Y. Cleavage efficient 2A peptides for high level monoclonal antibody expression in CHO cells. MAbs 2015, 7, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Lee, S.R.; Li, L.H.; Park, H.J.; Park, J.H.; Lee, K.Y.; Kim, M.K.; Shin, B.A.; Choi, S.Y. High cleavage efficiency of a 2A peptide derived from porcine teschovirus-1 in human cell lines, zebrafish and mice. PLoS ONE 2011, 6, e18556. [Google Scholar] [CrossRef] [PubMed]
- Lechardeur, D.; Lukacs, G.L. Nucleocytoplasmic transport of plasmid DNA: A perilous journey from the cytoplasm to the nucleus. Hum. Gene Ther. 2006, 17, 882–889. [Google Scholar] [CrossRef] [PubMed]
- Dean, D.A.; Dean, B.S.; Muller, S.; Smith, L.C. Sequence requirements for plasmid nuclear import. Exp. Cell Res. 1999, 253, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Kanazawa, T.; Yamazaki, M.; Fukuda, T.; Takashima, Y.; Okada, H. Versatile nuclear localization signal-based oligopeptide as a gene vector. Biol. Pharm. Bull. 2015, 38, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Bogacheva, M.; Egorova, A.; Slita, A.; Maretina, M.; Baranov, V.; Kiselev, A. Arginine-rich cross-linking peptides with different SV40 nuclear localization signal content as vectors for intranuclear DNA delivery. Bioorg. Med. Chem. Lett. 2017, 27, 4781–4785. [Google Scholar] [CrossRef] [PubMed]
- Cai, P.; Zhang, X.; Wang, M.; Wu, Y.L.; Chen, X. Combinatorial Nano-Bio Interfaces. ACS Nano 2018. [Google Scholar] [CrossRef]
- Schupp, J.; Krebs, F.K.; Zimmer, N.; Trzeciak, E.; Schuppan, D.; Tuettenberg, A. Targeting myeloid cells in the tumor sustaining microenvironment. Cell. Immunol. 2017. [Google Scholar] [CrossRef]
- Deshantri, A.K.; Varela Moreira, A.; Ecker, V.; Mandhane, S.N.; Schiffelers, R.M.; Buchner, M.; Fens, M. Nanomedicines for the treatment of hematological malignancies. J. Control. Release 2018, 287, 194–215. [Google Scholar] [CrossRef] [PubMed]
- Lorden, E.R.; Levinson, H.M.; Leong, K.W. Integration of drug, protein, and gene delivery systems with regenerative medicine. Drug Deliv. Transl. Res. 2015, 5, 168–186. [Google Scholar] [CrossRef] [PubMed]
- Angell, C.; Xie, S.; Zhang, L.; Chen, Y. DNA Nanotechnology for Precise Control over Drug Delivery and Gene Therapy. Small 2016, 12, 1117–1132. [Google Scholar] [CrossRef] [PubMed]
- Shao, K.; Singha, S.; Clemente-Casares, X.; Tsai, S.; Yang, Y.; Santamaria, P. Nanoparticle-based immunotherapy for cancer. ACS Nano 2015, 9, 16–30. [Google Scholar] [CrossRef] [PubMed]
- Blackman, L.D.; Varlas, S.; Arno, M.C.; Houston, Z.H.; Fletcher, N.L.; Thurecht, K.J.; Hasan, M.; Gibson, M.I.; O’Reilly, R.K. Confinement of Therapeutic Enzymes in Selectively Permeable Polymer Vesicles by Polymerization-Induced Self-Assembly (PISA) Reduces Antibody Binding and Proteolytic Susceptibility. ACS Central Sci. 2018, 4, 718–723. [Google Scholar] [CrossRef] [PubMed]
- Steen, E.J.L.; Edem, P.E.; Norregaard, K.; Jorgensen, J.T.; Shalgunov, V.; Kjaer, A.; Herth, M.M. Pretargeting in nuclear imaging and radionuclide therapy: Improving efficacy of theranostics and nanomedicines. Biomaterials 2018, 179, 209–245. [Google Scholar] [CrossRef] [PubMed]
- Rather, A.M.; Mahato, S.; Maji, K.; Gogoi, N.; Manna, U. ‘Reactive’ nano-complex coated medical cotton: A facile avenue for tailored release of small molecules. Nanoscale 2017, 9, 16154–16165. [Google Scholar] [CrossRef] [PubMed]
- Wolfram, R.K.; Heller, L.; Csuk, R. Targeting mitochondria: Esters of rhodamine B with triterpenoids are mitocanic triggers of apoptosis. Eur. J. Med. Chem. 2018, 152, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Malekkhaiat Haffner, S.; Malmsten, M. Membrane interactions and antimicrobial effects of inorganic nanoparticles. Adv. Colloid Interface Sci. 2017, 248, 105–128. [Google Scholar] [CrossRef] [PubMed]
- Radis-Baptista, G.; Campelo, I.S.; Morlighem, J.R.L.; Melo, L.M.; Freitas, V.J.F. Cell-penetrating peptides (CPPs): From delivery of nucleic acids and antigens to transduction of engineered nucleases for application in transgenesis. J. Biotechnol. 2017, 252, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Falanga, A.; Galdiero, S. Peptide chemistry encounters nanomedicine: Recent applications and upcoming scenarios in cancer. Future Med. Chem. 2018, 10, 1877–1880. [Google Scholar] [CrossRef] [PubMed]
- Bros, M.; Nuhn, L.; Simon, J.; Moll, L.; Mailander, V.; Landfester, K.; Grabbe, S. The Protein Corona as a Confounding Variable of Nanoparticle-Mediated Targeted Vaccine Delivery. Front. Immunol. 2018, 9, 1760. [Google Scholar] [CrossRef] [PubMed]
