Correlation between Diabetes Mellitus and Knee Osteoarthritis: A Dry-To-Wet Lab Approach
Abstract
1. Introduction
2. Results
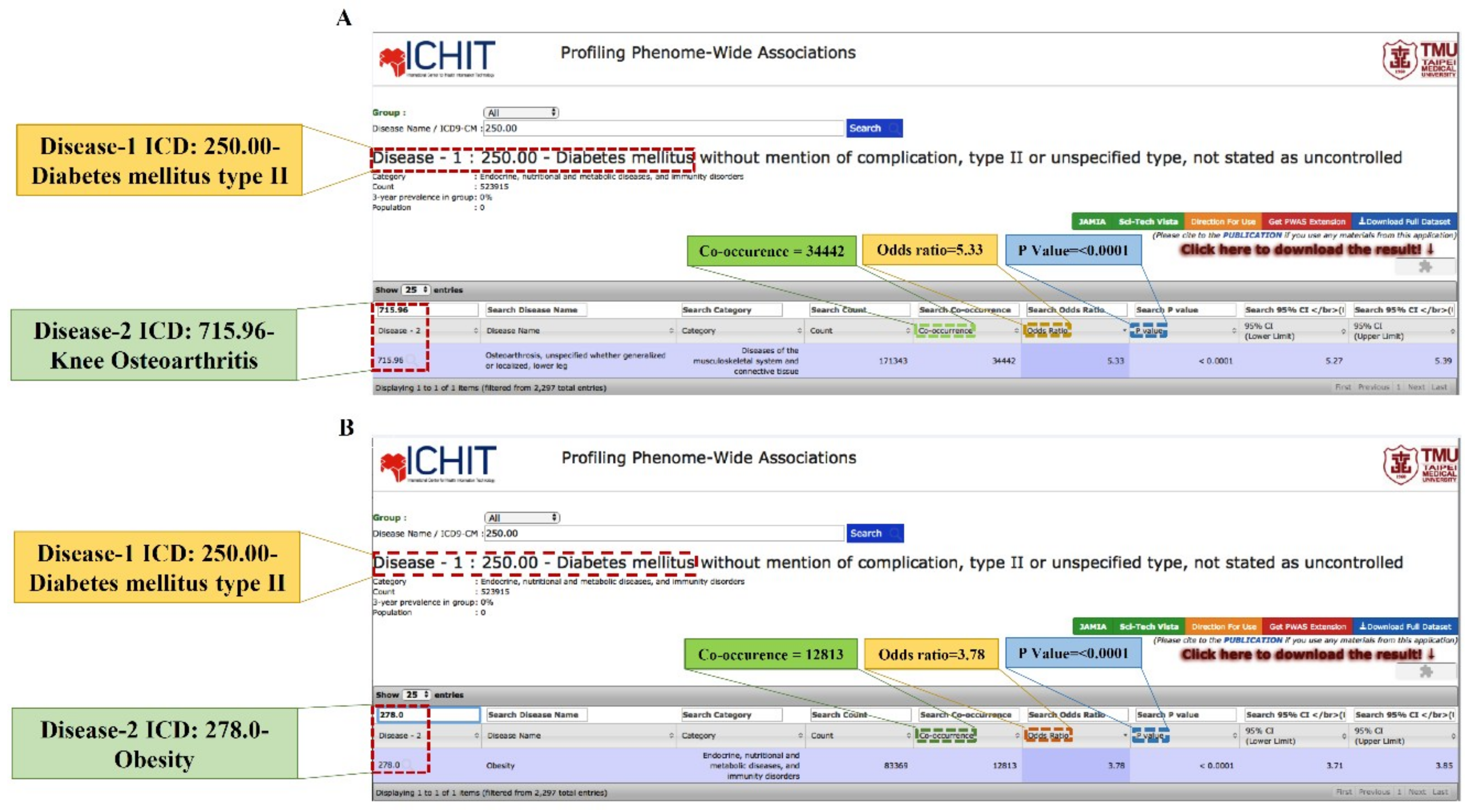
2.1. Association between T1DM and KOA
2.2. Association between T2DM and KOA
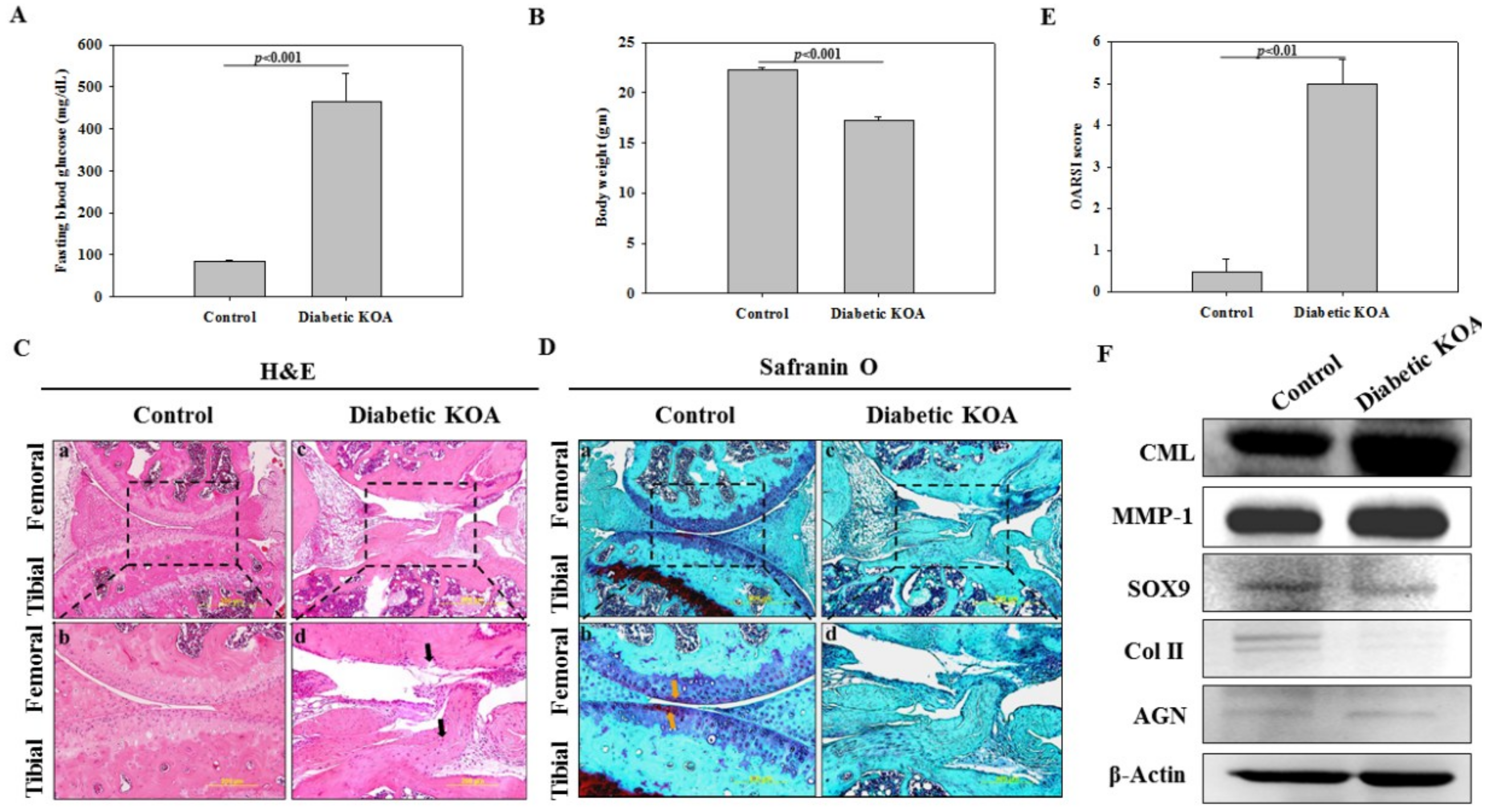
2.3. Characterization of DM
2.4. Staining-Based Assessment of KOA-Like Phenotype in Diabetic Mice
2.5. Determination of Accumulated Advanced Glycation End Product (AGE) and Inflammatory Degradative Enzyme
2.6. Expression of Cartilage-Specific Proteins
3. Discussion
4. Materials and Methods
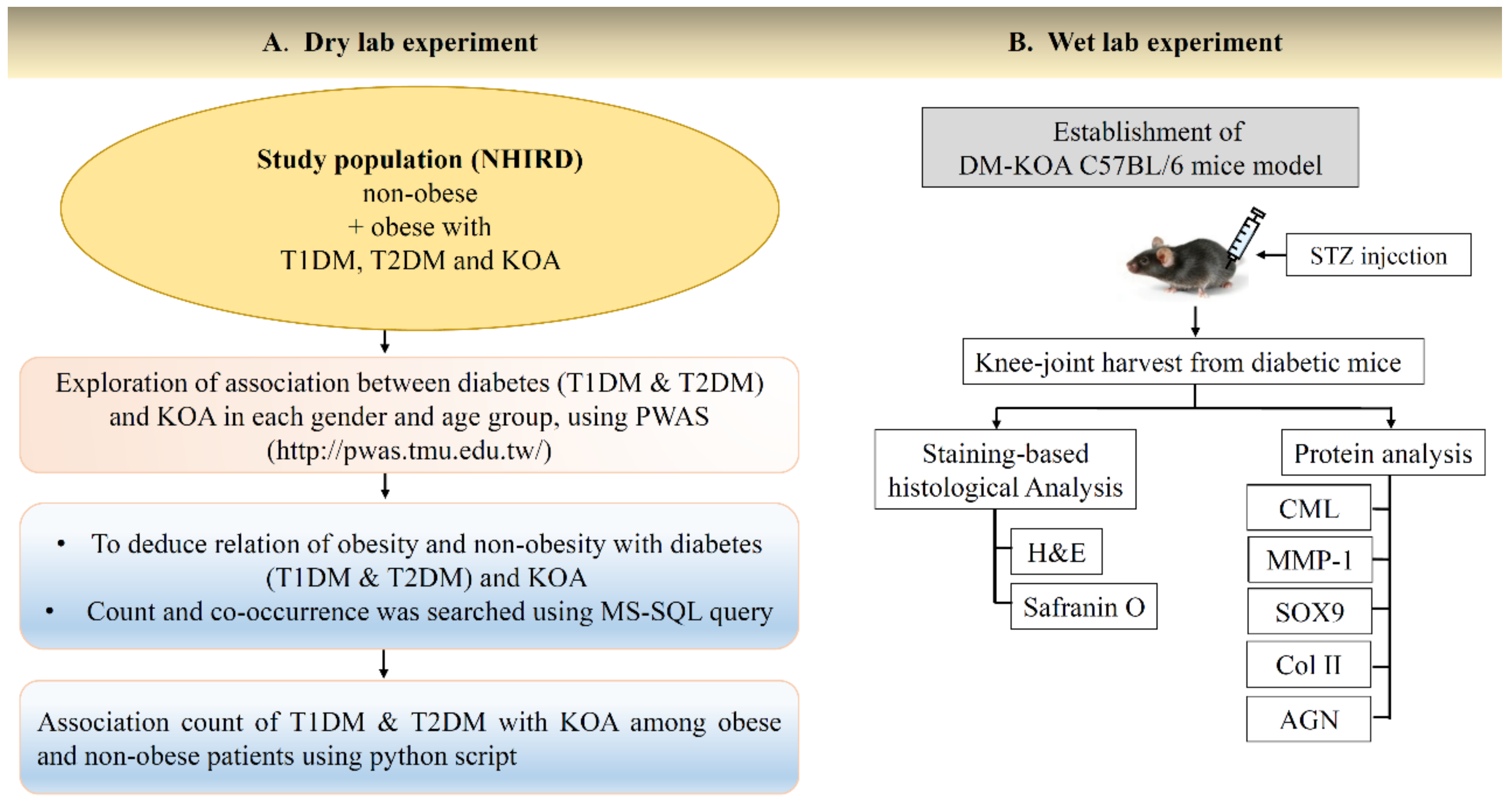
4.1. Dry-Lab Experiments
Data Source
4.2. Wet-Lab Experiments
4.2.1. Induction of Experimental Diabetes in Mice
4.2.2. Monitoring of Blood Glucose Level
4.2.3. Histologic Analysis
4.2.4. Western Blot Analysis
5. Ethical Statement
6. Statistical Analysis
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| DM | Diabetes mellitus |
| KOA | Knee osteoarthritis |
| AGE | Advanced glycation end product |
| CML | Carboxymethyl lysine |
| NHIRD | National Health Insurance Research Database |
| PWAS | Phenome-wide association study |
| Col II | Type II collagen |
| AGN | Aggrecan |
References
- Yu, S.H.; Chen, S.Y.; Li, W.S.; Dubey, N.K.; Chen, W.H.; Chuu, J.J.; Leu, S.J.; Deng, W.P. Hypoglycemic Activity through a Novel Combination of Fruiting Body and Mycelia of Cordyceps militaris in High-Fat Diet-Induced Type 2 Diabetes Mellitus Mice. J. Diabetes Res. 2015, 2015, 723190. [Google Scholar] [PubMed]
- Shaw, J.E.; Sicree, R.A.; Zimmet, P.Z. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res. Clin. Pract. 2010, 87, 4–14. [Google Scholar] [PubMed]
- Shumway, J.T.; Gambert, S.R. Diabetic nephropathy-pathophysiology and management. Int. Urol. Nephrol. 2002, 34, 257–264. [Google Scholar] [PubMed]
- Laakso, M.; Pyorala, K. Age of onset and type of diabetes. Diabetes Care 1985, 8, 114–117. [Google Scholar] [PubMed]
