A Novel High Sensitivity Type II Collagen Blood-Based Biomarker, PRO-C2, for Assessment of Cartilage Formation
Abstract
1. Introduction
2. Results
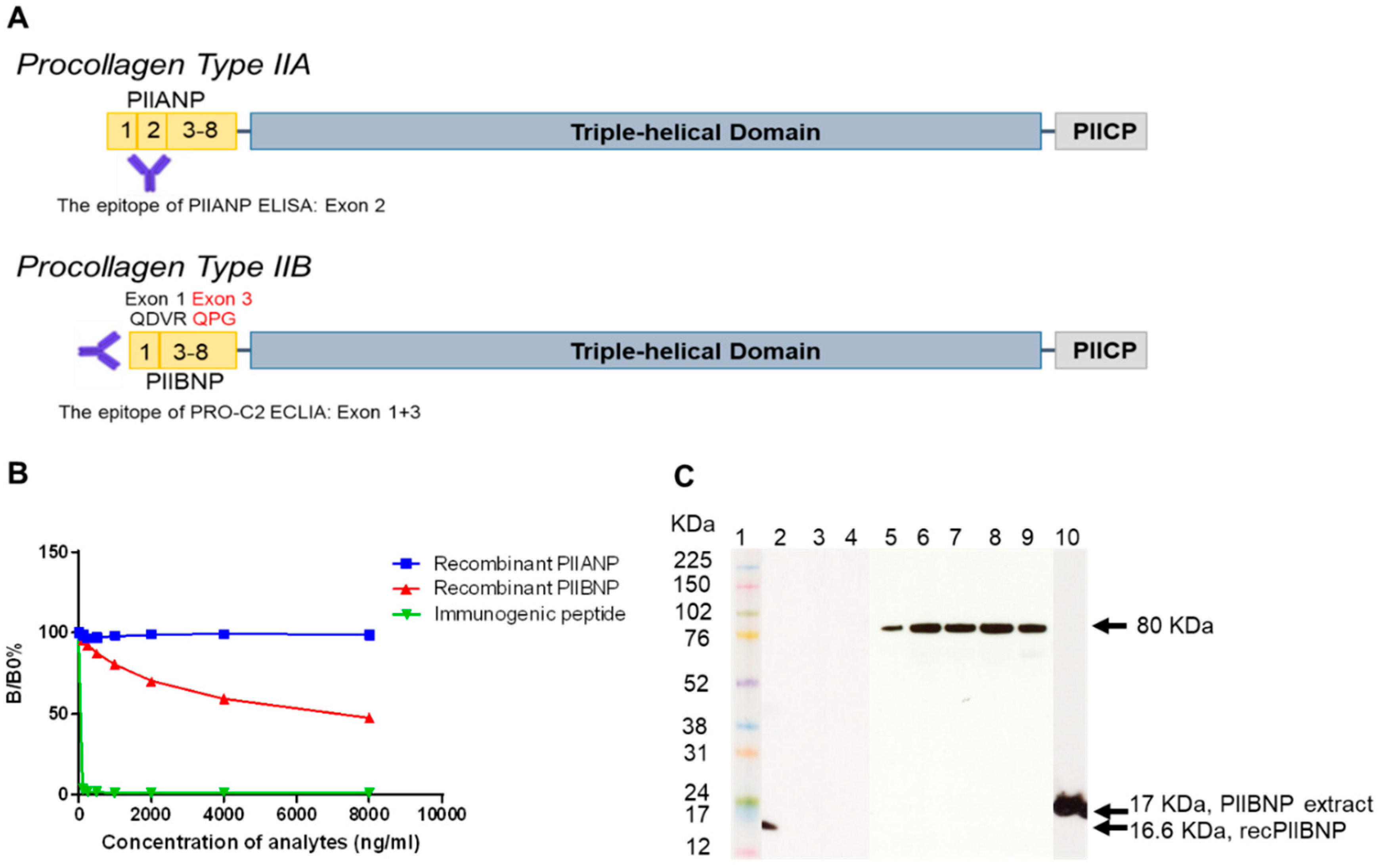
2.1. Characterization of a Monoclonal Antibody
2.1.1. Binding Specificity
2.1.2. Western Blot
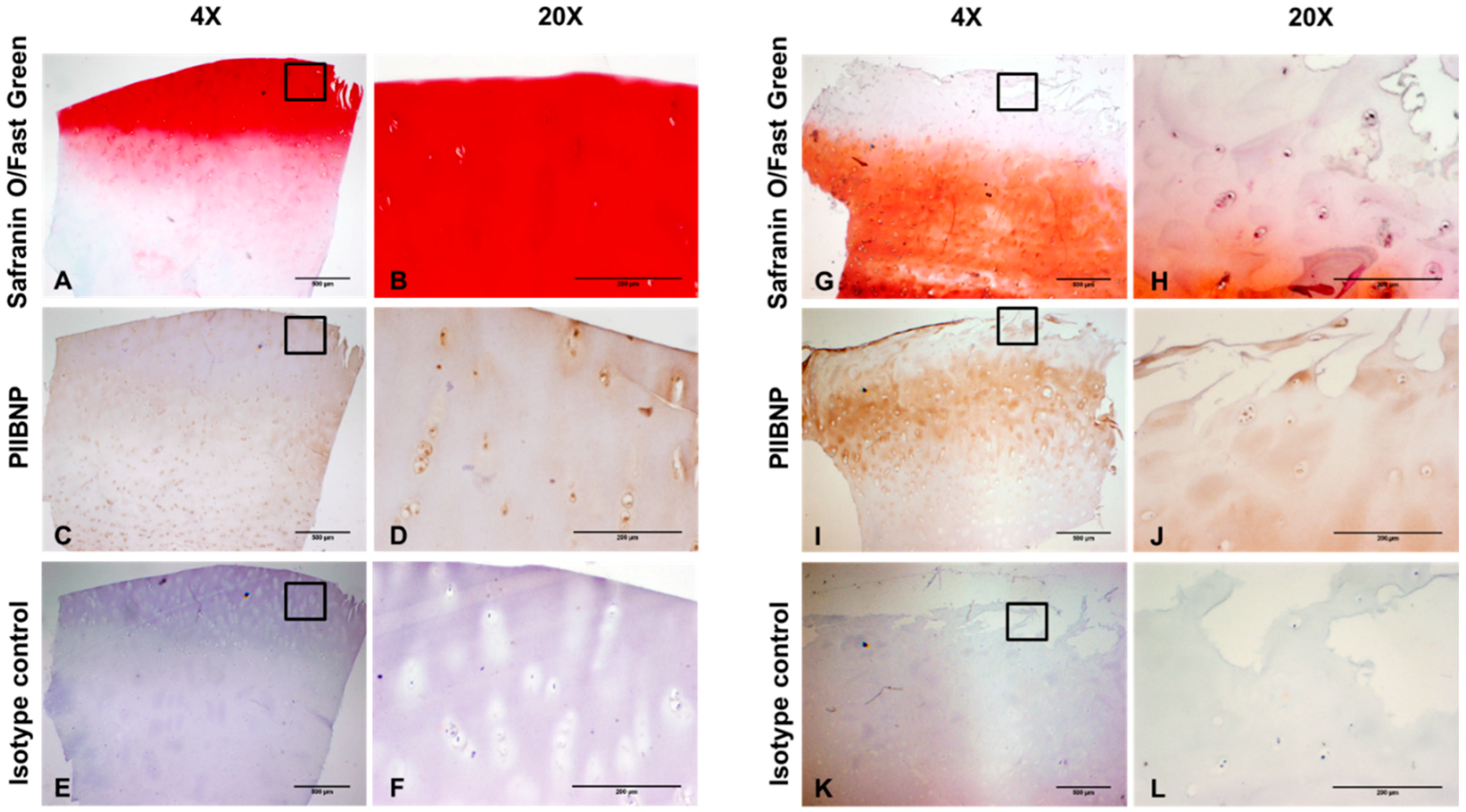
2.2. Immunolocalization of PIIBNP in Human Articular Cartilage
2.3. Development and Validation of hsPRO-C2 ElectroChemiLuminescence ImmunoAssay (ECLIA)
2.3.1. Technical Performance of hsPRO-C2 ECLIA
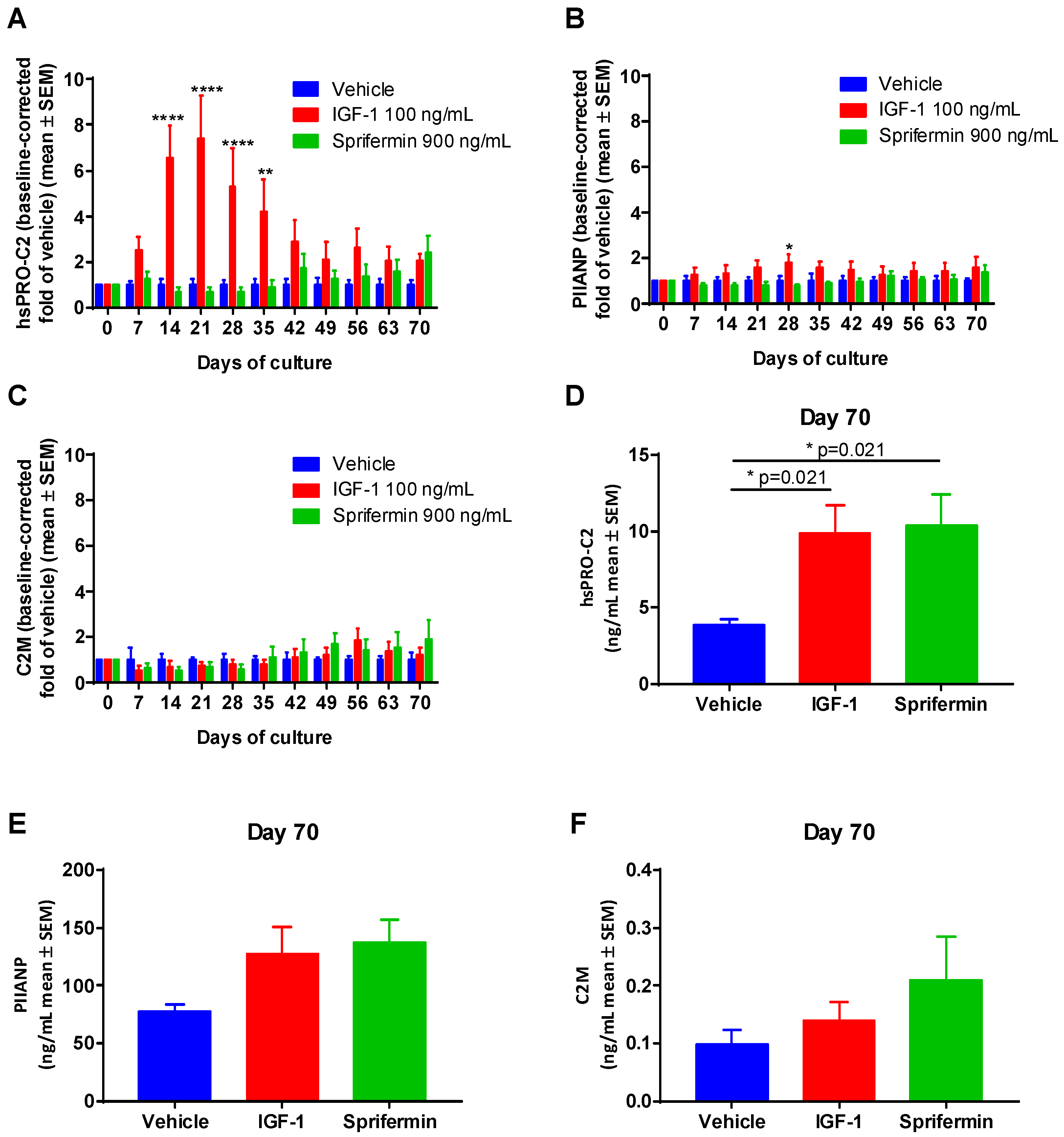
2.3.2. Induction of PIIANP and PIIBNP Release in Human OA Cartilage Explants by Growth Factors
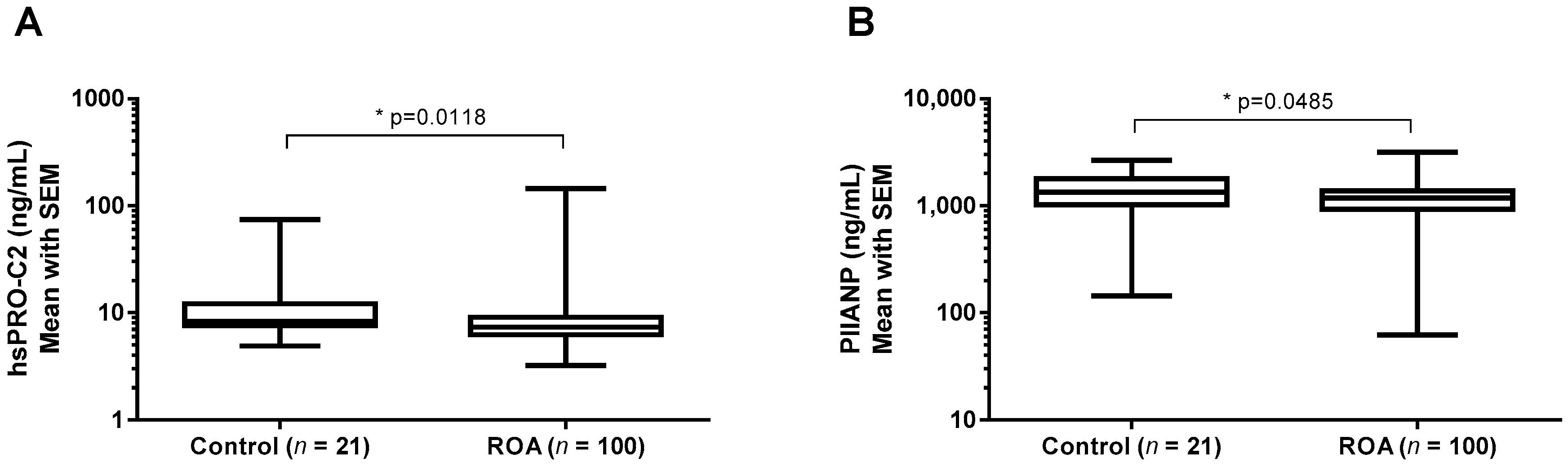
2.3.3. hsPRO-C2 Concentration in Healthy Subjects and Knee OA Patients
3. Discussion
4. Methods
4.1. Materials
4.2. Human PIIBNP Extraction from Articular Cartilage
4.3. Western Blot
4.4. Histology and Immunohistochemistry
4.5. Assay Protocol
4.6. Technical Evaluation
4.7. Comparison of hsPRO-C2 ECLIA and PIIANP ELISA
4.7.1. PIIANP and PIIBNP Profiling in Human OA Cartilage Explants Ex Vivo Model
4.7.2. Healthy Subjects and Knee OA Patients Population
4.7.3. Correlation of PIIANP and PIIBNP in Human Sera
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| BMI | Body mass index |
| DMOAD | Disease-modifying osteoarthritis drug |
| ECLIA | Electrochemiluminescence immunoassay |
| ECM | Extracellular matrix |
| IGF-1 | Insulin-like growth factor 1 |
| LLOD | Lower limit of detection |
| LLOQ | Lower limit of quantification |
| MRI | Magnetic resonance imaging |
| OA | Osteoarthritis |
| PBS | Phosphate buffered saline |
| PINP | Procollagen type I N-terminal propeptides |
| PIIANP | Procollagen type IIA N-terminal propeptides |
| PIIBNP | Procollagen type IIB N-terminal propeptides |
| RA | Rheumatoid arthritis |
| SD | Standard deviation |
| SDS-PAGE | Sodium dodecyl sulfate-polyacrylamide gel electrophoresis |
| ULOD | Upper limit of detection |
References
- Abramson, S.B.; Attur, M.; Yazici, Y. Prospects for disease modification in osteoarthritis. Nat. Clin. Pract. Rheumatol. 2006, 2, 304–312. [Google Scholar] [CrossRef] [PubMed]
