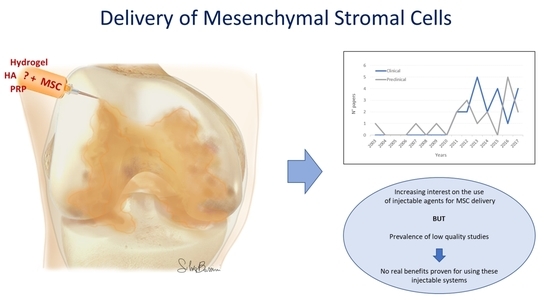
Injectable Systems for Intra-Articular Delivery of Mesenchymal Stromal Cells for Cartilage Treatment: A Systematic Review of Preclinical and Clinical Evidence
Abstract
1. Introduction
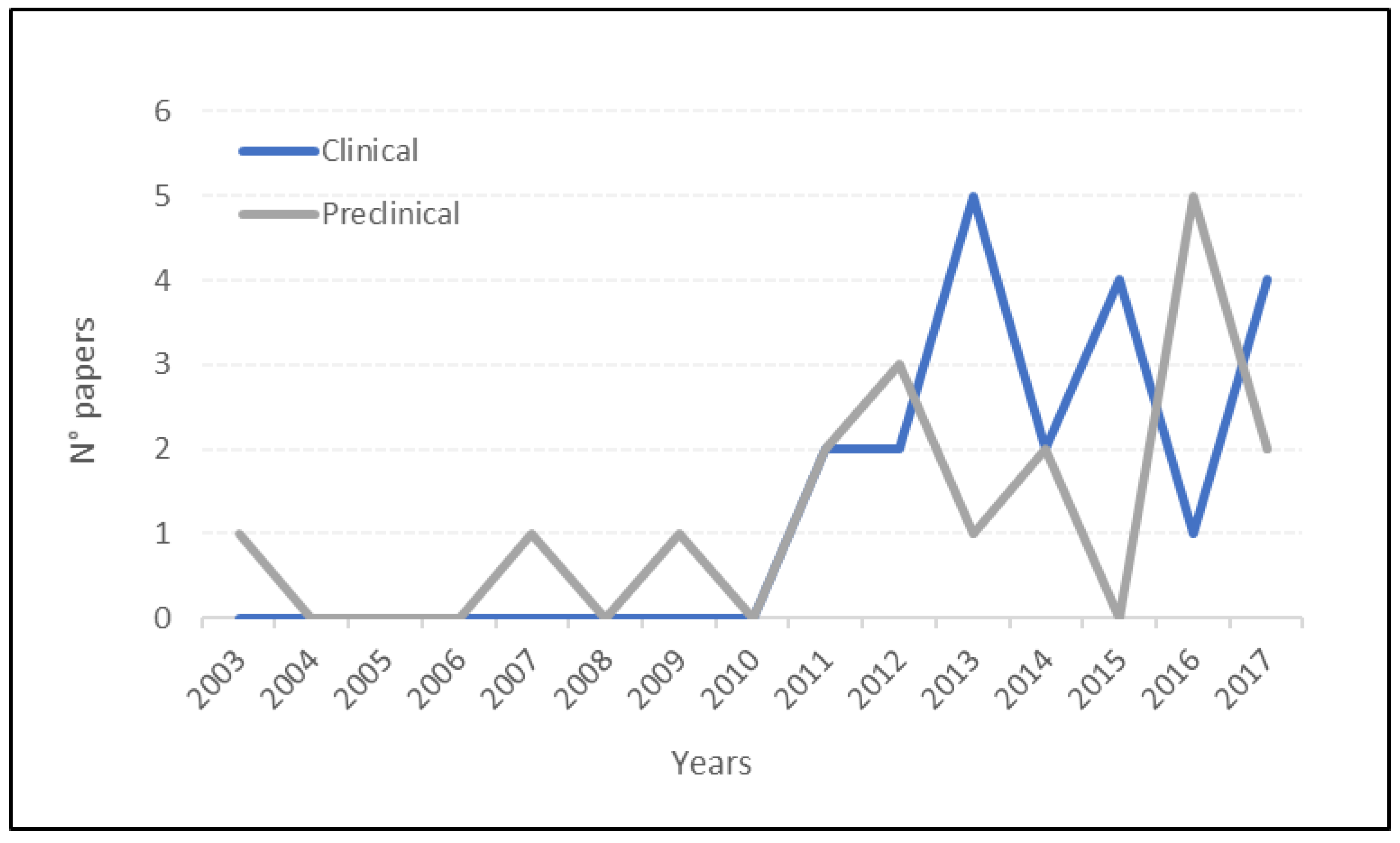
2. Results
2.1. Preclinical Studies
2.1.1. MSC Injection with PRP
2.1.2. MSC Injection with HA
2.1.3. MSC Injection with Hydrogels
2.2. Clinical Studies
2.2.1. MSC Injection with PRP
2.2.2. MSC Injection with HA
2.2.3. MSC Injection with PRP and HA
3. Discussion
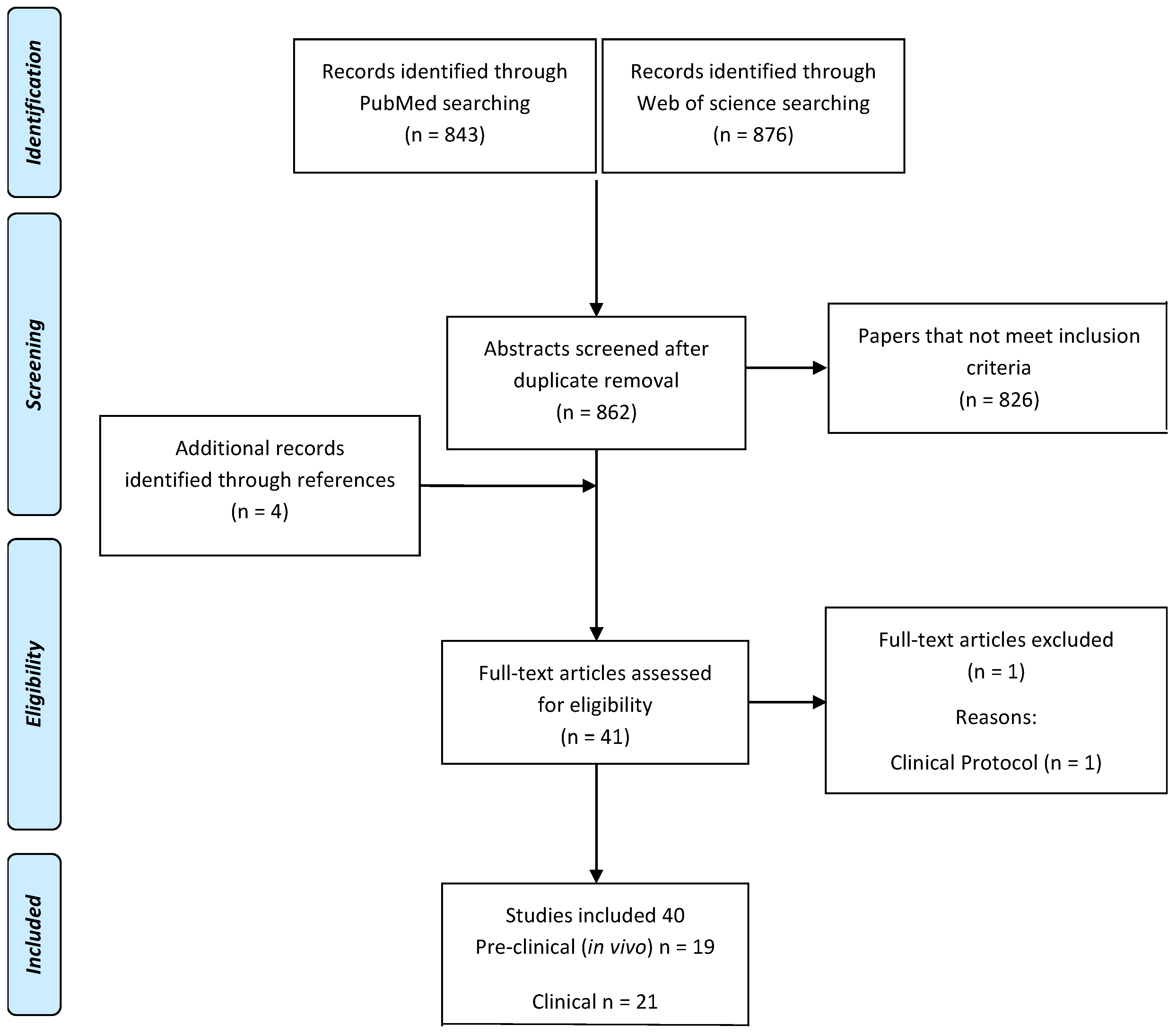
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hunziker, E.B. Articular Cartilage Repair: Basic Science and Clinical Progress. A Review of the Current Status and Prospects. Osteoarthr. Cartil. 2002, 10, 432–463. [Google Scholar] [CrossRef] [PubMed]
- Loeser, R.F.; Goldring, S.R.; Scanzello, C.R.; Goldring, M.B. Osteoarthritis: A Disease of the Joint as an Organ. Arthritis Rheum. 2012, 64, 1697–1707. [Google Scholar] [CrossRef] [PubMed]
- Filardo, G.; Kon, E.; Longo, U.G.; Madry, H.; Marchettini, P.; Marmotti, A.; Van Assche, D.; Zanon, G.; Peretti, G.M. Non-Surgical Treatments for the Management of Early Osteoarthritis. Knee Surg. Sports Traumatol. Arthrosc. 2016, 24, 1775–1785. [Google Scholar] [CrossRef] [PubMed]
- Marcacci, M.; Filardo, G.; Kon, E. Treatment of Cartilage Lesions: What Works and Why? Injury 2013, 44 (Suppl. 1), S11–S15. [Google Scholar] [CrossRef]
- Brittberg, M.; Lindahl, A.; Nilsson, A.; Ohlsson, C.; Isaksson, O.; Peterson, L. Treatment of Deep Cartilage Defects in the Knee with Autologous Chondrocyte Transplantation. N. Engl. J. Med. 1994, 331, 889–895. [Google Scholar] [CrossRef] [PubMed]
- Hangody, L.; Kish, G.; Kárpáti, Z.; Udvarhelyi, I.; Szigeti, I.; Bély, M. Mosaicplasty for the Treatment of Articular Cartilage Defects: Application in Clinical Practice. Orthopedics 1998, 21, 751–756. [Google Scholar] [PubMed]
- Kon, E.; Filardo, G.; Drobnic, M.; Madry, H.; Jelic, M.; van Dijk, N.; Della Villa, S. Non-Surgical Management of Early Knee Osteoarthritis. Knee Surg. Sports Traumatol. Arthrosc. Off. J. ESSKA 2012, 20, 436–449. [Google Scholar] [CrossRef] [PubMed]
- Madry, H.; Grün, U.W.; Knutsen, G. Cartilage Repair and Joint Preservation: Medical and Surgical Treatment Options. Dtsch. Arztebl. Int. 2011, 108, 669–677. [Google Scholar] [PubMed]
- Benya, P.D.; Padilla, S.R.; Nimni, M.E. The Progeny of Rabbit Articular Chondrocytes Synthesize Collagen Types I and III and Type I Trimer, but Not Type II. Verifications by Cyanogen Bromide Peptide Analysis. Biochemistry (Mosc.) 1977, 16, 865–872. [Google Scholar] [CrossRef]
- Caplan, A.I. Mesenchymal Stem Cells. J. Orthop. Res. 1991, 9, 641–650. [Google Scholar] [CrossRef] [PubMed]
- Caplan, A.I. The Mesengenic Process. Clin. Plast. Surg. 1994, 21, 429–435. [Google Scholar] [PubMed]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage Potential of Adult Human Mesenchymal Stem Cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Liechty, K.W.; MacKenzie, T.C.; Shaaban, A.F.; Radu, A.; Moseley, A.M.; Deans, R.; Marshak, D.R.; Flake, A.W. Human Mesenchymal Stem Cells Engraft and Demonstrate Site-Specific Differentiation after in Utero Transplantation in Sheep. Nat. Med. 2000, 6, 1282–1286. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, S.; Pittenger, M.F. Human Mesenchymal Stem Cells Modulate Allogeneic Immune Cell Responses. Blood 2005, 105, 1815–1822. [Google Scholar] [CrossRef] [PubMed]
- Caplan, A.I.; Dennis, J.E. Mesenchymal Stem Cells as Trophic Mediators. J. Cell. Biochem. 2006, 98, 1076–1084. [Google Scholar] [CrossRef] [PubMed]
- Horwitz, E.M.; Le Blanc, K.; Dominici, M.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Deans, R.J.; Krause, D.S.; Keating, A.; International Society for Cellular Therapy. Clarification of the Nomenclature for MSC: The International Society for Cellular Therapy Position Statement. Cytotherapy 2005, 7, 393–395. [Google Scholar] [CrossRef] [PubMed]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal Criteria for Defining Multipotent Mesenchymal Stromal Cells. The International Society for Cellular Therapy Position Statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Olee, T.; Grogan, S.P.; Lotz, M.K.; Colwell, C.W.; D’Lima, D.D.; Snyder, E.Y. Repair of Cartilage Defects in Arthritic Tissue with Differentiated Human Embryonic Stem Cells. Tissue Eng. Part A 2014, 20, 683–692. [Google Scholar] [CrossRef] [PubMed]
- Diekman, B.O.; Christoforou, N.; Willard, V.P.; Sun, H.; Sanchez-Adams, J.; Leong, K.W.; Guilak, F. Cartilage Tissue Engineering Using Differentiated and Purified Induced Pluripotent Stem Cells. Proc. Natl. Acad. Sci. USA 2012, 109, 19172–19177. [Google Scholar] [CrossRef] [PubMed]
- Blum, B.; Benvenisty, N. Clonal Analysis of Human Embryonic Stem Cell Differentiation into Teratomas. Stem Cells Dayt. Stem Cells 2007, 25, 1924–1930. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, A.; Liu, S.; Woltjen, K.; Thomas, B.; Meng, G.; Hotta, A.; Takahashi, K.; Ellis, J.; Yamanaka, S.; Rancourt, D.E. Cartilage Tissue Engineering Identifies Abnormal Human Induced Pluripotent Stem Cells. Sci. Rep. 2013, 3, 1978. [Google Scholar] [CrossRef] [PubMed]