- Libman, I.; Arslanian, S.A. Type II diabetes mellitus: No longer just adults. Pediatr. Ann. 1999, 28, 589–593. [Google Scholar] [PubMed]
- Rosenbloom, A.L.; Joe, J.R.; Young, R.S.; Winter, W.E. Emerging epidemic of type 2 diabetes in youth. Diabetes Care 1999, 22, 345–354. [Google Scholar] [PubMed]
- Brownlee, M. Biochemistry and molecular cell biology of diabetic complications. Nature 2001, 414, 813–820. [Google Scholar] [PubMed]
- Vogt, B.W.; Schleicher, E.D.; Wieland, O.H. epsilon-Amino-lysine-bound glucose in human tissues obtained at autopsy. Increase in diabetes mellitus. Diabetes 1982, 31, 1123–1127. [Google Scholar] [PubMed]
- Steffes, M.W. Affecting the decline of renal function in diabetes mellitus. Kidney Int. 2001, 60, 378–379. [Google Scholar] [PubMed]
- Fox, C.S. Cardiovascular disease risk factors, type 2 diabetes mellitus, and the Framingham Heart Study. Trends Cardiovasc. Med. 2010, 20, 90–95. [Google Scholar] [PubMed]
- Smith, A.G.; Singleton, J.R. Diabetic neuropathy. Continuum (Minneap Minn) 2012, 18, 60–84. [Google Scholar] [PubMed]
- Onur, T.; Wu, R.; Metz, L.; Dang, A. Characterisation of osteoarthritis in a small animal model of type 2 diabetes mellitus. Bone Joint Res. 2014, 3, 203–211. [Google Scholar] [PubMed]
- Berenbaum, F. Diabetes-induced osteoarthritis: From a new paradigm to a new phenotype. Ann. Rheum. Dis. 2011, 70, 1354–1356. [Google Scholar] [PubMed]
- Mayer-Davis, E.J.; Lawrence, J.M.; Dabelea, D.; Divers, J.; Isom, S.; Dolan, L.; Imperatore, G.; Linder, B.; Marcovina, S.; Pettitt, D.J.; et al. Incidence Trends of Type 1 and Type 2 Diabetes among Youths, 2002–2012. N. Engl. J. Med. 2017, 376, 1419–1429. [Google Scholar] [PubMed]
- Olsson, S.E. Degenerative joint disease (osteoarthrosis): A review with special reference to the dog. J. Small Anim. Pract. 1971, 12, 333–342. [Google Scholar] [PubMed]
- Stupina, T.A.; Shchudlo, N.A.; Stepanov, M.A. Structural reorganization of the main joint components during the experimental modeling of osteoarthrosis with reduced blood supply. Morfologiia 2014, 146, 61–65. [Google Scholar] [PubMed]
