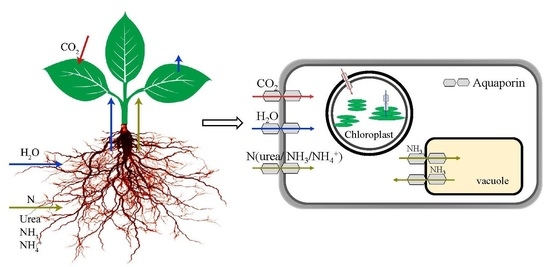
Role of Aquaporins in Determining Carbon and Nitrogen Status in Higher Plants
Abstract
1. Introduction
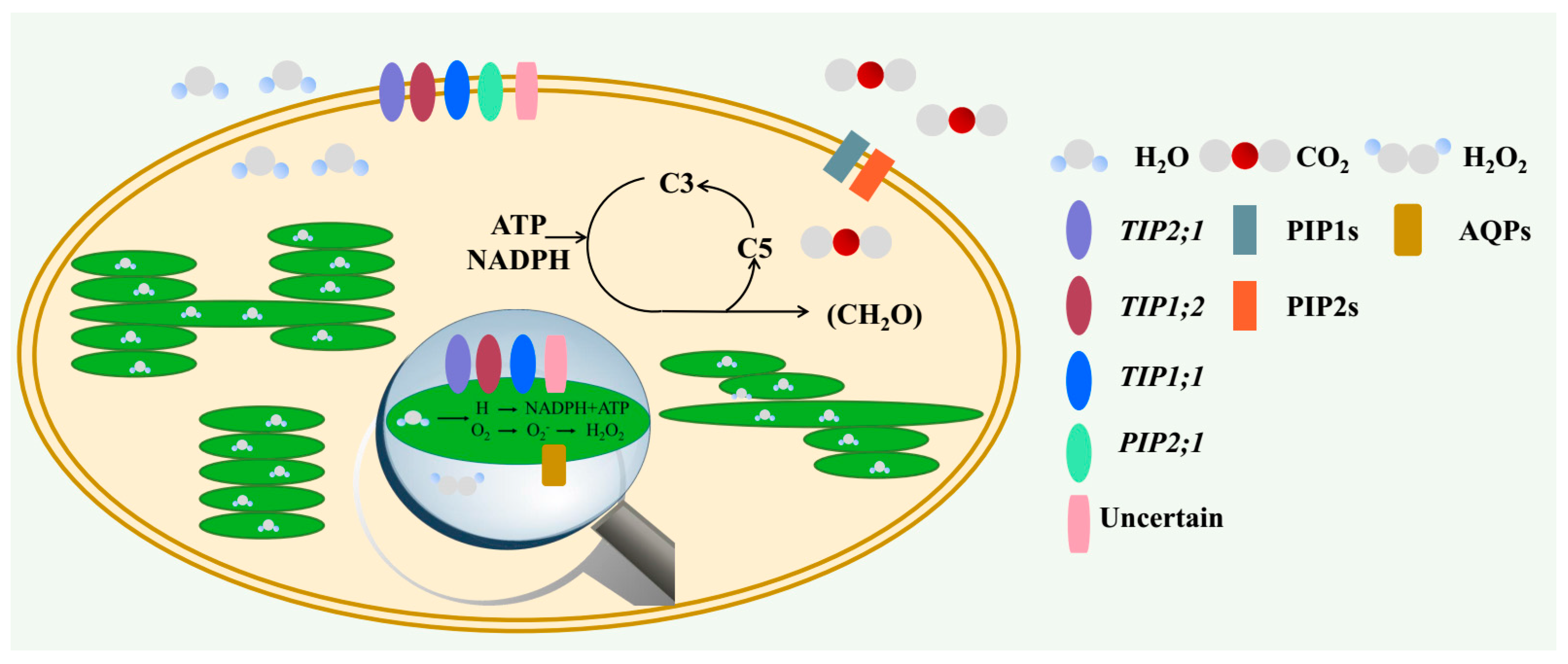
2. The Role of AQPs in Regulating Carbon Status
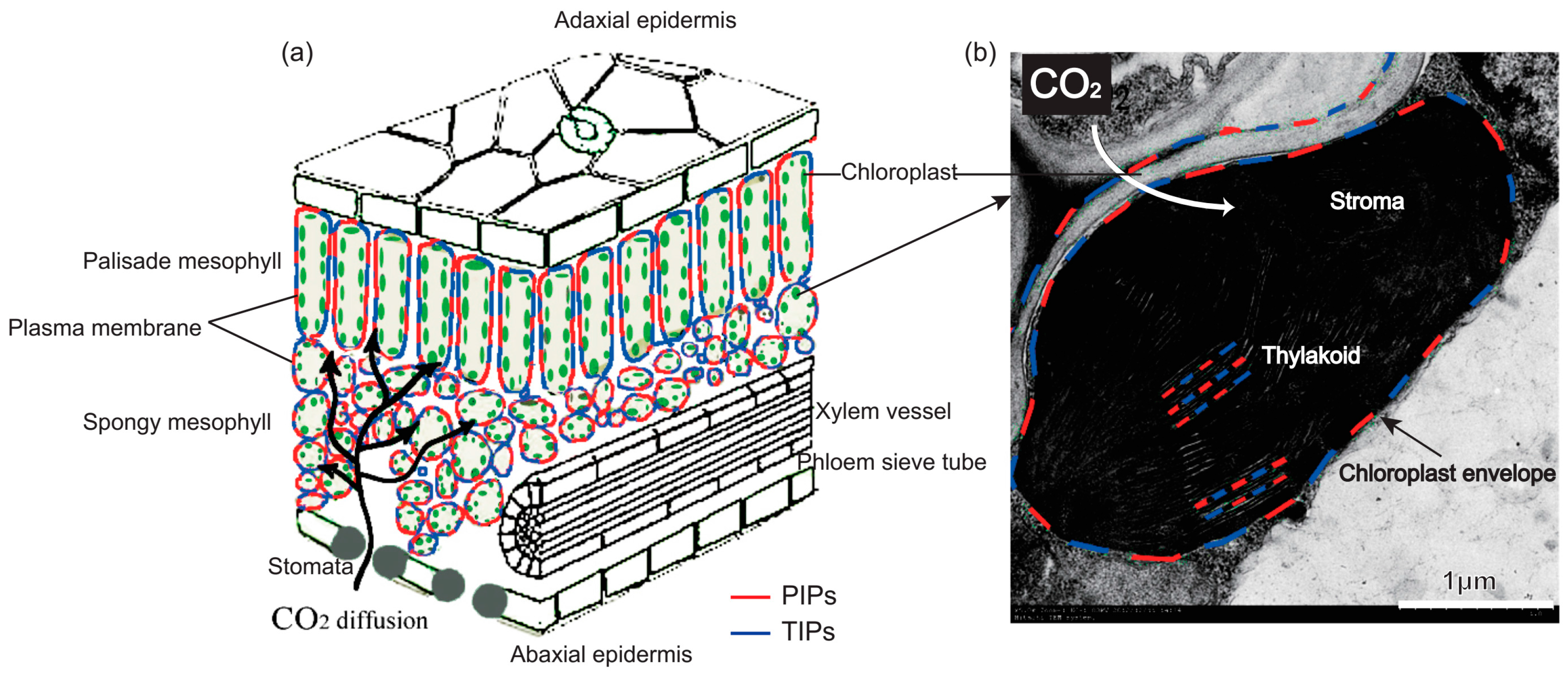
2.1. Water Transport Across the Membrane of Chloroplast and Thylakoid
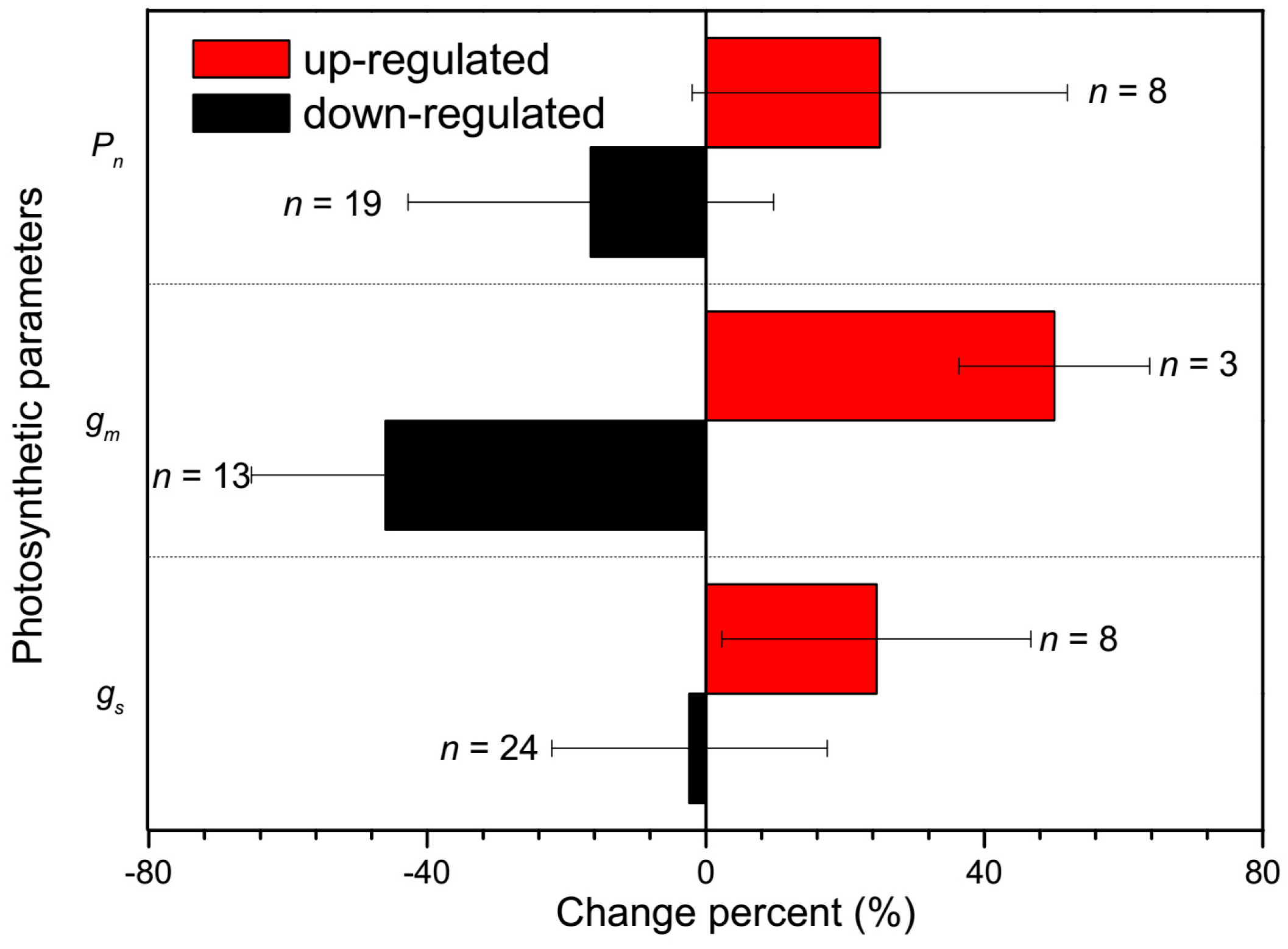
2.2. CO2 Transport Facilitator
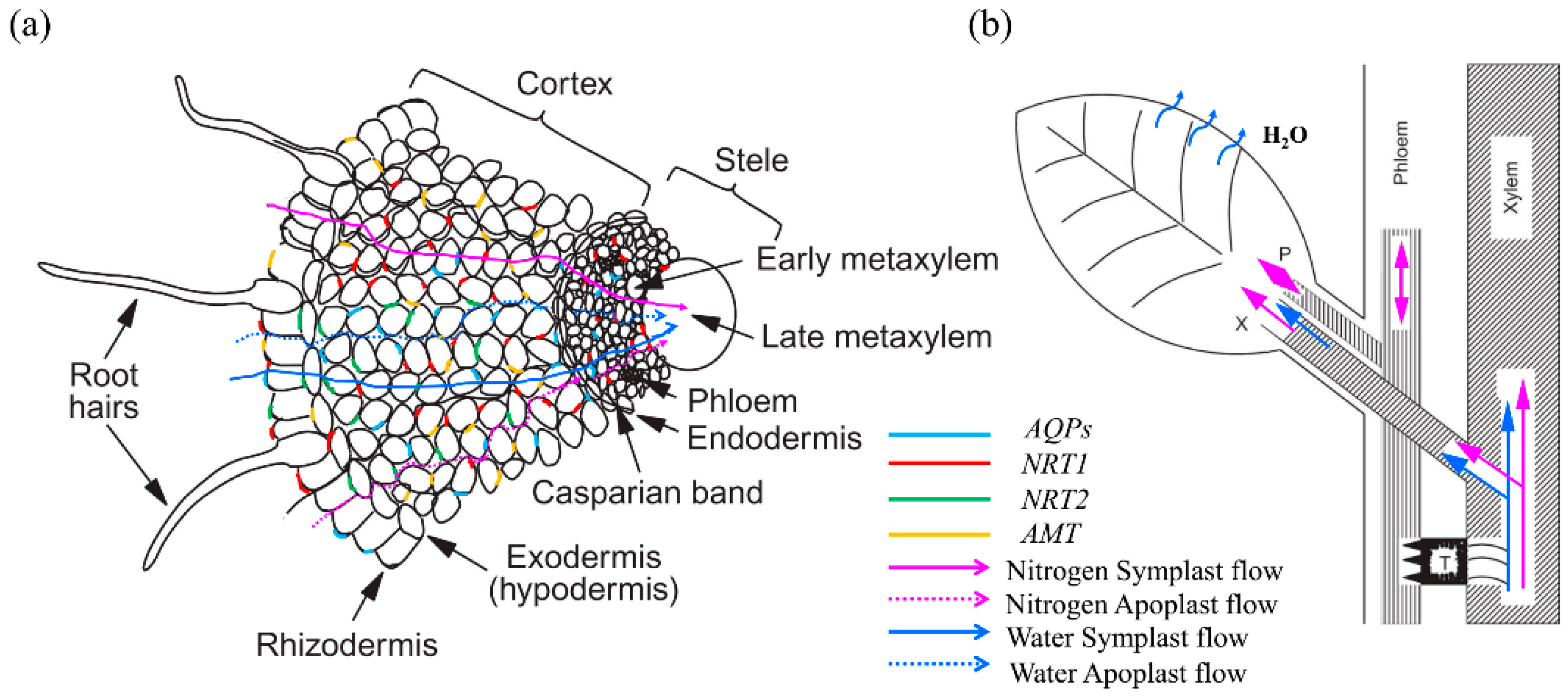
3. Role of AQPs in Regulating Nitrogen Status
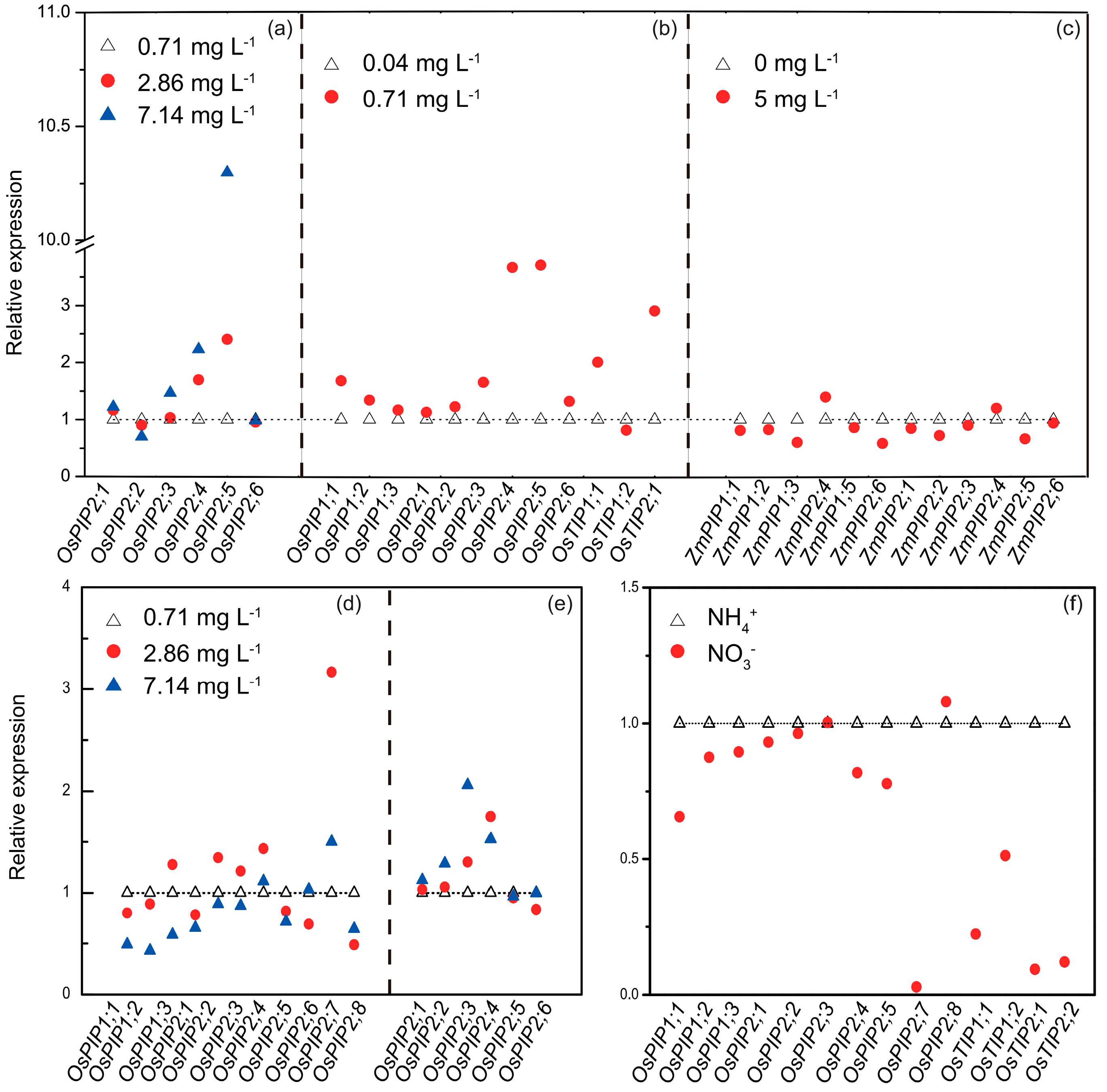
4. Responses of AQPs to Nitrogen Supply
5. Conclusions and Future Perspective
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Denker, B.M.; Smith, B.L.; Kuhajda, F.P.; Agre, P. Identification, purification, and partial characterization of a novel Mr 28,000 integral membrane protein from erythrocytes and renal tubules. J. Biol. Chem. 1988, 263, 15634–15642. [Google Scholar] [PubMed]
- Preston, G.M.; Carroll, T.P.; Guggino, W.B.; Agre, P. Appearance of water channels in Xenopus oocytes expressing red cell CHIP28 protein. Science 1992, 256, 385–387. [Google Scholar] [CrossRef] [PubMed]
- Maurel, C.; Javot, H.; Lauvergeat, V.; Gerbeau, P.; Tournaire, C.; Santoni, V.; Heyes, J. Molecular physiology of aquaporins in plants. Int. Rev. Cytol. Surv. Cell Biol. 2002, 215, 105–148. [Google Scholar]
- Wallace, I.S.; Choi, W.G.; Roberts, D.M. The structure, function and regulation of the nodulin 26-like intrinsic protein family of plant aquaglyceroporins. Biochim. Biophys. Acta Biomembr. 2006, 1758, 1165–1175. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, F.; Suga, S.; Uemura, T.; Sato, M.H.; Maeshima, M. Novel type aquaporin SIPs are mainly localized to the ER membrane and show cell-specific expression in Arabidopsis thaliana. FEBS Lett. 2005, 579, 5814–5820. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, R.K.; Vivancos, J.; Ramakrishnan, G.; Guerin, V.; Carpentier, G.; Sonah, H.; Labbe, C.; Isenring, P.; Belzile, F.J.; Belanger, R.R. A precise spacing between the NPA domains of aquaporins is essential for silicon permeability in plants. Plant J. 2015, 83, 489–500. [Google Scholar] [CrossRef] [PubMed]
- Flexas, J.; Ribas-Carbo, M.; Hanson, D.T.; Bota, J.; Otto, B.; Cifre, J.; McDowell, N.; Medrano, H.; Kaldenhoff, R. Tobacco aquaporin NtAQP1 is involved in mesophyll conductance to CO2 in vivo. Plant J. 2006, 48, 427–439. [Google Scholar] [CrossRef] [PubMed]
- Kato, Y.; Miwa, K.; Takano, J.; Wada, M.; Fujiwara, T.; Fujiwara, T.; Matoh, T. Highly boron deficiency-tolerant plants generated by enhanced expression of NIP5;1, a boric acid channel. Plant Cell Physiol. 2009, 50, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Durbak, A.R.; Phillips, K.A.; Pike, S.; O’Neill, M.A.; Mares, J.; Gallavotti, A.; Malcomber, S.T.; Gassmann, W.; Mcsteen, P. Transport of boron by the tassel-less1 aquaporin is critical for vegetative and reproductive development in maize. Plant Cell 2014, 26, 2978–2995. [Google Scholar] [CrossRef] [PubMed]
- Maurel, C.; Verdoucq, L.; Luu, D.T.; Santoni, V. Plant aquaporins: Membrane channels with multiple integrated functions. Annu. Rev. Plant Biol. 2008, 59, 595–624. [Google Scholar] [CrossRef] [PubMed]