- O’Driscoll, S.W.; Recklies, A.D.; Poole, A.R. Chondrogenesis in Periosteal Explants. An Organ Culture Model for in Vitro Study. J. Bone Jt. Surg. Am. 1994, 76, 1042–1051. [Google Scholar] [CrossRef]
- Yoo, J.U.; Barthel, T.S.; Nishimura, K.; Solchaga, L.; Caplan, A.I.; Goldberg, V.M.; Johnstone, B. The Chondrogenic Potential of Human Bone-Marrow-Derived Mesenchymal Progenitor Cells. J. Bone Jt. Surg. Am. 1998, 80, 1745–1757. [Google Scholar] [CrossRef]
- Mackay, A.M.; Beck, S.C.; Murphy, J.M.; Barry, F.P.; Chichester, C.O.; Pittenger, M.F. Chondrogenic Differentiation of Cultured Human Mesenchymal Stem Cells from Marrow. Tissue Eng. 1998, 4, 415–428. [Google Scholar] [CrossRef] [PubMed]
- Zvaifler, N.J.; Marinova-Mutafchieva, L.; Adams, G.; Edwards, C.J.; Moss, J.; Burger, J.A.; Maini, R.N. Mesenchymal Precursor Cells in the Blood of Normal Individuals. Arthritis Res. 2000, 2, 477–488. [Google Scholar] [CrossRef] [PubMed]
- De Bari, C.; Dell’Accio, F.; Tylzanowski, P.; Luyten, F.P. Multipotent Mesenchymal Stem Cells from Adult Human Synovial Membrane. Arthritis Rheum. 2001, 44, 1928–1942. [Google Scholar] [CrossRef]
- Chong, P.-P.; Selvaratnam, L.; Abbas, A.A.; Kamarul, T. Human Peripheral Blood Derived Mesenchymal Stem Cells Demonstrate Similar Characteristics and Chondrogenic Differentiation Potential to Bone Marrow Derived Mesenchymal Stem Cells. J. Orthop. Res. 2012, 30, 634–642. [Google Scholar] [CrossRef] [PubMed]
- Filardo, G.; Perdisa, F.; Roffi, A.; Marcacci, M.; Kon, E. Stem Cells in Articular Cartilage Regeneration. J. Orthop. Surg. 2016, 11, 42. [Google Scholar] [CrossRef] [PubMed]
- Sekiya, I.; Muneta, T.; Horie, M.; Koga, H. Arthroscopic Transplantation of Synovial Stem Cells Improves Clinical Outcomes in Knees with Cartilage Defects. Clin. Orthop. 2015, 473, 2316–2326. [Google Scholar] [CrossRef] [PubMed]
- Jo, C.H.; Chai, J.W.; Jeong, E.C.; Oh, S.; Shin, J.S.; Shim, H.; Yoon, K.S. Intra-Articular Injection of Mesenchymal Stem Cells for the Treatment of Osteoarthritis of the Knee: A 2-Year Follow-up Study. Am. J. Sports Med. 2017, 45, 2774–2783. [Google Scholar] [CrossRef] [PubMed]
- Yokota, N.; Yamakawa, M.; Shirata, T.; Kimura, T.; Kaneshima, H. Clinical Results Following Intra-Articular Injection of Adipose-Derived Stromal Vascular Fraction Cells in Patients with Osteoarthritis of the Knee. Regen. Ther. 2017, 6, 108–112. [Google Scholar] [CrossRef] [PubMed]
- Hindle, P.; Hall, A.C.; Biant, L.C. Viability of Chondrocytes Seeded onto a Collagen I/III Membrane for Matrix-Induced Autologous Chondrocyte Implantation. J. Orthop. Res. 2014, 32, 1495–1502. [Google Scholar] [CrossRef] [PubMed]
- Broeckx, S.Y.; Spaas, J.H.; Chiers, K.; Duchateau, L.; Van Hecke, L.; Van Brantegem, L.; Dumoulin, M.; Martens, A.M.; Pille, F. Equine Allogeneic Chondrogenic Induced Mesenchymal Stem Cells: A GCP Target Animal Safety and Biodistribution Study. Res. Vet. Sci. 2017, 117, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Desando, G.; Bartolotti, I.; Cavallo, C.; Schiavinato, A.; Secchieri, C.; Kon, E.; Filardo, G.; Paro, M.; Grigolo, B. Short-Term Homing of Hyaluronan-Primed Cells: Therapeutic Implications for Osteoarthritis Treatment. Tissue Eng. Part C Methods 2017, 24, 121–133. [Google Scholar] [CrossRef] [PubMed]
- Broeckx, S.; Zimmerman, M.; Crocetti, S.; Suls, M.; Mariën, T.; Ferguson, S.J.; Chiers, K.; Duchateau, L.; Franco-Obregón, A.; Wuertz, K.; et al. Regenerative Therapies for Equine Degenerative Joint Disease: A Preliminary Study. PLoS ONE 2014, 9, e85917. [Google Scholar] [CrossRef] [PubMed]
- Bembo, F.; Eraud, J.; Philandrianos, C.; Bertrand, B.; Silvestre, A.; Veran, J.; Sabatier, F.; Magalon, G.; Magalon, J. Combined Use of Platelet Rich Plasma & Micro-Fat in Sport and Race Horses with Degenerative Joint Disease: Preliminary Clinical Study in Eight Horses. Muscles Ligaments Tendons J. 2016, 6, 198–204. [Google Scholar] [PubMed]
- Mifune, Y.; Matsumoto, T.; Takayama, K.; Ota, S.; Li, H.; Meszaros, L.B.; Usas, A.; Nagamune, K.; Gharaibeh, B.; Fu, F.H.; et al. The Effect of Platelet-Rich Plasma on the Regenerative Therapy of Muscle Derived Stem Cells for Articular Cartilage Repair. Osteoarthr. Cartil. 2013, 21, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Hermeto, L.C.; DeRossi, R.; Oliveira, R.J.; Pesarini, J.R.; Antoniolli-Silva, A.C.M.B.; Jardim, P.H.A.; Santana, A.E.; Deffune, E.; Rinaldi, J.C.; Justulin, L.A. Effects of Intra-Articular Injection of Mesenchymal Stem Cells Associated with Platelet-Rich Plasma in a Rabbit Model of Osteoarthritis. Genet Mol. Res. GMR 2016, 15, 3. [Google Scholar] [CrossRef] [PubMed]
- Yun, S.; Ku, S.-K.; Kwon, Y.-S. Adipose-Derived Mesenchymal Stem Cells and Platelet-Rich Plasma Synergistically Ameliorate the Surgical-Induced Osteoarthritis in Beagle Dogs. J. Orthop. Surg. 2016, 11, 9. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.B.L.; Hui, J.H.P.; Song, I.C.; Ardany, L.; Lee, E.H. Injectable Mesenchymal Stem Cell Therapy for Large Cartilage Defects--a Porcine Model. Stem Cells Dayt. Stem Cells 2007, 25, 2964–2971. [Google Scholar] [CrossRef] [PubMed]
