Neural Stem Cells and Its Derivatives as a New Material for Melanin Inhibition
Abstract
1. Introduction
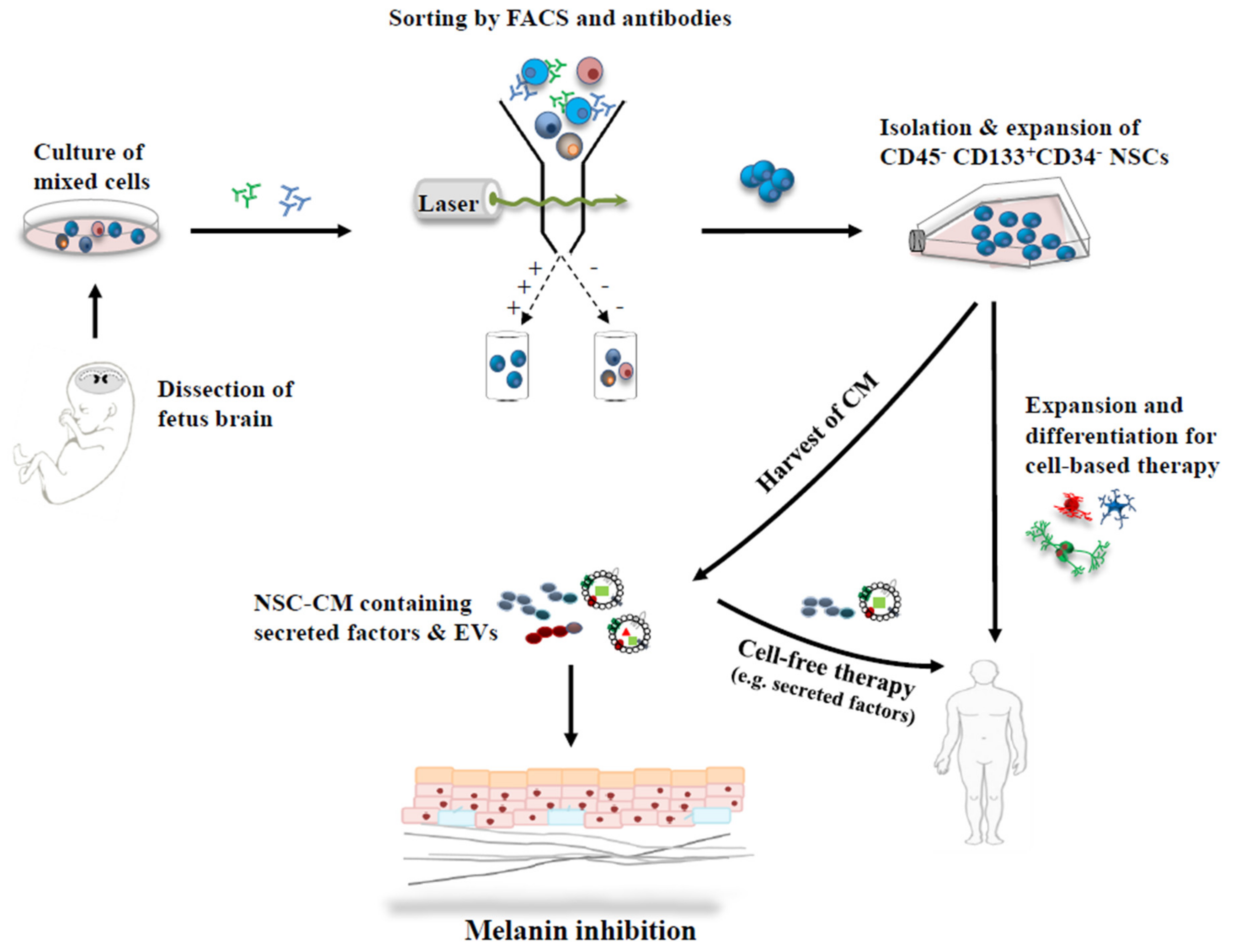
2. Neural Stem Cell (NSC) Characteristics
2.1. NSCs—Isolation and Characterization
2.2. NSC—Culture and Expansion
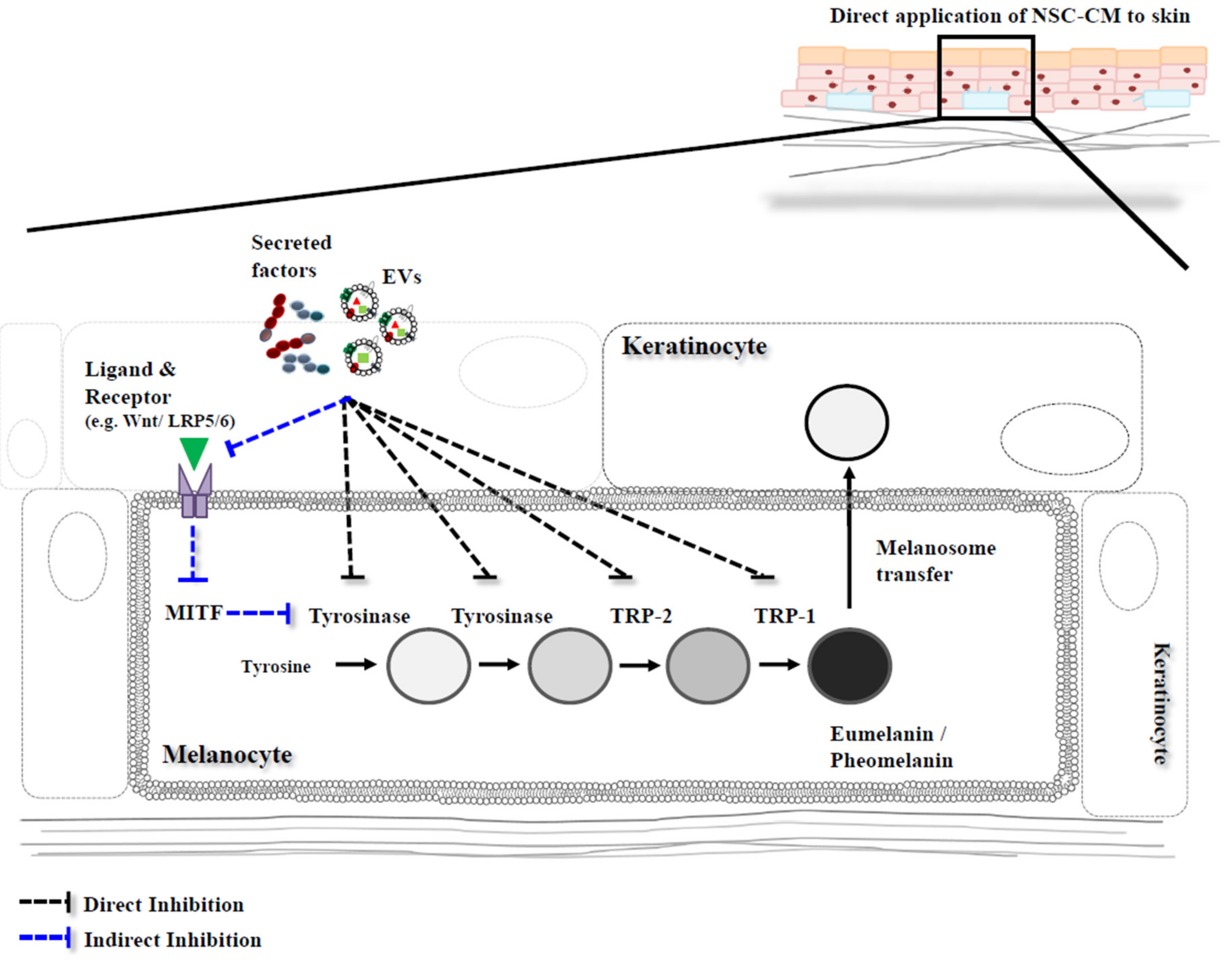
3. Melanin Inhibition with Conditioned Medium (CM) Derived from Neural Stem Cells
3.1. Conditioned Medium
3.2. NSC-Derived CM
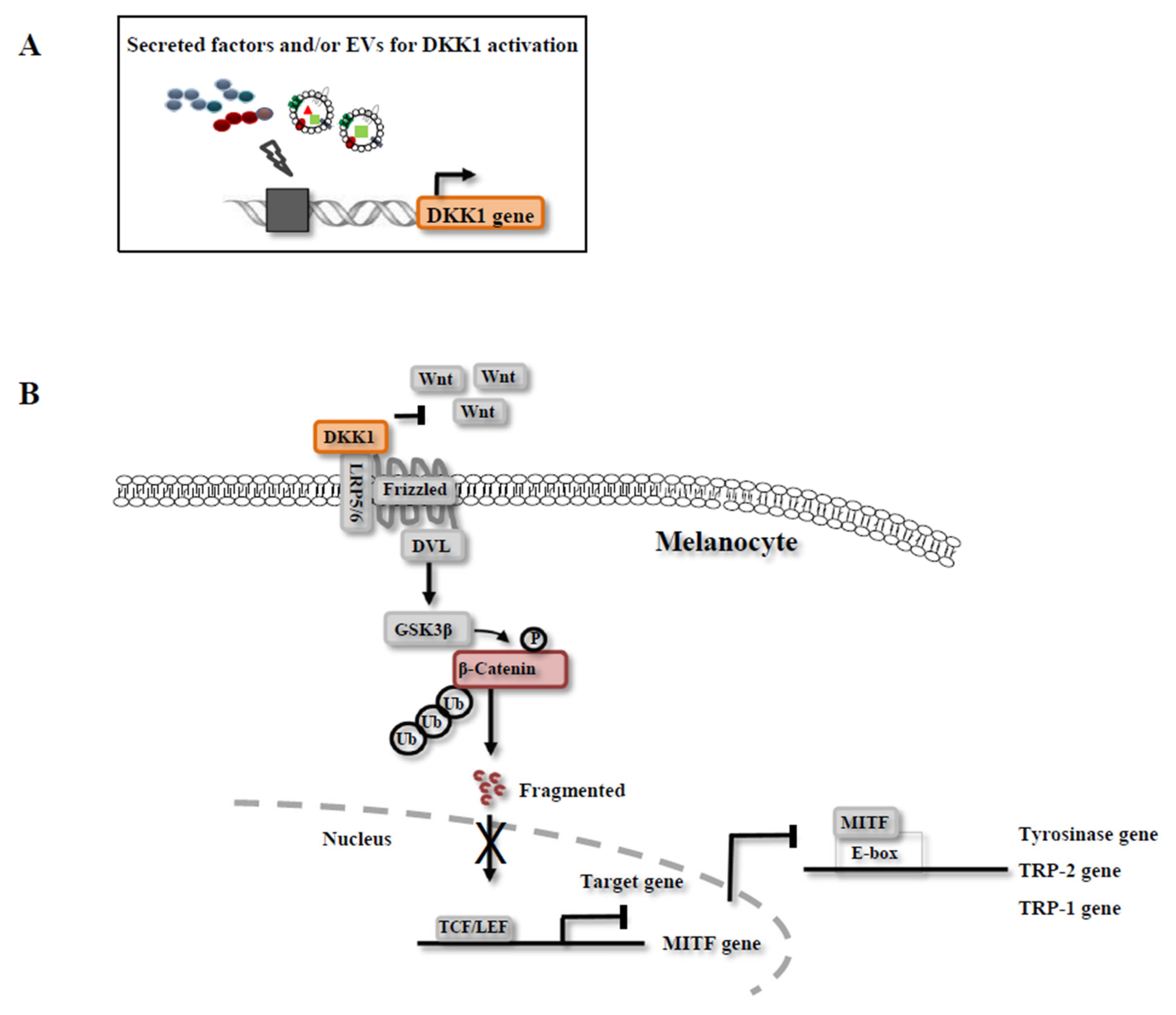
3.3. Mechanisms of Melanin Inhibition by CM
4. Exosomes in CM
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of interest
References
- Slominski, A.; Tobin, D.J.; Shibahara, S.; Wortsman, J. Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol. Rev. 2004, 84, 1155–1228. [Google Scholar] [CrossRef] [PubMed]
- Halaban, R.; Patton, R.S.; Cheng, E.; Svedine, S.; Trombetta, E.S.; Wahl, M.L.; Ariyan, S.; Hebert, D.N. Abnormal acidification of melanoma cells induces tyrosinase retention in the early secretory pathway. J. Biol. Chem. 2002, 277, 14821–14828. [Google Scholar] [CrossRef] [PubMed]
- Ancans, J.; Tobin, D.J.; Hoogduijn, M.J.; Smit, N.P.; Wakamatsu, K.; Thody, A.J. Melanosomal pH controls rate of melanogenesis, eumelanin/phaeomelanin ratio and melanosome maturation in melanocytes and melanoma cells. Exp. Cell Res. 2001, 268, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Del Marmol, V.; Beermann, F. Tyrosinase and related proteins in mammalian pigmentation. FEBS Lett. 1996, 381, 165–168. [Google Scholar] [CrossRef]
- Kuzumaki, T.; Matsuda, A.; Wakamatsu, K.; Ito, S.; Ishikawa, K. Eumelanin biosynthesis is regulated by coordinate expression of tyrosinase and tyrosinase-related protein-1 genes. Exp. Cell Res. 1993, 207, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Wakamatsu, K. Quantitative analysis of eumelanin and pheomelanin in humans, mice, and other animals: A comparative review. Pigment Cell Res. 2003, 16, 523–531. [Google Scholar] [CrossRef] [PubMed]
- Jimbow, K.; Alena, F.; Dixon, W.; Hara, H. Regulatory factors of pheo- and eumelanogenesis in melanogenic compartments. Pigment Cell Res. 1992, 3 (Suppl. S2), 36–42. [Google Scholar] [CrossRef]
- Slominski, A.; Moellmann, G.; Kuklinska, E. l-tyrosine, l-dopa, and tyrosinase as positive regulators of the subcellular apparatus of melanogenesis in bomirski Ab amelanotic melanoma cells. Pigment Cell Res. 1989, 2, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Videira, I.F.; Moura, D.F.; Magina, S. Mechanisms regulating melanogenesis. An. Bras. Dermatol. 2013, 88, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Unver, N.; Freyschmidt-Paul, P.; Horster, S.; Wenck, H.; Stab, F.; Blatt, T.; Elsasser, H.P. Alterations in the epidermal-dermal melanin axis and factor XIIIa melanophages in senile lentigo and ageing skin. Br. J. Dermatol. 2006, 155, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Brenner, M.; Hearing, V.J. The protective role of melanin against UV damage in human skin. Photochem. Photobiol. 2008, 84, 539–549. [Google Scholar] [CrossRef] [PubMed]
- Pillaiyar, T.; Manickam, M.; Namasivayam, V. Skin whitening agents: Medicinal chemistry perspective of tyrosinase inhibitors. J. Enzyme Inhib. Med. Chem. 2017, 32, 403–425. [Google Scholar] [CrossRef] [PubMed]
- Kligman, A.M.; Willis, I. A new formula for depigmenting human skin. Arch. Dermatol. 1975, 111, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Briganti, S.; Camera, E.; Picardo, M. Chemical and instrumental approaches to treat hyperpigmentation. Pigment Cell Res. 2003, 16, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Maeda, K.; Fukuda, M. Arbutin: Mechanism of its depigmenting action in human melanocyte culture. J. Pharmacol. Exp. Ther. 1996, 276, 765–769. [Google Scholar] [PubMed]
- Boissy, R.E.; Visscher, M.; DeLong, M.A. Deoxyarbutin: A novel reversible tyrosinase inhibitor with effective in vivo skin lightening potency. Exp. Dermatol. 2005, 14, 601–608. [Google Scholar] [CrossRef] [PubMed]
- Goncalez, M.L.; Correa, M.A.; Chorilli, M. Skin delivery of kojic acid-loaded nanotechnology-based drug delivery systems for the treatment of skin aging. Biomed. Res. Int. 2013, 2013, 271276. [Google Scholar] [CrossRef] [PubMed]
- Breathnach, A.C.; Nazzaro-Porro, M.; Passi, S.; Zina, G. Azelaic acid therapy in disorders of pigmentation. Clin. Dermatol. 1989, 7, 106–119. [Google Scholar] [CrossRef]