- Groszmann, M.; Osborn, H.L.; Evans, J.R. Carbon dioxide and water transport through plant aquaporins. Plant Cell Environ. 2017, 40, 938–961. [Google Scholar] [CrossRef] [PubMed]
- Maurel, C.; Verdoucq, L.; Rodrigues, O. Aquaporins and plant transpiration. Plant Cell Environ. 2016, 39, 2580–2587. [Google Scholar] [CrossRef] [PubMed]
- Maurel, C.; Boursiac, Y.; Luu, D.T.; Santoni, V.; Shahzad, Z.; Verdoucq, L. Aquaporins in plants. Physiol. Rev. 2015, 95, 1321–1358. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Santoni, V.; Maurel, C. Plant aquaporins: Roles in plant physiology. Biochim. Biophys. Acta 2014, 1840, 1574–1582. [Google Scholar] [CrossRef] [PubMed]
- Kaldenhoff, R.; Kai, L.; Uehlein, N. Aquaporins and membrane diffusion of CO2 in living organisms. Biochim. Biophys. Acta 2014, 1840, 1592–1595. [Google Scholar] [CrossRef] [PubMed]
- Stitt, M. Nitrate regulation of metabolism and growth. Curr. Opin. Plant Biol. 1999, 2, 178–186. [Google Scholar] [CrossRef]
- Saarinen, T. Internal C:N balance and biomass partitioning of Carex rostrata grown at three levels of nitrogen supply. Can. J. Bot. 1998, 76, 762–768. [Google Scholar] [CrossRef]
- Saarinen, T.; Haansuu, P. Shoot density of Carex rostrata stokes in relation to internal Carbon: Nitrogen balance. Oecologia 2000, 122, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Beebo, A.; Schoefs, B.; Spetea, C. Photosynthetic water oxidation requires water transport across the thylakoid membrane: Are aquaporins involved? Curr. Chem. Biol. 2012, 6, 244–253. [Google Scholar] [CrossRef]
- Finkelstein, A. Water transport mechanisms. (book reviews: Water movement through lipid bilayers, pores, and plasma membranes). Science 1988, 240, 228. [Google Scholar]
- Heinen, R.B.; Ye, Q.; Chaumont, F. Role of aquaporins in leaf physiology. J. Exp. Bot. 2009, 60, 2971–2985. [Google Scholar] [CrossRef] [PubMed]
- Zybailov, B.; Rutschow, H.; Friso, G.; Rudella, A.; Emanuelsson, O.; Sun, Q.; van Wijk, K.J. Sorting signals, N-terminal modifications and abundance of the chloroplast proteome. PLoS ONE 2008, 3, e1994. [Google Scholar] [CrossRef] [PubMed]
- Ferro, M.; Brugière, S.; Salvi, D.; Seigneurinberny, D.; Court, M.; Moyet, L.; Ramus, C.; Miras, S.; Mellal, M.; Gall, S.L. At_CHLORO, a comprehensive chloroplast proteome database with subplastidial localization and curated information on envelope proteins. Mol. Cell. Proteom. 2010, 9, 1063–1084. [Google Scholar] [CrossRef] [PubMed]
- Simm, S.; Papasotiriou, D.G.; Ibrahim, M.; Leisegang, M.S.; Müller, B.; Schorge, T.; Karas, M.; Mirus, O.; Sommer, M.S.; Schleiff, E. Defining the core proteome of the chloroplast envelope membranes. Front. Plant Sci. 2013, 4. [Google Scholar] [CrossRef] [PubMed]
- Uehlein, N.; Otto, B.; Hanson, D.T.; Fischer, M.; McDowell, N.; Kaldenhoff, R. Function of Nicotiana tabacum aquaporins as chloroplast gas pores challenges the concept of membrane CO2 permeability. Plant Cell 2008, 20, 648–657. [Google Scholar] [CrossRef] [PubMed]