- Bay-Jensen, A.-C.; Hoegh-Madsen, S.; Dam, E.; Henriksen, K.; Sondergaard, B.C.; Pastoureau, P.; Qvist, P.; Karsdal, M.A. Which elements are involved in reversible and irreversible cartilage degradation in osteoarthritis? Rheumatol. Int. 2010, 30, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Sandberg, M.; Vuorio, E. Localization of types I, II, and III collagen mRNAs in developing human skeletal tissues by in situ hybridization. J. Cell Biol. 1987, 104, 1077–1084. [Google Scholar] [CrossRef] [PubMed]
- Poole, A.R.; Rizkalla, G.; Ionescu, M.; Reiner, A.; Brooks, E.; Rorabeck, C.; Bourne, R.; Bogoch, E. Osteoarthritis in the human knee: A dynamic process of cartilage matrix degradation, synthesis and reorganization. Agents Actions Suppl. 1993, 39, 3–13. [Google Scholar] [PubMed]
- Garnero, P.; Ayral, X.; Rousseau, J.-C.; Christgau, S.; Sandell, L.J.; Dougados, M.; Delmas, P.D. Uncoupling of type II collagen synthesis and degradation predicts progression of joint damage in patients with knee osteoarthritis. Arthritis Rheumatol. 2002, 46, 2613–2624. [Google Scholar] [CrossRef] [PubMed]
- Tchetina, E.V. Developmental mechanisms in articular cartilage degradation in osteoarthritis. Arthritis 2011, 2011, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Tchetina, E.V.; Squires, G.; Poole, A.R. Increased type II collagen degradation and very early focal cartilage degeneration is associated with upregulation of chondrocyte differentiation related genes in early human articular cartilage lesions. J. Rheumatol. 2005, 32, 876–886. [Google Scholar] [PubMed]
- Karsdal, M.A.; Michaelis, M.; Ladel, C.; Siebuhr, A.S.; Bihlet, A.R.; Andersen, J.R.; Guehring, H.; Christiansen, C.; Bay-Jensen, A.C.; Kraus, V.B. Disease-modifying treatments for osteoarthritis (DMOADs) of the knee and hip: lessons learned from failures and opportunities for the future. Osteoarthr. Cartil. 2016, 24, 2013–2021. [Google Scholar] [CrossRef] [PubMed]