- Piao, L.Z.; Park, H.R.; Park, Y.K.; Lee, S.K.; Park, J.H.; Park, M.K. Mushroom tyrosinase inhibition activity of some chromones. Chem. Pharm. Bull. 2002, 50, 309–311. [Google Scholar] [CrossRef] [PubMed]
- Fu, B.; Li, H.; Wang, X.; Lee, F.S.; Cui, S. Isolation and identification of flavonoids in licorice and a study of their inhibitory effects on tyrosinase. J. Agric. Food Chem. 2005, 53, 7408–7414. [Google Scholar] [CrossRef] [PubMed]
- Huh, C.H.; Seo, K.I.; Park, J.Y.; Lim, J.G.; Eun, H.C.; Park, K.C. A randomized, double-blind, placebo-controlled trial of vitamin C iontophoresis in melasma. Dermatology 2003, 206, 316–320. [Google Scholar] [CrossRef] [PubMed]
- Shimogaki, H.; Tanaka, Y.; Tamai, H.; Masuda, M. In vitro and in vivo evaluation of ellagic acid on melanogenesis inhibition. Int. J. Cosmet. Sci. 2000, 22, 291–303. [Google Scholar] [CrossRef] [PubMed]
- Tse, T.W.; Hui, E. Tranexamic acid: An important adjuvant in the treatment of melasma. J. Cosmet. Dermatol. 2013, 12, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Engasser, P.G. Ochronosis caused by bleaching creams. J. Am. Acad. Dermatol. 1984, 10, 1072–1073. [Google Scholar] [CrossRef]
- Fisher, A.A. Current contact news. Hydroquinone uses and abnormal reactions. Cutis 1983, 31, 240–244. [Google Scholar] [PubMed]
- Zhou, H.; Kepa, J.K.; Siegel, D.; Miura, S.; Hiraki, Y.; Ross, D. Benzene metabolite hydroquinone up-regulates chondromodulin-I and inhibits tube formation in human bone marrow endothelial cells. Mol. Pharmacol. 2009, 76, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, N.; Onodera, H.; Mitsumori, K.; Tamura, T.; Maruyama, S.; Ito, A. Changes in thyroid function during development of thyroid hyperplasia induced by kojic acid in F344 rats. Carcinogenesis 1999, 20, 1567–1571. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.S.; Park, S.H.; Ahn, S.J.; Kim, H.K.; Park, J.S.; Lee, G.Y.; Kim, K.J.; Whang, K.K.; Kang, S.H.; Park, B.S.; et al. Whitening effect of adipose-derived stem cells: A critical role of TGF-β 1. Biol. Pharm. Bull. 2008, 31, 606–610. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.S.; Jeon, H.B.; Lim, H.; Shin, J.H.; Park, S.J.; Jo, Y.K.; Oh, W.; Yang, Y.S.; Cho, D.H.; Kim, J.Y. Conditioned media from human umbilical cord blood-derived mesenchymal stem cells inhibits melanogenesis by promoting proteasomal degradation of MITF. PLoS ONE 2015, 10, e0128078. [Google Scholar] [CrossRef] [PubMed]
- Hwang, I.; Park, J.H.; Park, H.S.; Choi, K.A.; Seol, K.C.; Oh, S.I.; Kang, S.; Hong, S. Neural stem cells inhibit melanin production by activation of Wnt inhibitors. J. Dermatol. Sci. 2013, 72, 274–283. [Google Scholar] [CrossRef] [PubMed]
- Carletti, B.; Piemonte, F.; Rossi, F. Neuroprotection: The emerging concept of restorative neural stem cell biology for the treatment of neurodegenerative diseases. Curr. Neuropharmacol. 2011, 9, 313–317. [Google Scholar] [PubMed]
- Redmond, D.E., Jr.; Bjugstad, K.B.; Teng, Y.D.; Ourednik, V.; Ourednik, J.; Wakeman, D.R.; Parsons, X.H.; Gonzalez, R.; Blanchard, B.C.; Kim, S.U.; et al. Behavioral improvement in a primate parkinson’s model is associated with multiple homeostatic effects of human neural stem cells. Proc. Natl. Acad. Sci. USA 2007, 104, 12175–12180. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.K.; Kim, J.; Cho, S.J.; Hatori, K.; Nagai, A.; Choi, H.B.; Lee, M.C.; McLarnon, J.G.; Kim, S.U. Proactive transplantation of human neural stem cells prevents degeneration of striatal neurons in a rat model of huntington disease. Neurobiol. Dis. 2004, 16, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.S.; Park, B.S.; Park, S.H.; Kim, H.K.; Sung, J.H. Antiwrinkle effect of adipose-derived stem cell: Activation of dermal fibroblast by secretory factors. J. Dermatol. Sci. 2009, 53, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.S.; Park, B.S.; Sung, J.H.; Yang, J.M.; Park, S.B.; Kwak, S.J.; Park, J.S. Wound healing effect of adipose-derived stem cells: A critical role of secretory factors on human dermal fibroblasts. J. Dermatol. Sci. 2007, 48, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.A.; Chung, H.M.; Won, C.H.; Sung, J.H. Potential application of adipose-derived stem cells and their secretory factors to skin: Discussion from both clinical and industrial viewpoints. Expert Opin. Biol. Ther. 2010, 10, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Ratajczak, J.; Wysoczynski, M.; Hayek, F.; Janowska-Wieczorek, A.; Ratajczak, M.Z. Membrane-derived microvesicles: Important and underappreciated mediators of cell-to-cell communication. Leukemia 2006, 20, 1487–1495. [Google Scholar] [CrossRef] [PubMed]