- Beebo, A.; Mathai, J.C.; Schoefs, B.; Spetea, C. Assessment of the requirement for aquaporins in the thylakoid membrane of plant chloroplasts to sustain photosynthetic water oxidation. FEBS Lett. 2013, 587, 2083–2089. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, M.; Asada, K. Superoxide production in aprotic interior of chloroplast thylakoids. Arch. Biochem. Biophys. 1988, 267, 714–722. [Google Scholar] [CrossRef]
- Mubarakshina Borisova, M.M.; Kozuleva, M.A.; Rudenko, N.N.; Naydov, I.A.; Klenina, I.B.; Ivanov, B.N. Photosynthetic electron flow to oxygen and diffusion of hydrogen peroxide through the chloroplast envelope via aquaporins. Biochim. Biophys. Acta 2012, 1817, 1314–1321. [Google Scholar] [CrossRef] [PubMed]
- Mubarakshina, M.; Khorobrykh, S.; Ivanov, B. Oxygen reduction in chloroplast thylakoids results in production of hydrogen peroxide inside the membrane. Biochim. Biophys. Acta 2006, 1757, 1496–1503. [Google Scholar] [CrossRef] [PubMed]
- Bienert, G.P.; Chaumont, F. Aquaporin-facilitated transmembrane diffusion of hydrogen peroxide. Biochim. Biophys. Acta 2014, 1840, 1596–1604. [Google Scholar] [CrossRef] [PubMed]
- Bienert, G.P.; Heinen, R.B.; Berny, M.C.; Chaumont, F. Maize plasma membrane aquaporin ZmPIP2;5, but not ZmPIP1;2, facilitates transmembrane diffusion of hydrogen peroxide. Biochim. Biophys. Acta 2014, 1838, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Bienert, G.P.; Moller, A.L.; Kristiansen, K.A.; Schulz, A.; Moller, I.M.; Schjoerring, J.K.; Jahn, T.P. Specific aquaporins facilitate the diffusion of hydrogen peroxide across membranes. J. Biol. Chem. 2007, 282, 1183–1192. [Google Scholar] [CrossRef] [PubMed]
- Sage, R.F.; Sharkey, T.D.; Pearcy, R.W. The effect of leaf nitrogen and temperature on the CO2 response of photosynthesis in the C3 dicot chenopodium-albumm L. Aust. J. Plant Physiol. 1990, 17, 135–148. [Google Scholar] [CrossRef]
- Barbour, M.M.; Kaiser, B.N. The response of mesophyll conductance to nitrogen and water availability differs between wheat genotypes. Plant Sci. 2016, 251, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Flexas, J.; Barbour, M.M.; Brendel, O.; Cabrera, H.M.; Carriqui, M.; Diaz-Espejo, A.; Douthe, C.; Dreyer, E.; Ferrio, J.P.; Gago, J.; et al. Mesophyll diffusion conductance to CO2: An unappreciated central player in photosynthesis. Plant Sci. 2012, 193–194, 70–84. [Google Scholar] [CrossRef] [PubMed]
- Hassiotou, F.; Ludwig, M.; Renton, M.; Veneklaas, E.J.; Evans, J.R. Influence of leaf dry mass per area, CO2, and irradiance on mesophyll conductance in sclerophylls. J. Exp. Bot. 2009, 60, 2303–2314. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.F.; Lu, J.W.; Pan, Y.H.; Lu, P.P.; Li, X.K.; Cong, R.H.; Ren, T. Anatomical variation of mesophyll conductance under potassium deficiency has a vital role in determining leaf photosynthesis. Plant Cell Environ. 2016, 39, 2428–2439. [Google Scholar] [CrossRef] [PubMed]