- Olsen, A.K.; Sondergaard, B.C.; Byrjalsen, I.; Tanko, L.B.; Christiansen, C.; Müller, A.; Hein, G.E.; Karsdal, M.A.; Qvist, P. Anabolic and catabolic function of chondrocyte ex vivo is reflected by the metabolic processing of type II collagen. Osteoarthr. Cartil. 2007, 15, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Madsen, S.H.; Sondergaard, B.C.; Bay-Jensen, A.-C.; Karsdal, M.A. Cartilage formation measured by a novel PIINP assay suggests that IGF-I does not stimulate but maintains cartilage formation ex vivo. Scand. J. Rheumatol. 2009, 38, 222–226. [Google Scholar] [CrossRef] [PubMed]
- Karsdal, M.A.; Madsen, S.H.; Christiansen, C.; Henriksen, K.; Fosang, A.J.; Sondergaard, B.C. Cartilage degradation is fully reversible in the presence of aggrecanase but not matrix metalloproteinase activity. Arthr. Res. Ther. 2008, 10, R63. [Google Scholar] [CrossRef] [PubMed]
- Gigout, A.; Guehring, H.; Froemel, D.; Meurer, A.; Ladel, C.; Reker, D.; Bay-Jensen, A.C.; Karsdal, M.A.; Lindemann, S. Sprifermin (rhFGF18) enables proliferation of chondrocytes producing a hyaline cartilage matrix. Osteoarthr. Cartil. 2017, 25, 1858–1867. [Google Scholar] [CrossRef] [PubMed]
- Reker, D.; Kjelgaard-Petersen, C.F.; Siebuhr, A.S.; Michaelis, M.; Gigout, A.; Karsdal, M.A.; Ladel, C.; Bay-Jensen, A.C. Sprifermin (rhFGF18) modulates extracellular matrix turnover in cartilage explants ex vivo. J. Transl. Med. 2017, 15, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Onuora, S. Sprifermin shows cartilage-protective effects in knee OA. Nat. Rev. Rheumatol. 2014, 10, 322. [Google Scholar] [CrossRef] [PubMed]
- Lohmander, L.S.; Hellot, S.; Dreher, D.; Krantz, E.F.W.; Kruger, D.S.; Guermazi, A.; Eckstein, F. Intraarticular sprifermin (recombinant human fibroblast growth factor 18) in knee osteoarthritis: A randomized, double-blind, placebo-controlled trial. Arthritis Rheumatol. 2014, 66, 1820–1831. [Google Scholar] [CrossRef] [PubMed]
- Eckstein, F.; Wirth, W.; Guermazi, A.; Maschek, S.; Aydemir, A. Brief report: Intraarticular sprifermin not only increases cartilage thickness, but also reduces cartilage loss: Location-independent post hoc analysis using magnetic resonance imaging. Arthritis Rheumatol. 2015, 67, 2916–2922. [Google Scholar] [CrossRef] [PubMed]