- Khalil, I.A.; Harashima, H. An efficient PEGylated gene delivery system with improved targeting: Synergism between octaarginine and a fusogenic peptide. Int. J Pharm. 2018, 538, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Hua, S.; de Matos, M.B.C.; Metselaar, J.M.; Storm, G. Current Trends and Challenges in the Clinical Translation of Nanoparticulate Nanomedicines: Pathways for Translational Development and Commercialization. Front. Pharm. 2018, 9, 790. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Sun, F.; Liu, S.; Jiang, S. Anti-PEG antibodies in the clinic: Current issues and beyond PEGylation. J. Control. Release 2016, 244, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Das, S.K.; Menezes, M.E.; Bhatia, S.; Wang, X.Y.; Emdad, L.; Sarkar, D.; Fisher, P.B. Gene Therapies for Cancer: Strategies, Challenges and Successes. J. Cell. Physiol. 2015, 230, 259–271. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Chen, X.; Jia, J.; Zhang, W.; Yang, T.; Wang, L.; Ma, G. pH-Responsive Poly(d,l-lactic-co-glycolic acid) Nanoparticles with Rapid Antigen Release Behavior Promote Immune Response. ACS Nano 2015, 9, 4925–4938. [Google Scholar] [CrossRef] [PubMed]
- Foged, C.; Brodin, B.; Frokjaer, S.; Sundblad, A. Particle size and surface charge affect particle uptake by human dendritic cells in an in vitro model. Int. J. Pharm. 2005, 298, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Xiang, S.D.; Scholzen, A.; Minigo, G.; David, C.; Apostolopoulos, V.; Mottram, P.L.; Plebanski, M. Pathogen recognition and development of particulate vaccines: Does size matter? Methods 2006, 40, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Huo, S.; Mizuhara, T.; Das, R.; Lee, Y.W.; Hou, S.; Moyano, D.F.; Duncan, B.; Liang, X.J.; Rotello, V.M. The Interplay of Size and Surface Functionality on the Cellular Uptake of Sub-10 nm Gold Nanoparticles. ACS Nano 2015, 9, 9986–9993. [Google Scholar] [CrossRef] [PubMed]
- Goodman, C.M.; McCusker, C.D.; Yilmaz, T.; Rotello, V.M. Toxicity of gold nanoparticles functionalized with cationic and anionic side chains. Bioconjug. Chem. 2004, 15, 897–900. [Google Scholar] [CrossRef] [PubMed]
- Niikura, K.; Matsunaga, T.; Suzuki, T.; Kobayashi, S.; Yamaguchi, H.; Orba, Y.; Kawaguchi, A.; Hasegawa, H.; Kajino, K.; Ninomiya, T.; et al. Gold nanoparticles as a vaccine platform: Influence of size and shape on immunological responses in vitro and in vivo. ACS Nano 2013, 7, 3926–3938. [Google Scholar] [CrossRef] [PubMed]
- Thomann-Harwood, L.J.; Kaeuper, P.; Rossi, N.; Milona, P.; Herrmann, B.; McCullough, K.C. Nanogel vaccines targeting dendritic cells: Contributions of the surface decoration and vaccine cargo on cell targeting and activation. J. Control. Release 2013, 166, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Mohsen, M.O.; Zha, L.; Cabral-Miranda, G.; Bachmann, M.F. Major findings and recent advances in virus-like particle (VLP)-based vaccines. Semin. Immunol. 2017, 34, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Chang, D.; Lim, M.; Goos, J.; Qiao, R.; Ng, Y.Y.; Mansfeld, F.M.; Jackson, M.; Davis, T.P.; Kavallaris, M. Biologically Targeted Magnetic Hyperthermia: Potential and Limitations. Front. Pharm. 2018, 9, 831. [Google Scholar] [CrossRef] [PubMed]
- Ghassami, E.; Varshosaz, J.; Taymouri, S. Redox sensitive polysaccharide based nanoparticles for improved cancer treatment: A comprehensive review. Curr. Pharm. Des. 2018. [Google Scholar] [CrossRef]
- Lammers, T.; Kiessling, F.; Ashford, M.; Hennink, W.; Crommelin, D.; Storm, G. Cancer nanomedicine: Is targeting our target? Nat. Rev. Mater. 2016, 1. [Google Scholar] [CrossRef] [PubMed]
- Lachelt, U.; Wagner, E. Nucleic Acid Therapeutics Using Polyplexes: A Journey of 50 Years (and Beyond). Chem. Rev. 2015, 115, 11043–11078. [Google Scholar] [CrossRef] [PubMed]
- Keeney, M.; Ong, S.G.; Padilla, A.; Yao, Z.; Goodman, S.; Wu, J.C.; Yang, F. Development of poly(beta-amino ester)-based biodegradable nanoparticles for nonviral delivery of minicircle DNA. ACS Nano 2013, 7, 7241–7250. [Google Scholar] [CrossRef] [PubMed]
