Apoptotic and Nonapoptotic Activities of Pterostilbene against Cancer
Abstract
1. Introduction
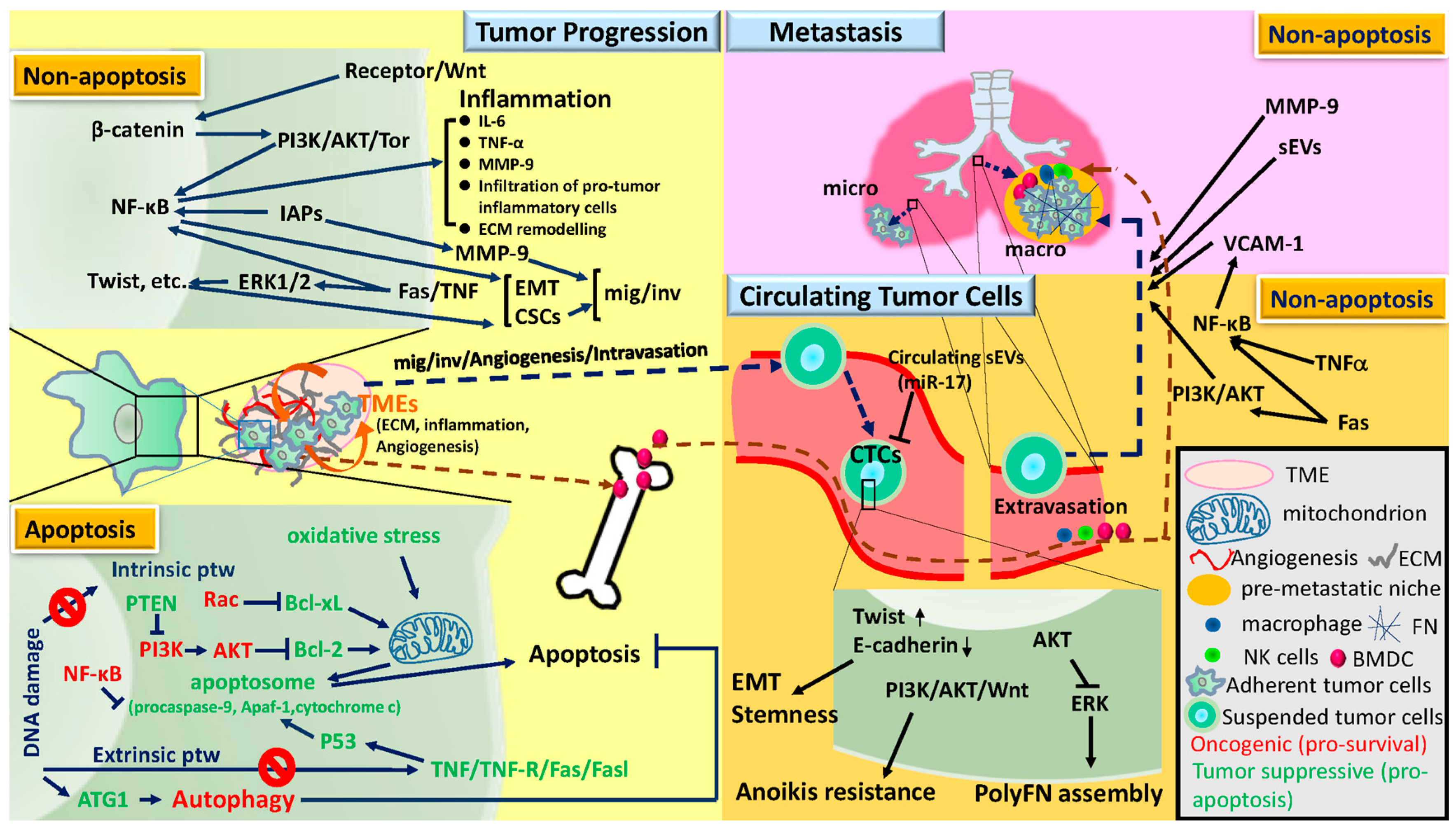
2. Tumor Progression, Circulating Tumor Cells, and Metastasis
2.1. Tumor Progression
2.2. Circulating Tumor Cells
2.3. Metastasis
3. PS Emerges as a Potent Alternative Medicine
4. Pro-Apoptotic Effects of PS against Cancer
4.1. Enhancement of Endoplasmic Reticulum Stress (ERS) Signaling
4.2. Induction of Autophagy
4.3. Regulation of miRNA Profiles
4.4. Inhibiting the Function of Growth Receptors
4.5. Affecting Epigenetic Pathway
5. Nonapoptotic Effects of PS against Cancer
5.1. Effects in Senescence Induction
5.2. Effects against EMT
5.3. Effects against Inflammation
5.4. Effects against Cell Migration and Invasion
5.5. Effects against Cancer Stemness
5.6. Effects against PolyFN Assembly on CTCs
5.7. Effects against the Diminished Circulating miR-17-92 Cluster
6. Potential Prevention and Treatment of Various Types of Cancer by PS
6.1. Lung Cancer
6.2. Breast Cancer
6.3. Prostate Cancer
6.4. Hepatocellular Melanoma and Myeloma Cancers
6.5. Colon Cancer and Pancreatic Cancer
7. Future Perspective
7.1. Potential Drug Packing and Delivery System for PS
7.2. FN-Targeting and PS Combined Therapy
7.3. PS-Enhanced Immunotherapy
7.4. PS Modification
7.5. Future Prospect of PS in Clinical Approach
8. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wong, A.S.; Che, C.M.; Leung, K.W. Recent advances in ginseng as cancer therapeutics: A functional and mechanistic overview. Nat. Prod. Rep. 2015, 32, 256–572. [Google Scholar] [CrossRef] [PubMed]
- Gotwals, P.; Cameron, S.; Cipolletta, D.; Cremasco, V.; Crystal, A.; Hewes, B.; Mueller, B.; Quaratino, S.; Sabatos-Peyton, C.; Petruzzelli, L.; et al. Prospects for combining targeted and conventional cancer therapy with immunotherapy. Nat. Rev. Cancer 2017, 17, 286–301. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.D.; Siegel, R.L.; Lin, C.C.; Mariotto, A.B.; Kramer, J.L.; Rowland, J.H.; Stein, K.D.; Alteri, R.; Jemal, A. Cancer treatment and survivorship statistics. CA Cancer J. Clin. 2016, 66, 271–289. [Google Scholar] [CrossRef] [PubMed]
- Mathiyalagan, R.; Yang, D.C. Ginseng nanoparticles: A budding tool for cancer treatment. Nanomedicine 2017, 12, 1091–1094. [Google Scholar] [CrossRef] [PubMed]
- Nagao, K.; Jinnouchi, T.; Kai, S.; Yanagita, T. Pterostilbene, a dimethylated analog of resveratrol, promotes energy metabolism in obese rats. J. Nutr. Biochem. 2017, 43, 151–155. [Google Scholar] [CrossRef] [PubMed]
- McCormack, D.; McFadden, D. Pterostilbene and cancer: Current review. J. Surg. Res. 2012, 173, e53–e61. [Google Scholar] [CrossRef] [PubMed]
- Chaffer, C.L.; Weinberg, R.A. A perspective on cancer cell metastasis. Science 2011, 331, 1559–1564. [Google Scholar] [CrossRef] [PubMed]
- Tam, W.L.; Weinberg, R.A. The epigenetics of epithelial-mesenchymal plasticity in cancer. Nat. Med. 2013, 19, 1438–1449. [Google Scholar] [CrossRef] [PubMed]
- Massague, J.; Obenauf, A.C. Metastatic colonization by circulating tumour cells. Nature 2016, 529, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Roos, W.P.; Thomas, A.D.; Kaina, B. DNA damage and the balance between survival and death in cancer biology. Nat. Rev. Cancer 2016, 16, 20–33. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Riggi, N.; Aguet, M.; Stamenkovic, I. Cancer Metastasis: A Reappraisal of Its Underlying Mechanisms and Their Relevance to Treatment. Annu. Rev. Pathol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Letai, A. Apoptosis and Cancer. Annu. Rev. Cancer Biol. 2017, 1, 275–294. [Google Scholar] [CrossRef]
- Bai, L.; Wang, S. Targeting apoptosis pathways for new cancer therapeutics. Annu. Rev. Med. 2014, 65, 139–155. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, S.; Panda, P.K.; Sinha, N.; Das, D.N.; Bhutia, S.K. Autophagy and apoptosis: Where do they meet? Apoptosis 2014, 19, 555–566. [Google Scholar] [CrossRef] [PubMed]
