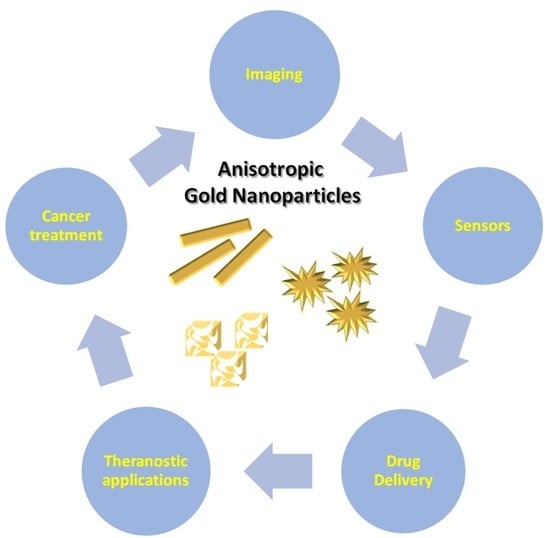
Anisotropic Gold Nanoparticles in Biomedical Applications
Abstract
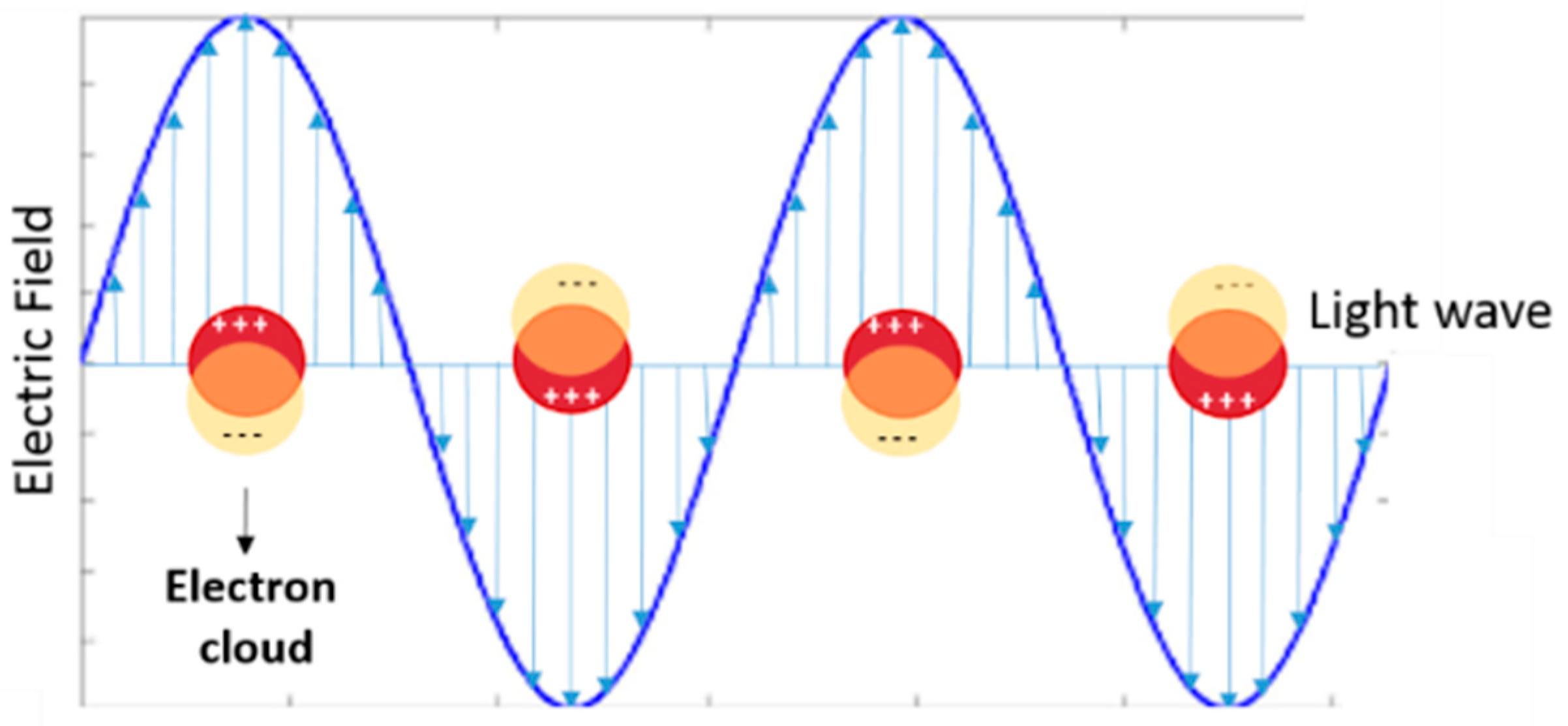
1. Introduction
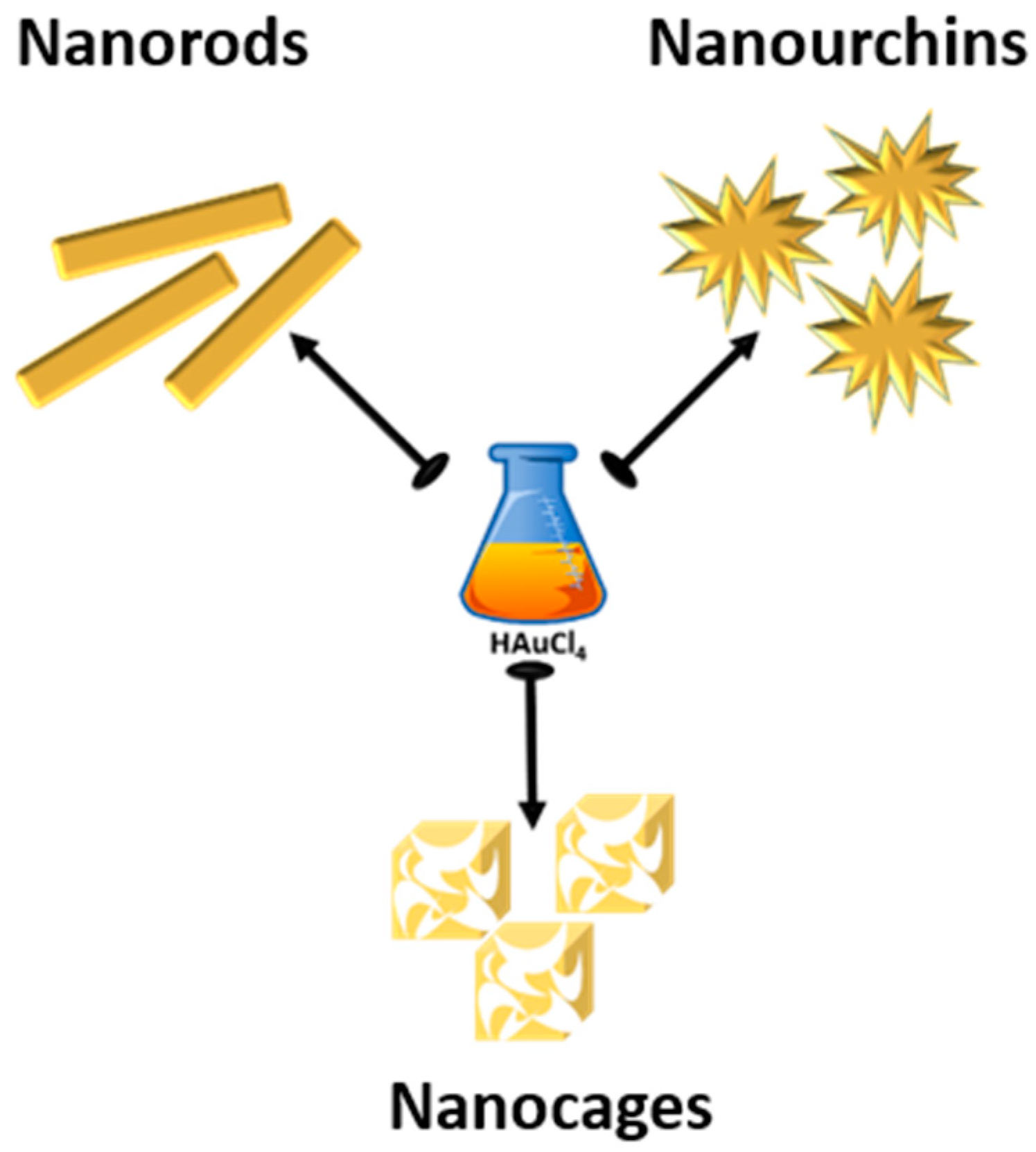
2. Synthesis
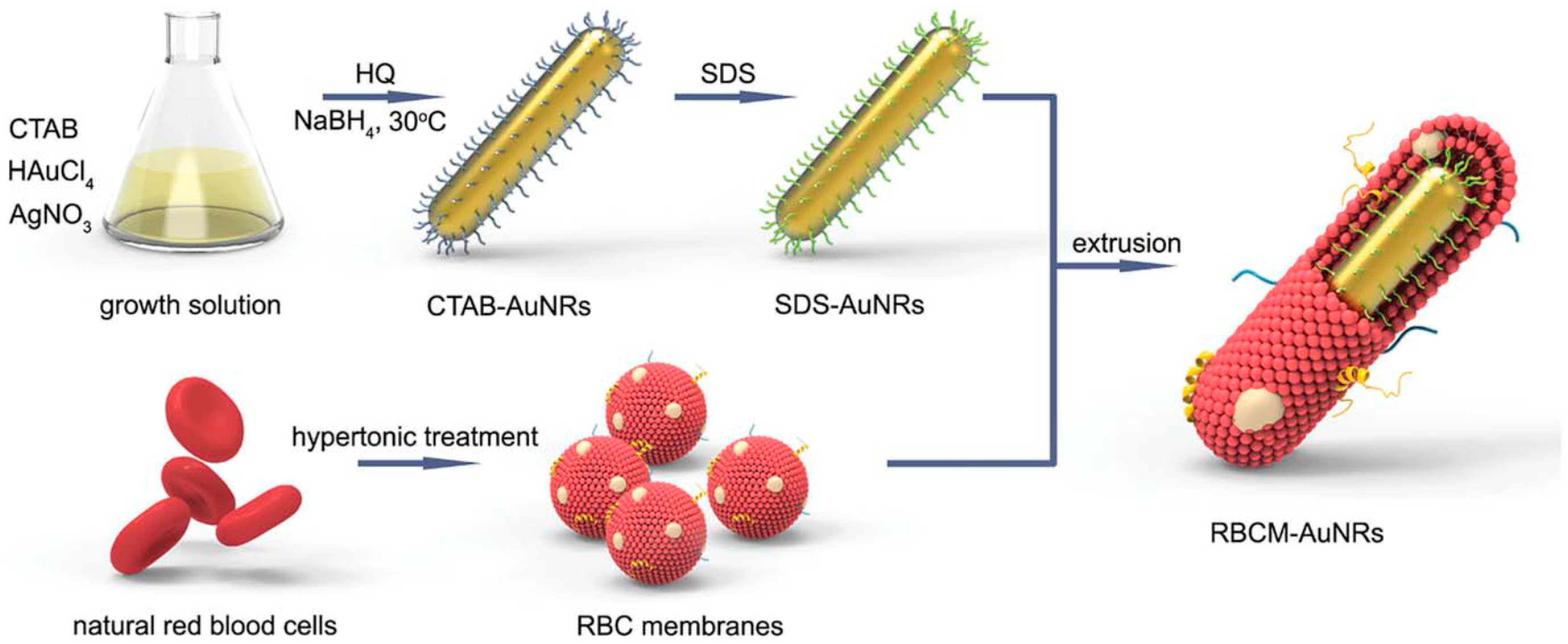
2.1. Synthesis of Gold Nanorods (AuNRs)
2.2. Synthesis of Gold Nanourchins (AuNUs)
2.3. Synthesis of Gold Nanocages (AuNCs)
3. Anisotropic Gold Nanoparticles in Bio-Sensing
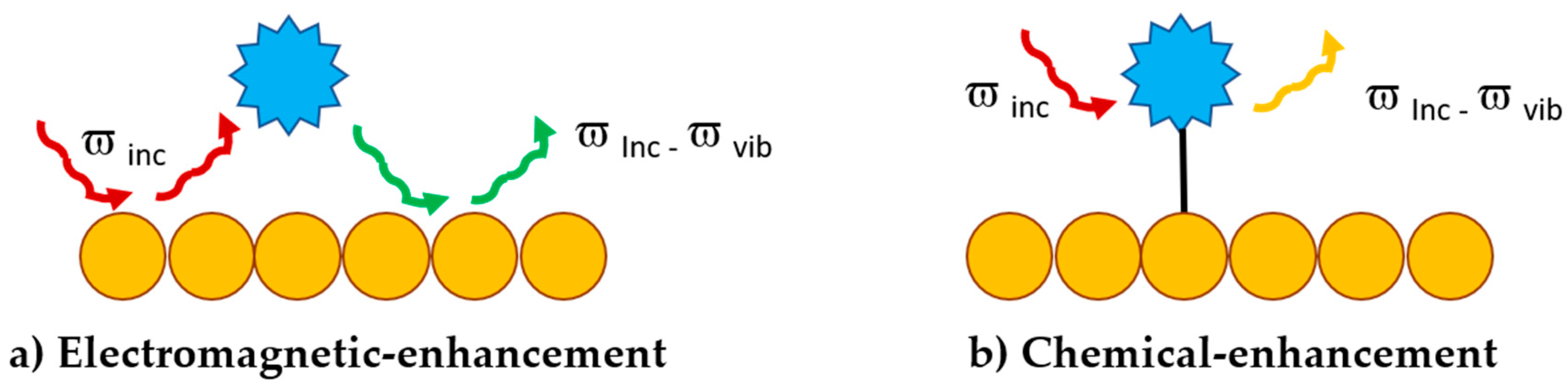
3.1. Surface Enhanced Raman Scattering (SERS)
3.2. Colorimetry
3.3. Surface Plasmon Resonance-Based and Fluorescence-Based Sensors
4. Anisotropic Gold Nanoparticles in Therapeutics and Imaging
4.1. The Photothermal and Photodynamic Therapy (PTT and PDT)
4.2. The Radio-Therapy
4.3. Anisotropic Gold Nanoparticles in Medical Imaging
4.4. Anisotropic Gold Nanoparticles as Theranostic Agents
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Yang, X.; Yang, M.; Pang, B.; Vara, M.; Xia, Y. Gold Nanomaterials at Work in Biomedicine. Chem. Rev. 2015, 115, 10410–10488. [Google Scholar] [CrossRef] [PubMed]
- Compostella, F.; Pitirollo, O.; Silvestri, A.; Polito, L. Glyco-gold nanoparticles: Synthesis and applications. Beilstein J. Org. Chem. 2017, 13. [Google Scholar] [CrossRef] [PubMed]
- Louis, C.; Pluchery, O. Gold Nanoparticles for Physics, Chemistry and Biology; Louis, C., Pluchery, O., Eds.; Imperial College Press: London, UK, 2017. [Google Scholar]
- Attia, Y.A.; Buceta, D.; Requejo, F.G.; Giovanetti, L.J.; López-Quintela, M.A. Photostability of gold nanoparticles with different shapes: The role of Ag clusters. Nanoscale 2015, 7, 11273–11279. [Google Scholar] [CrossRef] [PubMed]
- Mie, G. Beiträge zur Optik trüber Medien, speziell kolloidaler Metallösungen. Ann. Phys. 1908, 330, 377–405. [Google Scholar] [CrossRef]
- Szunerits, S.; Spadavecchia, J.; Boukherroub, R. Surface plasmon resonance: Signal amplification using colloidal gold nanoparticles for enhanced sensitivity. Rev. Anal. Chem. 2014, 33, 153–164. [Google Scholar] [CrossRef]
- Amendola, V.; Pilot, R.; Frasconi, M.; Maragò, O.M.; Iatì, M.A. Surface plasmon resonance in gold nanoparticles: A review. J. Phys. Condens. Matter 2017, 29, 3002–3050. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Zhao, P.; Astruc, D. Anisotropic gold nanoparticles: Synthesis, properties, applications, and toxicity. Angew. Chem. Int. Ed. 2014, 53, 1756–1789. [Google Scholar] [CrossRef] [PubMed]
- Xue, C.; Li, Q. Anisotropic Nanomaterials; Li, Q., Ed.; Springer International Publishing: Berlin, Germany, 2015. [Google Scholar]
- Dykman, L.; Khlebtsov, N. Gold Nanoparticles in Biomedical Applications, 1st ed.; Dykman, L., Khlebtsov, N., Eds.; CRC Press: Boca Raton, FL, USA, 2017; ISBN 9781138560741. [Google Scholar]
- Burrows, N.D.; Vartanian, A.M.; Abadeer, N.S.; Grzincic, E.M.; Jacob, L.M.; Lin, W.; Li, J.; Dennison, J.M.; Hinman, J.G.; Murphy, C.J. Anisotropic Nanoparticles and Anisotropic Surface Chemistry. J. Phys. Chem. Lett. 2016, 7, 632–641. [Google Scholar] [CrossRef] [PubMed]
- Niu, C.; Song, Q.; He, G.; Na, N.; Ouyang, J. Near-infrared-fluorescent probes for bioapplications based on silica-coated gold nanobipyramids with distance-dependent plasmon-enhanced fluorescence. Anal. Chem. 2016, 88, 11062–11069. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, J.A.; Meneghetti, M.R. New Aspects of the Gold Nanorod Formation Mechanism via Seed-Mediated Methods Revealed by Molecular Dynamics Simulations. Langmuir 2018, 34, 366–375. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.Y.; Chang, S.-S.; Lee, C.-L.; Wang, C.R.C. Gold Nanorods: Electrochemical Synthesis and Optical Properties. J. Phys. Chem. B 1997, 101, 6661–6664. [Google Scholar] [CrossRef]
- Jana, N.R.; Gearheart, L.; Murphy, C.J. Wet chemical synthesis of high aspect ratio cylindrical gold nanorods. J. Phys. Chem. B 2001, 105, 4065–4067. [Google Scholar] [CrossRef]
- Nanorods, G.; Busbee, B.B.D.; Obare, S.O.; Murphy, C.J. An Improved Synthesis of High-Aspect-Ratio Gold Nanorods. Adv. Mater. 2003, 15, 414–416. [Google Scholar]
- Nik, B.; Sayed, A.E. Preparation and Growth Mechanism of Gold Nanorods (NRs) Using Seed-Mediated Growth Method. Chem. Mater. 2003, 15, 1957–1962. [Google Scholar] [CrossRef]
- Ye, X.; Zheng, C.; Chen, J.; Gao, Y.; Murray, C.B. Using binary surfactant mixtures to simultaneously improve the dimensional tunability and monodispersity in the seeded growth of gold nanorods. Nano Lett. 2013, 13, 765–771. [Google Scholar] [CrossRef] [PubMed]
- Williams, M.G.; Boyne, D.A.; Griep, M.H. Rapid synthesis of high purity gold nanorods via microwave irradiation. Mater. Res. Express 2017, 4, 035040. [Google Scholar] [CrossRef]
- Wustoni, S.; Phan, D.T. Sustainable synthesis of gold nanorods assisted by cubic-shaped seeds as intermediate particles. Inorg. Chem. Commun. 2018, 93, 78–82. [Google Scholar] [CrossRef]