- Ryan, M.C.; Sandell, L.J. Differential expression of a cysteine-rich domain in the amino-terminal propeptide of type II (cartilage) procollagen by alternative splicing of mRNA. J. Biol. Chem. 1990, 265, 10334–10339. [Google Scholar] [PubMed]
- Nah, H.D.; Upholt, W.B. Type II collagen mRNA containing an alternatively spliced exon predominates in the chick limb prior to chondrogenesis. J. Biol. Chem. 1991, 266, 23446–23452. [Google Scholar] [PubMed]
- McAlinden, A.; Havlioglu, N.; Liang, L.; Davies, S.R.; Sandell, L.J. Alternative splicing of type II procollagen exon 2 is regulated by the combination of a weak 5’ splice site and an adjacent intronic stem-loop cis element. J. Biol. Chem. 2005, 280, 32700–32711. [Google Scholar] [CrossRef] [PubMed]
- Aigner, T.; Zhu, Y.; Chansky, H.H.; Matsen, F.A.; Maloney, W.J.; Sandell, L.J. Reexpression of type IIA procollagen by adult articular chondrocytes in osteoarthritic cartilage. Arthritis Rheum. 1999, 42, 1443–1450. [Google Scholar] [CrossRef]
- Sandell, L.J.; Morris, N.; Robbins, J.R.; Goldring, M.B. Alternatively spliced type II procollagen mRNAs define distinct populations of cells during vertebral development: differential expression of the amino-propeptide. J. Cell Biol. 1991, 114, 1307–1319. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Mcalinden, A.; Sandell, L.J. Type IIA procollagen in development of the human intervertebral disc: Regulated expression of the NH2-propeptide by enzymic processing reveals a unique developmental pathway. Dev. Dyn. 2001, 220, 350–362. [Google Scholar] [CrossRef] [PubMed]
- Rousseau, J.-C.; Sandell, L.J.; Delmas, P.D.; Garnero, P. Development and clinical application in arthritis of a new immunoassay for serum type IIA procollagen NH2 propeptide. Methods Mol. Med. 2004, 101, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Peltonen, L.; Halila, R.; Ryhänen, L. Enzymes converting procollagens to collagens. J. Cell. Biochem. 