- Tosens, T.; Niinemets, U.; Vislap, V.; Eichelmann, H.; Castro Diez, P. Developmental changes in mesophyll diffusion conductance and photosynthetic capacity under different light and water availabilities in populus tremula: How structure constrains function. Plant Cell Environ. 2012, 35, 839–856. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Gao, L.; Liu, W.; Wang, M.; Gu, M.; Ren, B.; Xu, G.; Shen, Q.; Guo, S. Aquaporin plays an important role in mediating chloroplastic CO2 concentration under high-N supply in rice (Oryza sativa L.) plants. Physiol. Plant. 2015, 156, 215–226. [Google Scholar] [CrossRef] [PubMed]
- Nakhoul, N.L.; Davis, B.A.; Romero, M.F.; Boron, W.F. Effect of expressing the water channel aquaporin-1 on the CO2 permeability of Xenopus oocytes. Am. J. Physiol. 1998, 274, 297–298. [Google Scholar]
- Terashima, I.; Ono, K. Effects of HgCl2 on CO2 dependence of leaf photosynthesis: Evidence indicating involvement of aquaporins in CO2 diffusion across the plasma membrane. Plant Cell Physiol. 2002, 43, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Uehlein, N.; Lovisolo, C.; Siefritz, F.; Kaldenhoff, R. The tobacco aquaporin NtAQP1 is a membrane CO2 pore with physiological functions. Nature 2003, 425, 734–737. [Google Scholar] [CrossRef] [PubMed]
- Heckwolf, M.; Pater, D.; Hanson, D.T.; Kaldenhoff, R. The Arabidopsis thaliana aquaporin AtPIP1;2 is a physiologically relevant CO2 transport facilitator. Plant J. 2011, 67, 795–804. [Google Scholar] [CrossRef] [PubMed]
- Uehlein, N.; Sperling, H.; Heckwolf, M.; Kaldenhoff, R. The Arabidopsis aquaporin PIP1;2 rules cellular CO2 uptake. Plant Cell Environ. 2012, 35, 1077–1083. [Google Scholar] [CrossRef] [PubMed]
- Hanba, Y.T.; Shibasaka, M.; Hayashi, Y.; Hayakawa, T.; Kasamo, K.; Terashima, I.; Katsuhara, M. Overexpression of the barley aquaporin HvPIP2;1 increases internal CO2 conductance and CO2 assimilation in the leaves of transgenic rice plants. Plant Cell Physiol. 2004, 45, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Katsuhara, M.; Hanba, Y.T. Barley plasma membrane intrinsic proteins (PIP aquaporins) as water and CO2 transporters. Pflugers Arch. 2008, 456, 687–691. [Google Scholar] [CrossRef] [PubMed]
- Mori, I.C.; Rhee, J.; Shibasaka, M.; Sasano, S.; Kaneko, T.; Horie, T.; Katsuhara, M. CO2 transport by PIP2 aquaporins of barley. Plant Cell Physiol. 2014, 55, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Heinen, R.B.; Bienert, G.P.; Cohen, D.; Chevalier, A.S.; Uehlein, N.; Hachez, C.; Kaldenhoff, R.; Thiec, D.L.; Chaumont, F. Expression and characterization of plasma membrane aquaporins in stomatal complexes of Zea mays. Plant Mol. Biol. 2014, 86, 335–350. [Google Scholar] [CrossRef] [PubMed]
- A, N.-R.; JM, R.-L.; Kaldenhoff, R.; Morte, A. The aquaporin TcAQP1 of the desert Truffle terfezia claveryi is a membrane pore for water and CO2 transport. Mol. Plant-Microbe Interact. 2012, 25, 259–266. [Google Scholar]
- Secchi, F.; Zwieniecki, M.A. The physiological response of populus Tremula × alba leaves to the down-regulation of PIP1 aquaporin gene expression under no water stress. Front. Plant Sci. 2013, 4. [Google Scholar] [CrossRef] [PubMed]
- Tsuchihira, A.; Hanba, Y.T.; Kato, N.; Doi, T.; Kawazu, T.; Maeshima, M. Effect of overexpression of radish plasma membrane aquaporins on water-use efficiency, photosynthesis and growth of eucalyptus trees. Tree Physiol. 2010, 30, 417–430. [Google Scholar] [CrossRef] [PubMed]