- Chhatre, A.; Thaokar, R.; Mehra, A. Formation of Gold Nanorods by Seeded Growth: Mechanisms and Modeling. Cryst. Growth Des. 2017, 18, 3269–3282. [Google Scholar] [CrossRef]
- Burrows, N.D.; Harvey, S.; Idesis, F.A.; Murphy, C.J. Understanding the Seed-Mediated Growth of Gold Nanorods through a Fractional Factorial Design of Experiments. Langmuir 2017, 33, 1891–1907. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yao, J.; Luo, J.; Duan, X.; Yao, Y.; Liu, T. Effect of Growth Temperature on Tailoring the Size and Aspect Ratio of Gold Nanorods. Langmuir 2017, 33, 7479–7485. [Google Scholar] [CrossRef] [PubMed]
- Walsh, M.J.; Tong, W.; Katz-Boon, H.; Mulvaney, P.; Etheridge, J.; Funston, A.M. A Mechanism for Symmetry Breaking and Shape Control in Single-Crystal Gold Nanorods. Acc. Chem. Res. 2017, 50, 2925–2935. [Google Scholar] [CrossRef] [PubMed]
- Orendorff, C.J.; Murphy, C.J. Quantitation of metal content in the silver-assisted growth of gold nanorods. J. Phys. Chem. B 2006, 110, 3990–3994. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Juste, J.; Liz-Marzán, L.M.; Carnie, S.; Chan, D.Y.C.; Mulvaney, P. Electric-field-directed growth of gold nanorods in aqueous surfactant solutions. Adv. Funct. Mater. 2004, 14, 571–579. [Google Scholar] [CrossRef]
- Tong, W.; Walsh, M.J.; Mulvaney, P.; Etheridge, J.; Funston, A.M. Control of Symmetry Breaking Size and Aspect Ratio in Gold Nanorods: Underlying Role of Silver Nitrate. J. Phys. Chem. C 2017, 121, 3549–3559. [Google Scholar] [CrossRef]
- Liu, M.; Guyot-Sionnest, P. Mechanism of silver(I)-assisted growth of gold nanorods and bipyramids. J. Phys. Chem. B 2005, 109, 22192–22200. [Google Scholar] [CrossRef] [PubMed]
- Jessl, S.; Tebbe, M.; Guerrini, L.; Fery, A.; Alvarez-Puebla, R.A.; Pazos-Perez, N. Silver-Assisted Synthesis of Gold Nanorods: The Relation between Silver Additive and Iodide Impurities. Small 2018, 14, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Park, K.; Hsiao, M.S.; Yi, Y.J.; Izor, S.; Koerner, H.; Jawaid, A.; Vaia, R.A. Highly Concentrated Seed-Mediated Synthesis of Monodispersed Gold Nanorods. ACS Appl. Mater. Interfaces 2017, 9, 26363–26371. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.M.; Xu, J.; Blahove, M.; Canonico-May, S.A.; Santaloci, T.J.; Braselton, M.E.; Stone, J.W. Synthesis of less toxic gold nanorods by using dodecylethyldimethylammonium bromide as an alternative growth-directing surfactant. J. Colloid Interface Sci. 2017, 505, 1172–1176. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.H.; Murphy, C.J. Mini Gold Nanorods with Tunable Plasmonic Peaks beyond 1000 nm. Chem. Mater. 2018, 30, 1427–1435. [Google Scholar] [CrossRef]
- Silvestri, A.; Zambelli, V.; Ferretti, A.M.; Salerno, D.; Bellani, G.; Polito, L. Design of functionalized gold nanoparticle probes for computed tomography imaging. Contrast Media Mol. Imaging 2016. [Google Scholar] [CrossRef] [PubMed]
- Jana, N.R. Gram-scale synthesis of soluble, near-monodisperse gold nanorods and other anisotropic nanoparticles. Small 2005, 1, 875–882. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Cao, Z.; Panwar, N.; Hu, R.; Wang, X.; Qu, J.; Tjin, S.C.; Xu, G.; Yong, K.T. Functionalized gold nanorods for nanomedicine: Past, present and future. Coord. Chem. Rev. 2017, 352, 15–66. [Google Scholar] [CrossRef]
- Requejo, K.I.; Liopo, A.V.; Derry, P.J.; Zubarev, E.R. Accelerating Gold Nanorod Synthesis with Nanomolar Concentrations of Poly(vinylpyrrolidone). Langmuir 2017, 33, 12681–12688. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, W.; Zhang, X.; Yao, H.; Wei, Z.; Li, X.; Mu, X.; Jiang, J.; Zhang, H. Seedless preparation of Au nanorods by hydroquinone assistant and red blood cell membrane camouflage. RSC Adv. 2018, 8, 21316–21325. [Google Scholar] [CrossRef]
- Liu, K.; Bu, Y.; Zheng, Y.; Jiang, X.; Yu, A.; Wang, H. Seedless Synthesis of Monodispersed Gold Nanorods with Remarkably High Yield: Synergistic Effect of Template Modification and Growth Kinetics Regulation. Chem. A Eur. J. 2017, 23, 3291–3299. [Google Scholar] [CrossRef] [PubMed]
- El Kurdi, R.; Patra, D. Capping of supramolecular curcubit[7]uril facilitates formation of Au nanorods during pre-reduction by curcumin. Colloids Surfaces A Physicochem. Eng. Asp. 2018, 553, 97–104. [Google Scholar] [CrossRef]
- Chen, S.; Wang, Z.L.; Ballato, J.; Foulger, S.H.; Carroll, D.L. Monopod, Bipod, Tripod, and Tetrapod Gold Nanocrystals. J. Am. Chem. Soc. 2003, 125, 16186–16187. [Google Scholar] [CrossRef] [PubMed]
- Sau, T.K.; Murphy, C.J. Room temperature, high-yield synthesis of multiple shapes of gold nanoparticles in aqueous solution. J. Am. Chem. Soc. 2004, 126, 8648–8649. [Google Scholar] [CrossRef] [PubMed]