- Saw, K.-Y.; Hussin, P.; Loke, S.-C.; Azam, M.; Chen, H.-C.; Tay, Y.-G.; Low, S.; Wallin, K.-L.; Ragavanaidu, K. Articular Cartilage Regeneration with Autologous Marrow Aspirate and Hyaluronic Acid: An Experimental Study in a Goat Model. Arthrosc. J. Arthrosc. Relat. Surg. 2009, 25, 1391–1400. [Google Scholar] [CrossRef] [PubMed]
- McIlwraith, C.W.; Frisbie, D.D.; Rodkey, W.G.; Kisiday, J.D.; Werpy, N.M.; Kawcak, C.E.; Steadman, J.R. Evaluation of Intra-Articular Mesenchymal Stem Cells to Augment Healing of Microfractured Chondral Defects. Arthrosc. J. Arthrosc. Relat. Surg. 2011, 27, 1552–1561. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.S.; Kang, M.S.; Lee, K.Y.; Lee, M.J.; Wang, L.; Kim, H.J. Therapeutic Effects of Mesenchymal Stem Cells and Hyaluronic Acid Injection on Osteochondral Defects in Rabbits’ Knees. Knee Surg. Relat. Res. 2012, 24, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Murphy, J.M.; Fink, D.J.; Hunziker, E.B.; Barry, F.P. Stem Cell Therapy in a Caprine Model of Osteoarthritis. Arthritis Rheum. 2003, 48, 3464–3474. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.; Luo, X.; He, N.; Xia, H.; Lv, X.; Zhang, X.; Li, D.; Wang, F.; He, J.; Zhang, L.; et al. Efficacy and Persistence of Allogeneic Adipose-Derived Mesenchymal Stem Cells Combined with Hyaluronic Acid in Osteoarthritis After Intra-Articular Injection in a Sheep Model. Tissue Eng. Part A 2017, 24, 219–233. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; He, J.; Zhang, X.; Luo, X.; He, N.; Sun, Z.; Xia, H.; Liu, V.; Zhang, L.; Lin, X.; et al. Comparative Efficacy of Autologous Stromal Vascular Fraction and Autologous Adipose-Derived Mesenchymal Stem Cells Combined with Hyaluronic Acid for the Treatment of Sheep Osteoarthritis. Cell Transpl. 2018, 27, 1111–1125. [Google Scholar] [CrossRef] [PubMed]
- Mokbel, A.N.; El Tookhy, O.S.; Shamaa, A.A.; Rashed, L.A.; Sabry, D.; El Sayed, A.M. Homing and Reparative Effect of Intra-Articular Injection of Autologus Mesenchymal Stem Cells in Osteoarthritic Animal Model. BMC Musculoskelet. Disord. 2011, 12, 259. [Google Scholar] [CrossRef] [PubMed]
- Sato, M.; Uchida, K.; Nakajima, H.; Miyazaki, T.; Guerrero, A.R.; Watanabe, S.; Roberts, S.; Baba, H. Direct Transplantation of Mesenchymal Stem Cells into the Knee Joints of Hartley Strain Guinea Pigs with Spontaneous Osteoarthritis. Arthritis Res. Ther. 2012, 14, R31. [Google Scholar] [CrossRef] [PubMed]
- Chiang, E.-R.; Ma, H.-L.; Wang, J.-P.; Liu, C.-L.; Chen, T.-H.; Hung, S.-C. Allogeneic Mesenchymal Stem Cells in Combination with Hyaluronic Acid for the Treatment of Osteoarthritis in Rabbits. PLoS ONE 2016, 11, e0149835. [Google Scholar] [CrossRef] [PubMed]
- Suhaeb, A.M.; Naveen, S.; Mansor, A.; Kamarul, T. Hyaluronic Acid with or without Bone Marrow Derived-Mesenchymal Stem Cells Improves Osteoarthritic Knee Changes in Rat Model: A Preliminary Report. Indian J. Exp. Biol. 2012, 50, 383–390. [Google Scholar] [PubMed]
- Kim, S.J.; Kim, J.E.; Kim, S.H.; Kim, S.J.; Jeon, S.J.; Kim, S.H.; Jung, Y. Therapeutic Effects of Neuropeptide Substance P Coupled with Self-Assembled Peptide Nanofibers on the Progression of Osteoarthritis in a Rat Model. Biomaterials 2016, 74, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.E.; Lee, S.M.; Kim, S.H.; Tatman, P.; Gee, A.O.; Kim, D.-H.; Lee, K.E.; Jung, Y.; Kim, S.J. Effect of Self-Assembled Peptide-Mesenchymal Stem Cell Complex on the Progression of Osteoarthritis in a Rat Model. Int. J. Nanomed. 2014, 9 (Suppl. 1), 141–157. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Choi, Y.J.; Suh, D.S.; Heo, D.B.; Kim, Y.I.; Ryu, J.-S.; Koh, Y.G. Mesenchymal Stem Cell Implantation in Osteoarthritic Knees: Is Fibrin Glue Effective as a Scaffold? Am. J. Sports Med. 2015, 43, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Bastos, R.; Mathias, M.; Andrade, R.; Bastos, R.; Balduino, A.; Schott, V.; Rodeo, S.; Espregueira-Mendes, J. Intra-Articular Injections of Expanded Mesenchymal Stem Cells with and without Addition of Platelet-Rich Plasma Are Safe and Effective for Knee Osteoarthritis. Knee Surg. Sports Traumatol. Arthrosc. Off. J. ESSKA 2018. [Google Scholar] [CrossRef] [PubMed]
- Bansal, H.; Comella, K.; Leon, J.; Verma, P.; Agrawal, D.; Koka, P.; Ichim, T. Intra-Articular Injection in the Knee of Adipose Derived Stromal Cells (Stromal Vascular Fraction) and Platelet Rich Plasma for Osteoarthritis. J. Transl. Med. 2017, 15, 141. [Google Scholar] [CrossRef] [PubMed]