1985, 28, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Fukui, N.; McAlinden, A.; Zhu, Y.; Crouch, E.; Broekelmann, T.J.; Mecham, R.P.; Sandell, L.J. Processing of type II procollagen amino propeptide by matrix metalloproteinases. J. Biol. Chem. 2002, 277, 2193–2201. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Oganesian, A.; Keene, D.R.; Sandell, L.J. Type IIA procollagen containing the cysteine-rich amino propeptide is deposited in the extracellular matrix of prechondrogenic tissue and binds to TGF-β1 and BMP-2. J. Cell Biol. 1999, 144, 1069–1080. [Google Scholar] [CrossRef] [PubMed]
- Rousseau, J.-C.; Zhu, Y.; Miossec, P.; Vignon, E.; Sandell, L.J.; Garnero, P.; Delmas, P.D. Serum levels of type IIA procollagen amino terminal propeptide (PIIANP) are decreased in patients with knee osteoarthritis and rheumatoid arthritis. Osteoarthr. Cartil. 2004, 12, 440–447. [Google Scholar] [CrossRef] [PubMed]
- Gudmann, N.; Wang, J.; Hoielt, S.; Chen, P.; Siebuhr, A.; He, Y.; Christiansen, T.; Karsdal, M.; Bay-Jensen, A. Cartilage turnover reflected by metabolic processing of type II collagen: A novel marker of anabolic function in chondrocytes. Int. J. Mol. Sci. 2014, 15, 18789–18803. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Bryan, J.; Franz, C.; Havlioglu, N.; Sandell, L.J. Type IIB procollagen NH2-propeptide induces death of tumor cells via interaction with integrins αvβ3 and αvβ5. J. Biol. Chem. 2010, 285, 20806–20817. [Google Scholar] [CrossRef] [PubMed]
- Aubert-Foucher, E.; Mayer, N.; Pasdeloup, M.; Pagnon, A.; Hartmann, D.; Mallein-Gerin, F. A unique tool to selectively detect the chondrogenic IIB form of human type II procollagen protein. Matrix Biol. 2014, 34, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Sinkeviciute, D.; He, Y.; Karsdal, M.; Henrotin, Y.; Mobasheri, A.; Önnerfjord, P.; Bay-Jensen, A. The minor collagens in articular cartilage. Protein Cell 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Sharif, M.; Kirwan, J.; Charni, N.; Sandell, L.J.; Whittles, C.; Garnero, P. A 5-yr longitudinal study of type IIA collagen synthesis and total type II collagen degradation in patients with knee osteoarthritis--association with disease progression. Rheumatology 2007, 46, 938–943. [Google Scholar] [CrossRef] [PubMed]