- Letai, A.; Bassik, M.C.; Walensky, L.D.; Sorcinelli, M.D.; Weiler, S.; Korsmeyer, S.J. Distinct BH3 domains either sensitize or activate mitochondrial apoptosis, serving as prototype cancer therapeutics. Cancer Cell 2002, 2, 183–192. [Google Scholar] [CrossRef]
- Anantharaju, P.G.; Gowda, P.C.; Vimalambike, M.G.; Madhunapantula, S.V. An overview on the role of dietary phenolics for the treatment of cancers. Nutr. J. 2016, 15, 99. [Google Scholar] [CrossRef] [PubMed]
- Su, Z.; Yang, Z.; Xu, Y.; Chen, Y.; Yu, Q. Apoptosis, autophagy, necroptosis, and cancer metastasis. Mol. Cancer 2015, 14, 48. [Google Scholar] [CrossRef] [PubMed]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef] [PubMed]
- Labi, V.; Erlacher, M. How cell death shapes cancer. Cell Death Dis. 2015, 6, e1675. [Google Scholar] [CrossRef] [PubMed]
- Frances, D.; Sharma, N.; Pofahl, R.; Maneck, M.; Behrendt, K.; Reuter, K.; Krieg, T.; Klein, C.A.; Haase, I.; Niemann, C. A role for Rac1 activity in malignant progression of sebaceous skin tumors. Oncogene 2015, 34, 5505–5512. [Google Scholar] [CrossRef] [PubMed]
- Hein, A.L.; Post, C.M.; Sheinin, Y.M.; Lakshmanan, I.; Natarajan, A.; Enke, C.A.; Batra, S.K.; Ouellette, M.M.; Yan, Y. RAC1 GTPase promotes the survival of breast cancer cells in response to hyper-fractionated radiation treatment. Oncogene 2016, 35, 6319–6329. [Google Scholar] [CrossRef] [PubMed]
- Molinari, F.; Frattini, M. Functions and Regulation of the PTEN Gene in Colorectal Cancer. Front. Oncol. 2013, 3, 326. [Google Scholar] [CrossRef] [PubMed]
- McCubrey, J.A.; Steelman, L.S.; Bertrand, F.E.; Davis, N.M.; Sokolosky, M.; Abrams, S.L.; Montalto, G.; D’Assoro, A.B.; Libra, M.; Nicoletti, F.; et al. GSK-3 as potential target for therapeutic intervention in cancer. Oncotarget 2014, 5, 2881–2911. [Google Scholar] [CrossRef] [PubMed]
- Czarny, P.; Pawlowska, E.; Bialkowska-Warzecha, J.; Kaarniranta, K.; Blasiak, J. Autophagy in DNA damage response. Int. J. Mol. Sci. 2015, 16, 2641–2662. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.J.; Lee, Y.H.; Yeh, Y.L.; Wu, W.S.; Ho, C.T.; Li, C.Y.; Wang, B.J.; Wang, Y.J. Autophagy-inducing effect of pterostilbene: A prospective therapeutic/preventive option for skin diseases. J. Food Drug. Anal. 2017, 25, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Varga, J.; De Oliveira, T.; Greten, F.R. The architect who never sleeps: Tumor-induced plasticity. FEBS Lett. 2014, 588, 2422–2427. [Google Scholar] [CrossRef] [PubMed]
- Batlle, E.; Clevers, H. Cancer stem cells revisited. Nat. Med. 2017, 23, 1124–1134. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Yang, Y.; Di, S.; Feng, X.; Liu, D.; Jiang, S.; Hu, W.; Qin, Z.; Li, Y.; Lv, J.; et al. Pterostilbene exerts anticancer activity on non-small-cell lung cancer via activating endoplasmic reticulum stress. Sci. Rep. 2017, 7, 8091. [Google Scholar] [CrossRef] [PubMed]
- Abnave, P.; Aboukhatwa, E.; Kosaka, N.; Thompson, J.; Hill, M.A.; Aboobaker, A.A. Epithelial-mesenchymal transition transcription factors control pluripotent adult stem cell migration in vivo in planarians. Development 2017, 144, 3440–3453. [Google Scholar] [CrossRef] [PubMed]
- Restifo, N.P. Not so Fas: Re-evaluating the mechanisms of immune privilege and tumor escape. Nat. Med. 2000, 6, 493–495. [Google Scholar] [CrossRef] [PubMed]
- Wajant, H.; Pfizenmaier, K.; Scheurich, P. Non-apoptotic Fas signaling. Cytokine Growth Factor Rev. 2003, 14, 53–66. [Google Scholar] [CrossRef]
- Zheng, H.X.; Cai, Y.D.; Wang, Y.D.; Cui, X.B.; Xie, T.T.; Li, W.J.; Peng, L.; Zhang, Y.; Wang, Z.Q.; Wang, J.; et al. Fas signaling promotes motility and metastasis through epithelial-mesenchymal transition in gastrointestinal cancer. Oncogene 2013, 32, 1183–1192. [Google Scholar] [CrossRef] [PubMed]
- Whiteside, T.L. The tumor microenvironment and its role in promoting tumor growth. Oncogene 2008, 27, 5904–5912. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-kappaB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2. [Google Scholar] [CrossRef] [PubMed]