- Pintat, J.; Silvestre, A.; Magalon, G.; Gadeau, A.P.; Pesquer, L.; Perozziello, A.; Peuchant, A.; Mounayer, C.; Dallaudière, B. Intra-Articular Injection of Mesenchymal Stem Cells and Platelet-Rich Plasma to Treat Patellofemoral Osteoarthritis: Preliminary Results of a Long-Term Pilot Study. J. Vasc. Int. Radiol. 2017, 28, 1708–1713. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, S.A.; Kazmerchak, S.E.; Heckman, M.G.; Zubair, A.C.; O’Connor, M.I. A Prospective, Single-Blind, Placebo-Controlled Trial of Bone Marrow Aspirate Concentrate for Knee Osteoarthritis. Am. J. Sports Med. 2017, 45, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, N.; Diamond, R.; Sekyere, E.O.; Thomas, W.D. Management of Knee Osteoarthritis by Combined Stromal Vascular Fraction Cell Therapy, Platelet-Rich Plasma, and Musculoskeletal Exercises: A Case Series. J. Pain Res. 2015, 8, 799–806. [Google Scholar] [CrossRef] [PubMed]
- Srinivas, P.L.; Kumar, P.P. Role of PRP and Stem Cell Injections in Osteoarthritic Patients of Knee Joint. Journal of evolution of medical and dental sciences. J. Evolution. Med. Dent. Sci. 2015, 4, 9468–9474. [Google Scholar]
- Koh, Y.-G.; Choi, Y.-J.; Kwon, S.-K.; Kim, Y.-S.; Yeo, J.-E. Clinical Results and Second-Look Arthroscopic Findings after Treatment with Adipose-Derived Stem Cells for Knee Osteoarthritis. Knee Surg. Sports Traumatol. Arthrosc. Off. J. ESSKA 2015, 23, 1308–1316. [Google Scholar] [CrossRef] [PubMed]
- Pham, P.V.; Bui, K.H.-T.; Duong, T.D.; Nguyen, N.T.; Nguyen, T.D.; Le, V.T.; Mai, V.T.; Phan, N.L.-C.; Le, D.M.; Ngoc, N.K. Symptomatic Knee Osteoarthritis Treatment Using Autologous Adipose Derived Stem Cells and Platelet-Rich Plasma: A Clinical Study. Biomed. Res. Ther. 2014, 1, 1–7. [Google Scholar]
- Koh, Y.-G.; Kwon, O.-R.; Kim, Y.-S.; Choi, Y.-J. Comparative Outcomes of Open-Wedge High Tibial Osteotomy with Platelet-Rich Plasma Alone or in Combination with Mesenchymal Stem Cell Treatment: A Prospective Study. Arthrosc. J. Arthrosc. Relat. Surg. 2014, 30, 1453–1460. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Park, E.H.; Kim, Y.C.; Koh, Y.G. Clinical Outcomes of Mesenchymal Stem Cell Injection with Arthroscopic Treatment in Older Patients with Osteochondral Lesions of the Talus. Am. J. Sports Med. 2013, 41, 1090–1099. [Google Scholar] [CrossRef] [PubMed]
- Koh, Y.-G.; Jo, S.-B.; Kwon, O.-R.; Suh, D.-S.; Lee, S.-W.; Park, S.-H.; Choi, Y.-J. Mesenchymal Stem Cell Injections Improve Symptoms of Knee Osteoarthritis. Arthrosc. J. Arthrosc. Relat. Surg. 2013, 29, 748–755. [Google Scholar] [CrossRef] [PubMed]
- Koh, Y.-G.; Choi, Y.-J. Infrapatellar Fat Pad-Derived Mesenchymal Stem Cell Therapy for Knee Osteoarthritis. Knee 2012, 19, 902–907. [Google Scholar] [CrossRef] [PubMed]
- Saw, K.-Y.; Anz, A.; Siew-Yoke Jee, C.; Merican, S.; Ching-Soong Ng, R.; Roohi, S.A.; Ragavanaidu, K. Articular Cartilage Regeneration with Autologous Peripheral Blood Stem Cells versus Hyaluronic Acid: A Randomized Controlled Trial. Arthrosc. J. Arthrosc. Relat. Surg. 2013, 29, 684–694. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.B.L.; Wang, V.T.Z.; Chan, Y.H.; Hui, J.H.P. A Novel, Minimally-Invasive Technique of Cartilage Repair in the Human Knee Using Arthroscopic Microfracture and Injections of Mesenchymal Stem Cells and Hyaluronic Acid—A Prospective Comparative Study on Safety and Short-Term Efficacy. Ann. Acad. Med. Singapore 2012, 41, 511–517. [Google Scholar] [PubMed]
- Saw, K.-Y.; Anz, A.; Merican, S.; Tay, Y.-G.; Ragavanaidu, K.; Jee, C.S.Y.; McGuire, D.A. Articular Cartilage Regeneration with Autologous Peripheral Blood Progenitor Cells and Hyaluronic Acid after Arthroscopic Subchondral Drilling: A Report of 5 Cases with Histology. Arthrosc. J. Arthrosc. Relat. Surg. 2011, 27, 493–506. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.L.; Lee, K.B.L.; Tai, B.C.; Law, P.; Lee, E.H.; Hui, J.H.P. Injectable Cultured Bone Marrow-Derived Mesenchymal Stem Cells in Varus Knees with Cartilage Defects Undergoing High Tibial Osteotomy: A Prospective, Randomized Controlled Clinical Trial with 2 Years’ Follow-Up. Arthrosc. J. Arthrosc. Relat. Surg. 2013, 29, 2020–2028. [Google Scholar] [CrossRef] [PubMed]
- Turajane, T.; Chaveewanakorn, U.; Fongsarun, W.; Aojanepong, J.; Papadopoulos, K.I. Avoidance of Total Knee Arthroplasty in Early Osteoarthritis of the Knee with Intra-Articular Implantation of Autologous Activated Peripheral Blood Stem Cells versus Hyaluronic Acid: A Randomized Controlled Trial with Differential Effects of Growth Factor Addition. Stem Cells Int. 2017, 2017, 8925132. [Google Scholar] [PubMed]
- Pak, J.; Lee, J.H.; Park, K.S.; Jeong, B.C.; Lee, S.H. Regeneration of Cartilage in Human Knee Osteoarthritis with Autologous Adipose Tissue-Derived Stem Cells and Autologous Extracellular Matrix. BioResearch Open Access 2016, 5, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Pak, J.; Chang, J.-J.; Lee, J.H.; Lee, S.H. Safety Reporting on Implantation of Autologous Adipose Tissue-Derived Stem Cells with Platelet-Rich Plasma into Human Articular Joints. BMC Musculoskelet. Disord. 2013, 14, 337. [Google Scholar] [CrossRef] [PubMed]
- Pak, J. Regeneration of Human Bones in Hip Osteonecrosis and Human Cartilage in Knee Osteoarthritis with Autologous Adipose-Tissue-Derived Stem Cells: A Case Series. J. Med. Case Rep. 2011, 5, 296. [Google Scholar] [CrossRef] [PubMed]