- Cohen, R.N.; van der Aa, M.A.; Macaraeg, N.; Lee, A.P.; Szoka, F.C., Jr. Quantification of plasmid DNA copies in the nucleus after lipoplex and polyplex transfection. J. Control. Release 2009, 135, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Selby, L.I.; Cortez-Jugo, C.M.; Such, G.K.; Johnston, A.P.R. Nanoescapology: Progress toward understanding the endosomal escape of polymeric nanoparticles. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2017, 9. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Liang, W.; Qiu, Y.; Cespi, M.; Palmieri, G.F.; Mason, A.J.; Lam, J.K. Incorporation of a Nuclear Localization Signal in pH Responsive LAH4-L1 Peptide Enhances Transfection and Nuclear Uptake of Plasmid DNA. Mol. Pharm. 2016, 13, 3141–3152. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.S.; Hung, Y.C.; Lin, W.H.; Huang, G.S. Assessment of gold nanoparticles as a size-dependent vaccine carrier for enhancing the antibody response against synthetic foot-and-mouth disease virus peptide. Nanotechnology 2010, 21, 195101. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.H.; Huang, Y.F.; Zhang, C.Z.; Niu, J.; Chen, Y.; Chu, Y.; Jiang, Z.H.; Gao, J.Q.; Mao, Z.W. Integration of antimicrobial peptides with gold nanoparticles as unique non-viral vectors for gene delivery to mesenchymal stem cells with antibacterial activity. Biomaterials 2016, 103, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Fogli, S.; Montis, C.; Paccosi, S.; Silvano, A.; Michelucci, E.; Berti, D.; Bosi, A.; Parenti, A.; Romagnoli, P. Inorganic nanoparticles as potential regulators of immune response in dendritic cells. Nanomedicine 2017, 12, 1647–1660. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.H.; Niu, J.; Zhang, C.Z.; Yu, W.; Wu, J.H.; Shan, Y.H.; Wang, X.R.; Shen, Y.Q.; Mao, Z.W.; Liang, W.Q.; et al. TAT conjugated cationic noble metal nanoparticles for gene delivery to epidermal stem cells. Biomaterials 2014, 35, 5605–5618. [Google Scholar] [CrossRef] [PubMed]
- Niut, Y.; Popatt, A.; Yu, M.; Karmakar, S.; Gu, W.; Yu, C. Recent advances in the rational design of silica-based nanoparticles for gene therapy. Ther. Deliv. 2012, 3, 1217–1237. [Google Scholar] [PubMed]
- Pasqua, L.; Leggio, A.; Sisci, D.; Ando, S.; Morelli, C. Mesoporous Silica Nanoparticles in Cancer Therapy: Relevance of the Targeting Function. Mini Rev. Med. Chem. 2016, 16, 743–753. [Google Scholar] [CrossRef] [PubMed]
- Xiong, L.; Qiao, S.Z. A mesoporous organosilica nano-bowl with high DNA loading capacity - a potential gene delivery carrier. Nanoscale 2016, 8, 17446–17450. [Google Scholar] [CrossRef] [PubMed]
- Mody, K.T.; Popat, A.; Mahony, D.; Cavallaro, A.S.; Yu, C.; Mitter, N. Mesoporous silica nanoparticles as antigen carriers and adjuvants for vaccine delivery. Nanoscale 2013, 5, 5167–5179. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Zhou, X.; Xing, D. Controlled release of doxorubicin from graphene oxide based charge-reversal nanocarrier. Biomaterials 2014, 35, 4185–4194. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.P.; Herrera, C.E.; Singh, B.; Singh, S.; Singh, R.K.; Kumar, R. Graphene oxide: An efficient material and recent approach for biotechnological and biomedical applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 86, 173–197. [Google Scholar] [CrossRef] [PubMed]
- Arayachukiat, S.; Seemork, J.; Pan-In, P.; Amornwachirabodee, K.; Sangphech, N.; Sansureerungsikul, T.; Sathornsantikun, K.; Vilaivan, C.; Shigyou, K.; Pienpinijtham, P.; et al. Bringing macromolecules into cells and evading endosomes by oxidized carbon nanoparticles. Nano Lett. 2015, 15, 3370–3376. [Google Scholar] [CrossRef] [PubMed]
- Mehra, N.K.; Jain, N.K. Development, characterization and cancer targeting potential of surface engineered carbon nanotubes. J. Drug Target. 2013, 21, 745–758. [Google Scholar] [CrossRef] [PubMed]
- Sajid, M.I.; Jamshaid, U.; Jamshaid, T.; Zafar, N.; Fessi, H.; Elaissari, A. Carbon nanotubes from synthesis to in vivo biomedical applications. Int. J. Pharm. 2016, 501, 278–299. [Google Scholar] [CrossRef] [PubMed]
- Angelakeris, M. Magnetic nanoparticles: A multifunctional vehicle for modern theranostics. Biochim. Biophys. Acta 2017, 1861, 1642–1651. [Google Scholar] [CrossRef] [PubMed]
- Seemann, K.M.; Luysberg, M.; Revay, Z.; Kudejova, P.; Sanz, B.; Cassinelli, N.; Loidl, A.; Ilicic, K.; Multhoff, G.; Schmid, T.E. Magnetic heating properties and neutron activation of tungsten-oxide coated biocompatible FePt core-shell nanoparticles. J. Control. Release 2015, 197, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Svoboda, O.; Fohlerova, Z.; Baiazitova, L.; Mlynek, P.; Samouylov, K.; Provaznik, I.; Hubalek, J. Transfection by Polyethyleneimine-Coated Magnetic Nanoparticles: Fine-Tuning the Condition for Electrophysiological Experiments. J. Biomed. Nanotechnol. 2018, 14, 1505–1514. [Google Scholar] [CrossRef] [PubMed]