- Kawase, M.; Hanba, Y.T.; Katsuhara, M. The photosynthetic response of tobacco plants overexpressing ice plant aquaporin McMIPb to a soil water deficit and high vapor pressure deficit. J. Plant Res. 2013, 126, 517–527. [Google Scholar] [CrossRef] [PubMed]
- Sade, N.; Shatil-Cohen, A.; Attia, Z.; Maurel, C.; Boursiac, Y.; Kelly, G.; Granot, D.; Yaaran, A.; Lerner, S.; Moshelion, M. The role of plasma membrane aquaporins in regulating the bundle sheath-mesophyll continuum and leaf hydraulics. Plant Physiol. 2014, 166, 1609–1620. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wang, H.; Gago, J.; Cui, H.; Qian, Z.; Kodama, N.; Ji, H.; Tian, S.; Shen, D.; Chen, Y.; et al. Harpin HPA1 interacts with aquaporin PIP1;4 to promote the substrate transport and photosynthesis in Arabidopsis. Sci. Rep. 2015, 5. [Google Scholar] [CrossRef] [PubMed]
- Dechorgnat, J.; Nguyen, C.T.; Armengaud, P.; Jossier, M.; Diatloff, E.; Filleur, S.; Daniel-Vedele, F. From the soil to the seeds: The long journey of nitrate in plants. J. Exp. Bot. 2011, 62, 1349–1359. [Google Scholar] [CrossRef] [PubMed]
- Marschner, H. Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Academic Press: New York, NY, USA, 2011. [Google Scholar]
- Von Wirén, N.; Gazzarrini, S.; Gojon, A.; Frommer, W.B. The molecular physiology of ammonium uptake and retrieval. Curr. Opin. Plant Biol. 2000, 3, 254–261. [Google Scholar] [CrossRef]
- Shatil-Cohen, A.; Attia, Z.; Moshelion, M. Bundle-sheath cell regulation of xylem-mesophyll water transport via aquaporins under drought stress: A target of xylem-borne aba? Plant J. 2011, 67, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Vandeleur, R.K.; Sullivan, W.; Athman, A.; Jordans, C.; Gilliham, M.; Kaiser, B.N.; Tyerman, S.D. Rapid shoot-to-root signalling regulates root hydraulic conductance via aquaporins. Plant Cell Environ. 2014, 37, 520–538. [Google Scholar] [CrossRef] [PubMed]
- Sadok, W.; Sinclair, T.R. Transpiration response of ‘slow-wilting’ and commercial soybean (Glycine max (L.) Merr.) genotypes to three aquaporin inhibitors. J. Exp. Bot. 2010, 61, 821–829. [Google Scholar] [CrossRef] [PubMed]
- Aharon, R. Overexpression of a plasma membrane aquaporin in transgenic tobacco improves plant vigor under favorable growth conditions but not under drought or salt stress. Plant Cell Online 2003, 15, 439–447. [Google Scholar] [CrossRef]
- EP, M.; JE, E.; EL, K. Can decreased transpiration limit plant nitrogen acquisition in elevated CO2? Funct. Plant Biol. 2002, 29, 1115–1120. [Google Scholar]
- Tanguilig, V.C.; Yambao, E.B.; O’toole, J.C.; De Datta, S.K. Water stress effects on leaf elongation, leaf water potential, transpiration, and nutrient uptake of rice, maize, and soybean. Plant Soil 1987, 103, 155–168. [Google Scholar] [CrossRef]
- Loque, D.; Ludewig, U.; Yuan, L.; von Wiren, N. Tonoplast intrinsic proteins AtTip2;1 and AtTip2;3 facilitate NH3 transport into the vacuole. Plant Physiol. 2005, 137, 671–680. [Google Scholar] [CrossRef] [PubMed]