- Chua, H.L.; Bhat-Nakshatri, P.; Clare, S.E.; Morimiya, A.; Badve, S.; Nakshatri, H. NF-kappaB represses E-cadherin expression and enhances epithelial to mesenchymal transition of mammary epithelial cells: Potential involvement of ZEB-1 and ZEB-2. Oncogene 2007, 26, 711–724. [Google Scholar] [CrossRef] [PubMed]
- Rinkenbaugh, A.L.; Baldwin, A.S. The NF-κB Pathway and Cancer Stem Cells. Cells 2016, 5, 16. [Google Scholar] [CrossRef] [PubMed]
- Doherty, M.R.; Smigiel, J.M.; Junk, D.J.; Jackson, M.W. Cancer Stem Cell Plasticity Drives Therapeutic Resistance. Cancers (Basel) 2016, 8, 8. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.H.; Hong, B.H.; Ho, C.T.; Yen, G.C. Targeting cancer stem cells in breast cancer: Potential anticancer properties of 6-shogaol and pterostilbene. J. Agric. Food Chem. 2015, 63, 2432–2441. [Google Scholar] [CrossRef] [PubMed]
- Bai, D.; Ueno, L.; Vogt, P.K. Akt-mediated regulation of NFkappaB and the essentialness of NFkappaB for the oncogenicity of PI3K and Akt. Int. J. Cancer 2009, 125, 2863–2870. [Google Scholar] [CrossRef] [PubMed]
- Fulda, S. Regulation of apoptosis pathways in cancer stem cells. Cancer Lett. 2013, 338, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Douma, S.; Van Laar, T.; Zevenhoven, J.; Meuwissen, R.; Van Garderen, E.; Peeper, D.S. Suppression of anoikis and induction of metastasis by the neurotrophic receptor TrkB. Nature 2004, 430, 1034–1039. [Google Scholar] [CrossRef] [PubMed]
- Stupack, D.G.; Puente, X.S.; Boutsaboualoy, S.; Storgard, C.M.; Cheresh, D.A. Apoptosis of adherent cells by recruitment of caspase-8 to unligated integrins. J. Cell Biol. 2001, 155, 459–470. [Google Scholar] [CrossRef] [PubMed]
- Wendel, H.G.; De Stanchina, E.; Fridman, J.S.; Malina, A.; Ray, S.; Kogan, S.; Cordon-Cardo, C.; Pelletier, J.; Lowe, S.W. Survival signalling by Akt and eIF4E in oncogenesis and cancer therapy. Nature 2004, 428, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Ting, D.T.; Stott, S.L.; Wittner, B.S.; Ozsolak, F.; Paul, S.; Ciciliano, J.C.; Smas, M.E.; Winokur, D.; Gilman, A.J.; et al. RNA sequencing of pancreatic circulating tumour cells implicates WNT signalling in metastasis. Nature 2012, 487, 510–513. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.C.; Abdel-Ghany, M.; Elble, R.C.; Pauli, B.U. Lung endothelial dipeptidyl peptidase IV promotes adhesion and metastasis of rat breast cancer cells via tumor cell surface-associated fibronectin. J. Biol. Chem. 1998, 273, 24207–24215. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.C.; Abdel-Ghany, M.; Pauli, B.U. A novel consensus motif in fibronectin mediates dipeptidyl peptidase IV adhesion and metastasis. J. Biol. Chem. 2003, 278, 24600–24607. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Cheng, H.C.; Isom, R.; Chen, C.S.; Levine, R.A.; Pauli, B.U. Protein kinase Cepsilon mediates polymeric fibronectin assembly on the surface of blood-borne rat breast cancer cells to promote pulmonary metastasis. J. Biol. Chem. 2008, 283, 7616–7627. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.J.; Lin, J.F.; Cheng, L.H.; Chang, W.T.; Kao, Y.H.; Chang, M.M.; Wang, B.J.; Cheng, H.C. Pterostilbene prevents AKT-ERK axis-mediated polymerization of surface fibronectin on suspended lung cancer cells independently of apoptosis and suppresses metastasis. J. Hematol. Oncol. 2017, 10, 72. [Google Scholar] [CrossRef] [PubMed]
- Gopal, S.; Veracini, L.; Grall, D.; Butori, C.; Schaub, S.; Audebert, S.; Camoin, L.; Baudelet, E.; Radwanska, A.; Beghelli-de la Forest Divonne, S.; et al. Fibronectin-guided migration of carcinoma collectives. Nat. Commun. 2017, 8, 14105. [Google Scholar] [CrossRef] [PubMed]
- Robinson, B.D.; Sica, G.L.; Liu, Y.F.; Rohan, T.E.; Gertler, F.B.; Condeelis, J.S.; Jones, J.G. Tumor microenvironment of metastasis in human breast carcinoma: A potential prognostic marker linked to hematogenous dissemination. Clin. Cancer Res. 2009, 15, 2433–2441. [Google Scholar] [CrossRef] [PubMed]
- Moy, P.M.; Holmes, E.C.; Golub, S.H. Depression of natural killer cytotoxic activity in lymphocytes infiltrating human pulmonary tumors. Cancer Res. 1985, 45, 57–60. [Google Scholar] [PubMed]