- Felgner, P.L.; Gadek, T.R.; Holm, M.; Roman, R.; Chan, H.W.; Wenz, M.; Northrop, J.P.; Ringold, G.M.; Danielsen, M. Lipofection: A highly efficient, lipid-mediated DNA-transfection procedure. Proc. Natl. Acad. Sci. USA 1987, 84, 7413–7417. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Huang, L. In vivo gene transfer via intravenous administration of cationic lipid-protamine-DNA (LPD) complexes. Gene Ther. 1997, 4, 891–900. [Google Scholar] [CrossRef] [PubMed]
- Rezaee, M.; Oskuee, R.K.; Nassirli, H.; Malaekeh-Nikouei, B. Progress in the development of lipopolyplexes as efficient non-viral gene delivery systems. J. Control. Release 2016, 236, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Zuhorn, I.S.; Hoekstra, D. On the mechanism of cationic amphiphile-mediated transfection. To fuse or not to fuse: Is that the question? J. Membr. Biol. 2002, 189, 167–179. [Google Scholar] [CrossRef] [PubMed]
- Mevel, M.; Haudebourg, T.; Colombani, T.; Peuziat, P.; Dallet, L.; Chatin, B.; Lambert, O.; Berchel, M.; Montier, T.; Jaffres, P.A.; et al. Important role of phosphoramido linkage in imidazole-based dioleyl helper lipids for liposome stability and primary cell transfection. J. Gene Med. 2016, 18, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Cardarelli, F.; Pozzi, D.; Bifone, A.; Marchini, C.; Caracciolo, G. Cholesterol-dependent macropinocytosis and endosomal escape control the transfection efficiency of lipoplexes in CHO living cells. Mol. Pharm. 2012, 9, 334–340. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Bahreman, A.; Daudey, G.; Bussmann, J.; Olsthoorn, R.C.; Kros, A. Drug Delivery via Cell Membrane Fusion Using Lipopeptide Modified Liposomes. ACS Central Sci. 2016, 2, 621–630. [Google Scholar] [CrossRef] [PubMed]
- Alving, C.R.; Beck, Z.; Matyas, G.R.; Rao, M. Liposomal adjuvants for human vaccines. Expert Opin. Drug Del. 2016, 13, 807–816. [Google Scholar] [CrossRef] [PubMed]
- Chappuis, F.; Farinelli, T.; Deckx, H.; Sarnecki, M.; Go, O.; Salzgeber, Y.; Stals, C. Immunogenicity and estimation of antibody persistence following vaccination with an inactivated virosomal hepatitis A vaccine in adults: A 20-year follow-up study. Vaccine 2017, 35, 1448–1454. [Google Scholar] [CrossRef] [PubMed]
- Levin, Y.; Kochba, E.; Shukarev, G.; Rusch, S.; Herrera-Taracena, G.; van Damme, P. A phase 1, open-label, randomized study to compare the immunogenicity and safety of different administration routes and doses of virosomal influenza vaccine in elderly. Vaccine 2016, 34, 5262–5272. [Google Scholar] [CrossRef] [PubMed]
- Lohcharoenkal, W.; Wang, L.; Chen, Y.C.; Rojanasakul, Y. Protein nanoparticles as drug delivery carriers for cancer therapy. BioMed Res. Int. 2014, 2014, 180549. [Google Scholar] [CrossRef] [PubMed]
- Moran, M.C.; Rosell, N.; Ruano, G.; Busquets, M.A.; Vinardell, M.P. Gelatin-based nanoparticles as DNA delivery systems: Synthesis, physicochemical and biocompatible characterization. Colloids Surf. B Biointerfaces 2015, 134, 156–168. [Google Scholar] [CrossRef] [PubMed]
- Merlot, A.M.; Kalinowski, D.S.; Richardson, D.R. Unraveling the mysteries of serum albumin-more than just a serum protein. Front. Physiol. 2014, 5, 299. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Shuai, Y.; Li, H.; Gao, Z. Tyrosine residues play an important role in heme detoxification by serum albumin. Biochim. Biophys. Acta 2014, 1840, 970–976. [Google Scholar] [CrossRef] [PubMed]
- Karimi, M.; Avci, P.; Mobasseri, R.; Hamblin, M.R.; Naderi-Manesh, H. The novel albumin-chitosan core-shell nanoparticles for gene delivery: Preparation, optimization and cell uptake investigation. J. Nanopart. Res. 2013, 15, 1651. [Google Scholar] [CrossRef] [PubMed]
- Kumari, M.; Liu, C.H.; Wu, W.C. Efficient gene delivery by oligochitosan conjugated serum albumin: Facile synthesis, polyplex stability, and transfection. Carbohyd. Polym. 2018, 183, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Wang, Q.; Zhang, Z.; Gong, T.; Sun, X. Cationic bovine serum albumin based self-assembled nanoparticles as siRNA delivery vector for treating lung metastatic cancer. Small 2014, 10, 524–535. [Google Scholar] [CrossRef] [PubMed]
- Neddermeyer, A.H.; Hultenby, K.; Paidikondala, M.; Schuchman, R.M.; Bidokhti, M.R.M. Investigating Tick-borne Flaviviral-like Particles as a Delivery System for Gene Therapy. Curr. Ther. Res. Clin. Exp. 2018, 88, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Fric, J.; Marek, M.; Hruskova, V.; Holan, V.; Forstova, J. Cellular and humoral immune responses to chimeric EGFP-pseudocapsids derived from the mouse polyomavirus after their intranasal administration. Vaccine 2008, 26, 3242–3251. [Google Scholar] [CrossRef] [PubMed]