- Benito, M.J.; Veale, D.J.; FitzGerald, O.; van den Berg, W.B.; Bresnihan, B. Synovial tissue inflammation in early and late osteoarthritis. Ann. Rheum. Dis. 2005, 64, 1263–1267. [Google Scholar] [PubMed]
- Zhuo, Q.; Yang, W.; Chen, J.; Wang, Y. Metabolic syndrome meets osteoarthritis. Nat. Rev. Rheumatol. 2012, 8, 729–737. [Google Scholar] [PubMed]
- American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2009, 32 (Suppl. 1), S62–S67. [Google Scholar]
- Polsky, S.; Ellis, S.L. Obesity, insulin resistance, and type 1 diabetes mellitus. Curr. Opin. Endocrinol. Diabetes Obes. 2015, 22, 277–282. [Google Scholar] [PubMed]
- Messier, S.P. Obesity and osteoarthritis: Disease genesis and nonpharmacologic weight management. Rheum. Dis. Clin. N. Am. 2008, 34, 713–729. [Google Scholar]
- Reijman, M.; Pols, H.A.; Bergink, A.P.; Hazes, J.M.; Belo, J.N.; Lievense, A.M.; Bierma-Zeinstra, S.M. Body mass index associated with onset and progression of osteoarthritis of the knee but not of the hip: The Rotterdam Study. Ann. Rheum. Dis. 2007, 66, 158–162. [Google Scholar] [PubMed]
- Berenbaum, F.; Eymard, F.; Houard, X. Osteoarthritis, inflammation and obesity. Curr. Opin. Rheumatol. 2013, 25, 114–118. [Google Scholar] [PubMed]
- Golden, S.H.; Brown, A.; Cauley, J.A.; Chin, M.H.; Gary-Webb, T.L.; Kim, C.; Sosa, J.A.; Sumner, A.E.; Anton, B. Health disparities in endocrine disorders: Biological, clinical, and nonclinical factors—An Endocrine Society scientific statement. J. Clin. Endocrinol. Metab. 2012, 97, E1579–E1639. [Google Scholar] [PubMed]
- Syed-Abdul, S.; Moldovan, M.; Nguyen, P.A.; Enikeev, R.; Jian, W.S.; Iqbal, U.; Hsu, M.H.; Li, Y.C. Profiling phenome-wide associations: A population-based observational study. J. Am. Med. Inform. Assoc. 2015, 22, 896–899. [Google Scholar] [PubMed]