- Kusuma, G.D.; Carthew, J.; Lim, R.; Frith, J.E. Effect of the microenvironment on mesenchymal stem cell paracrine signaling: Opportunities to engineer the therapeutic effect. Stem Cells Dev. 2017, 26, 617–631. [Google Scholar] [CrossRef] [PubMed]
- Drago, D.; Cossetti, C.; Iraci, N.; Gaude, E.; Musco, G.; Bachi, A.; Pluchino, S. The stem cell secretome and its role in brain repair. Biochimie 2013, 95, 2271–2285. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.S.; Park, S.H.; Park, K.C. Transforming growth factor-β1 decreases melanin synthesis via delayed extracellular signal-regulated kinase activation. Int. J. Biochem. Cell Biol. 2004, 36, 1482–1491. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Esparza, M.; Solano, F.; Garcia-Borron, J.C. Independent regulation of tyrosinase by the hypopigmenting cytokines TGF β1 and TNF alpha and the melanogenic hormone alpha-MSH in B16 mouse melanocytes. Cell. Mol. Biol. 1999, 45, 991–1000. [Google Scholar] [PubMed]
- Choi, H.; Ahn, S.; Lee, B.G.; Chang, I.; Hwang, J.S. Inhibition of skin pigmentation by an extract of lepidium apetalum and its possible implication in IL-6 mediated signaling. Pigment Cell Res. 2005, 18, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, Y.; Itami, S.; Watabe, H.; Yasumoto, K.; Abdel-Malek, Z.A.; Kubo, T.; Rouzaud, F.; Tanemura, A.; Yoshikawa, K.; Hearing, V.J. Mesenchymal-epithelial interactions in the skin: Increased expression of dickkopf1 by palmoplantar fibroblasts inhibits melanocyte growth and differentiation. J. Cell Biol. 2004, 165, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Solano, F.; Briganti, S.; Picardo, M.; Ghanem, G. Hypopigmenting agents: An updated review on biological, chemical and clinical aspects. Pigment Cell Res. 2006, 19, 550–571. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.C.; Yang, C.H.; Chang, N.F.; Wu, P.S.; Chen, Y.S.; Lee, S.M.; Chen, C.W. Study on the stability of deoxyarbutin in an anhydrous emulsion system. Int. J. Mol. Sci. 2011, 12, 5946–5954. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, K.; Nishimura, T.; Nomura, K.; Sugimoto, K.; Kuriki, T. Syntheses of arbutin-α-glycosides and a comparison of their inhibitory effects with those of α-arbutin and arbutin on human tyrosinase. Chem. Pharm. Bull. 2003, 51, 798–801. [Google Scholar] [CrossRef] [PubMed]
- Curto, E.V.; Kwong, C.; Hermersdorfer, H.; Glatt, H.; Santis, C.; Virador, V.; Hearing, V.J., Jr.; Dooley, T.P. Inhibitors of mammalian melanocyte tyrosinase: In vitro comparisons of alkyl esters of gentisic acid with other putative inhibitors. Biochem. Pharmacol. 1999, 57, 663–672. [Google Scholar] [CrossRef]
- Kim, D.W.; Jeon, B.J.; Hwang, N.H.; Kim, M.S.; Park, S.H.; Dhong, E.S.; Yoon, E.S.; Lee, B.I. Adipose-derived stem cells inhibit epidermal melanocytes through an interleukin-6-mediated mechanism. Plast. Reconstr. Surg. 2014, 134, 470–480. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Guo, S.; Wei, C.; Li, H.; Chen, L.; Yin, C.; Zhang, C. The comparison of adipose stem cell and placental stem cell in secretion characteristics and in facial antiaging. Stem Cells Int. 2016, 2016, 14. [Google Scholar] [CrossRef] [PubMed]
- Palmer, T.D.; Takahashi, J.; Gage, F.H. The adult rat hippocampus contains primordial neural stem cells. Mol. Cell. Neurosci. 1997, 8, 389–404. [Google Scholar] [CrossRef] [PubMed]
- Doetsch, F. A niche for adult neural stem cells. Curr. Opin. Genet. Dev. 2003, 13, 543–550. [Google Scholar] [CrossRef] [PubMed]
- Uchida, N.; Buck, D.W.; He, D.; Reitsma, M.J.; Masek, M.; Phan, T.V.; Tsukamoto, A.S.; Gage, F.H.; Weissman, I.L. Direct isolation of human central nervous system stem cells. Proc. Natl. Acad. Sci. USA 2000, 97, 14720–14725. [Google Scholar] [CrossRef] [PubMed]
- Golebiewska, A.; Atkinson, S.P.; Lako, M.; Armstrong, L. Epigenetic landscaping during hESC differentiation to neural cells. Stem Cells 2009, 27, 1298–1308. [Google Scholar] [CrossRef] [PubMed]