- Cimica, V.; Galarza, J.M. Adjuvant formulations for virus-like particle (VLP) based vaccines. Clin. Immunol. 2017, 183, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Quader, S.; Kataoka, K. Nanomaterial-Enabled Cancer Therapy. Mol. Ther. 2017, 25, 1501–1513. [Google Scholar] [CrossRef] [PubMed]
- McCutchan, J.H.; Pagano, J.S. Enchancement of the infectivity of simian virus 40 deoxyribonucleic acid with diethylaminoethyl-dextran. J. Natl. Cancer Inst. 1968, 41, 351–357. [Google Scholar] [PubMed]
- Hatakeyama, H.; Akita, H.; Harashima, H. The polyethyleneglycol dilemma: Advantage and disadvantage of PEGylation of liposomes for systemic genes and nucleic acids delivery to tumors. Biol. Pharm. Bull. 2013, 36, 892–899. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.K.; Jones, C.H.; Mistriotis, P.; Yu, Y.; Ma, X.; Ravikrishnan, A.; Jiang, M.; Andreadis, S.T.; Pfeifer, B.A.; Cheng, C. Poly(ethylene glycol)-block-cationic polylactide nanocomplexes of differing charge density for gene delivery. Biomaterials 2013, 34, 9688–9699. [Google Scholar] [CrossRef] [PubMed]
- Laemmli, U.K. Characterization of DNA condensates induced by poly(ethylene oxide) and polylysine. Proc. Natl. Acad. Sci. USA 1975, 72, 4288–4292. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.J.; Jeong, E.J.; Lee, K.Y. Carbon Dioxide-Generating PLG Nanoparticles for Controlled Anti-Cancer Drug Delivery. Pharm. Res. 2018, 35, 59. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Xiong, F.; He, J.; Dai, X.; Wang, G. Surface-functionalized, pH-responsive poly(lactic-co-glycolic acid)-based microparticles for intranasal vaccine delivery: Effect of surface modification with chitosan and mannan. Eur. J. Pharm. Biopharm. 2016, 109, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Danhier, F.; Ansorena, E.; Silva, J.M.; Coco, R.; Le Breton, A.; Preat, V. PLGA-based nanoparticles: An overview of biomedical applications. J. Control. 2012, 161, 505–522. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.J.; Lee, W.C. Polysaccharide-modified nanoparticles with intelligent CD44 receptor targeting ability for gene delivery. Int. J. Nanomed. 2018, 13, 3989–4002. [Google Scholar] [CrossRef] [PubMed]
- Merdan, T.; Kopecek, J.; Kissel, T. Prospects for cationic polymers in gene and oligonucleotide therapy against cancer. Adv. Drug Deliv. Rev. 2002, 54, 715–758. [Google Scholar] [CrossRef]
- Wu, G.Y.; Wu, C.H. Receptor-mediated gene delivery and expression in vivo. J. Biol. Chem. 1988, 263, 14621–14624. [Google Scholar] [PubMed]
- Curiel, D.T.; Agarwal, S.; Wagner, E.; Cotten, M. Adenovirus enhancement of transferrin-polylysine-mediated gene delivery. Proc. Natl. Acad. Sci. USA 1991, 88, 8850–8854. [Google Scholar] [CrossRef] [PubMed]
- Zauner, W.; Blaas, D.; Kuechler, E.; Wagner, E. Rhinovirus-mediated endosomal release of transfection complexes. J. Virol. 1995, 69, 1085–1092. [Google Scholar] [PubMed]
- Saito, G.; Amidon, G.L.; Lee, K.D. Enhanced cytosolic delivery of plasmid DNA by a sulfhydryl-activatable listeriolysin O/protamine conjugate utilizing cellular reducing potential. Gene Ther. 2003, 10, 72–83. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.X.; Szoka, F.C. The influence of polymer structure on the interactions of cationic polymers with DNA and morphology of the resulting complexes. Gene Ther. 1997, 4, 823–832. [Google Scholar] [CrossRef] [PubMed]
- Itaka, K.; Harada, A.; Yamasaki, Y.; Nakamura, K.; Kawaguchi, H.; Kataoka, K. In situ single cell observation by fluorescence resonance energy transfer reveals fast intra-cytoplasmic delivery and easy release of plasmid DNA complexed with linear polyethylenimine. J. Gene Med. 2004, 6, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Grandinetti, G.; Ingle, N.P.; Reineke, T.M. Interaction of poly(ethylenimine)-DNA polyplexes with mitochondria: Implications for a mechanism of cytotoxicity. Mol. Pharm. 2011, 8, 1709–1719. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.L.; Ewert, K.K.; Majzoub, R.N.; Hwu, Y.K.; Liang, K.S.; Leal, C.; Safinya, C.R. Optimizing cationic and neutral lipids for efficient gene delivery at high serum content. J. Gene Med. 2014, 16, 84–96. [Google Scholar] [CrossRef] [PubMed]
- Erbacher, P.; Zou, S.; Bettinger, T.; Steffan, A.M.; Remy, J.S. Chitosan-based vector/DNA complexes for gene delivery: Biophysical characteristics and transfection ability. Pharm. Res. 1998, 15, 1332–1339. [Google Scholar] [CrossRef] [PubMed]