- Barzana, G.; Aroca, R.; Bienert, G.P.; Chaumont, F.; Ruiz-Lozano, J.M. New insights into the regulation of aquaporins by the arbuscular mycorrhizal symbiosis in maize plants under drought stress and possible implications for plant performance. Mol. Plant-Microbe Interact. 2014, 27, 349–363. [Google Scholar] [CrossRef] [PubMed]
- Holm, L.M.; Jahn, T.P.; Moller, A.L.; Schjoerring, J.K.; Ferri, D.; Klaerke, D.A.; Zeuthen, T. NH3 and NH4+ permeability in aquaporin-expressing Xenopus oocytes. Pflugers Arch. 2005, 450, 415–428. [Google Scholar] [CrossRef] [PubMed]
- Bertl, A.; Kaldenhoff, R. Function of a separate NH3-pore in aquaporin TIP2;2 from wheat. FEBS Lett. 2007, 581, 5413–5417. [Google Scholar] [CrossRef] [PubMed]
- Engelsberger, W.R.; Schulze, W.X. Nitrate and ammonium lead to distinct global dynamic phosphorylation patterns when resupplied to nitrogen-starved Arabidopsis seedlings. Plant J. 2012, 69, 978–995. [Google Scholar] [CrossRef] [PubMed]
- Lanquar, V.; Loque, D.; Hormann, F.; Yuan, L.; Bohner, A.; Engelsberger, W.R.; Lalonde, S.; Schulze, W.X.; von Wiren, N.; Frommer, W.B. Feedback inhibition of ammonium uptake by a phospho-dependent allosteric mechanism in Arabidopsis. Plant Cell 2009, 21, 3610–3622. [Google Scholar] [CrossRef] [PubMed]
- Neuhauser, B.; Dynowski, M.; Mayer, M.; Ludewig, U. Regulation of NH4+ transport by essential cross talk between amt monomers through the Carboxyl tails. Plant Physiol. 2007, 143, 1651–1659. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhao, Y.; Luo, W.; Li, R.; He, Q.; Fang, X.; De Michele, R.; Ast, C.; von Wirén, N.; Lin, J. Single-particle analysis reveals shutoff control of the Arabidopsis ammonium transporter AMT1;3 by clustering and internalization. Proc. Natl. Acad. Sci. USA 2013, 110, 13204–13209. [Google Scholar] [CrossRef] [PubMed]
- Coskun, D.; Britto, D.T.; Li, M.; Becker, A.; Kronzucker, H.J. Rapid ammonia gas transport accounts for futile transmembrane cycling under NH3/NH4+ toxicity in plant roots. Plant Physiol. 2013, 163, 1859–1867. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Zhou, Y.; Ma, X.; Gao, L.; Song, C.P. Arabidopsis CAP1-mediated ammonium sensing required reactive oxygen species in plant cell growth. Plant Signal. Behav. 2014, 9, e29582. [Google Scholar] [CrossRef] [PubMed]
- Eckert, M.; Biela, A.; Siefritz, F.; Kaldenhoff, R. New aspects of plant aquaporin regulation and specificity. J. Exp. Bot. 1999, 50, 1541–1545. [Google Scholar] [CrossRef]
- Gaspar, M. Cloning and characterization of ZmPIP1-5b, an aquaporin transporting water and urea. Plant Sci. 2003, 165, 21–31. [Google Scholar] [CrossRef]
- Klebl, F.; Wolf, M.; Sauer, N. A defect in the yeast plasma membrane urea transporter Dur3p is complemented by CpNIP1, a nod26-like protein from Zucchini (cucurbita pepo L.), and by Arabidopsis thaliana δ-tip or γ-tip. FEBS Lett. 2003, 547, 69–74. [Google Scholar] [CrossRef]