- Anitua, E.; Sánchez, M.; Orive, G. Potential of Endogenous Regenerative Technology for in Situ Regenerative Medicine. Adv. Drug Deliv. Rev. 2010, 62, 741–752. [Google Scholar] [CrossRef] [PubMed]
- Filardo, G.; Kon, E.; Roffi, A.; Di Matteo, B.; Merli, M.L.; Marcacci, M. Platelet-Rich Plasma: Why Intra-Articular? A Systematic Review of Preclinical Studies and Clinical Evidence on PRP for Joint Degeneration. Knee Surg. Sports Traumatol. Arthrosc. Off. J. ESSKA 2015, 23, 2459–2474. [Google Scholar] [CrossRef] [PubMed]
- Bellamy, N.; Campbell, J.; Robinson, V.; Gee, T.; Bourne, R.; Wells, G. Viscosupplementation for the Treatment of Osteoarthritis of the Knee; The Cochrane Library: London, UK, 2006. [Google Scholar]
- Eddhahak, A.; Zidi, M. Influence of Viscoelastic Properties of an Hyaluronic Acid-Based Hydrogel on Viability of Mesenchymal Stem Cells. Biomed. Mater. Eng. 2015, 26, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Kavalkovich, K.W.; Boynton, R.E.; Murphy, J.M.; Barry, F. Chondrogenic Differentiation of Human Mesenchymal Stem Cells within an Alginate Layer Culture System. In Vitro Cell. Dev. Biol. Anim. 2002, 38, 457–466. [Google Scholar] [CrossRef]
- Maniwa, S.; Ochi, M.; Motomura, T.; Nishikori, T.; Chen, J.; Naora, H. Effects of Hyaluronic Acid and Basic Fibroblast Growth Factor on Motility of Chondrocytes and Synovial Cells in Culture. Acta Orthop. Scand. 2001, 72, 299–303. [Google Scholar] [CrossRef] [PubMed]
- Visser, J.; Levett, P.A.; te Moller, N.C.R.; Besems, J.; Boere, K.W.M.; van Rijen, M.H.P.; de Grauw, J.C.; Dhert, W.J.A.; van Weeren, P.R.; Malda, J. Crosslinkable Hydrogels Derived from Cartilage, Meniscus, and Tendon Tissue. Tissue Eng. Part A 2015, 21, 1195–1206. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.G.; Kim, T.K.; Taboas, A.; Malik, A.; Manson, P.; Elisseeff, J. In Vitro Chondrogenesis of Bone Marrow-Derived Mesenchymal Stem Cells in a Photopolymerizing Hydrogel. Tissue Eng. 2003, 9, 679–688. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Han, Q.; Chen, W.; Song, J.; Zhao, X.; Ouyang, Y.; Yuan, W.; Fan, C. Platelet-Rich Plasma Derived Growth Factors Contribute to Stem Cell Differentiation in Musculoskeletal Regeneration. Front. Chem. 2017, 5, 89. [Google Scholar] [CrossRef] [PubMed]
- Kreuz, P.C.; Krüger, J.P.; Metzlaff, S.; Freymann, U.; Endres, M.; Pruss, A.; Petersen, W.; Kaps, C. Platelet-Rich Plasma Preparation Types Show Impact on Chondrogenic Differentiation, Migration, and Proliferation of Human Subchondral Mesenchymal Progenitor Cells. Arthrosc. J. Arthrosc. Relat. Surg. 2015, 31, 1951–1961. [Google Scholar] [CrossRef] [PubMed]
- Caplan, A.I. New Era of Cell-Based Orthopedic Therapies. Tissue Eng. Part B Rev. 2009, 15, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Foster, A.A.; Marquardt, L.M.; Heilshorn, S.C. The Diverse Roles of Hydrogel Mechanics in Injectable Stem Cell Transplantation. Curr. Opin. Chem. Eng. 2017, 15, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Andia, I.; Abate, M. Knee Osteoarthritis: Hyaluronic Acid, Platelet-Rich Plasma or Both in Association? Expert Opin. Biol. Ther. 2014, 14, 635–649. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.-H.; Lo, W.-C.; Hsu, W.-C.; Wei, H.-J.; Liu, H.-Y.; Lee, C.-H.; Tina Chen, S.-Y.; Shieh, Y.-H.; Williams, D.F.; Deng, W.-P. Synergistic Anabolic Actions of Hyaluronic Acid and Platelet-Rich Plasma on Cartilage Regeneration in Osteoarthritis Therapy. Biomaterials 2014, 35, 9599–9607. [Google Scholar] [CrossRef] [PubMed]
- Lana, J.F.S.D.; Weglein, A.; Sampson, S.E.; Vicente, E.F.; Huber, S.C.; Souza, C.V.; Ambach, M.A.; Vincent, H.; Urban-Paffaro, A.; Onodera, C.M.K.; et al. Randomized Controlled Trial Comparing Hyaluronic Acid, Platelet-Rich Plasma and the Combination of Both in the Treatment of Mild and Moderate Osteoarthritis of the Knee. J. Stem Cells Regen. Med. 2016, 12, 69–78. [Google Scholar] [PubMed]
- Russo, F.; D’Este, M.; Vadalà, G.; Cattani, C.; Papalia, R.; Alini, M.; Denaro, V. Platelet Rich Plasma and Hyaluronic Acid Blend for the Treatment of Osteoarthritis: Rheological and Biological Evaluation. PLoS ONE 2016, 11, e0157048. [Google Scholar] [CrossRef] [PubMed]
- Kamei, G.; Kobayashi, T.; Ohkawa, S.; Kongcharoensombat, W.; Adachi, N.; Takazawa, K.; Shibuya, H.; Deie, M.; Hattori, K.; Goldberg, J.L.; et al. Articular Cartilage Repair with Magnetic Mesenchymal Stem Cells. Am. J. Sports Med. 2013, 41, 1255–1264. [Google Scholar] [CrossRef] [PubMed]
| Publication | Animal Model | Lesion Type | MSC Type | Delivery System | Study Design | Results |
|---|---|---|---|---|---|---|
| PRP | ||||||
| Bembo 2016 [36] Muscles, ligaments and tendon J | Sport horses | OA | Micro fat suspension | PRP Plts 4.3 ± 1.1 × 109/L Activation: No Leucocytes: 20 ± 9 × 106/L | Micro fat+PRP Experimental time: 3 months | Significant improvement of the lameness score 3 months after treatment; returned to competition for 4 horses which 3 resumed intensive training; no adverse events |