- Slutter, B.; Plapied, L.; Fievez, V.; Sande, M.A.; des Rieux, A.; Schneider, Y.J.; Van Riet, E.; Jiskoot, W.; Preat, V. Mechanistic study of the adjuvant effect of biodegradable nanoparticles in mucosal vaccination. J. Control. Release 2009, 138, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Saade, F.; Honda-Okubo, Y.; Trec, S.; Petrovsky, N. A novel hepatitis B vaccine containing Advax, a polysaccharide adjuvant derived from delta inulin, induces robust humoral and cellular immunity with minimal reactogenicity in preclinical testing. Vaccine 2013, 31, 1999–2007. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Yu, S.; Zhao, D.; Guo, S.; Wang, X.; Zhao, K. Polysaccharides as vaccine adjuvants. Vaccine 2018, 36, 5226–5234. [Google Scholar] [CrossRef] [PubMed]
- Glass, J.J.; Kent, S.J.; De Rose, R. Enhancing dendritic cell activation and HIV vaccine effectiveness through nanoparticle vaccination. Exp. Rev. Vac. 2016, 15, 719–729. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yang, W.; Hu, C.; Wang, Q.; Wu, Y. Properties and applications of nanoparticle/microparticle conveyors with adjuvant characteristics suitable for oral vaccination. Int. J. Nanomed. 2018, 13, 2973–2987. [Google Scholar] [CrossRef] [PubMed]
- Laube, B.L. Aerosolized Medications for Gene and Peptide Therapy. Respir. Care 2015, 60, 806–821. [Google Scholar] [CrossRef] [PubMed]
- Fernando, G.J.; Zhang, J.; Ng, H.I.; Haigh, O.L.; Yukiko, S.R.; Kendall, M.A. Influenza nucleoprotein DNA vaccination by a skin targeted, dry coated, densely packed microprojection array (Nanopatch) induces potent antibody and CD8(+) T cell responses. J. Control. Release 2016, 237, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Jorritsma, S.H.T.; Gowans, E.J.; Grubor-Bauk, B.; Wijesundara, D.K. Delivery methods to increase cellular uptake and immunogenicity of DNA vaccines. Vaccine 2016, 34, 5488–5494. [Google Scholar] [CrossRef] [PubMed]
- Dif, F.; Djediat, C.; Alegria, O.; Demeneix, B.; Levi, G. Transfection of multiple pulmonary cell types following intravenous injection of PEI-DNA in normal and CFTR mutant mice. J. Gene Med. 2006, 8, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Ukawa, M.; Akita, H.; Hayashi, Y.; Ishiba, R.; Tange, K.; Arai, M.; Kubo, K.; Higuchi, Y.; Shimizu, K.; Konishi, S.; et al. Neutralized nanoparticle composed of SS-cleavable and pH-activated lipid-like material as a long-lasting and liver-specific gene delivery system. Adv. Healthc. Mater. 2014, 3, 1222–1229. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Tenzer, S.; Storck, W.; Hobernik, D.; Raker, V.K.; Fischer, K.; Decker, S.; Dzionek, A.; Krauthauser, S.; Diken, M.; et al. Protein corona-mediated targeting of nanocarriers to B cells allows redirection of allergic immune responses. J. Allergy Clin. Immunol. 2018. [Google Scholar] [CrossRef]
- Wang, M.; Gao, Z.; Zhang, Y.; Pan, L. Lactic acid bacteria as mucosal delivery vehicles: A realistic therapeutic option. Appl. Microbiol. Biotechnol. 2016, 100, 5691–5701. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.Z.; Li, Y.M.; Yu, X.; Li, Y.; Li, W.K.; Wang, Q.L.; Liang, A.X.; Li, X.; Yang, L.G.; Han, L. The efficacy, biodistribution and safety of an inhibin DNA vaccine delivered by attenuated Salmonella choleraesuis. Microbiol. Biotechnol. 2018, 11, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; Lira, V.; Hudson, T.E.; Lemmens, E.E.; Hanson, W.G.; Flores, R.; Barajas, G.; Katibah, G.E.; Desbien, A.L.; Lauer, P.; et al. Recombinant Listeria promotes tumor rejection by CD8(+) T cell-dependent remodeling of the tumor microenvironment. Proc. Natl. Acad. Sci. USA 2018, 115, 8179–8184. [Google Scholar] [CrossRef] [PubMed]
- Celec, P.; Gardlik, R. Gene therapy using bacterial vectors. Front. Biosci. 2017, 22, 81–95. [Google Scholar] [CrossRef]
- Lin, I.Y.; Van, T.T.; Smooker, P.M. Live-Attenuated Bacterial Vectors: Tools for Vaccine and Therapeutic Agent Delivery. Vaccines 2015, 3, 940–972. [Google Scholar] [CrossRef] [PubMed]
- Jiao, H.; Yang, H.; Zhao, D.; Chen, J.; Zhang, Q.; Liang, J.; Yin, Y.; Kong, G.; Li, G. Design and immune characterization of a novel Neisseria gonorrhoeae DNA vaccine using bacterial ghosts as vector and adjuvant. Vaccine 2018, 36, 4532–4539. [Google Scholar] [CrossRef] [PubMed]
- Hou, R.; Li, M.; Tang, T.; Wang, R.; Li, Y.; Xu, Y.; Tang, L.; Wang, L.; Liu, M.; Jiang, Y.; et al. Construction of Lactobacillus casei ghosts by Holin-mediated inactivation and the potential as a safe and effective vehicle for the delivery of DNA vaccines. BMC Microbiol. 2018, 18, 80. [Google Scholar] [CrossRef] [PubMed]