- Griffin, T.M.; Guilak, F. Why is obesity associated with osteoarthritis? Insights from mouse models of obesity. Biorheology 2008, 45, 387–398. [Google Scholar] [PubMed]
- Al-Goblan, A.S.; Al-Alfi, M.A.; Khan, M.Z. Mechanism linking diabetes mellitus and obesity. Diabetes Metab. Syndr. Obes. 2014, 7, 587–591. [Google Scholar] [PubMed]
- Buckwalter, J.A.; Mankin, H.J.; Grodzinsky, A.J. Articular cartilage and osteoarthritis. Instr. Course Lect. 2005, 54, 465–480. [Google Scholar] [PubMed]
- Glasson, S.S.; Chambers, M.G.; Van Den Berg, W.B.; Little, C.B. The OARSI histopathology initiative—Recommendations for histological assessments of osteoarthritis in the mouse. Osteoarthritis Cartilage 2010, 18 (Suppl. 3), S17–S23. [Google Scholar] [PubMed]
- Zeng, G.Q.; Chen, A.B.; Li, W.; Song, J.H.; Gao, C.Y. High MMP-1, MMP-2, and MMP-9 protein levels in osteoarthritis. Genet. Mol. Res. 2015, 14, 14811–14822. [Google Scholar] [PubMed]
- Louati, K.; Vidal, C.; Berenbaum, F.; Sellam, J. Association between diabetes mellitus and osteoarthritis: Systematic literature review and meta-analysis. RMD Open 2015, 1, e000077. [Google Scholar] [PubMed]
- Saxena, R.; Elbers, C.C.; Guo, Y.; Peter, I.; Gaunt, T.R.; Mega, J.L.; Lanktree, M.B.; Tare, A.; Castillo, B.A.; Li, Y.R.; et al. Large-scale gene-centric meta-analysis across 39 studies identifies type 2 diabetes loci. Am. J. Hum. Genet. 2012, 90, 410–425. [Google Scholar] [PubMed]
- Zhang, Y.; Xu, L.; Nevitt, M.C.; Aliabadi, P.; Yu, W.; Qin, M.; Lui, L.Y.; Felson, D.T. Comparison of the prevalence of knee osteoarthritis between the elderly Chinese population in Beijing and whites in the United States: The Beijing Osteoarthritis Study. Arthritis Rheum. 2001, 44, 2065–2071. [Google Scholar] [PubMed]
- Scavini, M.; Stidley, C.A.; Shah, V.O.; Narva, A.S.; Tentori, F.; Kessler, D.S.; Bobelu, A.; Albert, C.P.; Bobelu, J.; Jamon, E.; et al. Prevalence of diabetes is higher among female than male Zuni indians. Diabetes Care 2003, 26, 55–60. [Google Scholar] [PubMed]