- Pastoriza-Santos, I.; Liz-Marzán, L.M. N,N-Dimethylformamide as a Reaction Medium for Metal Nanoparticle Synthesis. Adv. Funct. Mater. 2009, 19, 679–688. [Google Scholar] [CrossRef]
- Senthil Kumar, P.; Pastoriza-Santos, I.; Rodríguez-González, B.; Javier García De Abajo, F.; Liz-Marzán, L.M. High-yield synthesis and optical response of gold nanostars. Nanotechnology 2008, 19. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.Y.; Yu, T.; Cho, E.C.; Ma, Y.; Park, O.O.; Xia, Y. Synthesis of gold nano-hexapods with controllable arm lengths and their tunable optical properties. Angew. Chem. Int. Ed. 2011, 50, 6328–6331. [Google Scholar] [CrossRef] [PubMed]
- Pu, Y.; Zhao, Y.; Zheng, P.; Li, M. Elucidating the Growth Mechanism of Plasmonic Gold Nanostars with Tunable Optical and Photothermal Properties. Inorg. Chem. 2018, 57, 8599–8607. [Google Scholar] [CrossRef] [PubMed]
- Sheen Mers, S.V.; Umadevi, S.; Ganesh, V. Controlled Growth of Gold Nanostars: Effect of Spike Length on SERS Signal Enhancement. ChemPhysChem 2017, 18, 1358–1369. [Google Scholar] [CrossRef] [PubMed]
- Pallavicini, P.; Basile, S.; Chirico, G.; Dacarro, G.; D’Alfonso, L.; Donà, A.; Patrini, M.; Falqui, A.; Sironi, L.; Taglietti, A. Monolayers of gold nanostars with two near-IR LSPRs capable of additive photothermal response. Chem. Commun. 2015, 51, 12928–12930. [Google Scholar] [CrossRef] [PubMed]
- Dacarro, G.; Pallavicini, P.; Maria, S.; Chirico, G.; Alfonso, L.D.; Falqui, A.; Marchesi, N.; Pascale, A.; Sironi, L.; Taglietti, A.; et al. Synthesis of reduced-size gold nanostars and internalization in SH-SY5Y cells. J. Colloid Interface Sci. 2017, 505, 1055–1064. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Khoury, C.G.; Hwang, H.; Wilson, C.M.; Grant, G.A.; Vo-Dinh, T. Gold nanostars: Surfactant-free synthesis, 3D modelling, and two-photon photoluminescence imaging. Nanotechnology 2012, 23. [Google Scholar] [CrossRef] [PubMed]
- De Silva Indrasekara, A.S.; Johnson, S.F.; Odion, R.A.; Vo-Dinh, T. Manipulation of the Geometry and Modulation of the Optical Response of Surfactant-Free Gold Nanostars: A Systematic Bottom-Up Synthesis. ACS Omega 2018, 3, 2202–2210. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; Liu, L.; Walsh, T.R.; Gong, Y.; El-Khoury, P.Z.; Zhang, Y.; Zhu, Z.; De Yoreo, J.J.; Engelhard, M.H.; Zhang, X.; et al. Controlled synthesis of highly-branched plasmonic gold nanoparticles through peptoid engineering. Nat. Commun. 2018, 9, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Sasidharan, S.; Bahadur, D.; Srivastava, R. Rapid, One-Pot, Protein-Mediated Green Synthesis of Gold Nanostars for Computed Tomographic Imaging and Photothermal Therapy of Cancer. ACS Sustain. Chem. Eng. 2017, 5, 10163–10175. [Google Scholar] [CrossRef]
- Kariuki, V.M.; Hoffmeier, J.C.; Yazgan, I.; Sadik, O.A. Seedless synthesis and SERS characterization of multi-branched gold nanoflowers using water soluble polymers. Nanoscale 2017, 9, 8330–8340. [Google Scholar] [CrossRef] [PubMed]
- Fortuni, B.; Fujita, Y.; Ricci, M.; Inose, T.; Aubert, R.; Lu, G.; Hutchison, J.A.; Hofkens, J.; Latterini, L.; Uji-I, H. A novel method for in situ synthesis of SERS-active gold nanostars on polydimethylsiloxane film. Chem. Commun. 2017, 53, 5121–5124. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Fan, C.; Wang, J.; He, J.; Liang, E. Surface-enhanced Raman scattering of 4-mercaptobenzoic acid and hemoglobin adsorbed on self-assembled Ag monolayer films with different shapes. Appl. Phys. A Mater. Sci. Process. 2014, 117, 1075–1083. [Google Scholar] [CrossRef]
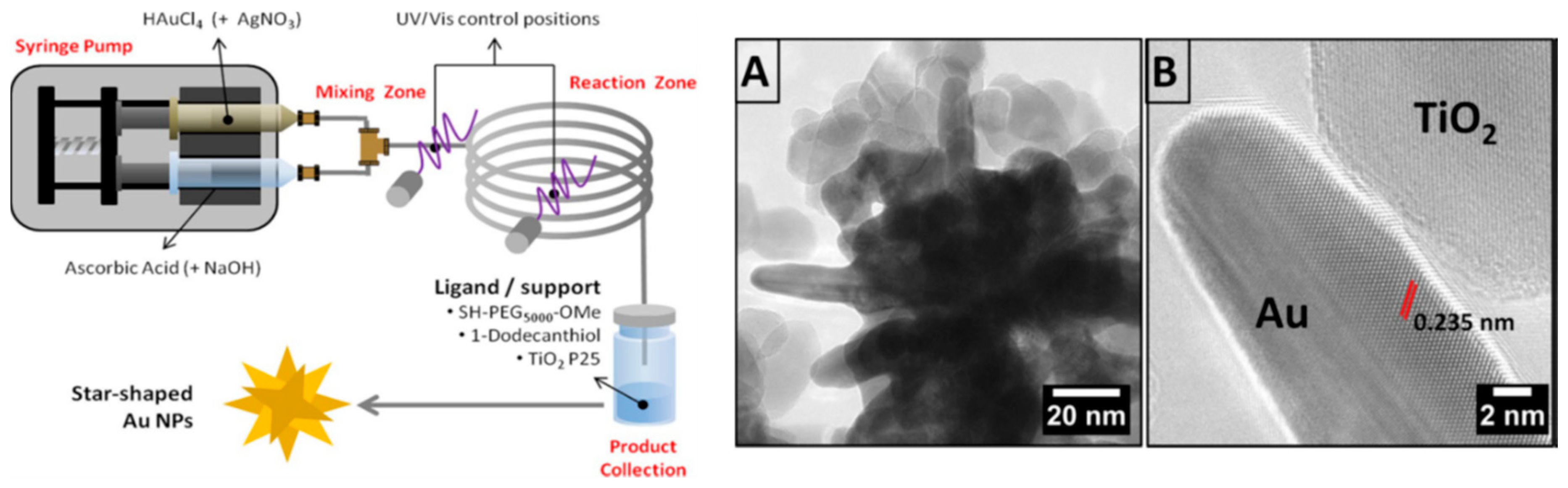
- Silvestri, A.; Lay, L.; Psaro, R.; Polito, L.; Evangelisti, C. Fluidic Manufacture of Star-Shaped Gold Nanoparticles. Chem. A Eur. J. 2017, 23, 9732–9735. [Google Scholar] [CrossRef] [PubMed]
- Sahandi Zangabad, P.; Karimi, M.; Mehdizadeh, F.; Malekzad, H.; Ghasemi, A.; Bahrami, S.; Zare, H.; Moghoofei, M.; Hekmatmanesh, A.; Hamblin, M.R. Nanocaged platforms: Modification, drug delivery and nanotoxicity. Opening synthetic cages to release the tiger. Nanoscale 2017, 9, 1356–1392. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Xia, Y. Mechanistic Study on the Replacement Reaction between Silver Nanostructures and Chloroauric Acid in Aqueous Medium. J. Am. Chem. Soc. 2004, 126, 3892–3901. [Google Scholar] [CrossRef] [PubMed]
- Ogunyankin, M.O.; Shin, J.E.; Lapotko, D.O.; Ferry, V.E.; Zasadzinski, J.A. Optimizing the NIR Fluence Threshold for Nanobubble Generation by Controlled Synthesis of 10–40 nm Hollow Gold Nanoshells. Adv. Funct. Mater. 2018, 28, 1–13. [Google Scholar] [CrossRef]