| Broeckx 2014 [35] PLoS ONE | Sport horses | Allogeneic PBMSCs | PRP Plts 200 × 106; stored at −80 °C before use Activation: N/A Leukocytes: N/A | PRP vs. MSCs vs. MSCs+PRP vs. chondrogenic induced MSCs+PRP Experimental time: 6 and 12 weeks, 6 and 12 months | Significant function improvement up to 12 months after treatment in MSCs+PRP group compared with PRP alone. Highest short-term clinical scores were obtained with chondrogenic induced MSCs+PRP | |
| Hermeto 2016 [38] Genet Mol Res | Rabbits | AD-MSCs (4 × 106 cells) | PRP Plt: 997.42 ± 48.01/μL; Activation: 10% Ca gluconate Leukocytes: N/A | Saline vs. PRP vs. undifferentiated MSCs+PRP vs. differentiated MSCs+PRP Experimental time: 2 months | Improved tissue repair in both MSCs group at macroscopic and histological examinations; any improvements in PRP alone group | |
| Yun 2016 [39] J Orthop Surg Res | Dogs | AD-MSCs (107 cells) | PRP Plts: 106/μL Activation: N/A Leukocytes: N/A | Saline vs. PRP vs. MSCs+saline vs. MSCs+PRP Experimental time: 2, 3, 4 months | Decreased lameness score at 2 and at 3 months in both PRP alone and MSCs+PRP groups; significant increases in focal compressive strength in all treatments groups with highest value in MSCs+PRP group; inflammation reduction in both PRP and MSCs+PRP groups | |
| Mifune 2013 [37] Osteoarthritis Cartilage | Rats | MDSCs (5 × 105 cells) | PRP Plts: 230 × 104/mL) Activation: N/A Leukocytes: N/A | Saline vs. PRP vs. MDSCs vs. MDSCs expressing BMP-4/sFlt1+PRP vs. MDSCs expressing BMP-4/sFlt1 vs. MDSCs+PRP Experimental time: 4 and 12 weeks | Significant AC repair at histology in MDSCs expressing BMP-4/sFlt1+ PRP at 4 weeks compared with MDSCs expressing BMP-4/sFlt1, with higher numbers of cells producing type-II collagen and lower levels of chondrocyte apoptosis | |
| HA | ||||||
| Kim 2012 [43] Knee Surg Relat Res | Rabbits | Osteochondral defect | BMSCs (106 cells) | HA | No treatment vs. HA vs. MSCs vs. MSCs+HA vs. MSCs+HA inj vs. MSCs+HA+1 HA inj vs. MSCs+HA+2 HA inj Experimental time: 7 weeks | Significant improvements in osteochondral defect healing at macroscopic and histological evaluation in all treatment groups compared with untreated defects; at histology, MSCs+HA+2 HA inj showed better results than other groups |
| McIlwraith 2011 [42] Arthroscopy | Horses | BMSCs (20 × 106 cells) | High molecular weight HA | MFX+HA or MFX+HA+MSCs Experimental time: 6 and 12 months | No difference in clinical and histological analysis, but significant increase in repair tissue firmness and better repair tissue quality at arthroscopic and macroscopic analysis in MSCs group with greater levels of aggrecan than in HA alone group | |
| Saw 2009 [41] Arthroscopy | Goats | BMC | High molecular weight HA | No treatment vs. subchondral drilling + 3 HA inj vs. subchondral drilling + 3 HA + BMC inj Experimental time: 24 weeks | Better cartilage repair in MSCs group at histology, with hyaline cartilage regeneration | |
| Lee 2007 [40] Stem Cells | Minipigs | BMSCs (3.5–10.1 × 106 cells) | High molecular weight HA | Saline vs. HA vs. MSCs+HA Experimental time: 6 and 12 weeks | Improvement in cartilage healing at histologic and macroscopic analysis at both 6 and 12 weeks in MSCs+HA group compared with controls | |
| Lv 2018 [46] Cell Transplant | Sheep | OA | SVF vs. cultured AD-MSCs | Medium molecular weight HA | Saline vs. HA vs. SVF/HA vs. low dose AD-MSCs/HA vs. high dose AD-MSCs/HA Experimental time: | Better results in AD-MSCs/HA than SVF/HA in blocking OA progression and promoting cartilage regeneration |
| Feng 2017 [45] Tissue Eng Part A | Sheep | Allogeneic AD-MSCs (5 × 107 cells vs. 107 cells) | Medium molecular weight HA | High dosage AD-MSCs or low dosage + HA vs. HA alone vs. saline Experimental time: 14 weeks | Typical articular cartilage feature in both AD-MSCs groups and presence of AD-MSCs at synovium at 14 weeks at MRI; lower inflammatory factors from synovial fluid of AD-MSCs groups than HA alone | |
| Desando 2017 [34] Tissue Eng Part C | Rabbits | BMSCs (2 × 106 cells) and BMC | High molecular weight HA | BMSCs+saline vs. BMSCs+HA vs. BMC+saline vs. BMC+HA Experimental time: 2 months | Joint repair evidence in all treatments, superior results for BMC-HA than other groups; BMSCs migrate to the meniscus while BMC in cartilage, but HA favor cells migration to cartilage | |
| Chiang 2016 [49] Plos ONE | Rabbits | Allogeneic BMSCs (106 cells) | High molecular weight HA | Untreated vs. Sham vs. HA vs. MSCs+HA Experimental time: 6 and 12 weeks | Less cartilage loss and surface abrasion with better histological scores and cartilage content in MSCs group compared with HA alone; engraftment of allogenic MSCs were evident in surface cartilage | |
| Suhaeb 2012 [50] Indian J Exp Biol | Rat | BMSCs (3-5 × 106 cells) | High molecular weight HA | HA vs. BMSCs vs. BMSCs+HA | Better results with HA and BMSCs alone in counteracting OA progression with respect to their combination | |
| Sato 2012 [48] Arthritis Res Ther | Pigs | Xenogeneic hMSCs (7 × 106 cells) | Low molecular weight HA | Saline vs. HA vs. MSCs+saline vs. MSCs+HA Experimental time: 5 weeks | Histological partial defect repair only in MSCs+HA group at 5 weeks with an increase in type-II collagen content and low levels of MMP-13 | |