- Hame, S.L.; Alexander, R.A. Knee osteoarthritis in women. Curr. Rev. Musculoskelet. Med. 2013, 6, 182–187. [Google Scholar] [PubMed]
- Hazari, A.; Maiya, A.G.; Shivashankara, K.N.; Agouris, I.; Monteiro, A.; Jadhav, R.; Kumar, S.; Shashi Kumar, C.G.; Mayya, S.S. Kinetics and kinematics of diabetic foot in type 2 diabetes mellitus with and without peripheral neuropathy: A systematic review and meta-analysis. Springerplus 2016, 5, 1819. [Google Scholar] [PubMed]
- Bijlsma, J.W.; Knahr, K. Strategies for the prevention and management of osteoarthritis of the hip and knee. Best Pract. Res. Clin. Rheumatol. 2007, 21, 59–76. [Google Scholar] [PubMed]
- Li, J.; Cesari, M.; Liu, F.; Dong, B.; Vellas, B. Effects of Diabetes Mellitus on Cognitive Decline in Patients with Alzheimer Disease: A Systematic Review. Can. J. Diabetes 2017, 41, 114–119. [Google Scholar] [PubMed]
- Twig, G.; Afek, A.; Derazne, E.; Tzur, D.; Cukierman-Yaffe, T.; Gerstein, H.C.; Tirosh, A. Diabetes risk among overweight and obese metabolically healthy young adults. Diabetes Care 2014, 37, 2989–2995. [Google Scholar] [PubMed]
- King, L.K.; March, L.; Anandacoomarasamy, A. Obesity & osteoarthritis. Indian J. Med. Res. 2013, 138, 185–193. [Google Scholar] [PubMed]
- Peng, B.Y.; Chiou, C.S.; Dubey, N.K.; Yu, S.H.; Deng, Y.H.; Tsai, F.C.; Chiang, H.S.; Shieh, Y.H.; Chen, W.H.; Deng, W.P. Non-invasive in vivo molecular imaging of intra-articularly transplanted immortalized bone marrow stem cells for osteoarthritis treatment. Oncotarget 2017, 8, 97153–97164. [Google Scholar] [PubMed]
- Dubey, N.K.; Mishra, V.K.; Dubey, R.; Syed-Abdul, S.; Wang, J.R.; Wang, P.D.; Deng, W.-P. Combating Osteoarthritis through Stem Cell Therapies by Rejuvenating Cartilage: A Review. Stem Cells Int. 2018, 2018, 13. [Google Scholar]
- Vlassara, H.; Uribarri, J. Advanced glycation end products (AGE) and diabetes: Cause, effect, or both? Curr. Diabetes Rep. 2014, 14, 453. [Google Scholar]