- Erlebacher, J.; Aziz, M.J.; Karma, A.; Dimitrov, N.; Sieradzki, K. Evolution of nanoporosity in dealloying. Nature 2001, 410, 450–453. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Schaaf, P. Nanoporous gold nanoparticles. J. Mater. Chem. 2012, 22, 5344–5348. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, H.; Zhang, F.; Wu, Y.; Guo, Z.; Zhou, L.; Li, J. Investigation of halide-induced aggregation of Au nanoparticles into spongelike gold. Langmuir 2014, 30, 2648–2659. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.J.; Lim, S.H.; Ha, J.M.; Choi, S.M. Green Synthesis of High-Purity Mesoporous Gold Sponges Using Self-Assembly of Gold Nanoparticles Induced by Thiolated Poly(ethylene glycol). Langmuir 2016, 32, 5937–5945. [Google Scholar] [CrossRef] [PubMed]
- Xue, W.; Luo, L.; Li, Y.; Yin, T.; Bian, K.; Zhu, R.; Gao, D. Fabrication of gold nanocages and nanoshells using lanreotide acetate and a comparison study of their photothermal antitumor therapy. J. Mater. Chem. B 2017. [Google Scholar] [CrossRef]
- Lee, D.H.; Park, J.S.; Hwang, J.H.; Kang, D.; Yim, S.Y.; Kim, J.H. Fabrication of hollow nanoporous gold nanoshells with high structural tunability based on the plasma etching of polymer colloid templates. J. Mater. Chem. C 2018, 6, 6194–6199. [Google Scholar] [CrossRef]
- Wang, L.; Chen, Y.; Lin, H.Y.; Hou, Y.T.; Yang, L.C.; Sun, A.Y.; Liu, J.Y.; Chang, C.W.; Wan, D. Near-IR-absorbing gold nanoframes with enhanced physiological stability and improved biocompatibility for in vivo biomedical applications. ACS Appl. Mater. Interfaces 2017, 9, 3873–3884. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Yang, X.; Yang, Z.; Lin, L.; Liu, Y.; Zhou, Z.; Shen, Z.; Yu, G.; Dai, Y.; Jacobson, O.; et al. Rational Design of Branched Nanoporous Gold Nanoshells with Enhanced Physico-Optical Properties for Optical Imaging and Cancer Therapy. ACS Nano 2017, 11, 6102–6113. [Google Scholar] [CrossRef] [PubMed]
- Silvestri, A.; Mondini, S.; Marelli, M.; Pifferi, V.; Falciola, L.; Ponti, A.; Ferretti, A.M.; Polito, L. Synthesis of Water Dispersible and Catalytically Active Gold-Decorated Cobalt Ferrite Nanoparticles. Langmuir 2016, 32. [Google Scholar] [CrossRef] [PubMed]
- Bora, T. Recent Developments on Metal Nanoparticles for SERS Applications. Noble Precious Met. Prop. Nanoscale Eff. Appl. 2018. [Google Scholar] [CrossRef]
- Schreiber, B.; Gkogkou, D.; Dedelaite, L.; Kerbusch, J.; Hübner, R.; Sheremet, E.; Zahn, D.R.T.; Ramanavicius, A.; Facsko, S.; Rodriguez, R.D. Large-scale self-organized gold nanostructures with bidirectional plasmon resonances for SERS. RSC Adv. 2018, 8, 22569–22576. [Google Scholar] [CrossRef]
- Fleischmann, M.; Hendra, P.J.; McQuillan, A.J. Raman spectra of pyridine adsorbed at a silver electrode. Chem. Phys. Lett. 1974, 26, 163–166. [Google Scholar] [CrossRef]
- Kneipp, K.; Kneipp, K.; Kneipp, H.; Kneipp, H.; Itzkan, I.; Itzkan, I.; Dasari, R.R.; Dasari, R.R.; Feld, M.S.; Feld, M.S. Ultrasensitive chemical analysis by Raman spectroscopy. Chem. Rev. 1999, 99, 2957–2976. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Trujillo, M.J.; Olson, J.E.; Camden, J.P. SERS Sensors: Recent Developments and a Generalized Classification Scheme Based on the Signal Origin. Annu. Rev. Anal. Chem. 2018, 11, 147–169. [Google Scholar] [CrossRef] [PubMed]
- Jensen, L.; Aikens, C.M.; Schatz, G.C. Electronic structure methods for studying surface-enhanced Raman scattering. Chem. Soc. Rev. 2008, 37, 1061–1073. [Google Scholar] [CrossRef] [PubMed]
- Le Ru, E.C.; Blackie, E.J.; Meyer, M.; Etchegoin, P.G. Surface Enhanced Raman Scattering Enhancement Factors: A Comprehensive Study. J. Phys. Chem. C 2007, 111, 13794–13803. [Google Scholar] [CrossRef]
- Ou, Y.; Webb, J.A.; Brien, C.M.O.; Pence, I.J.; Lin, E.C.; Paul, E.P.; Cole, D.; Ou, S.; Lapierre-landry, M.; Delapp, R.C.; et al. Diagnosis of immunomarkers in vivo via multiplexed surface enhanced Raman spectroscopy with gold nanostars. Nanoscale 2018, 10, 13092–13105. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Kyaw, Y.M.E.; Tan, E.K.M.; Bekale, L.; Kang, M.W.C.; Kim, S.S.Y.; Tan, I.; Lam, K.P.; Kah, J.C.Y. Quantitative and Label-Free Detection of Protein Kinase A Activity Based on Surface-Enhanced Raman Spectroscopy with Gold Nanostars. Anal. Chem. 2018, 90, 6071–6080. [Google Scholar] [CrossRef] [PubMed]
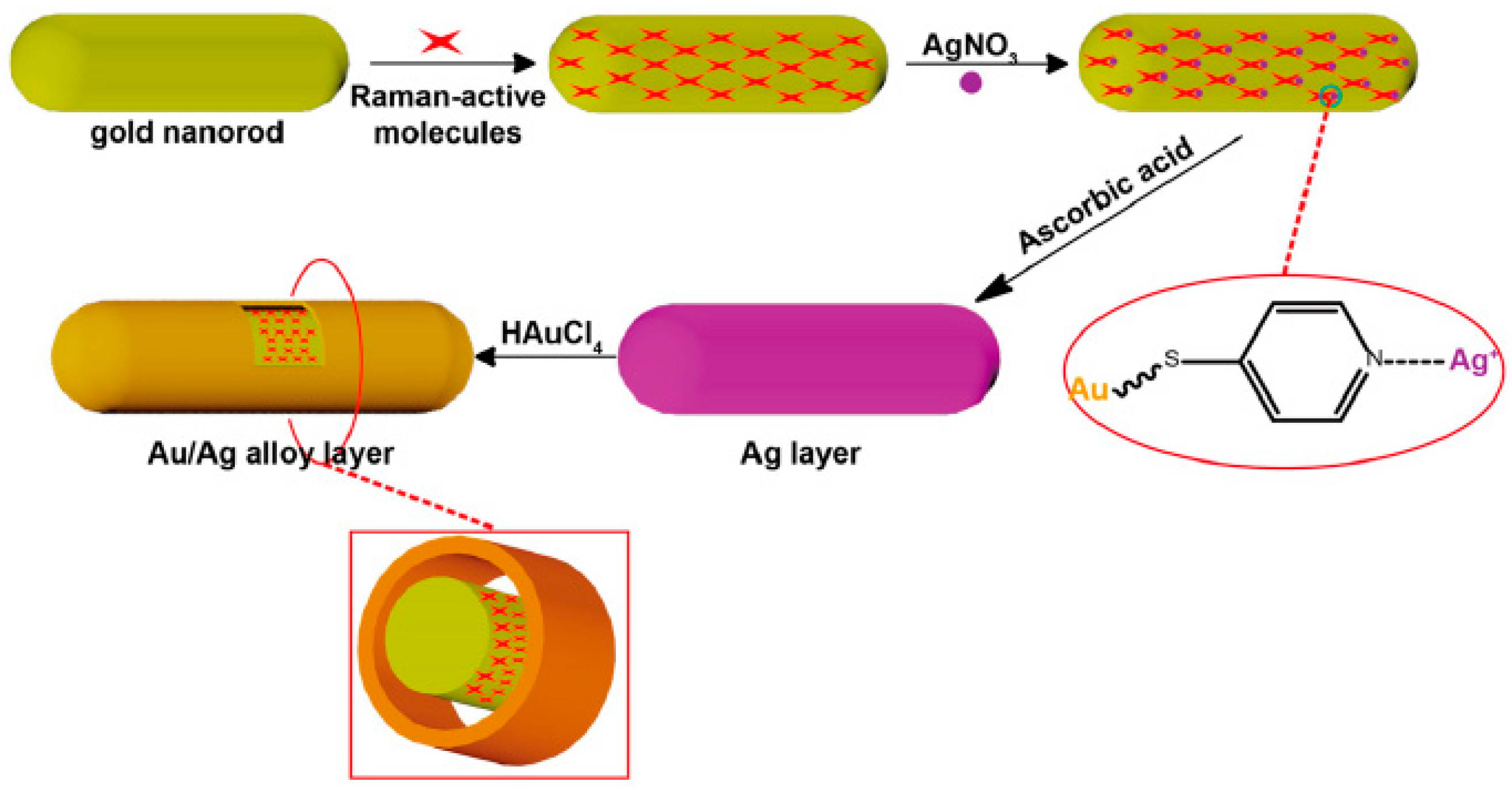
- Pissuwan, D.; Hattori, Y. Detection of adhesion molecules on inflamed macrophages at early-stage using SERS probe gold nanorods. Nano-Micro Lett. 2017, 9, 1–9. [Google Scholar] [CrossRef]
- Gao, X.; Zheng, P.; Kasani, S.; Wu, S.; Yang, F.; Lewis, S.; Nayeem, S.; Engler-Chiurazzi, E.B.; Wigginton, J.G.; Simpkins, J.W.; et al. Paper-Based Surface-Enhanced Raman Scattering Lateral Flow Strip for Detection of Neuron-Specific Enolase in Blood Plasma. Anal. Chem. 2017, 89, 10104–10110. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, P.; Habeeb Muhammed, M.A.; Alsaiari, S.K.; Moosa, B.; Almalik, A.; Kumar, A.; Ringe, E.; Khashab, N.M. Tunable and Linker Free Nanogaps in Core-Shell Plasmonic Nanorods for Selective and Quantitative Detection of Circulating Tumor Cells by SERS. ACS Appl. Mater. Interfaces 2017, 9, 37597–37605. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Shan, Y.; Tan, L.; Yu, X.; Bao, M.; Li, W.; Shi, H. Hollow Au nanoflower substrates for identification and discrimination of the differentiation of bone marrow mesenchymal stem cells by surface-enhanced Raman spectroscopy. J. Mater. Chem. B 2017, 5, 5983–5995. [Google Scholar] [CrossRef]