- Martin-Fontecha, A.; Thomsen, L.L.; Brett, S.; Gerard, C.; Lipp, M.; Lanzavecchia, A.; Sallusto, F. Induced recruitment of NK cells to lymph nodes provides IFN-gamma for T(H)1 priming. Nat. Immunol. 2004, 5, 1260–1265. [Google Scholar] [CrossRef] [PubMed]
- Spiegel, A.; Brooks, M.W.; Houshyar, S.; Reinhardt, F.; Ardolino, M.; Fessler, E.; Chen, M.B.; Krall, J.A.; DeCock, J.; Zervantonakis, I.K.; et al. Neutrophils Suppress Intraluminal NK Cell-Mediated Tumor Cell Clearance and Enhance Extravasation of Disseminated Carcinoma Cells. Cancer Discov. 2016, 6, 630–649. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.T.; Steelman, L.S.; Chappell, W.H.; McCubrey, J.A. Akt inactivates ERK causing decreased response to chemotherapeutic drugs in advanced CaP cells. Cell Cycle 2008, 7, 631–636. [Google Scholar] [PubMed]
- Sher, Y.P.; Shih, J.Y.; Yang, P.C.; Roffler, S.R.; Chu, Y.W.; Wu, C.W.; Yu, C.L.; Peck, K. Prognosis of non-small cell lung cancer patients by detecting circulating cancer cells in the peripheral blood with multiple marker genes. Clin. Cancer Res. 2005, 11, 173–179. [Google Scholar] [PubMed]
- Bae, Y.K.; Kim, A.; Kim, M.K.; Choi, J.E.; Kang, S.H.; Lee, S.J. Fibronectin expression in carcinoma cells correlates with tumor aggressiveness and poor clinical outcome in patients with invasive breast cancer. Hum. Pathol. 2013, 44, 2028–2037. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.P.; Huang, M.D.; Xia, R.; Liu, X.X.; Sun, M.; Yin, L.; Chen, W.M.; Han, L.; Zhang, E.B.; Kong, R.; et al. Decreased expression of the long non-coding RNA FENDRR is associated with poor prognosis in gastric cancer and FENDRR regulates gastric cancer cell metastasis by affecting fibronectin1 expression. J. Hematol. Oncol. 2014, 7, 63. [Google Scholar] [CrossRef] [PubMed]
- Schwarzenbach, H.; Nishida, N.; Calin, G.A.; Pantel, K. Clinical relevance of circulating cell-free microRNAs in cancer. Nat. Rev. Clin. Oncol. 2014, 11, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Boelens, M.C.; Wu, T.J.; Nabet, B.Y.; Xu, B.; Qiu, Y.; Yoon, T.; Azzam, D.J.; Twyman-Saint Victor, C.; Wiemann, B.Z.; Ishwaran, H.; et al. Exosome transfer from stromal to breast cancer cells regulates therapy resistance pathways. Cell 2014, 159, 499–513. [Google Scholar] [CrossRef] [PubMed]
- Tkach, M.; Thery, C. Communication by Extracellular Vesicles: Where We Are and Where We Need to Go. Cell 2016, 164, 1226–1232. [Google Scholar] [CrossRef] [PubMed]
- Hoshino, A.; Costa-Silva, B.; Shen, T.L.; Rodrigues, G.; Hashimoto, A.; Tesic Mark, M.; Molina, H.; Kohsaka, S.; Di Giannatale, A.; Ceder, S.; et al. Tumour exosome integrins determine organotropic metastasis. Nature 2015, 527, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-P.; He, L. Noncoding RNAs in Cancer Development. Annu. Rev. Cancer Biol. 2017, 1, 163–184. [Google Scholar] [CrossRef]
- Hayashita, Y.; Osada, H.; Tatematsu, Y.; Yamada, H.; Yanagisawa, K.; Tomida, S.; Yatabe, Y.; Kawahara, K.; Sekido, Y.; Takahashi, T. A polycistronic microRNA cluster, miR-17-92, is overexpressed in human lung cancers and enhances cell proliferation. Cancer Res. 2005, 65, 9628–9632. [Google Scholar] [CrossRef] [PubMed]
- Manier, S.; Liu, C.J.; Avet-Loiseau, H.; Park, J.; Shi, J.; Campigotto, F.; Salem, K.Z.; Huynh, D.; Glavey, S.V.; Rivotto, B.; et al. Prognostic role of circulating exosomal miRNAs in multiple myeloma. Blood 2017, 129, 2429–2436. [Google Scholar] [CrossRef] [PubMed]
- Shan, S.W.; Lee, D.Y.; Deng, Z.; Shatseva, T.; Jeyapalan, Z.; Du, W.W.; Zhang, Y.; Xuan, J.W.; Yee, S.P.; Siragam, V.; et al. MicroRNA MiR-17 retards tissue growth and represses fibronectin expression. Nat. Cell Biol. 2009, 11, 1031–1038. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; King, M.R. Adhesion receptors as therapeutic targets for circulating tumor cells. Front. Oncol. 2012, 2, 79. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Mu, E.; Wei, Y.; Riethdorf, S.; Yang, Q.; Yuan, M.; Yan, J.; Hua, Y.; Tiede, B.J.; Lu, X.; et al. VCAM-1 promotes osteolytic expansion of indolent bone micrometastasis of breast cancer by engaging alpha4beta1-positive osteoclast progenitors. Cancer Cell 2011, 20, 701–714. [Google Scholar] [CrossRef] [PubMed]