- Yoon, H.S.; Baik, S.H.; Oh, C.H. Quantitative measurement of desquamation and skin elasticity in diabetic patients. Skin Res. Technol. 2002, 8, 250–254. [Google Scholar] [PubMed]
- Yu, S.H.; Dubey, N.K.; Li, W.S.; Liu, M.C.; Chiang, H.S.; Leu, S.J.; Shieh, Y.H.; Tsai, F.C.; Deng, W.P. Cordyceps militaris Treatment Preserves Renal Function in Type 2 Diabetic Nephropathy Mice. PLoS ONE 2016, 11, e0166342. [Google Scholar]
- Rose, B.J.; Kooyman, D.L. A Tale of Two Joints: The Role of Matrix Metalloproteases in Cartilage Biology. Dis. Mark. 2016, 2016, 4895050. [Google Scholar]
- Maldonado, M.; Nam, J. The role of changes in extracellular matrix of cartilage in the presence of inflammation on the pathology of osteoarthritis. Biomed. Res. Int. 2013, 2013, 284873. [Google Scholar] [PubMed]
- Duquette, J.J.; Grigg, P.; Hoffman, A.H. The effect of diabetes on the viscoelastic properties of rat knee ligaments. J. Biomech. Eng. 1996, 118, 557–564. [Google Scholar] [PubMed]
- Lin, H.C.; Lee, H.C.; Kuo, N.W.; Chu, C.H. Hospital characteristics associated with post-discharge suicide of severely depressed patients. J. Affect. Disord. 2008, 110, 215–221. [Google Scholar] [PubMed]

| Age Group (Years) | Female | Male | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Unadjusted | Adjusted Obese | Adjusted Non-Obese | Unadjusted | Adjusted Obese | Adjusted Non-Obese | |||||||
| Co. | OR (CI 95%) | Co. | OR (CI 95%) | Co. | OR (CI 95%) | Co. | OR (CI 95%) | Co. | OR (CI 95%) | Co. | OR (CI 95%) | |
| 50–59 | 191 | 1.28 * (1.11–1.48) | 2 | 0.46 (0.11–1.89) | 189 | 1.30 ** (1.12–1.50) | 80 | 1.46 * (1.17–1.82) | 1 | 1.65 (0.22–12.26) | 79 | 1.45 * (1.16–1.81) |
| 60–69 | 462 | 1.23 ** (1.12–1.36) | 8 | 1.28 (0.61–2.68) | 454 | 1.23 ** (1.12–1.35) | 189 | 1.45 ** (1.25–1.68) | 1 | 0.75 (0.10–5.57) | 188 | 1.45 ** (1.26–1.68) |
| 70–79 | 357 | 1.07 (0.96–1.19) | 2 | 0.64 (0.15–2.72) | 355 | 1.07 (0.96–1.19) | 206 | 1.31 ** (1.14–1.50) | 1 | 2.29 (0.28–18.70) | 205 | 1.30 ** (1.13–1.50) |