- Gu, R.; Chen, X.; Zhou, Y.; Yuan, L. Isolation and characterization of three maize aquaporin genes, ZmNIP2;1, ZmNIP2;4 and ZmTIP4;4 involved in urea transport. BMB Rep. 2012, 45, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Wallace, I.S.; Roberts, D.M. Distinct transport selectivity of two structural subclasses of the nodulin-like intrinsic protein family of plant aquaglyceroporin channels. Biochemistry 2005, 44, 16826–16834. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yan, J.; Vatamaniuk, O.K.; Du, X. CsNIP2;1 is a plasma membrane transporter from Cucumis sativus that facilitates urea uptake when expressed in Saccharomyces cerevisiae and Arabidopsis thaliana. Plant Cell Physiol. 2016, 57, 616–629. [Google Scholar] [CrossRef] [PubMed]
- Gerbeau, P.; Guclu, J.; Ripoche, P.; Maurel, C. Aquaporin Nt-TIPa can account for the high permeability of tobacco cell vacuolar membrane to small neutral solutes. Plant J. 1999, 18, 577–587. [Google Scholar] [CrossRef] [PubMed]
- Soto, G.; Alleva, K.; Mazzella, M.A.; Amodeo, G.; Muschietti, J.P. AtTIP1;3 and AtTIP5;1, the only highly expressed Arabidopsis pollen-specific aquaporins, transport water and urea. FEBS Lett. 2008, 582, 4077–4082. [Google Scholar] [CrossRef] [PubMed]
- Soto, G.; Fox, R.; Ayub, N.; Alleva, K.; Guaimas, F.; Erijman, E.J.; Mazzella, A.; Amodeo, G.; Muschietti, J. Tip5;1 is an aquaporin specifically targeted to pollen mitochondria and is probably involved in nitrogen remobilization in Arabidopsis thaliana. Plant J. 2010, 64, 1038–1047. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.H. Urea transport by nitrogen-regulated tonoplast intrinsic proteins in Arabidopsis. Plant Physiol. 2003, 133, 1220–1228. [Google Scholar] [CrossRef] [PubMed]
- Clarkson, D.T.; Carvajal, M.; Henzler, T.; Waterhouse, R.N.; Smyth, A.J.; Cooke, D.T.; Steudle, E. Root hydraulic conductance: Diurnal aquaporin expression and the effects of nutrient stress. J. Exp. Bot. 2000, 51, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Ren, B.B.; Wang, M.; Chen, Y.P.; Sun, G.M.; Li, Y.; Shen, Q.R.; Guo, S.W. Water absorption is affected by the nitrogen supply to rice plants. Plant Soil 2015, 396, 397–410. [Google Scholar] [CrossRef]
- Gorska, A.; Zwieniecki, M.A. Nitrate induction of root hydraulic conductivity in maize is not correlated with aquaporin expression. Planta 2008, 228, 989–998. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Okamoto, M.; Xing, X.; Crawford, N.M. Microarray analysis of the nitrate response in Arabidopsis roots and shoots reveals over 1000 rapidly responding genes and new linkages to glucose, trehalose-6-phosphate, iron, and sulfate metabolism. Plant Physiol. 2003, 132, 556–567. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-H.; Garvin, D.F.; Kochian, L.V. Nitrate-induced genes in tomato roots. Array analysis reveals novel genes that may play a role in nitrogen nutrition. Plant Physiol. 2001, 127, 345–359. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa-Sakurai, J.; Hayashi, H.; Murai-Hatano, M. Nitrogen availability affects hydraulic conductivity of rice roots, possibly through changes in aquaporin gene expression. Plant Soil 2014, 379, 289–300. [Google Scholar] [CrossRef]
- Di Pietro, M.; Vialaret, J.; Li, G.W.; Hem, S.; Prado, K.; Rossignol, M.; Maurel, C.; Santoni, V. Coordinated post-translational responses of aquaporins to abiotic and nutritional stimuli in Arabidopsis roots. Mol. Cell. Proteom. 2013, 12, 3886–3897. [Google Scholar] [CrossRef] [PubMed]