- Reyes, M.; Piotrowski, M.; Ang, S.K.; Chan, J.; He, S.; Chu, J.J.H.; Kah, J.C.Y. Exploiting the Anti-Aggregation of Gold Nanostars for Rapid Detection of Hand, Foot, and Mouth Disease Causing Enterovirus 71 Using Surface-Enhanced Raman Spectroscopy. Anal. Chem. 2017, 89, 5373–5381. [Google Scholar] [CrossRef] [PubMed]
- Kang, W.C.M.; Wong, Y.L.; Piotrowski, M.; Teo, W.P.J.; He, S.; Kah, J.C. A gold nanostar-based SERS platform for point-of-care diagnostics of carbapenemase-producing enterobacteriacae. Proc. SPIE 2017, 10340, 1034011. [Google Scholar] [CrossRef]
- Jazayeri, M.H.; Aghaie, T.; Avan, A.; Vatankhah, A.; Ghaffari, M.R.S. Colorimetric detection based on gold nano particles (GNPs): An easy, fast, inexpensive, low-cost and short time method in detection of analytes (protein, DNA, and ion). Sens. Bio-Sensing Res. 2018, 20, 1–8. [Google Scholar] [CrossRef]
- Saha, K.; Agasti, S.S.; Kim, C.; Li, X.; Rotello, V.M. Gold nanoparticles in chemical and biological sensing. Chem. Rev. 2012, 112, 2739–2779. [Google Scholar] [CrossRef] [PubMed]
- Khanna, V.K. Nanoparticle-based sensors. Def. Sci. J. 2008, 58, 608–616. [Google Scholar] [CrossRef]
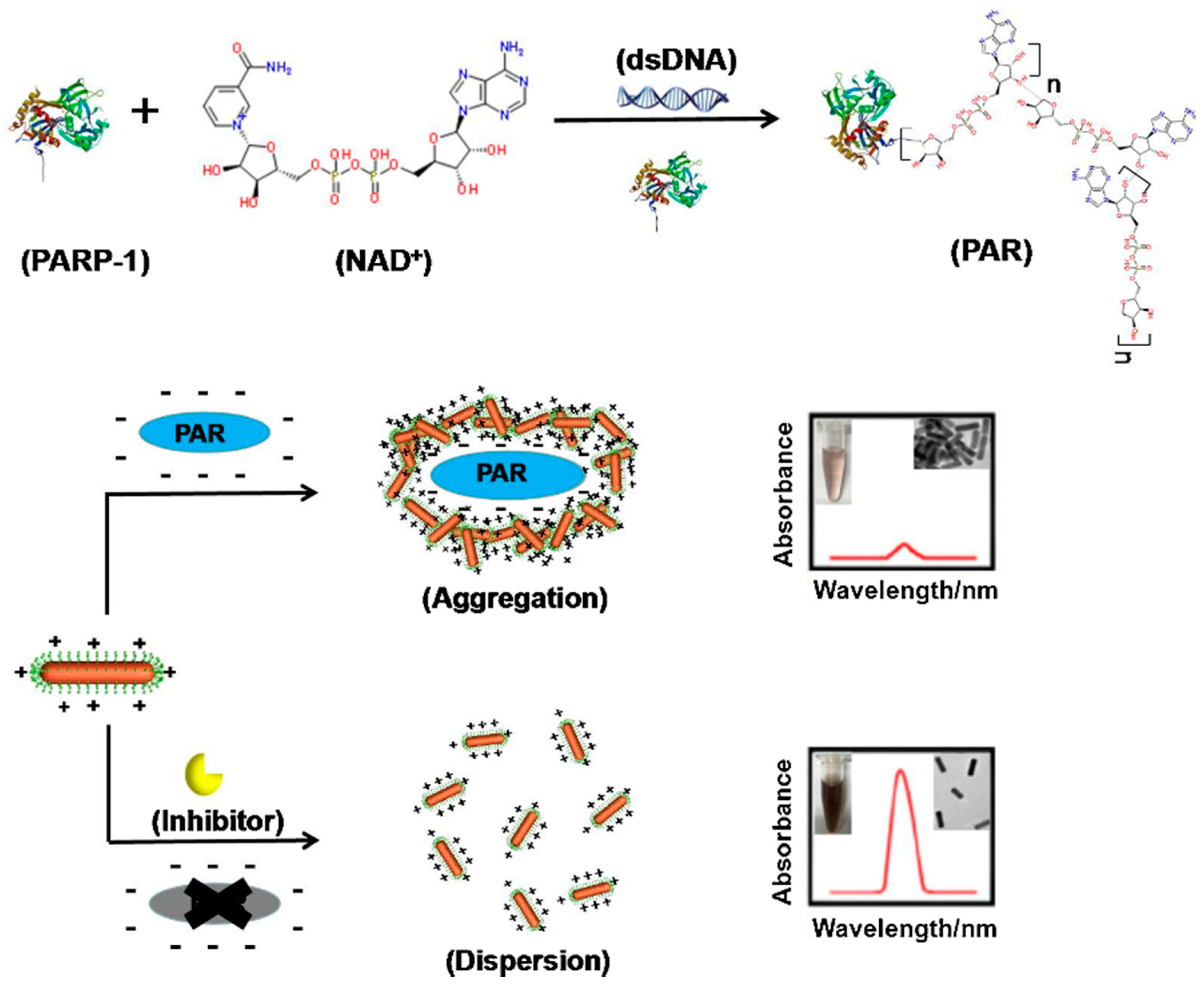
- Wu, S.; Wei, M.; Yang, H.; Fan, J.; Wei, W.; Zhang, Y.; Liu, S. Counterions-mediated gold nanorods-based sensor for label-free detection of poly(ADP-ribose) polymerase-1 activity and its inhibitor. Sensors Actuators B Chem. 2018, 259, 565–572. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, J.; Cao, Y.; Li, G. Gold nanoparticles based colorimetric assay of protein poly(ADP-ribosyl)ation. Analyst 2011, 136, 2044. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Ouyang, W.; Xie, P.; Lin, Y.; Qiu, B.; Lin, Z.; Chen, G.; Guo, L. Highly Uniform Gold Nanobipyramids for Ultrasensitive Colorimetric Detection of Influenza Virus. Anal. Chem. 2017, 89, 1617–1623. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, C.M.; Shimizu, F.M.; Ferreira, M. 6-Surface Plasmon Resonance (SPR) for Sensors and Biosensors. In Nanocharacterization Techniques; Róz, A.L., Da Ferreira, M., de Lima Leite, F., Oliveira, O.N., Eds.; Micro and Nano Technologies; William Andrew Publishing: Norwich, NY, USA, 2017; pp. 183–200. ISBN 978-0-323-49778-7. [Google Scholar]
- Victoria, S. Application of Surface Plasmon Resonance (SPR) for the Detection of Single Viruses and Single Biological Nano-objects. J. Bacteriol. Parasitol. 2012, 3. [Google Scholar] [CrossRef]
- Law, W.-C.; Yong, K.-T.; Baev, A.; Hu, R.; Prasad, P.N. Nanoparticle enhanced surface plasmon resonance biosensing: Application of gold nanorods. Opt. Express 2009, 17, 19041–19046. [Google Scholar] [CrossRef] [PubMed]
- Hao, K.; He, Y.; Lu, H.; Pu, S.; Zhang, Y.; Dong, H.; Zhang, X. High-sensitive surface plasmon resonance microRNA biosensor based on streptavidin functionalized gold nanorods-assisted signal amplification. Anal. Chim. Acta 2017, 954, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Xing, T.Y.; Zhao, J.; Weng, G.J.; Zhu, J.; Li, J.J.; Zhao, J.W. Specific Detection of Carcinoembryonic Antigen Based on Fluorescence Quenching of Hollow Porous Gold Nanoshells with Roughened Surface. ACS Appl. Mater. Interfaces 2017, 9, 36632–36641. [Google Scholar] [CrossRef] [PubMed]
- Niidome, Y.; Haine, A.T.; Niidome, T. Anisotropic Gold-based Nanoparticles: Preparation, Properties, and Applications. Chem. Lett. 2016, 45, 488–498. [Google Scholar] [CrossRef]
- Singh, P.; Pandit, S.; Mokkapati, V.R.S.S.; Garg, A.; Ravikumar, V.; Mijakovic, I. Gold Nanoparticles in Diagnostics and Therapeutics for Human Cancer. Int. J. Mol. Sci. 2018, 19, 1979. [Google Scholar] [CrossRef] [PubMed]
- Ong, Z.Y.; Chen, S.; Nabavi, E.; Regoutz, A.; Payne, D.J.; Elson, D.S.; Dexter, D.T.; Dunlop, I.E.; Porter, A.E. Multibranched Gold Nanoparticles with Intrinsic LAT-1 Targeting Capabilities for Selective Photothermal Therapy of Breast Cancer. ACS Appl. Mater. Interfaces 2017, 9, 39259–39270. [Google Scholar] [CrossRef] [PubMed]
- Manivasagan, P.; Bharathiraja, S.; Moorthy, M.S.; Mondal, S.; Nguyen, T.P.; Kim, H.; Phan, T.T.V.; Lee, K.D.; Oh, J. Biocompatible chitosan oligosaccharide modified gold nanorods as highly effective photothermal agents for ablation of breast cancer cells. Polymers 2018, 10, 232. [Google Scholar] [CrossRef]