- Huhn, S.L.; Yung, Y.; Cheshier, S.; Harsh, G.; Ailles, L.; Weissman, I.; Vogel, H.; Tse, V. Identification of phenotypic neural stem cells in a pediatric astroblastoma. J. Neurosurg. 2005, 103, 446–450. [Google Scholar] [CrossRef] [PubMed]
- Flax, J.D.; Aurora, S.; Yang, C.; Simonin, C.; Wills, A.M.; Billinghurst, L.L.; Jendoubi, M.; Sidman, R.L.; Wolfe, J.H.; Kim, S.U.; et al. Engraftable human neural stem cells respond to developmental cues, replace neurons, and express foreign genes. Nat. Biotechnol. 1998, 16, 1033–1039. [Google Scholar] [PubMed]
- Donato, R.; Miljan, E.A.; Hines, S.J.; Aouabdi, S.; Pollock, K.; Patel, S.; Edwards, F.A.; Sinden, J.D. Differential development of neuronal physiological responsiveness in two human neural stem cell lines. BMC Neurosci. 2007, 8, 36. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.U. Human neural stem cells genetically modified for brain repair in neurological disorders. Neuropathology 2004, 24, 159–171. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.C.; Lee, S.T.; Chu, K.; Joo, K.M.; Kang, L.; Im, W.S.; Park, J.E.; Kim, S.U.; Kim, M.; Cha, C.I. Neuroprotective effect of neural stem cell-conditioned media in in vitro model of huntington’s disease. Neurosci. Lett. 2008, 435, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.O.; Choi, S.M.; Kim, H.S. Mesenchymal stem cell-derived secretome and microvesicles as a cell-free therapeutics for neurodegenerative disorders. Tissue Eng. Regen. Med. 2013, 10, 93–101. [Google Scholar] [CrossRef]
- Chaput, N.; Thery, C. Exosomes: Immune properties and potential clinical implementations. Semin. Immunopathol. 2011, 33, 419–440. [Google Scholar] [CrossRef] [PubMed]
- Marote, A.; Teixeira, F.G.; Mendes-Pinheiro, B.; Salgado, A.J. Mscs-derived exosomes: Cell-secreted nanovesicles with regenerative potential. Front. Pharmacol. 2016, 7, 231. [Google Scholar] [CrossRef] [PubMed]
- Park, C.W.; Kim, K.S.; Bae, S.; Son, H.K.; Myung, P.K.; Hong, H.J.; Kim, H. Cytokine secretion profiling of human mesenchymal stem cells by antibody array. Int. J. Stem Cells 2009, 2, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Van Laake, L.W.; Passier, R.; Monshouwer-Kloots, J.; Verkleij, A.J.; Lips, D.J.; Freund, C.; den Ouden, K.; Ward-van Oostwaard, D.; Korving, J.; Tertoolen, L.G.; et al. Human embryonic stem cell-derived cardiomyocytes survive and mature in the mouse heart and transiently improve function after myocardial infarction. Stem Cell Res. 2007, 1, 9–24. [Google Scholar] [CrossRef] [PubMed]
- Uemura, R.; Xu, M.; Ahmad, N.; Ashraf, M. Bone marrow stem cells prevent left ventricular remodeling of ischemic heart through paracrine signaling. Circ. Res. 2006, 98, 1414–1421. [Google Scholar] [CrossRef] [PubMed]
- Katsha, A.M.; Ohkouchi, S.; Xin, H.; Kanehira, M.; Sun, R.; Nukiwa, T.; Saijo, Y. Paracrine factors of multipotent stromal cells ameliorate lung injury in an elastase-induced emphysema model. Mol. Ther. 2011, 19, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Ben-Hur, T. Immunomodulation by neural stem cells. J. Neurol. Sci. 2008, 265, 102–104. [Google Scholar] [CrossRef] [PubMed]
- Bacigaluppi, M.; Pluchino, S.; Peruzzotti-Jametti, L.; Kilic, E.; Kilic, U.; Salani, G.; Brambilla, E.; West, M.J.; Comi, G.; Martino, G.; et al. Delayed post-ischaemic neuroprotection following systemic neural stem cell transplantation involves multiple mechanisms. Brain 2009, 132, 2239–2251. [Google Scholar] [CrossRef] [PubMed]
- Madhavan, L.; Ourednik, V.; Ourednik, J. Neural stem/progenitor cells initiate the formation of cellular networks that provide neuroprotection by growth factor-modulated antioxidant expression. Stem Cells 2008, 26, 254–265. [Google Scholar] [CrossRef] [PubMed]
- Chu, K.; Kim, M.; Park, K.I.; Jeong, S.W.; Park, H.K.; Jung, K.H.; Lee, S.T.; Kang, L.; Lee, K.; Park, D.K.; et al. Human neural stem cells improve sensorimotor deficits in the adult rat brain with experimental focal ischemia. Brain Res. 2004, 1016, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Kokaia, Z.; Martino, G.; Schwartz, M.; Lindvall, O. Cross-talk between neural stem cells and immune cells: The key to better brain repair? Nat. Neurosci. 2012, 15, 1078–1087. [Google Scholar] [CrossRef] [PubMed]