- Chapin, F.S.; Walter, C.H.; Clarkson, D.T. Growth response of barley and tomato to nitrogen stress and its control by abscisic acid, water relations and photosynthesis. Planta 1988, 173, 352–366. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Kaldenhoff, R.; Uehlein, N.; Sattelmacher, B.; Brueck, H. Relationship between water and nitrogen uptake in nitrate- and ammonium-supplied Phaseolus vulgaris L. Plants. J. Plant Nutr. Soil Sci. 2007, 170, 73–80. [Google Scholar] [CrossRef]
- Wang, M.; Ding, L.; Gao, L.; Li, Y.; Shen, Q.; Guo, S. The interactions of aquaporins and mineral nutrients in higher plants. Int. J. Mol. Sci. 2016, 17, 1229. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Gao, C.; Li, Y.; Li, Y.; Zhu, Y.; Xu, G.; Shen, Q.; Kaldenhoff, R.; Kai, L.; Guo, S. The enhanced drought tolerance of rice plants under ammonium is related to aquaporin (AQP). Plant Sci. 2015, 234, 14–21. [Google Scholar] [CrossRef] [PubMed]
| Substrate | Family | Name | Expression System | References |
|---|---|---|---|---|
| NH3 | TIPs | AtTIP2;1 | yeast | Loque et al. [64] |
| AtTIP2;3 | yeast | Loque et al. [64] | ||
| ZmTIP1;1 | yeast | Barzana et al. [65] | ||
| ZmTIP1;2 | yeast | Barzana et al. [65] | ||
| TaTIP2;1 | Xenopus oocytes | Holm et al. [66] | ||
| TaTIP2;2 | yeast | Bertl and Kaldenhoff [67] | ||
| Urea | PIPs | NtAQP1 | Xenopus oocytes | Eckert et al. [74] |
| ZmPIP1;5 | Xenopus oocytes | Gaspar [75] | ||
| NIPs | CpNIP1 | yeast | Klebl et al. [76] | |
| ZmNIP2;1 | yeast | Gu et al. [77] | ||
| ZmNIP2;4 | yeast | Gu et al. [77] | ||
| AtNIP5;1 | Xenopus oocytes | Wallace and Roberts [78] | ||
| AtNIP6;1 | Xenopus oocytes | Wallace and Roberts [78] | ||
| CsNIP2;1 | yeast | Zhang et al. [79] | ||
| TIPs | NtTIPa | Xenopus oocytes | Gerbeau et al. [80] | |
| AtTIP1;1 | yeast | Liu [83] | ||
| AtTIP1;2 | yeast | Liu [83] | ||
| AtTIP2;1 | yeast | Liu [83] | ||
| AtTIP4;1 | yeast | Liu [83] | ||
| AtTIP1;3 | Xenopus oocytes | Soto et al. [81] | ||
| AtTIP5;1 | Xenopus oocytes | Soto et al. [82] | ||
| ZmTIP4;4 | yeast | Gu et al. [77] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, L.; Lu, Z.; Ding, L.; Guo, J.; Wang, M.; Ling, N.; Guo, S.; Shen, Q. Role of Aquaporins in Determining Carbon and Nitrogen Status in Higher Plants. Int. J. Mol. Sci. 2018, 19, 35. https://doi.org/10.3390/ijms19010035
Gao L, Lu Z, Ding L, Guo J, Wang M, Ling N, Guo S, Shen Q. Role of Aquaporins in Determining Carbon and Nitrogen Status in Higher Plants. International Journal of Molecular Sciences. 2018; 19(1):35. https://doi.org/10.3390/ijms19010035
Chicago/Turabian StyleGao, Limin, Zhifeng Lu, Lei Ding, Junjie Guo, Min Wang, Ning Ling, Shiwei Guo, and Qirong Shen. 2018. "Role of Aquaporins in Determining Carbon and Nitrogen Status in Higher Plants" International Journal of Molecular Sciences 19, no. 1: 35. https://doi.org/10.3390/ijms19010035
APA StyleGao, L., Lu, Z., Ding, L., Guo, J., Wang, M., Ling, N., Guo, S., & Shen, Q. (2018). Role of Aquaporins in Determining Carbon and Nitrogen Status in Higher Plants. International Journal of Molecular Sciences, 19(1), 35. https://doi.org/10.3390/ijms19010035