- Mancha-Agresti, P.; de Castro, C.P.; Dos Santos, J.S.C.; Araujo, M.A.; Pereira, V.B.; LeBlanc, J.G.; Leclercq, S.Y.; Azevedo, V. Recombinant Invasive Lactococcus lactis Carrying a DNA Vaccine Coding the Ag85A Antigen Increases INF-gamma, IL-6, and TNF-alpha Cytokines after Intranasal Immunization. Front. Microbiol. 2017, 8, 1263. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Wu, M.; Fang, C.; Cheng, C.; Zhao, M.; Fang, W.; Chu, P.K.; Ping, Y.; Tang, G. Engineering nanoparticle-coated bacteria as oral DNA vaccines for cancer immunotherapy. Nano Lett. 2015, 15, 2732–2739. [Google Scholar] [CrossRef] [PubMed]
- Mottais, A.; Le Gall, T.; Sibiril, Y.; Ravel, J.; Laurent, V.; d’Arbonneau, F.; Montier, T. Enhancement of lung gene delivery after aerosol: A new strategy using non-viral complexes with antibacterial properties. Biosci. Rep. 2017, 37. [Google Scholar] [CrossRef] [PubMed]
- Rajapaksa, A.E.; Ho, J.J.; Qi, A.; Bischof, R.; Nguyen, T.H.; Tate, M.; Piedrafita, D.; McIntosh, M.P.; Yeo, L.Y.; Meeusen, E.; et al. Effective pulmonary delivery of an aerosolized plasmid DNA vaccine via surface acoustic wave nebulization. Respir. Res. 2014, 15, 60. [Google Scholar] [CrossRef] [PubMed]
- Davies, L.A.; Nunez-Alonso, G.A.; McLachlan, G.; Hyde, S.C.; Gill, D.R. Aerosol delivery of DNA/liposomes to the lung for cystic fibrosis gene therapy. Hum. Gene Ther. Clin. Dev. 2014, 25, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Yuan, J.; Wang, Y.; Silvers, W.K. Distribution of ATPase-positive Langerhans cells in normal adult human skin. Br. J. Dermatol. 1985, 113, 707–711. [Google Scholar] [CrossRef] [PubMed]
- Russo, E.; Nitschke, M.; Halin, C. Dendritic cell interactions with lymphatic endothelium. Lymphatic Res. Biol. 2013, 11, 172–182. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Liu, H.; Shi, S.; Dong, L.; Wang, H.; Wu, N.; Gao, H.; Cheng, Z.; Zheng, Q.; Cai, J.; et al. A novel dendritic-cell-targeting DNA vaccine for hepatitis B induces T cell and humoral immune responses and potentiates the antivirus activity in HBV transgenic mice. Immunol. Lett. 2015, 168, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, R.D.; Huh, W.K.; Bae, S.; Lamb, L.S., Jr.; Conner, M.G.; Boyer, J.; Wang, C.; Hung, C.F.; Sauter, E.; Paradis, M.; et al. A pilot study of pNGVL4a-CRT/E7(detox) for the treatment of patients with HPV16+ cervical intraepithelial neoplasia 2/3 (CIN2/3). Gynecol. Oncol. 2016, 140, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Larregina, A.T.; Watkins, S.C.; Erdos, G.; Spencer, L.A.; Storkus, W.J.; Beer Stolz, D.; Falo, L.D., Jr. Direct transfection and activation of human cutaneous dendritic cells. Gene Ther. 2001, 8, 608–617. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zeng, M.; Shan, H.; Tong, C. Microneedle Patches as Drug and Vaccine Delivery Platform. Curr. Med. Chem. 2017, 24, 2413–2422. [Google Scholar] [CrossRef] [PubMed]
- Platteel, A.C.M.; Nieuwenhuizen, N.E.; Domaszewska, T.; Schurer, S.; Zedler, U.; Brinkmann, V.; Sijts, A.; Kaufmann, S.H.E. Efficacy Testing of H56 cDNA Tattoo Immunization against Tuberculosis in a Mouse Model. Front. Immunol. 2017, 8, 1744. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Danishmalik, S.N.; Sin, J.I. DNA vaccines, electroporation and their applications in cancer treatment. Hum. Vacc. Immunotherap. 2015, 11, 1889–1900. [Google Scholar] [CrossRef] [PubMed]
- Schultheis, K.; Smith, T.R.F.; Kiosses, W.B.; Kraynyak, K.A.; Wong, A.; Oh, J.; Broderick, K.E. Delineating the Cellular Mechanisms Associated with Skin Electroporation. Hum. Gene Ther. Methods 2018, 29, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Lamolinara, A.; Stramucci, L.; Hysi, A.; Iezzi, M.; Marchini, C.; Mariotti, M.; Amici, A.; Curcio, C. Intradermal DNA Electroporation Induces Cellular and Humoral Immune Response and Confers Protection against HER2/neu Tumor. J. Immunol. Res. 2015, 2015, 159145. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, V.; Rosati, M.; Jalah, R.; Ganneru, B.; Alicea, C.; Yu, L.; Guan, Y.; LaBranche, C.; Montefiori, D.C.; King, A.D.; et al. DNA vaccination by intradermal electroporation induces long-lasting immune responses in rhesus macaques. J. Med. Primatol. 2014, 43, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Weiss, R.; Scheiblhofer, S.; Freund, J.; Ferreira, F.; Livey, I.; Thalhamer, J. Gene gun bombardment with gold particles displays a particular Th2-promoting signal that over-rules the Th1-inducing effect of immunostimulatory CpG motifs in DNA vaccines. Vaccine 2002, 20, 3148–3154. [Google Scholar] [CrossRef]