- Nelson, F.; Dahlberg, L.; Laverty, S.; Reiner, A.; Pidoux, I.; Ionescu, M.; Fraser, G.L.; Brooks, E.; Tanzer, M.; Rosenberg, L.C.; et al. Evidence for altered synthesis of type II collagen in patients with osteoarthritis. J. Clin. Investig. 1998, 102, 2115–2125. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Yoshihara, Y.; Samura, A.; Yamada, H.; Shinmei, M.; Roos, H.; Lohmander, L.S. Synovial fluid concentrations of the C-propeptide of type II collagen correlate with body mass index in primary knee osteoarthritis. Ann. Rheum. Dis. 1997, 56, 500–503. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, S.; Itokazu, M.; Suzuki, Y.; Shimizu, K. Procollagen II C propeptide level in the synovial fluid as a predictor of radiographic progression in early knee osteoarthritis. Ann. Rheum. Dis. 2003, 62, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Conrozier, T.; Ferrand, F.; Poole, A.R.; Verret, C.; Mathieu, P.; Ionescu, M.; Vincent, F.; Piperno, M.; Spiegel, A.; Vignon, E. Differences in biomarkers of type II collagen in atrophic and hypertrophic osteoarthritis of the hip: implications for the differing pathobiologies. Osteoarthr. Cartil. 2007, 15, 462–467. [Google Scholar] [CrossRef] [PubMed]
- Antoniou, J.; Steffen, T.; Nelson, F.; Winterbottom, N.; Hollander, A.P.; Poole, R.A.; Aebi, M.; Alini, M. The human lumbar intervertebral disc: evidence for changes in the biosynthesis and denaturation of the extracellular matrix with growth, maturation, ageing, and degeneration. J. Clin. Investig. 1996, 98, 996–1003. [Google Scholar] [CrossRef] [PubMed]
- Burgeson, R.E.; Nimni, M.E. Collagen types. Molecular structure and tissue distribution. Clin. Orthop. Relat. Res. 1992, 250–272. [Google Scholar]