| 80–89 | 76 | 1.43 * (1.13–1.80) | 0 | 0 (n/a) | 76 | 1.44 * (1.14–1.81) | 23 | 0.74 (0.49–1.12) | 0 | 0 (n/a) | 23 | 0.74 (0.49–1.12) |
| Total | 1086 | 1.32 ** (1.24–1.40) | 12 | 0.93 (0.47–1.67) | 1074 | 1.32 ** (1.24–1.40) | 498 | 1.44 ** (1.31–1.58) | 3 | 1.31 (0.26–4.06) | 495 | 1.44 ** (1.31–1.58) |
| Age Group (Years) | Male & Female | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Unadjusted | Adjusted Obese | Adjusted Non-Obese | |||||||
| Co. | OR (CI 95%) | p-Value | Co. | OR (CI 95%) | p-Value | Co. | OR (CI 95%) | p-Value | |
| All age groups | 1584 | 1.40 ** (1.33–1.47) | <0.0001 | 15 | 0.99 (0.54–1.67) | 0.0477 | 1569 | 1.40 ** (1.33–1.48) | <0.0001 |
| Age Group (Years) | Female | Male | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Unadjusted | Adjusted Obese | Adjusted Non-Obese | Unadjusted | Adjusted Obese | Adjusted Non-Obese | |||||||
| Co. | OR (CI 95%) | Co. | OR (CI 95%) | Co. | OR (CI 95%) | Co. | OR (CI 95%) | Co. | OR (CI 95%) | Co. | OR (CI 95%) | |
| 30–39 | 298 | 2.77 ** (2.46–3.12) | 21 | 1.40 (0.86–2.27) | 277 | 2.74 ** (2.43–3.10) | 230 | 2.68 ** (2.34–3.06) | 9 | 1.56 (0.72–3.37) | 221 | 2.65 ** (2.31–3.04) |
| 40–49 | 1896 | 2.05 ** (1.95–2.15) | 98 | 1.22 (0.96–1.54) | 1798 | 2.03 ** (1.93–2.14) | 997 | 1.90 ** (1.78–2.03) | 15 | 1.02 (0.56–1.88) | 982 | 1.90 ** (1.78–2.03) |
| 50–59 | 5650 | 1.52 ** (1.47–1.56) | 182 | 1.12 (0.92–1.35) | 5468 | 1.51 ** (1.46–1.56) | 2067 | 1.55 ** (1.47–1.62) | 23 | 1.20 (0.70–2.07) | 2044 | 1.54 ** (1.47–1.62) |
| 60–69 | 10,602 | 1.3 1** (1.28–1.34) | 199 | 1.41 * (1.15–1.74) | 10,403 | 1.30 ** (1.27–1.34) | 4113 | 1.44 ** (1.39–1.48) | 38 | 1.03 (0.66–1.60) | 4075 | 1.44 ** (1.39–1.49) |
| 70–79 | 8091 | 1.28 ** (1.24-1.31) | 53 | 1.03 (0.70–1.49) | 8038 | 1.28 ** (1.24–1.31) | 4944 | 1.43 ** (1.39-1.48) | 24 | 1.68 (0.91–3.12) | 4920 | 1.43 ** (1.38–1.48) |
| 80–89 | 1498 | 1.44 ** (1.35–1.53) | 5 | 1.37 (0.38–4.98) | 1493 | 1.44 ** (1.35–1.53) | 939 | 1.54 ** (1.43–1.65) | 0 | 0 (n/a) | 939 | 1.54 ** (1.43–1.66) |