- Chen, S.; Fan, J.; Qiu, W.; Liu, F.; Yan, G.; Zeng, X.; Zhang, X. A cellular/intranuclear dual-targeting nanoplatform based on gold nanostar for accurate tumor photothermal therapy. J. Mater. Chem. B 2018, 6, 1543–1551. [Google Scholar] [CrossRef]
- Yang, J.; Lei, Q.; Han, K.; Gong, Y.-H.; Chen, S.; Cheng, H.; Cheng, S.-X.; Zhuo, R.-X.; Zhang, X.-Z. Reduction-sensitive polypeptides incorporated with nuclear localization signal sequences for enhanced gene delivery. J. Mater. Chem. 2012, 22, 13591–13599. [Google Scholar] [CrossRef]
- Yin, D.; Li, X.; Ma, Y.; Liu, Z. Targeted cancer imaging and photothermal therapy via monosaccharide-imprinted gold nanorods. Chem. Commun. 2017, 53, 6716–6719. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhi, X.; Yang, M.; Zhang, J.; Lin, L.; Zhao, X.; Hou, W.; Zhang, C.; Zhang, Q.; Pan, F.; et al. Tumor-triggered drug release from calcium carbonate-encapsulated gold nanostars for near-infrared photodynamic/photothermal combination antitumor therapy. Theranostics 2017, 7, 1650–1662. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.; Sano, M.; Jenkins, C.H.; Zhang, G.; Vernekohl, D.; Zhao, W.; Wei, C.; Zhang, Y.; Zhang, Z.; Liu, Y.; et al. Synergistically Enhancing the Therapeutic Effect of Radiation Therapy with Radiation Activatable and Reactive Oxygen Species-Releasing Nanostructures. ACS Nano 2018, 12, 4946–4958. [Google Scholar] [CrossRef] [PubMed]
- Detappe, A.; Kunjachan, S.; Drané, P.; Kotb, S.; Myronakis, M.; Biancur, D.E.; Ireland, T.; Wagar, M.; Lux, F.; Tillement, O.; et al. Key clinical beam parameters for nanoparticle-mediated radiation dose amplification. Sci. Rep. 2016, 6, 34040. [Google Scholar] [CrossRef] [PubMed]
- Goel, S.; Ni, D.; Cai, W. Harnessing the Power of Nanotechnology for Enhanced Radiation Therapy. ACS Nano 2017, 11, 5233–5237. [Google Scholar] [CrossRef] [PubMed]
- Sharmah, A.; Yao, Z.; Lu, L.; Guo, T. X-ray-Induced Energy Transfer between Nanomaterials under X-ray Irradiation. J. Phys. Chem. C 2016, 120, 3054–3060. [Google Scholar] [CrossRef]
- Cheng, N.N.; Starkewolf, Z.; Davidson, R.A.; Sharmah, A.; Lee, C.; Lien, J.; Guo, T. Chemical Enhancement by Nanomaterials under X-ray Irradiation. J. Am. Chem. Soc. 2012, 134, 1950–1953. [Google Scholar] [CrossRef] [PubMed]
- Carter, J.D.; Cheng, N.N.; Qu, Y.; Suarez, G.D.; Guo, T. Nanoscale Energy Deposition by X-ray Absorbing Nanostructures. J. Phys. Chem. B 2007, 111, 11622–11625. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; DuBois, R.N. The role of COX-2 in intestinal inflammation and colorectal cancer. Oncogene 2009, 29, 781. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.K.K.; Yiu Sung, J.J.; Lee, C.W.; Yu, J.; Cho, C.H. Cyclooxygenase-2 in tumorigenesis of gastrointestinal cancers: An update on the molecular mechanisms. Cancer Lett. 2010, 295, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, N.; Chaki, R.; Mandal, V.; Mandal, S.C. COX-2 as a target for cancer chemotherapy. Pharmacol. Rep. 2010, 62, 233–244. [Google Scholar] [CrossRef]
- Xu, L.; Stevens, J.; Hilton, M.B.; Seaman, S.; Conrads, T.P.; Veenstra, T.D.; Logsdon, D.; Morris, H.; Swing, D.A.; Patel, N.L.; et al. COX-2 Inhibition Potentiates Antiangiogenic Cancer Therapy and Prevents Metastasis in Preclinical Models. Sci. Transl. Med. 2014, 6, 242ra84. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Liu, W.; Cheng, Z.; Yao, K.; Yang, Y.; Xu, B.; Su, G. Targeted delivery of siRNA with pH-responsive hybrid gold nanostars for cancer treatment. Int. J. Mol. Sci. 2017, 18, 2029. [Google Scholar] [CrossRef]
- Mankoff, D.A. A definition of molecular imaging. J. Nucl. Med. 2007, 48, 18N–21N. [Google Scholar] [PubMed]
- Silvestri, A.; Polito, L.; Bellani, G.; Zambelli, V.; Jumde, R.P.; Psaro, R.; Evangelisti, C. Gold nanoparticles obtained by aqueous digestive ripening: Their application as X-ray contrast agents. J. Colloid Interface Sci. 2015, 439, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Chaix, A.; Rajoua, K.; Stonajovic, V.; El Cheikh, K.; Bouffard, E.; Brocéro, A.; Garcia, M.; Maynadier, M.; Morère, A.; Gary-Bobo, M.; et al. Two-Photon Fluorescence Imaging and Therapy of Cancer Cells with Anisotropic Gold-Nanoparticle-Supported Porous Silicon Nanostructures. ChemNanoMat 2018, 4, 343–347. [Google Scholar] [CrossRef]
- Kim, T.; Zhang, Q.; Li, J.; Zhang, L.; Jokerst, J. V A Gold/Silver Hybrid Nanoparticle for Treatment and Photoacoustic Imaging of Bacterial Infection. ACS Nano 2018, 12, 5615–5625. [Google Scholar] [CrossRef] [PubMed]
- Andreev, O.A.; Karabadzhak, A.G.; Weerakkody, D.; Andreev, G.O.; Engelman, D.M.; Reshetnyak, Y.K. pH (low) insertion peptide (pHLIP) inserts across a lipid bilayer as a helix and exits by a different path. Proc. Natl. Acad. Sci. USA 2010, 107, 4081–4086. [Google Scholar] [CrossRef] [PubMed]