- Einstein, O.; Ben-Hur, T. The changing face of neural stem cell therapy in neurologic diseases. Arch. Neurol. 2008, 65, 452–456. [Google Scholar] [CrossRef] [PubMed]
- Hoffrogge, R.; Mikkat, S.; Scharf, C.; Beyer, S.; Christoph, H.; Pahnke, J.; Mix, E.; Berth, M.; Uhrmacher, A.; Zubrzycki, I.Z.; et al. 2-DE proteome analysis of a proliferating and differentiating human neuronal stem cell line (Rencell VM). Proteomics 2006, 6, 1833–1847. [Google Scholar] [CrossRef] [PubMed]
- Skalnikova, H.; Vodicka, P.; Gadher, S.J.; Kovarova, H. Proteomics of neural stem cells. Expert Rev. Proteom. 2008, 5, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.M.; Lee, S.T.; Chu, K.; Jung, K.H.; Song, E.C.; Kim, S.J.; Sinn, D.I.; Kim, J.H.; Park, D.K.; Kang, K.M.; et al. Systemic transplantation of human adipose stem cells attenuated cerebral inflammation and degeneration in a hemorrhagic stroke model. Brain Res. 2007, 1183, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Kubis, N.; Tomita, Y.; Tran-Dinh, A.; Planat-Benard, V.; Andre, M.; Karaszewski, B.; Waeckel, L.; Penicaud, L.; Silvestre, J.S.; Casteilla, L.; et al. Vascular fate of adipose tissue-derived adult stromal cells in the ischemic murine brain: A combined imaging-histological study. Neuroimage 2007, 34, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Caplan, A.I.; Dennis, J.E. Mesenchymal stem cells as trophic mediators. J. Cell. Biochem. 2006, 98, 1076–1084. [Google Scholar] [CrossRef] [PubMed]
- Widlund, H.R.; Fisher, D.E. Microphthalamia-associated transcription factor: A critical regulator of pigment cell development and survival. Oncogene 2003, 22, 3035–3041. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.Y.; Fisher, D.E. Melanocyte biology and skin pigmentation. Nature 2007, 445, 843–850. [Google Scholar] [CrossRef] [PubMed]
- Vachtenheim, J.; Borovansky, J. “Transcription physiology” of pigment formation in melanocytes: Central role of MITF. Exp. Dermatol. 2010, 19, 617–627. [Google Scholar] [CrossRef] [PubMed]
- Qiao, L.; Xu, Z.L.; Zhao, T.J.; Ye, L.H.; Zhang, X.D. DKK-1 secreted by mesenchymal stem cells inhibits growth of breast cancer cells via depression of Wnt signalling. Cancer Lett. 2008, 269, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, Y.; Passeron, T.; Watabe, H.; Yasumoto, K.; Rouzaud, F.; Hoashi, T.; Hearing, V.J. The effects of dickkopf 1 on gene expression and Wnt signaling by melanocytes: Mechanisms underlying its suppression of melanocyte function and proliferation. J. Investig. Dermatol. 2007, 127, 1217–1225. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, Y.; Passeron, T.; Hoashi, T.; Watabe, H.; Rouzaud, F.; Yasumoto, K.; Hara, T.; Tohyama, C.; Katayama, I.; Miki, T.; et al. Dickkopf 1 (DKK1) regulates skin pigmentation and thickness by affecting Wnt/β-catenin signaling in keratinocytes. FASEB J. 2008, 22, 1009–1020. [Google Scholar] [CrossRef] [PubMed]
- Aguilera, O.; Pena, C.; Garcia, J.M.; Larriba, M.J.; Ordonez-Moran, P.; Navarro, D.; Barbachano, A.; Lopez de Silanes, I.; Ballestar, E.; Fraga, M.F.; et al. The Wnt antagonist dickkopf-1 gene is induced by 1α,25-dihydroxyvitamin D3 associated to the differentiation of human colon cancer cells. Carcinogenesis 2007, 28, 1877–1884. [Google Scholar] [CrossRef] [PubMed]
- Pendas-Franco, N.; Garcia, J.M.; Pena, C.; Valle, N.; Palmer, H.G.; Heinaniemi, M.; Carlberg, C.; Jimenez, B.; Bonilla, F.; Munoz, A.; et al. Dickkopf-4 is induced by TCF/β-catenin and upregulated in human colon cancer, promotes tumour cell invasion and angiogenesis and is repressed by 1α,25-dihydroxyvitamin D3. Oncogene 2008, 27, 4467–4477. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.L.; Zinser, G.M.; Waltz, S.E. Vitamin D3-dependent VDR signaling delays RON-mediated breast tumorigenesis through suppression of β-catenin activity. Oncotarget 2015, 6, 16304–16320. [Google Scholar] [CrossRef] [PubMed]
- Tachida, Y.; Sakurai, H.; Okutsu, J. Proteomic comparison of the secreted factors of mesenchymal stem cells from bone marrow, adipose tissue and dental pulp. J. Proteom. Bioinform. 2015, 8, 266. [Google Scholar] [CrossRef]
- Ranganath, S.H.; Levy, O.; Inamdar, M.S.; Karp, J.M. Harnessing the mesenchymal stem cell secretome for the treatment of cardiovascular disease. Cell Stem Cell 2012, 10, 244–258. [Google Scholar] [CrossRef] [PubMed]