| Total | 28,035 | 2.76 ** (2.72–2.80) | 558 | 1.80 ** (1.61–201) | 27,477 | 2.77 ** (2.73–2.81) | 13,290 | 2.63 ** (2.58–2.68) | 109 | 1.62 ** (1.25–2.08) | 13181 | 2.64 ** (2.58–2.69) |
| Age Group (Years) | Male & Female | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Unadjusted | Adjusted Obese | Adjusted Non-Obese | |||||||
| Co. | OR (CI 95%) | p-Value | Co. | OR (CI 95%) | p-Value | Co. | OR (CI 95%) | p-Value | |
| All age group | 41,325 | 2.75 ** (2.72–2.78) | <0.0001 | 667 | 1.71 ** (1.55–1.89) | <0.0001 | 40658 | 2.75 ** (2.72–2.79) | <0.0001 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dubey, N.K.; Ningrum, D.N.A.; Dubey, R.; Deng, Y.-H.; Li, Y.-C.; Wang, P.D.; Wang, J.R.; Syed-Abdul, S.; Deng, W.-P. Correlation between Diabetes Mellitus and Knee Osteoarthritis: A Dry-To-Wet Lab Approach. Int. J. Mol. Sci. 2018, 19, 3021. https://doi.org/10.3390/ijms19103021
Dubey NK, Ningrum DNA, Dubey R, Deng Y-H, Li Y-C, Wang PD, Wang JR, Syed-Abdul S, Deng W-P. Correlation between Diabetes Mellitus and Knee Osteoarthritis: A Dry-To-Wet Lab Approach. International Journal of Molecular Sciences. 2018; 19(10):3021. https://doi.org/10.3390/ijms19103021
Chicago/Turabian StyleDubey, Navneet Kumar, Dina Nur Anggraini Ningrum, Rajni Dubey, Yue-Hua Deng, Yu-Chuan Li, Peter D. Wang, Joseph R. Wang, Shabbir Syed-Abdul, and Win-Ping Deng. 2018. "Correlation between Diabetes Mellitus and Knee Osteoarthritis: A Dry-To-Wet Lab Approach" International Journal of Molecular Sciences 19, no. 10: 3021. https://doi.org/10.3390/ijms19103021
APA StyleDubey, N. K., Ningrum, D. N. A., Dubey, R., Deng, Y.-H., Li, Y.-C., Wang, P. D., Wang, J. R., Syed-Abdul, S., & Deng, W.-P. (2018). Correlation between Diabetes Mellitus and Knee Osteoarthritis: A Dry-To-Wet Lab Approach. International Journal of Molecular Sciences, 19(10), 3021. https://doi.org/10.3390/ijms19103021