- Dane, K.Y.; Nembrini, C.; Tomei, A.A.; Eby, J.K.; O’Neil, C.P.; Velluto, D.; Swartz, M.A.; Inverardi, L.; Hubbell, J.A. Nano-sized drug-loaded micelles deliver payload to lymph node immune cells and prolong allograft survival. J. Control. Release 2011, 156, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Allen, T.M.; Hansen, C.B.; Guo, L.S. Subcutaneous administration of liposomes: A comparison with the intravenous and intraperitoneal routes of injection. Biochim. Biophys. Acta 1993, 1150, 9–16. [Google Scholar] [CrossRef]
- Longmire, M.; Choyke, P.L.; Kobayashi, H. Clearance properties of nano-sized particles and molecules as imaging agents: Considerations and caveats. Nanomedicine 2008, 3, 703–717. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Sheng, Y.; Shi, J.; Yu, B.; Yu, Z.; Liao, G. Long circulating polymeric nanoparticles for gene/drug delivery. Curr. Drug Metab. 2017. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Hu, Y.; Yin, L.; Tang, C.; Yin, C. Effects of particle size and surface charge on cellular uptake and biodistribution of polymeric nanoparticles. Biomaterials 2010, 31, 3657–3666. [Google Scholar] [CrossRef] [PubMed]
- Blank, F.; Stumbles, P.A.; Seydoux, E.; Holt, P.G.; Fink, A.; Rothen-Rutishauser, B.; Strickland, D.H.; von Garnier, C. Size-dependent uptake of particles by pulmonary antigen-presenting cell populations and trafficking to regional lymph nodes. Am. J. Respir. Cell Mol. Biol. 2013, 49, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Delcassian, D.; Patel, A.K.; Cortinas, A.B.; Langer, R. Drug delivery across length scales. J. Drug Target. 2018, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Frenz, T.; Grabski, E.; Duran, V.; Hozsa, C.; Stepczynska, A.; Furch, M.; Gieseler, R.K.; Kalinke, U. Antigen presenting cell-selective drug delivery by glycan-decorated nanocarriers. Eur. J. Pharm. Biopharm. 2015, 95, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Appelmelk, B.J.; van Die, I.; van Vliet, S.J.; Vandenbroucke-Grauls, C.M.; Geijtenbeek, T.B.; van Kooyk, Y. Cutting edge: Carbohydrate profiling identifies new pathogens that interact with dendritic cell-specific ICAM-3-grabbing nonintegrin on dendritic cells. J. Immunol. 2003, 170, 1635–1639. [Google Scholar] [CrossRef] [PubMed]
- Burgdorf, S.; Lukacs-Kornek, V.; Kurts, C. The mannose receptor mediates uptake of soluble but not of cell-associated antigen for cross-presentation. J. Immunol. 2006, 176, 6770–6776. [Google Scholar] [CrossRef] [PubMed]
- Qiao, C.; Liu, J.; Yang, J.; Li, Y.; Weng, J.; Shao, Y.; Zhang, X. Enhanced non-inflammasome mediated immune responses by mannosylated zwitterionic-based cationic liposomes for HIV DNA vaccines. Biomaterials 2016, 85, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.Y.; Zhou, G.; Li, Q.L.; Lu, J.S.; Shi, D.Y.; Pang, X.B.; Zhou, X.W.; Yu, Y.Z.; Huang, P.T. Enhanced effects of DNA vaccine against botulinum neurotoxin serotype A by targeting antigen to dendritic cells. Immunol. Let. 2017, 190, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Cao, W.; Yang, Z.G.; Zhao, G.F. DC targeting DNA vaccines induce protective and therapeutic antitumor immunity in mice. Int. J. Clin. Exp. Med. 2015, 8, 17565–17577. [Google Scholar] [PubMed]
- Walsh, K.P.; Mills, K.H. Dendritic cells and other innate determinants of T helper cell polarisation. Trends Immunol. 2013, 34, 521–530. [Google Scholar] [CrossRef] [PubMed]
- Polini, A.; Mercato, L.L.D.; Barra, A.; Zhang, Y.S.; Calabi, F.; Gigli, G. Towards the development of human immune-system-on-a-chip platforms. Drug Discov. Today 2018. [Google Scholar] [CrossRef] [PubMed]
- Marshall, H.T.; Djamgoz, M.B.A. Immuno-Oncology: Emerging Targets and Combination Therapies. Front. Oncol. 2018, 8, 315. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Zhang, S.; Deng, Y.; Wang, P.; Hou, Q.; Xu, H. Prognostic Factors for Checkpoint Inhibitor Based Immunotherapy: An Update With New Evidences. Front. Pharm. 2018, 9, 1050. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hobernik, D.; Bros, M. DNA Vaccines—How Far From Clinical Use? Int. J. Mol. Sci. 2018, 19, 3605. https://doi.org/10.3390/ijms19113605
Hobernik D, Bros M. DNA Vaccines—How Far From Clinical Use? International Journal of Molecular Sciences. 2018; 19(11):3605. https://doi.org/10.3390/ijms19113605
Chicago/Turabian StyleHobernik, Dominika, and Matthias Bros. 2018. "DNA Vaccines—How Far From Clinical Use?" International Journal of Molecular Sciences 19, no. 11: 3605. https://doi.org/10.3390/ijms19113605
APA StyleHobernik, D., & Bros, M. (2018). DNA Vaccines—How Far From Clinical Use? International Journal of Molecular Sciences, 19(11), 3605. https://doi.org/10.3390/ijms19113605