| Mokbel 2011 [47] BMC Musculoskeletal Disorders | Donkeys | BMSCs (1.8-2.3 × 106 cells/mL) | Low molecular weight HA | MSCs+HA vs. HA alone Experimental time: 1, 2, 6 months | Defect repair at clinical and radiological evaluation in MSCs+HA group compared with the control; MSCs integrated with healthy cartilage in the superficial and inner part | |
| Murphy 2003 [44] Arthritis Rheum | Goats | BMSCs (10 × 106 cells) | High molecular weight HA | HA vs. HA+BMSCs Experimental time: 12 and 26 weeks | No adverse events; stimulation of the regeneration of meniscal tissue and delay of OA progression in MSCs group | |
| Hydrogel | ||||||
| Kim 2016 [51] Biomaterials | Rat OA | OA | PBMSCs | SAP hydrogel | SAP hydrogel 0.5 SP vs. SAP hydrogel SP vs. SAP hydrogel 2SP vs. SAP hydrogel SP+MSCs Experimental time: 6 weeks | Markedly improved cartilage regeneration in the SAP-SP group showing recruitment of MSCs in the defect |
| Kim 2014 [52] Int J Nanomed | Rat OA | Allogeneic BMSCs | SAP hydrogel | MSCs vs. SAP hydrogel vs. SAP hydrogel+MSCs vs. no treatment Experimental time: 6 weeks | Evidence of chondroprotection at histological view and decrease of inflammation and apoptosis biomarkers in SAP+MSCs group; increased BMD in SAP hydrogel+MSCs groups relative to the controls | |
| Defect type | Publication | Study Type | MSC Type | Delivery System | Study Design | Results |
|---|---|---|---|---|---|---|
| PRP | ||||||
| Osteochondral lesion | Kim 2015 [53] Am J Sports Med | Comparative | SVF | PRP Plts: 1.28 × 106/μL Activation: CaCl2 Leukocytes: N/A | 40 pts (20 vs. 20) (knee) Age: mean 59.2 years Lesion size/degree: 5.44 ± 1.4 cm2 Treatment: SVF on FG scaffold vs. SVF+PRP Follow-up: 28.6 months | Significant improvement in both groups; better clinical results at final follow-up and 2nd look appearance at 12 months for SVF-FG |
| OA | Bastos 2018 [54] KSSTA | RCT | Cultured BMSCs | PRP Plts: 106/μL Activation: N/A Leukocytes: N/A | 18 pts (9 vs. 9) (knee) Age: mean 57.6 years Lesion size/degree: grade II-IV Treatment: BMSCs vs. BMSCs+PRP Follow-up: 12 months | Improvement in knee pain and function in both groups, without significance difference |
| Bansal 2017 [55] J Transl Med | Case series | SVF | PRP Plts: N/A Activation: N/A Leukocytes: N/A | 10 pts (knee) Age: mean 58.4 years Lesion size/degree: N/A Treatment: SVF+PRP Follow-up: 3, 6, 12, 18, 24 months | Functional improvement with pain reduction at 12 and 24 months; reduction of atypical cells in synovial fluid; unaltered haematological and biochemical analysis | |
| Pintat 2017 [56] J Vasc Interv Radiol | Case series | SVF | PRP Plts: 700,000/mm3 Activation: N/A Leukocytes: 200/mm3 | 19 pts (knee) Age: mean 42.1 years Lesion size/degree: N/A Treatment: SVF+PRP Follow-up: 6 and 12 months | Functional improvement at 6 and 12 month follow-ups with no complications but no relevant changes at MRI | |
| Shapiro 2017 [57] Am J Sports Med | RCT | BMAC | PPP | 25 pts (knee) Age: mean 60 years Lesion size/degree: 2.3 K-L Treatment: BMC+PPP vs. saline Follow-up: 6 months | No adverse events; similar pain relief in both group | |
| Gibbs 2015 [58] J Pain Res | Case series | SVF | PRP Plts: N/A Activation: N/A Leucocytes: N/A | 4 pts (7 knees) Age: mean 51.5 years Lesion size/degree: N/A Treatment: SVF+PRP and 3 monthly PRP inj Follow-up: 12 months | Functional, pain and quality of life score improvement at 12 months | |
| Srinivas 2015 [59] J of evolution of med and Dent Sci | Case series | BMC | PRP Plts: N/A Activation: N/A Leucocytes: N/A | 115 pts (knee) Age: 56–87 years Lesion size/degree: moderate to severe Treatment: 65 BMC+PRP and 50 corticosteroid Follow-up: 6 months | Pain improvement from 1 week up to 6 months after injection of PRP + BMC | |
| Koh 2015 [60] KSSTA | Case series | SVF | PRP Plts: 1.28 × 106 cells/μL Activation: N/A Leucocytes: N/A | 30 pts (knee) Age: mean 70.3 years Lesion size/degree: 2.3 K-L Treatment: SVF+PRP Follow-up: 24 months | Significant clinical improvement; 87.5% of 2nd look arthroscopy within 24 months improved or maintained cartilage status | |
| Pham 2014 [61] Biomed Res Ther | Case series | SVF | PRP Plts: N/A Activation: CaCl2 Leukocytes: N/A | 21 pts (knee) Age: N/A Lesion size/degree: II/III Treatment: SVF+PRP Follow-up: 6 months | Significant clinical scores improvement; no side effects; increased cartilage thickness at MRI | |
| Koh 2014 [62] Arthroscopy | RCT | SVF | PRP Plts: 1.303 × 103 mL Activation: N/A Leukocytes: N/A | 44 pts (21 vs. 23) (knee) Age: mean 53.2 years Lesion size/degree: 1–3 K–L Treatment: HTO+PRP vs. HTO+PRP+SVF Follow-up: 24 months | Better improvement of KOOS pain and symptoms and VAS pain in SVF+PRP than PRP alone | |
| Kim 2013 [63] Am J Sports Med | Comparative | SVF | PRP Plts: N/A Activation: N/A Leukocytes: N/A | 75 pts (ankle) Age: mean 56.8 years Lesion size/degree: 108.76 ± 34.6 mm2 Treatment: MFX vs. MFX+SVF+PRP Follow-up: 21.8 months | Clinical improvement in both groups with better results for SVF group | |