- Pileggi, A. Mesenchymal stem cells for the treatment of diabetes. Diabetes 2012, 61, 1355–1356. [Google Scholar] [CrossRef] [PubMed]
- Joyce, N.; Annett, G.; Wirthlin, L.; Olson, S.; Bauer, G.; Nolta, J.A. Mesenchymal stem cells for the treatment of neurodegenerative disease. Regen. Med. 2010, 5, 933–946. [Google Scholar] [CrossRef] [PubMed]
- Saito, H.; Yasumoto, K.; Takeda, K.; Takahashi, K.; Yamamoto, H.; Shibahara, S. Microphthalmia-associated transcription factor in the Wnt signaling pathway. Pigment Cell Res. 2003, 16, 261–265. [Google Scholar] [CrossRef] [PubMed]
- Krupnik, V.E.; Sharp, J.D.; Jiang, C.; Robison, K.; Chickering, T.W.; Amaravadi, L.; Brown, D.E.; Guyot, D.; Mays, G.; Leiby, K.; et al. Functional and structural diversity of the human dickkopf gene family. Gene 1999, 238, 301–313. [Google Scholar] [CrossRef]
- Niida, A.; Hiroko, T.; Kasai, M.; Furukawa, Y.; Nakamura, Y.; Suzuki, Y.; Sugano, S.; Akiyama, T. DKK1, a negative regulator of Wnt signaling, is a target of the β-catenin/TCF pathway. Oncogene 2004, 23, 8520–8526. [Google Scholar] [CrossRef] [PubMed]
- Mao, B.; Wu, W.; Li, Y.; Hoppe, D.; Stannek, P.; Glinka, A.; Niehrs, C. LDL-receptor-related protein 6 is a receptor for dickkopf proteins. Nature 2001, 411, 321–325. [Google Scholar] [CrossRef] [PubMed]
- Kawano, Y.; Kypta, R. Secreted antagonists of the Wnt signalling pathway. J. Cell Sci. 2003, 116, 2627–2634. [Google Scholar] [CrossRef] [PubMed]
- Busca, R.; Ballotti, R. Cyclic amp a key messenger in the regulation of skin pigmentation. Pigment Cell Res. 2000, 13, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Busca, R.; Abbe, P.; Mantoux, F.; Aberdam, E.; Peyssonnaux, C.; Eychene, A.; Ortonne, J.P.; Ballotti, R. Ras mediates the camp-dependent activation of extracellular signal-regulated kinases (ERKs) in melanocytes. EMBO J. 2000, 19, 2900–2910. [Google Scholar] [CrossRef] [PubMed]
- Bertolotto, C.; Abbe, P.; Hemesath, T.J.; Bille, K.; Fisher, D.E.; Ortonne, J.P.; Ballotti, R. Microphthalmia gene product as a signal transducer in camp-induced differentiation of melanocytes. J. Cell Biol. 1998, 142, 827–835. [Google Scholar] [CrossRef] [PubMed]
- Flaherty, K.T.; Hodi, F.S.; Fisher, D.E. From genes to drugs: Targeted strategies for melanoma. Nat. Rev. Cancer 2012, 12, 349–361. [Google Scholar] [CrossRef] [PubMed]
- Raposo, G.; Stoorvogel, W. Extracellular vesicles: Exosomes, microvesicles, and friends. J. Cell Biol. 2013, 200, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Yanez-Mo, M.; Siljander, P.R.; Andreu, Z.; Zavec, A.B.; Borras, F.E.; Buzas, E.I.; Buzas, K.; Casal, E.; Cappello, F.; Carvalho, J.; et al. Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles 2015, 4, 27066. [Google Scholar] [CrossRef] [PubMed]
- Lener, T.; Gimona, M.; Aigner, L.; Borger, V.; Buzas, E.; Camussi, G.; Chaput, N.; Chatterjee, D.; Court, F.A.; Del Portillo, H.A.; et al. Applying extracellular vesicles based therapeutics in clinical trials—An ISEV position paper. J. Extracell. Vesicles 2015, 4, 30087. [Google Scholar] [CrossRef] [PubMed]
- Lotvall, J.; Hill, A.F.; Hochberg, F.; Buzas, E.I.; Di Vizio, D.; Gardiner, C.; Gho, Y.S.; Kurochkin, I.V.; Mathivanan, S.; Quesenberry, P.; et al. Minimal experimental requirements for definition of extracellular vesicles and their functions: A position statement from the international society for extracellular vesicles. J. Extracell. Vesicles 2014, 3, 26913. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.B.; Moncivais, K.; Caplan, A.I. Mesenchymal stem cells: Environmentally responsive therapeutics for regenerative medicine. Exp. Mol. Med. 2013, 45, e54. [Google Scholar] [CrossRef] [PubMed]
- Sims, B.; Gu, L.; Krendelchtchikov, A.; Matthews, Q.L. Neural stem cell-derived exosomes mediate viral entry. Int. J. Nanomed. 2014, 9, 4893–4897. [Google Scholar] [CrossRef] [PubMed]
- Baglio, S.R.; Rooijers, K.; Koppers-Lalic, D.; Verweij, F.J.; Perez Lanzon, M.; Zini, N.; Naaijkens, B.; Perut, F.; Niessen, H.W.; Baldini, N.; et al. Human bone marrow- and adipose-mesenchymal stem cells secrete exosomes enriched in distinctive miRNA and tRNA species. Stem Cell. Res. Ther. 2015, 6, 127. [Google Scholar] [CrossRef] [PubMed]