- Barnhart, B.C.; Legembre, P.; Pietras, E.; Bubici, C.; Franzoso, G.; Peter, M.E. CD95 ligand induces motility and invasiveness of apoptosis-resistant tumor cells. EMBO J. 2004, 23, 3175–3185. [Google Scholar] [CrossRef] [PubMed]
- Kleber, S.; Sancho-Martinez, I.; Wiestler, B.; Beisel, A.; Gieffers, C.; Hill, O.; Thiemann, M.; Mueller, W.; Sykora, J.; Kuhn, A.; et al. Yes and PI3K bind CD95 to signal invasion of glioblastoma. Cancer Cell 2008, 13, 235–248. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Tan, Q.; Zheng, Y.; Chen, K.; Qian, C.; Li, N.; Wang, Q.; Cao, X. Blockade of Fas signaling in breast cancer cells suppresses tumor growth and metastasis via disruption of Fas signaling-initiated cancer-related inflammation. J. Biol. Chem. 2014, 289, 11522–11535. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, T.; Qian, B.Z.; Pollard, J.W. Immune cell promotion of metastasis. Nat. Rev. Immunol. 2015, 15, 73–86. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Fong, M.Y.; Min, Y.; Somlo, G.; Liu, L.; Palomares, M.R.; Yu, Y.; Chow, A.; O’Connor, S.T.; Chin, A.R.; et al. Cancer-secreted miR-105 destroys vascular endothelial barriers to promote metastasis. Cancer Cell 2014, 25, 501–515. [Google Scholar] [CrossRef] [PubMed]
- Costa-Silva, B.; Aiello, N.M.; Ocean, A.J.; Singh, S.; Zhang, H.; Thakur, B.K.; Becker, A.; Hoshino, A.; Mark, M.T.; Molina, H.; et al. Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nat. Cell Biol. 2015, 17, 816–826. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Tao, D.; Fang, Y.; Deng, C.; Xu, Q.; Zhou, J. TNF-Alpha Promotes Invasion and Metastasis via NF-Kappa B Pathway in Oral Squamous Cell Carcinoma. Med. Sci. Monit. Basic Res. 2017, 23, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Ran, R.; Lu, A.; Zhang, L.; Tang, Y.; Zhu, H.; Xu, H.; Feng, Y.; Han, C.; Zhou, G.; Rigby, A.C.; et al. Hsp70 promotes TNF-mediated apoptosis by binding IKK gamma and impairing NF-kappa B survival signaling. Genes Dev. 2004, 18, 1466–1481. [Google Scholar] [CrossRef] [PubMed]
- Mehner, C.; Hockla, A.; Miller, E.; Ran, S.; Radisky, D.C.; Radisky, E.S. Tumor cell-produced matrix metalloproteinase 9 (MMP-9) drives malignant progression and metastasis of basal-like triple negative breast cancer. Oncotarget 2014, 5, 2736–2749. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, M.; Welch, D.R.; Wynn, D.M.; Tsuruo, T.; Nicolson, G.L. Serum and plasma M(r) 92,000 progelatinase levels correlate with spontaneous metastasis of rat 13762NF mammary adenocarcinoma. Cancer Res. 1993, 53, 5802–5807. [Google Scholar] [PubMed]
- Deryugina, E.I.; Quigley, J.P. Matrix metalloproteinases and tumor metastasis. Cancer Metastasis Rev. 2006, 25, 9–34. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, R.N.; Psaila, B.; Lyden, D. Bone marrow cells in the ‘pre-metastatic niche’: Within bone and beyond. Cancer Metastasis Rev. 2006, 25, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Patel, S.; Tcyganov, E.; Gabrilovich, D.I. The Nature of Myeloid-Derived Suppressor Cells in the Tumor Microenvironment. Trends Immunol. 2016, 37, 208–220. [Google Scholar] [CrossRef] [PubMed]
- Ursini-Siegel, J.; Siegel, P.M. The influence of the pre-metastatic niche on breast cancer metastasis. Cancer Lett. 2016, 380, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, H.; Kalyanaraman, R. Pharmacology of Pterocarpus marsupium Roxb. Med. Plant Res. 2015, 5. [Google Scholar] [CrossRef]
- Achari, B.; Dutta, P.K.; Roy, S.K.; Chakraborty, P.; Sengupta, J.; Bandyopadhyay, D.; Maity, J.K.; Khan, I.A.; Ding, Y.; Ferreira, D. Fluorescent pigment and phenol glucosides from the heartwood of Pterocarpus marsupium. J. Nat. Prod. 2012, 75, 655–660. [Google Scholar] [CrossRef] [PubMed]
- Pterostilbene. Monograph. Altern. Med. Rev. 2010, 15, 159–163.