- Sosunov, E.A.; Anyukhovsky, E.P.; Sosunov, A.A.; Moshnikova, A.; Wijesinghe, D.; Engelman, D.M.; Reshetnyak, Y.K.; Andreev, O.A. pH (low) insertion peptide (pHLIP) targets ischemic myocardium. Proc. Natl. Acad. Sci. USA 2013, 110, 82–86. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Daniels, J.; Moshnikova, A.; Kuznetsov, S.; Ahmed, A.; Engelman, D.M.; Reshetnyak, Y.K.; Andreev, O.A. pHLIP peptide targets nanogold particles to tumors. Proc. Natl. Acad. Sci. USA 2013, 110, 465–470. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Zhang, Y.; Teng, Z.; Tian, W.; Luo, S.; Kong, X.; Su, X.; Tang, Y.; Wang, S.; Lu, G. pH-dependent transmembrane activity of peptide-functionalized gold nanostars for computed tomography/photoacoustic imaging and photothermal therapy. ACS Appl. Mater. Interfaces 2017, 9, 2114–2122. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhang, Y.; Li, Z.; Mei, E.; Lin, J.; Li, F.; Chen, C.; Qing, X.; Hou, L.; Xiong, L.; et al. Light-Responsive Biodegradable Nanorattles for Cancer Theranostics. Adv. Mater. 2018, 30, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, D.S.; Prasad, R.; Devrukhkar, J.; Selvaraj, K.; Srivastava, R. Disintegrable NIR Light Triggered Gold Nanorods Supported Liposomal Nanohybrids for Cancer Theranostics. Bioconjug. Chem. 2018, 29, 1510–1518. [Google Scholar] [CrossRef] [PubMed]
- Li Volsi, A.; Scialabba, C.; Vetri, V.; Cavallaro, G.; Licciardi, M.; Giammona, G. Near-Infrared Light Responsive Folate Targeted Gold Nanorods for Combined Photothermal-Chemotherapy of Osteosarcoma. ACS Appl. Mater. Interfaces 2017, 9, 14453–14469. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Chen, X.; Zhang, L.; Li, L.; Su, Z.M.; Wang, C. Spadix-Bract Structured Nanobowls for Bimodal Imaging-Guided Multidrug Chemo-Photothermal Synergistic Therapy. Chem. Mater. 2018, 30, 3722–3733. [Google Scholar] [CrossRef]
- Zhong, Y.; Goltsche, K.; Cheng, L.; Xie, F.; Meng, F.; Deng, C.; Zhong, Z.; Haag, R. Hyaluronic acid-shelled acid-activatable paclitaxel prodrug micelles effectively target and treat CD44-overexpressing human breast tumor xenografts in vivo. Biomaterials 2016, 84, 250–261. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.Y.; Yoon, H.Y.; Kim, J.-H.; Bae, S.M.; Park, R.-W.; Kang, Y.M.; Kim, I.-S.; Kwon, I.C.; Choi, K.; Jeong, S.Y.; et al. Smart Nanocarrier Based on PEGylated Hyaluronic Acid for Cancer Therapy. ACS Nano 2011, 5, 8591–8599. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Lei, Q.; Qiu, W.-X.; Liu, L.-H.; Zheng, D.-W.; Fan, J.-X.; Rong, L.; Sun, Y.-X.; Zhang, X.-Z. Mitochondria-targeting “Nanoheater” for enhanced photothermal/chemo-therapy. Biomaterials 2017, 117, 92–104. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Chen, Z.; Liu, Z.; Shi, P.; Dong, K.; Ju, E.; Ren, J.; Qu, X. A multi-stimuli responsive gold nanocage-hyaluronic platform for targeted photothermal and chemotherapy. Biomaterials 2014, 35, 9678–9688. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Qian, J.; Hou, G.; Suo, A.; Wang, Y.; Wang, J.; Sun, T.; Yang, M.; Wan, X.; Yao, Y. Hyaluronic Acid-Functionalized Gold Nanorods with pH/NIR Dual-Responsive Drug Release for Synergetic Targeted Photothermal Chemotherapy of Breast Cancer. ACS Appl. Mater. Interfaces 2017, 9, 36533–36547. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, C.; Wang, X.; Wang, X.; Cheng, L.; Li, Y.; Liu, Z. Mesoporous Silica Coated Single-Walled Carbon Nanotubes as a Multifunctional Light-Responsive Platform for Cancer Combination Therapy. Adv. Funct. Mater. 2014, 25, 384–392. [Google Scholar] [CrossRef]
- Wang, C.; Xu, H.; Liang, C.; Liu, Y.; Li, Z.; Yang, G.; Cheng, L.; Li, Y.; Liu, Z. Iron Oxide @ Polypyrrole Nanoparticles as a Multifunctional Drug Carrier for Remotely Controlled Cancer Therapy with Synergistic Antitumor Effect. ACS Nano 2013, 7, 6782–6795. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Liu, J.; Yu, J.; Wang, X.; Zheng, H.; Xu, Y.; Chen, M.; Han, J.; Liu, Z.; Zhang, Q. Synthesis of Janus Au@periodic mesoporous organosilica (PMO) nanostructures with precisely controllable morphology: A seed-shape defined growth mechanism. Nanoscale 2017, 9, 4826–4834. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.R.K.; Ali, H.R.; Rankin, C.R.; El-Sayed, M.A. Targeting heat shock protein 70 using gold nanorods enhances cancer cell apoptosis in low dose plasmonic photothermal therapy. Biomaterials 2016, 102, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhou, Z.; Zhang, F.; Xu, H.; Chen, W.; Jiang, T. A novel nanocomposite based on fluorescent turn-on gold nanostars for near-infrared photothermal therapy and self-theranostic caspase-3 imaging of glioblastoma tumor cell. Colloids Surfaces B Biointerfaces 2018, 170, 303–311. [Google Scholar] [CrossRef] [PubMed]