| Koh 2013 [64] Arthroscopy | Case series | SVF | PRP Plts: 1.28 × 106/μL Activation: CaCl2 Leukocytes: N/A | 18 pts (knee) Age: mean 54.6 Lesion size/degree: ICRS grade 3 or 4 Treatment: SVF+PRP Follow-up: 24.3 months | Function and pain improvement WOMAC and MRI correlate with cell numbers, better if OA < 3 | |
| Koh 2012 [65] Knee | Comparative | SVF | PRP Plts: 1.28 × 106/μL Activation: CaCl2 Leukocytes: N/A | 50 pts (knee) Age mean: N/A Lesion size/degree: ICRS grade mean 3.2 Treatment: debridement vs. debridement+SVF+PRP+2 weekly PRP inj Follow-up: 16.4 months | No major adverse events; improvement of clinical scores in both groups; SVF performed better at < 55 years and OA < 3 | |
| HA | ||||||
| Chondral lesion | Saw 2013 [66] Arthroscopy | RCT | PBPCs | High molecular weight HA | 50 pts (25 vs. 25) (knee) Age: mean 40 years Lesions size: ICRS grade 3 and 4 lesions Treatment: Subchondral drilling + 5 weekly inj of PBPCs+HA vs. HA alone+3 weekly inj after 6 months Follow-up: from 18 to 24 months | Improvement of the quality of articular cartilage repair in PBSC group at histologic and MRI evaluation |
| Lee 2012 [67] Ann Accad Med | Comparative | Cultured BMSCs | High molecular weight HA | 70 (35 vs. 35) (knee) Age: mean 44 Lesion size: N/A Treatment: MFX+inj of BMSCs+HA (+ 2 weekly inj HA) vs. BMSCs+periosteal patch Follow-up: 24.5 months | No significant difference between the two procedures, with less invasivity for BMSCs/HA IA inj | |
| Saw 2011 [68] Arthroscopy | Case series | PBPCs | High molecular weight HA | 5 pts (knee) Age: mean 39.4 years Lesions size: 2 grade IV kissing lesions–3 small Treatment: Subchondral drilling+PBPCs+HA 5 weekly inj Follow-up: from 10 to 26 months | No adverse events; hyaline cartilage regeneration at histology | |
| OA | Wong 2013 [69] Arthroscopy | RCT | Cultured BMSCs | N/A | 56 pts (28 vs. 28) (knee) Age mean: mean 51 years Lesion size/degree: Treatment: BMSCs+HA vs. HA inj after MFX+HTO Follow-up: 24 months | Clinical improvement at short term and MOCART outcomes at 1 year in cells group |
| Combination of delivery agents | ||||||
| OA | Turajane 2017 [70] Stem Cells Int | RCT | AAPBSCs | PRP Plts: N/A Activation: N/A Leukocytes: N/A High molecular weight HA | 60 pts (20 vs. 20 vs. 20) Age: mean 56.5 years Lesion size/degree: 2.3 K-L Treatment: MFX + 3 weekly inj of AAPBSCs+HA+PRP+hGCSF vs. MFX+3 weekly inj of AAPBSCs+HA+PRP vs. 3 weekly inj HA alone Follow-up: 12 months | Avoidance of TKA in the AAPBSC groups at 12 months and potent, early, and sustained symptom alleviation in GFA groups vs. HA alone |
| Pak 2016 [71] BioRes Open Access | Case series | SVF | PRP Plts: N/A Activation: CaCl2 Leukocytes: N/A | 3 pts (knee) Age: mean 71.6 years Lesion size/degree: stage 3 OA Treatment: SVF+PRP+HA+3 weekly PRP inj Follow-up: 3 months | Function and pain improvement at 3 months with signs of regenerating cartilage-like tissue at MRI | |
| Pak 2013 [72] BMC Musculoskeletal Disord | Case series | SVF | PRP Plts: N/A Activation: CaCl2 Leukocytes: N/A | 91 pts (various anatomic locations) Age: mean 51.23 years Lesion size/degree: N/A Treatment: SVF+PRP+HA+4 weekly PRP inj Follow-up: 26.62 months | SVF/PRP injections are safe; clinical improvement of knee and hip | |
| Pak 2011 [73] J Med Case Rep | Case report | SVF | PRP Plts: N/A Activation: CaCl2 Leukocytes: N/A | 2 pts (knee), 2 pts (hip) Age: 70, 79, and 29, 47 years Lesion size/degree: N/A Treatment: SVF+PRP+low dose dexamethasone inj Follow-up: 3 months | Clinical improvement; significant positive changes at MRI | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roffi, A.; Nakamura, N.; Sanchez, M.; Cucchiarini, M.; Filardo, G. Injectable Systems for Intra-Articular Delivery of Mesenchymal Stromal Cells for Cartilage Treatment: A Systematic Review of Preclinical and Clinical Evidence. Int. J. Mol. Sci. 2018, 19, 3322. https://doi.org/10.3390/ijms19113322
Roffi A, Nakamura N, Sanchez M, Cucchiarini M, Filardo G. Injectable Systems for Intra-Articular Delivery of Mesenchymal Stromal Cells for Cartilage Treatment: A Systematic Review of Preclinical and Clinical Evidence. International Journal of Molecular Sciences. 2018; 19(11):3322. https://doi.org/10.3390/ijms19113322
Chicago/Turabian StyleRoffi, Alice, Norimasa Nakamura, Mikel Sanchez, Magali Cucchiarini, and Giuseppe Filardo. 2018. "Injectable Systems for Intra-Articular Delivery of Mesenchymal Stromal Cells for Cartilage Treatment: A Systematic Review of Preclinical and Clinical Evidence" International Journal of Molecular Sciences 19, no. 11: 3322. https://doi.org/10.3390/ijms19113322
APA StyleRoffi, A., Nakamura, N., Sanchez, M., Cucchiarini, M., & Filardo, G. (2018). Injectable Systems for Intra-Articular Delivery of Mesenchymal Stromal Cells for Cartilage Treatment: A Systematic Review of Preclinical and Clinical Evidence. International Journal of Molecular Sciences, 19(11), 3322. https://doi.org/10.3390/ijms19113322