- McCormack, D.; McFadden, D. A review of pterostilbene antioxidant activity and disease modification. Oxid. Med. Cell. Longev. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Rimando, A.M.; Cuendet, M.; Desmarchelier, C.; Mehta, R.G.; Pezzuto, J.M.; Duke, S.O. Cancer chemopreventive and antioxidant activities of pterostilbene, a naturally occurring analogue of resveratrol. J. Agric. Food Chem. 2002, 50, 3453–3457. [Google Scholar] [CrossRef] [PubMed]
- Amorati, R.; Lucarini, M.; Mugnaini, V.; Pedulli, G.F.; Roberti, M.; Pizzirani, D. Antioxidant activity of hydroxystilbene derivatives in homogeneous solution. J. Org. Chem. 2004, 69, 7101–7107. [Google Scholar] [CrossRef] [PubMed]
- Adly, A.A. Oxidative Stress and Disease: An Updated Review. Res. J. Immunol. 2010, 3, 129–145. [Google Scholar]
- Kobayashi, M.; Yamamoto, M. Nrf2-Keap1 regulation of cellular defense mechanisms against electrophiles and reactive oxygen species. Adv. Enzym. Regul. 2006, 46, 113–140. [Google Scholar] [CrossRef] [PubMed]
- Chiou, Y.S.; Tsai, M.L.; Nagabhushanam, K.; Wang, Y.J.; Wu, C.H.; Ho, C.T.; Pan, M.H. Pterostilbene is more potent than resveratrol in preventing azoxymethane (AOM)-induced colon tumorigenesis via activation of the NF-E2-related factor 2 (Nrf2)-mediated antioxidant signaling pathway. J. Agric. Food Chem. 2011, 59, 2725–2733. [Google Scholar] [CrossRef] [PubMed]
- Sireesh, D.; Ganesh, M.R.; Dhamodharan, U.; Sakthivadivel, M.; Sivasubramanian, S.; Gunasekaran, P.; Ramkumar, K.M. Role of pterostilbene in attenuating immune mediated devastation of pancreatic beta cells via Nrf2 signaling cascade. J. Nutr. Biochem. 2017, 44, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Dvorakova, M.; Landa, P. Anti-inflammatory activity of natural stilbenoids: A review. Pharmacol. Res. 2017, 124, 126–145. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Wang, S.; Zhang, X.; Li, Y.; Zhao, Q.; Liu, T. Pterostilbene protects against myocardial ischemia/reperfusion injury via suppressing oxidative/nitrative stress and inflammatory response. Int. Immunopharmacol. 2017, 43, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Lv, M.; Liu, K.; Fu, S.; Li, Z.; Yu, X. Pterostilbene attenuates the inflammatory reaction induced by ischemia/reperfusion in rat heart. Mol. Med. Rep. 2015, 11, 724–728. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.J.; Ding, Y.; Wu, J.; Ning, B.T. Pterostilbene as treatment for severe acute pancreatitis. Genet. Mol. Res. 2016, 15, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Cichocki, M.; Paluszczak, J.; Szaefer, H.; Piechowiak, A.; Rimando, A.M.; Baer-Dubowska, W. Pterostilbene is equally potent as resveratrol in inhibiting 12-O-tetradecanoylphorbol-13-acetate activated NFkappaB, AP-1, COX-2, and iNOS in mouse epidermis. Mol. Nutr. Food Res. 2008, 52 (Suppl. S1), S62–S70. [Google Scholar] [PubMed]
- Luzzi, K.J.; MacDonald, I.C.; Schmidt, E.E.; Kerkvliet, N.; Morris, V.L.; Chambers, A.F.; Groom, A.C. Multistep nature of metastatic inefficiency: Dormancy of solitary cells after successful extravasation and limited survival of early micrometastases. Am. J. Pathol. 1998, 153, 865–873. [Google Scholar] [CrossRef]
- Kosuru, R.; Rai, U.; Prakash, S.; Singh, A.; Singh, S. Promising therapeutic potential of pterostilbene and its mechanistic insight based on preclinical evidence. Eur. J. Pharmacol. 2016, 789, 229–243. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Yang, Y.; Fan, C.; Di, S.; Hu, W.; Jiang, S.; Li, T.; Ma, Z.; Chao, D.; Feng, X.; et al. Pterostilbene Inhibits the Growth of Human Esophageal Cancer Cells by Regulating Endoplasmic Reticulum Stress. Cell. Physiol. Biochem. 2016, 38, 1226–1244. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.J.; Ho, C.T.; Wang, Y.J. Pterostilbene induces autophagy and apoptosis in sensitive and chemoresistant human bladder cancer cells. Mol. Nutr. Food Res. 2010, 54, 1819–1832. [Google Scholar] [CrossRef] [PubMed]
- Stoner, G.D.; Wang, L.S.; Seguin, C.; Rocha, C.; Stoner, K.; Chiu, S.; Kinghorn, A.D. Multiple berry types prevent N-nitrosomethylbenzylamine-induced esophageal cancer in rats. Pharm. Res. 2010, 27, 1138–1145. [Google Scholar] [CrossRef] [PubMed]
- Priego, S.; Feddi, F.; Ferrer, P.; Mena, S.; Benlloch, M.; Ortega, A.; Carretero, J.; Obrador, E.; Asensi, M.; Estrela, J.M. Natural polyphenols facilitate elimination of HT-29 colorectal cancer xenografts by chemoradiotherapy: A Bcl-2-and superoxide dismutase 2-dependent mechanism. Mol. Cancer Ther. 2008, 7, 3330–3342. [Google Scholar] [CrossRef] [PubMed]