- Katsuda, T.; Tsuchiya, R.; Kosaka, N.; Yoshioka, Y.; Takagaki, K.; Oki, K.; Takeshita, F.; Sakai, Y.; Kuroda, M.; Ochiya, T. Human adipose tissue-derived mesenchymal stem cells secrete functional neprilysin-bound exosomes. Sci. Rep. 2013, 3, 1197. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Zhang, J.; Yuan, H.; Xu, Z.; Li, Q.; Niu, X.; Hu, B.; Wang, Y.; Li, X. Exosomes secreted by human-induced pluripotent stem cell-derived mesenchymal stem cells repair critical-sized bone defects through enhanced angiogenesis and osteogenesis in osteoporotic rats. Int. J. Biol. Sci. 2016, 12, 836–849. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Xu, H.; Xu, W.; Wang, B.; Wu, H.; Tao, Y.; Zhang, B.; Wang, M.; Mao, F.; Yan, Y.; et al. Exosomes released by human umbilical cord mesenchymal stem cells protect against cisplatin-induced renal oxidative stress and apoptosis in vivo and in vitro. Stem Cell. Res. Ther. 2013, 4, 34. [Google Scholar] [CrossRef] [PubMed]
- Del Fattore, A.; Luciano, R.; Saracino, R.; Battafarano, G.; Rizzo, C.; Pascucci, L.; Alessandri, G.; Pessina, A.; Perrotta, A.; Fierabracci, A.; et al. Differential effects of extracellular vesicles secreted by mesenchymal stem cells from different sources on glioblastoma cells. Expert Opin. Biol. Ther. 2015, 15, 495–504. [Google Scholar] [CrossRef] [PubMed]
- Bobrie, A.; Colombo, M.; Raposo, G.; Thery, C. Exosome secretion: Molecular mechanisms and roles in immune responses. Traffic 2011, 12, 1659–1668. [Google Scholar] [CrossRef] [PubMed]
- Van der Pol, E.; Boing, A.N.; Harrison, P.; Sturk, A.; Nieuwland, R. Classification, functions, and clinical relevance of extracellular vesicles. Pharmacol. Rev. 2012, 64, 676–705. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.S.; Lai, R.C.; Lee, M.M.; Choo, A.B.; Lee, C.N.; Lim, S.K. Mesenchymal stem cell secretes microparticles enriched in pre-micrornas. Nucleic Acids Res. 2010, 38, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Lai, R.C.; Tan, S.S.; Teh, B.J.; Sze, S.K.; Arslan, F.; de Kleijn, D.P.; Choo, A.; Lim, S.K. Proteolytic potential of the MSC exosome proteome: Implications for an exosome-mediated delivery of therapeutic proteasome. Int. J. Proteom. 2012, 2012, 971907. [Google Scholar] [CrossRef] [PubMed]
- Mathivanan, S.; Fahner, C.J.; Reid, G.E.; Simpson, R.J. Exocarta 2012: Database of exosomal proteins, RNA and lipids. Nucleic Acids Res. 2012, 40, D1241–D1244. [Google Scholar] [CrossRef] [PubMed]
- Derkus, B.; Emregul, K.C.; Emregul, E. A new approach in stem cell research-exosomes: Their mechanism of action via cellular pathways. Cell Biol. Int. 2017, 41, 466–475. [Google Scholar] [CrossRef] [PubMed]
- Sarko, D.K.; McKinney, C.E. Exosomes: Origins and therapeutic potential for neurodegenerative disease. Front. Neurosci. 2017, 11, 82. [Google Scholar] [CrossRef] [PubMed]
- Hickman, R.A.; Faustin, A.; Wisniewski, T. Alzheimer disease and its growing epidemic: Risk factors, biomarkers, and the urgent need for therapeutics. Neurol. Clin. 2016, 34, 941–953. [Google Scholar] [CrossRef] [PubMed]
- Lo Cicero, A.; Delevoye, C.; Gilles-Marsens, F.; Loew, D.; Dingli, F.; Guere, C.; Andre, N.; Vie, K.; van Niel, G.; Raposo, G. Exosomes released by keratinocytes modulate melanocyte pigmentation. Nat. Commun. 2015, 6, 7506. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.D.; Johansson, H.J.; Graham, C.S.; Vesterlund, M.; Pham, M.T.; Bramlett, C.S.; Montgomery, E.N.; Mellema, M.S.; Bardini, R.L.; Contreras, Z.; et al. Comprehensive proteomic analysis of mesenchymal stem cell exosomes reveals modulation of angiogenesis via nuclear factor-kappab signaling. Stem Cells 2016, 34, 601–613. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Choi, D.Y.; Yun, S.J.; Choi, S.M.; Kang, J.W.; Jung, J.W.; Hwang, D.; Kim, K.P.; Kim, D.W. Proteomic analysis of microvesicles derived from human mesenchymal stem cells. J. Proteome Res. 2012, 11, 839–849. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Kim, E.; Choi, S.M.; Kim, D.W.; Kim, K.P.; Lee, I.; Kim, H.S. Microvesicles from brain-extract-treated mesenchymal stem cells improve neurological functions in a rat model of ischemic stroke. Sci. Rep. 2016, 6, 33038. [Google Scholar] [CrossRef] [PubMed]
| Source | Factor | Properties | Inhibition Effects | Advantages | Disadvantages | Ref. |
|---|---|---|---|---|---|---|
| Synthetic compounds | Deoxyarbutin | -Synthesized without every OH-group of arbutin -Reversible inhibition of tyrosinase activity | Strong | -A sustained depigmentation effect -Low cytotoxicity | -Thermolabile in aqueous solutions, where it decomposes to hydroquinone | [16,45] |