- He, Y.; Zheng, Q.; Simonsen, O.; Petersen, K.K.; Christiansen, T.G.; Karsdal, M.A.; Bay-Jensen, A.C. The development and characterization of a competitive ELISA for measuring active ADAMTS-4 in a bovine cartilage ex vivo model. Matrix Biol. 2013, 32, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Deaver, D.R. A new non-isotopic detection system for immunoassays. Nature 1995, 377, 758–760. [Google Scholar] [CrossRef] [PubMed]
- Blackburn, G.F.; Shah, H.P.; Kenten, J.H.; Leland, J.; Kamin, R.A.; Link, J.; Peterman, J.; Powell, M.J.; Shah, A.; Talley, D.B.; et al. Electrochemiluminescence detection for development of immunoassays and DNA probe assays for clinical diagnostics. Clin. Chem. 1991, 37, 1534–1539. [Google Scholar] [PubMed]
- Germaschewski, F.M.; Matheny, C.J.; Larkin, J.; Liu, F.; Thomas, L.R.; Saunders, J.S.; Sully, K.; Whittall, C.; Boyle, Y.; Peters, G.; et al. Quantitation of ARGS aggrecan fragments in synovial fluid, serum and urine from osteoarthritis patients. Osteoarthr. Cartil. 2014, 22, 690–697. [Google Scholar] [CrossRef] [PubMed]
- Larsson, S.; Lohmander, L.S.; Struglics, A. An ARGS-aggrecan assay for analysis in blood and synovial fluid. Osteoarthr. Cartil. 2014, 22, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Bay-Jensen, A.C.; Liu, Q.; Byrjalsen, I.; Li, Y.; Wang, J.; Pedersen, C.; Leeming, D.J.; Dam, E.B.; Zheng, Q.; Qvist, P.; et al. Enzyme-linked immunosorbent assay (ELISAs) for metalloproteinase derived type II collagen neoepitope, CIIM-Increased serum CIIM in subjects with severe radiographic osteoarthritis. Clin. Biochem. 2011, 44, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Oganesian, A.; Zhu, Y.; Sandell, L.J. Type IIA procollagen amino propeptide is localized in human embryonic tissues. J. Histochem. Cytochem. 1997. [Google Scholar] [CrossRef] [PubMed]
| Parameters | PRO-C2 Competition ELISA | hsPRO-C2 Competition ECLIA | PIIANP Competition ELISA |
|---|---|---|---|
| Linear range of standard (ng/mL) | 2.5–40 | 1.0–38.5 | 32–2000 |
| LLOD (ng/mL) | 1.0 | 0.13 | 30 |
| Intra-assay % CV (n = 10, 10 replicates) | 9.2 | 5.4 | 4.5 |
| Inter-assay % CV (n = 10, 10 replicates) | 10.8 | 5.5 | 6.7 |
| Analyte stability %, Recovery (n = 2, 4× FT, RT 24 h, 2–8 °C 24 h) | 110 | 104 | 100 |
| Spiking Recovery, % range | 102 (74–118) | 98 (82–123) | 101 |