- An, J.; Yang, X.Q.; Cheng, K.; Song, X.L.; Zhang, L.; Li, C.; Zhang, X.S.; Xuan, Y.; Song, Y.Y.; Fang, B.Y.; et al. In Vivo Computed Tomography/Photoacoustic Imaging and NIR-Triggered Chemo-Photothermal Combined Therapy Based on a Gold Nanostar-, Mesoporous Silica-, and Thermosensitive Liposome-Composited Nanoprobe. ACS Appl. Mater. Interfaces 2017, 9, 41748–41759. [Google Scholar] [CrossRef] [PubMed]





| NP Shape | Sensing Technique | Scope | References |
|---|---|---|---|
| AuNUs | SERS | Targeted diagnosis of immunomarker programmed death ligand 1 (PD-L1) and detection of epidermal growth factor receptor (EGFR) in breast cancer tumors in vivo. | [76] |
| AuNUs | SERS | Quantitative measurement of extracellular protein kinase A (PKA) activity as cancer biomarker. | [77] |
| AuNRs | SERS | Detection of intercellular adhesion molecule-1 (ICAM-1) in macrophages and in endothelial cells as indicators for clinical diagnosis of diseases correlated to inflammation. | [78] |
| AuNUs | SERS | Protein, lipid, nucleic acid, amino acids and carbohydrates to detect differentiation of BMSCs (bone marrow mesenchymal stem cells). | [81] |
| AuNUs | SERS | Development of a lateral flow strip for neuron-specific enolase (a traumatic brain injury protein biomarker) detection in blood plasma. | [79] |
| AuNRs | SERS | Circulating tumor cells detection. | [80] |
| AuNUs | SERS | Enterovirus 71 (EV71) detection. | [82] |
| AuNUs | SERS | Semi-quantitative methodology to detect carbapenemase activity in patients. | [83] |
| AuNRs | Colorimetry | Poly (ADP-ribose) measure, to detect poly(ADP-ribose) polymerase-1 (PARP-1) activity. | [87] |
| AuNUs | Colorimetry | Influenza virus detection. | [89] |
| AuNR | SPR | Design of a sensitive microRNA biosensor for disease diagnosis, treatment and prognosis. | [93] |
| AuNCs | Fluorescence quenching | Synthesis of a novel carcinoembryonic antigen (a cancer biomarker) probe. | [94] |
| Nanoparticle | NP Size | Outcome | Cell Line | Ref. |
|---|---|---|---|---|
| AuNCs or AuNShs | Diameter: 120 nm/150 nm | EPR targeting effect and PTT efficiency | HeLa cells | [64] |
| AuNRs-LA-COS | Length: 26 ± 3.1 nm Diameter: 6.8 ± 1.7 nm | PTT efficiency | MDA-MB-231 | [98] |
| AuNU-NLS@HA | Diameter: 93.2 nm | PTT efficiency | NIH3T3, MCF-7, 4T1 | [99] |
| SA-imprinted AuNRs@SiO2 | Length: 40 nm Width: 10 nm | Effective cancer-cell-targeting and PTT efficiency | HepG-2, L-02 | [101] |
| AuNUs | Diameter: 46, 70 and 90 nm | PTT and chemotherapeutic efficiency | MCF-7, MDA-MB-231, MDA-MB-468, MDA-MB-453, MCF-10A | [97] |
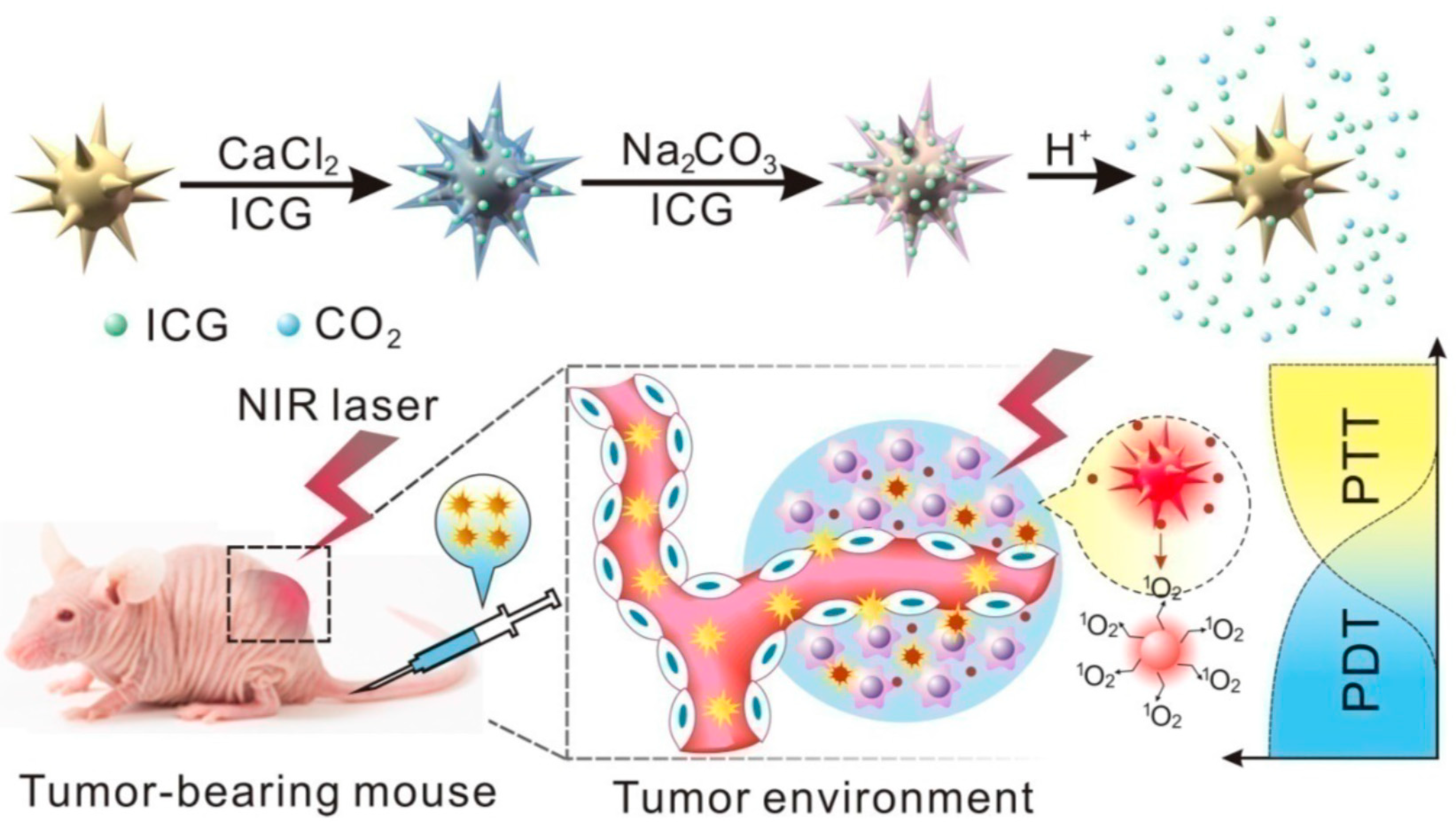
| AuNU@CaCO3/ICG | Diameter: 96 ± 7.5 nm | PDT/PTT efficiency | MGC803 | [102] |
| Au@TiO2 (DATs) | Diameter: 70.1 ± 4.9 nm | PDT efficiency | SUM159 | [103] |
| AuNUs, 9R-AuNUs, 9R/DG- AuNUs, 9R/DG-AuNUs hydrazone | Diameter: 72.5 ± 3.2, 77.8 ± 9.7, 230.7 ± 8.6, and 210.5 ± 10.3 nm | Delivery of siCOX-2; PTT efficiency | HepG2, SGC7901 | [113] |
| pSiNP-SH/AuNP pSiNP-SH/AuNP/Man | Diameter: 270 nm Diameter: 328 nm | Oxidative stress; 2-photon imaging | MCF-7 | [116] |
| Au/Ag hybrid nanoparticles | Width: 12−14 nm Length: 50 nm | PA imaging; antibacterial activity | SKOV3 | [117] |
| AuNU-pHLIP | Diameter: 60 nm | CT/PA-guided PTT efficiency | MCF-7 | [121] |
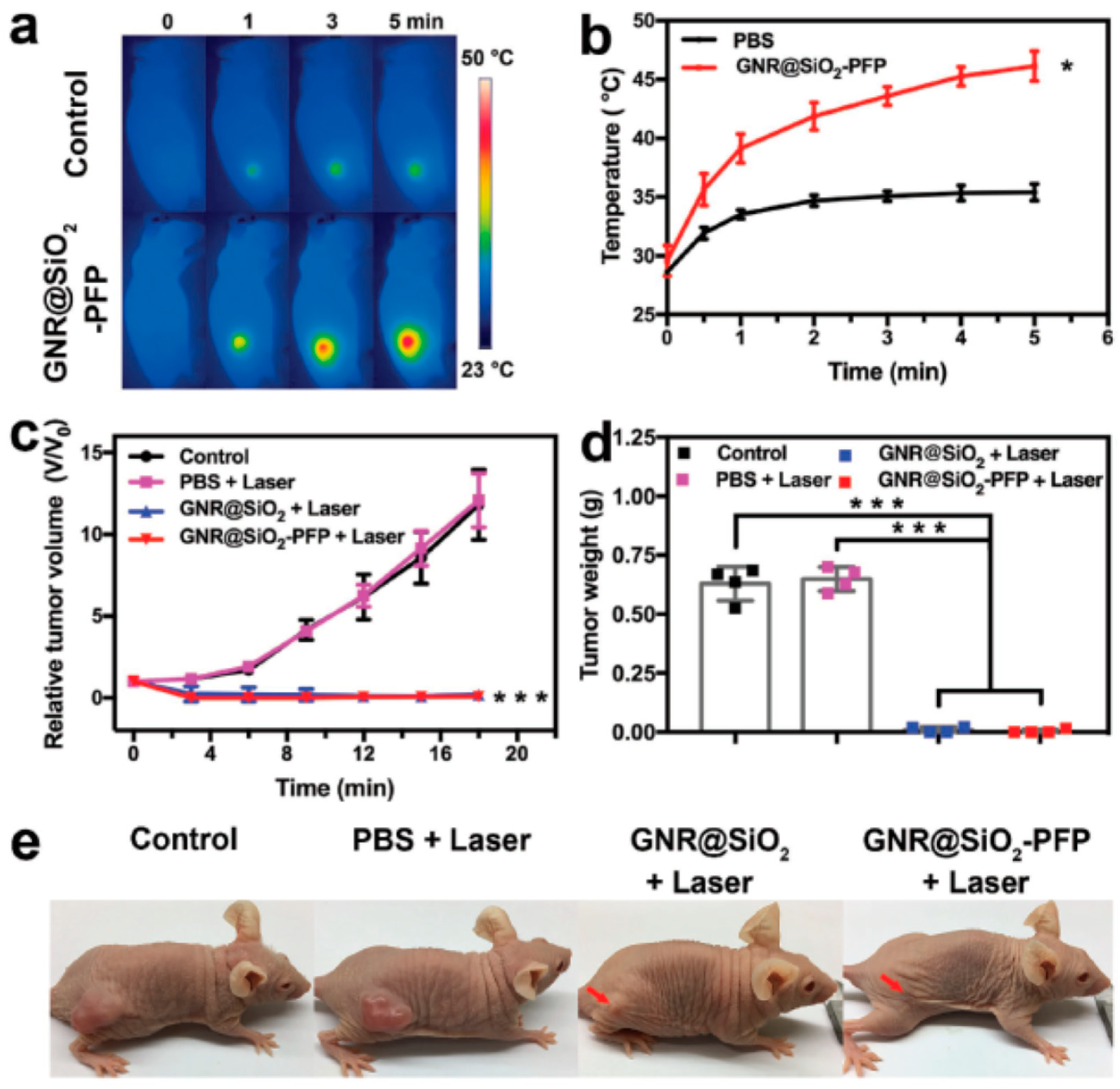
| AuNR@SiO2-PFP | Length: 52.24 ± 17.21 nm Width: 16.81 ± 3.97 nm | US/PA imaging guided PTT efficiency | A375 | [122] |
| AuNR-Lipos | Length: ~27 nm Width: ~9 nm | synergistic chemo/PTT | MDA-MB-231 | [123] |
| AuNRs@INU-LA- PEG-FA and AuNRs@PHEA-EDA-FA | Length: ~70 nm | synergistic chemo/PTT | U2OS | [124] |
| DT-AuNR/PDA bowl spadix-bract NP | Diameter: 90 ± 13 nm | Chemo/PTT therapy CT/PA imaging | Hep-G2, HeLa, MCF-7 | [125] |
| GNRs-HA-FA-DOX | Diameter: 70.9 ± 1.4 nm | Chemo/PTT therapy | MCF-7 | [130] |
| AuNR@PMO-PEG/DOX AuNR@PMO-PEG | Length: 578.5 ± 42.6 nm Diameter: 112.4 ± 12.8 nm | Drug delivery | 4T1 murine breast cancer cells | [133] |
| AuNU@probe | Diameter: 35 nm | PTT efficacy | U87-MG | [135] |
| GMS/DOX@SLB-FA | Diameter: 150 nm | Drug delivery | HeLa, A549 | [136] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kohout, C.; Santi, C.; Polito, L. Anisotropic Gold Nanoparticles in Biomedical Applications. Int. J. Mol. Sci. 2018, 19, 3385. https://doi.org/10.3390/ijms19113385
Kohout C, Santi C, Polito L. Anisotropic Gold Nanoparticles in Biomedical Applications. International Journal of Molecular Sciences. 2018; 19(11):3385. https://doi.org/10.3390/ijms19113385
Chicago/Turabian StyleKohout, Claudia, Cristina Santi, and Laura Polito. 2018. "Anisotropic Gold Nanoparticles in Biomedical Applications" International Journal of Molecular Sciences 19, no. 11: 3385. https://doi.org/10.3390/ijms19113385
APA StyleKohout, C., Santi, C., & Polito, L. (2018). Anisotropic Gold Nanoparticles in Biomedical Applications. International Journal of Molecular Sciences, 19(11), 3385. https://doi.org/10.3390/ijms19113385