- McCormack, D.E.; Mannal, P.; McDonald, D.; Tighe, S.; Hanson, J.; McFadden, D. Genomic analysis of pterostilbene predicts its antiproliferative effects against pancreatic cancer in vitro and in vivo. J. Gastrointest. Surg. 2012, 16, 1136–1143. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, M.J.; Lin, C.W.; Yang, S.F.; Sheu, G.T.; Yu, Y.Y.; Chen, M.K.; Chiou, H.L. A Combination of Pterostilbene with Autophagy Inhibitors Exerts Efficient Apoptotic Characteristics in Both Chemosensitive and Chemoresistant Lung Cancer Cells. Toxicol. Sci. 2017, 156, 549. [Google Scholar] [CrossRef] [PubMed]
- Mena, S.; Rodriguez, M.L.; Ponsoda, X.; Estrela, J.M.; Jaattela, M.; Ortega, A.L. Pterostilbene-induced tumor cytotoxicity: A lysosomal membrane permeabilization-dependent mechanism. PLoS ONE 2012, 7, e44524. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, M.T.; Chen, H.P.; Lu, C.C.; Chiang, J.H.; Wu, T.S.; Kuo, D.H.; Huang, L.J.; Kuo, S.C.; Yang, J.S. The novel pterostilbene derivative ANK-199 induces autophagic cell death through regulating PI3 kinase class III/beclin 1/Atgrelated proteins in cisplatinresistant CAR human oral cancer cells. Int. J. Oncol. 2014, 45, 782–794. [Google Scholar] [CrossRef] [PubMed]
- Principe, D.R.; Diaz, A.M.; Torres, C.; Mangan, R.J.; DeCant, B.; McKinney, R.; Tsao, M.S.; Lowy, A.; Munshi, H.G.; Jung, B.; et al. TGFbeta engages MEK/ERK to differentially regulate benign and malignant pancreas cell function. Oncogene 2017, 36, 4336–4348. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.L.; Shen, Y.; Xu, J.P.; Han, K.; Zhou, Y.; Yang, S.; Yin, J.Y.; Min, D.L.; Hu, H.Y. Pterostilbene suppresses human endometrial cancer cells in vitro by down-regulating miR-663b. Acta. Pharmacol. Sin. 2017, 38, 1394–1400. [Google Scholar] [CrossRef] [PubMed]
- Nikhil, K.; Sharan, S.; Chakraborty, A.; Roy, P. Pterostilbene-isothiocyanate conjugate suppresses growth of prostate cancer cells irrespective of androgen receptor status. PLoS ONE 2014, 9, e93335. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.; Hu, Y.; Li, J.; Wang, Z.; Huang, J.; Zhang, S.; Ding, L. Estrogen receptor-alpha36 is involved in pterostilbene-induced apoptosis and anti-proliferation in in vitro and in vivo breast cancer. PLoS ONE 2014, 9, e104459. [Google Scholar]
- Chen, R.J.; Tsai, S.J.; Ho, C.T.; Pan, M.H.; Ho, Y.S.; Wu, C.H.; Wang, Y.J. Chemopreventive effects of pterostilbene on urethane-induced lung carcinogenesis in mice via the inhibition of EGFR-mediated pathways and the induction of apoptosis and autophagy. J. Agric. Food Chem. 2012, 60, 11533–11541. [Google Scholar] [CrossRef] [PubMed]
- Kala, R.; Shah, H.N.; Martin, S.L.; Tollefsbol, T.O. Epigenetic-based combinatorial resveratrol and pterostilbene alters DNA damage response by affecting SIRT1 and DNMT enzyme expression, including SIRT1-dependent gamma-H2AX and telomerase regulation in triple-negative breast cancer. BMC Cancer 2015, 15, 672. [Google Scholar] [CrossRef] [PubMed]
- Lubecka, K.; Kurzava, L.; Flower, K.; Buvala, H.; Zhang, H.; Teegarden, D.; Camarillo, I.; Suderman, M.; Kuang, S.; Andrisani, O.; et al. Stilbenoids remodel the DNA methylation patterns in breast cancer cells and inhibit oncogenic NOTCH signaling through epigenetic regulation of MAML2 transcriptional activity. Carcinogenesis 2016, 37, 656–668. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Dias, S.J.; Rimando, A.M.; Dhar, S.; Mizuno, C.S.; Penman, A.D.; Lewin, J.R.; Levenson, A.S. Pterostilbene acts through metastasis-associated protein 1 to inhibit tumor growth, progression and metastasis in prostate cancer. PLoS ONE 2013, 8, e57542. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhou, G.; Song, W.; Tan, X.; Guo, Y.; Zhou, B.; Jing, H.; Zhao, S.; Chen, L. Pterostilbene protects vascular endothelial cells against oxidized low-density lipoprotein-induced apoptosis in vitro and in vivo. Apoptosis 2012, 17, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Amarnath Satheesh, M.; Pari, L. The antioxidant role of pterostilbene in streptozotocin-nicotinamide-induced type 2 diabetes mellitus in Wistar rats. J. Pharm. Pharmacol. 2006, 58, 1483–1490. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Rimando, A.; Pallas, M.; Camins, A.; Porquet, D.; Reeves, J.; Shukitt-Hale, B.; Smith, M.A.; Joseph, J.A.; Casadesus, G. Low-dose pterostilbene, but not resveratrol, is a potent neuromodulator in aging and Alzheimer’s disease. Neurobiol. Aging 2012, 33, 2062–2071. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.J.; Wu, P.H.; Ho, C.T.; Way, T.D.; Pan, M.H.; Chen, H.M.; Ho, Y.S.; Wang, Y.J. P53-dependent downregulation of hTERT protein expression and telomerase activity induces senescence in lung cancer cells as a result of pterostilbene treatment. Cell Death Dis. 2017, 8, e2985. [Google Scholar] [CrossRef] [PubMed]