| α-arbutin (α-glucosides of arbutin) | -Easily hydrolyzed to release hydroquinone | Strong | -Strong ability to inhibit tyrosinase | -N/A | [46] | |
| Magnesium l-ascorbyl-2-phosphate | -Inhibitor of tyrosinase | N/A | -Low adverse side-effects -Reduced cytotoxicity relative to hydroquinone | -Not tested in skin models | [47] | |
| Natural compounds | Hydroquinone | -Most effective inhibitor of melanin synthesis by glutathione depletion, melanosome degradation and melanocyte damage -Good tyrosinase inhibitor | Strong | -Gold-standard of depigmentation | -Permanent depigmentation and exogenous ochronosis following long-term use -Banned by the European Committee (24th Dir 2006/6/EC) | [13] |
| Arbutin | -Good tyrosinase inhibitor | Modest | -Low melanocyte cytotoxicity -Inhibition of melanin synthesis by competitive and reversible tyrosinase action | -Chemically unstable and can release hydroquinone, the potential toxicity | [15,26] | |
| Kojic acid | -Good tyrosinase inhibitor | Modest effect | -Acts as a free radical scavenger | -Causes allergies, such as irritant contact dermatitis | [17] | |
| Azelaic acid | -Competitive tyrosinase inhibitor | Weak | -No relevant side effects | -No induction of depigmentation on normal skin | [18] | |
| Ascorbic acid | -Good tyrosinase inhibitor | Modest | -Useful for health and beauty of skin-Good antioxidative, anti-inflammatory and photoprotective effects | -Instability and rapid oxidization in aqueous solution | [21] | |
| Stem cells | Human adipose-derived stem cells (ADSCs or ASCs) | -Inhibition of melanin synthesis is mainly mediated by highly secreted TGF-β1-CM greatly improved the facial indexes, such as Erythema and melanin | N/A | -CM contains biological effectors that decrease melanin, such as TGF-β1, TNF-α and IL-6-IL-6 and TNFα concentrations are lower than the IC50 value for tyrosinase activity | -No decrease of MITF expression in mouse B16 melanoma cell line-No defined key factors for melanin inhibition | [28] |
| -Melanin inhibition by highly secreted IL-6 | N/A | -Inhibition of cell proliferation of mouse melanocytes tyrosinase-Decreased MITF, TRP-1 and TRP-2 expression in melanocytes | -No significant decrease of tyrosinase expression in human melanocytes -No defined key factors for melanin inhibition | [48] | ||
| Human umbilical cord blood (hUCB) | -TGF-β1 was highly secreted, but the regulatory mechanism by which TGF-β1 causes depigmentation was not elucidated | N/A | -Proteasomal degradation of MITF in melan-a mouse melanocytes | tgf | ||
| Human placental stem cells (hPSCs) | -Only the Melanin index of the hPSC-CM group was significantly high compared to the ASC-CM group -CM greatly improved the facial indexes, such as Erythema and melanin | -Melanin index of hPSC-CM was improved in clinical research | -hPSC-CM are not reported in vitro and in vivo | [49] | ||
| Human neural stem cells (NSCs) | -Melanin inhibition by regulation of the gene and protein expression of TYR, TRP-1, TRP-2 and MITF, in mouse B16 melanoma cell line -Expression of DKK1 was significantly increased in CM-treated cells | N/A | -Inhibition of cell proliferationin mouse melanoma cell line -Inhibition of melanin production through high expression of Wnt/β-catenin inhibitors | -No defined key factors for melanin inhibition | [30] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hwang, I.; Hong, S. Neural Stem Cells and Its Derivatives as a New Material for Melanin Inhibition. Int. J. Mol. Sci. 2018, 19, 36. https://doi.org/10.3390/ijms19010036
Hwang I, Hong S. Neural Stem Cells and Its Derivatives as a New Material for Melanin Inhibition. International Journal of Molecular Sciences. 2018; 19(1):36. https://doi.org/10.3390/ijms19010036
Chicago/Turabian StyleHwang, Insik, and Sunghoi Hong. 2018. "Neural Stem Cells and Its Derivatives as a New Material for Melanin Inhibition" International Journal of Molecular Sciences 19, no. 1: 36. https://doi.org/10.3390/ijms19010036
APA StyleHwang, I., & Hong, S. (2018). Neural Stem Cells and Its Derivatives as a New Material for Melanin Inhibition. International Journal of Molecular Sciences, 19(1), 36. https://doi.org/10.3390/ijms19010036