| Dilution Recovery, % range (n = 5) | 94 (87–100) | 99 (95–102) | 102 |
| KL Grade | Number | Female % | Age (Mean, 95% CI) | VAS (Mean, 95% CI) | BMI (Mean, 95% CI) |
|---|---|---|---|---|---|
| 0, 1 (Control) | 22 | 40.9 | 62.2 (58.3–66.1) | 32.5 (21.3–43.6) | 26.0 (24.6–27.5) |
| 2, 3, 4 (OA) | 123 | 56.1 | 65.2 (63.9–66.5) | 49.4 (45.0–53.8) * | 28.3 (27.6–28.9) ** |
| Samples | R | Number | p Value |
|---|---|---|---|
| Pediatric serum | <0.1 | 18 | 0.7863 (ns) |
| Healthy adult serum | −0.146 | 62 | 0.1857 (ns) |
| OA serum | 0.148 | 155 | 0.0648 (ns) |
| RA serum | <0.1 | 10 | 0.7524 (ns) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, Y.; He, Y.; Reker, D.; Gudmann, N.S.; Henriksen, K.; Simonsen, O.; Ladel, C.; Michaelis, M.; Mobasheri, A.; Karsdal, M.; et al. A Novel High Sensitivity Type II Collagen Blood-Based Biomarker, PRO-C2, for Assessment of Cartilage Formation. Int. J. Mol. Sci. 2018, 19, 3485. https://doi.org/10.3390/ijms19113485
Luo Y, He Y, Reker D, Gudmann NS, Henriksen K, Simonsen O, Ladel C, Michaelis M, Mobasheri A, Karsdal M, et al. A Novel High Sensitivity Type II Collagen Blood-Based Biomarker, PRO-C2, for Assessment of Cartilage Formation. International Journal of Molecular Sciences. 2018; 19(11):3485. https://doi.org/10.3390/ijms19113485
Chicago/Turabian StyleLuo, Yunyun, Yi He, Ditte Reker, Natasja Stæhr Gudmann, Kim Henriksen, Ole Simonsen, Christoph Ladel, Martin Michaelis, Ali Mobasheri, Morten Karsdal, and et al. 2018. "A Novel High Sensitivity Type II Collagen Blood-Based Biomarker, PRO-C2, for Assessment of Cartilage Formation" International Journal of Molecular Sciences 19, no. 11: 3485. https://doi.org/10.3390/ijms19113485
APA StyleLuo, Y., He, Y., Reker, D., Gudmann, N. S., Henriksen, K., Simonsen, O., Ladel, C., Michaelis, M., Mobasheri, A., Karsdal, M., & Bay-Jensen, A.-C. (2018). A Novel High Sensitivity Type II Collagen Blood-Based Biomarker, PRO-C2, for Assessment of Cartilage Formation. International Journal of Molecular Sciences, 19(11), 3485. https://doi.org/10.3390/ijms19113485