- Joyce, J.A.; Pollard, J.W. Microenvironmental regulation of metastasis. Nat. Rev. Cancer 2009, 9, 239–252. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, P.; Wang, X.F. Microenvironmental regulation of cancer metastasis by miRNAs. Trends Cell Biol. 2014, 24, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Yang, A.; Schulte, B.A.; Wargovich, M.J.; Wang, G.Y. Resveratrol induces premature senescence in lung cancer cells via ROS-mediated DNA damage. PLoS ONE 2013, 8, e60065. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.C.; Hsu, K.Y.; Hung, C.M.; Lin, Y.C.; Yang, N.S.; Ho, C.T.; Kuo, S.C.; Way, T.D. The anti-tumor efficiency of pterostilbene is promoted with a combined treatment of Fas signaling or autophagy inhibitors in triple negative breast cancer cells. Food Funct. 2014, 5, 1856–1865. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Cui, H.; Huang, X.; Zhu, B.; Guan, S.; Cheng, W.; Lai, Y.; Zhang, X.; Hua, Z.C. MiR-7a is an important mediator in Fas-associated protein with death domain (FADD)-regulated expression of focal adhesion kinase (FAK). Oncotarget 2016, 7, 51393–51407. [Google Scholar] [CrossRef] [PubMed]
- Su, C.M.; Lee, W.H.; Wu, A.T.; Lin, Y.K.; Wang, L.S.; Wu, C.H.; Yeh, C.T. Pterostilbene inhibits triple-negative breast cancer metastasis via inducing microRNA-205 expression and negatively modulates epithelial-to-mesenchymal transition. J. Nutr. Biochem. 2015, 26, 675–685. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.H.; Chiou, Y.S.; Chen, W.J.; Wang, J.M.; Badmaev, V.; Ho, C.T. Pterostilbene inhibited tumor invasion via suppressing multiple signal transduction pathways in human hepatocellular carcinoma cells. Carcinogenesis 2009, 30, 1234–1242. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Rimando, A.M.; Lee, H.J.; Ji, Y.; Reddy, B.S.; Suh, N. Anti-inflammatory action of pterostilbene is mediated through the p38 mitogen-activated protein kinase pathway in colon cancer cells. Cancer Prev. Res. 2009, 2, 650–657. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.L.; Lin, Y.J.; Ho, C.T.; Yen, G.C. The inhibitory effect of pterostilbene on inflammatory responses during the interaction of 3T3-L1 adipocytes and RAW 264.7 macrophages. J. Agric. Food Chem. 2013, 61, 602–610. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.H.; Lin, Y.T.; Lin, C.L.; Wei, C.S.; Ho, C.T.; Chen, W.J. Suppression of Heregulin-beta1/HER2-Modulated Invasive and Aggressive Phenotype of Breast Carcinoma by Pterostilbene via Inhibition of Matrix Metalloproteinase-9, p38 Kinase Cascade and Akt Activation. Evid. Based Complement. Altern. Med. 2011, 2011, 562187. [Google Scholar] [CrossRef] [PubMed]
- Ko, H.S.; Kim, J.S.; Cho, S.M.; Lee, H.J.; Ahn, K.S.; Kim, S.H.; Lee, E.O. Urokinase-type plasminogen activator expression and Rac1/WAVE-2/Arp2/3 pathway are blocked by pterostilbene to suppress cell migration and invasion in MDA-MB-231 cells. Bioorg. Med. Chem. Lett. 2014, 24, 1176–1179. [Google Scholar] [CrossRef] [PubMed]
- Mak, K.K.; Wu, A.T.; Lee, W.H.; Chang, T.C.; Chiou, J.F.; Wang, L.S.; Wu, C.H.; Huang, C.Y.; Shieh, Y.S.; Chao, T.Y.; et al. Pterostilbene, a bioactive component of blueberries, suppresses the generation of breast cancer stem cells within tumor microenvironment and metastasis via modulating NF-kappaB/microRNA 448 circuit. Mol. Nutr. Food Res. 2013, 57, 1123–1134. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; DeCastro, A.J.; Lee, H.J.; Smolarek, A.K.; So, J.Y.; Simi, B.; Wang, C.X.; Zhou, R.; Rimando, A.M.; Suh, N. Dietary intake of pterostilbene, a constituent of blueberries, inhibits the beta-catenin/p65 downstream signaling pathway and colon carcinogenesis in rats. Carcinogenesis 2010, 31, 1272–1278. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.C.; Chan, M.L.; Chen, M.J.; Tsai, T.H.; Chen, Y.J. Modulation of macrophage polarization and lung cancer cell stemness by MUC1 and development of a related small-molecule inhibitor pterostilbene. Oncotarget 2016, 7, 39363–39375. [Google Scholar] [CrossRef] [PubMed]
- Huynh, T.T.; Lin, C.M.; Lee, W.H.; Wu, A.T.; Lin, Y.K.; Lin, Y.F.; Yeh, C.T.; Wang, L.S. Pterostilbene suppressed irradiation-resistant glioma stem cells by modulating GRP78/miR-205 axis. J. Nutr. Biochem. 2015, 26, 466–475. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.M.; Su, Y.H.; Huynh, T.T.; Lee, W.H.; Chiou, J.F.; Lin, Y.K.; Hsiao, M.; Wu, C.H.; Lin, Y.F.; Wu, A.T.; et al. BlueBerry Isolate, Pterostilbene, Functions as a Potential Anticancer Stem Cell Agent in Suppressing Irradiation-Mediated Enrichment of Hepatoma Stem Cells. Evid. Based Complement. Altern. Med. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Li Chew, C.; Lunardi, A.; Gulluni, F.; Ruan, D.T.; Chen, M.; Salmena, L.; Nishino, M.; Papa, A.; Ng, C.; Fung, J.; et al. In vivo Role of INPP4B in Tumor and Metastasis Suppression through Regulation of PI3K-AKT Signaling at Endosomes. Cancer Discov. 2015, 5, 740–751. [Google Scholar] [CrossRef] [PubMed]
- Dhar, S.; Kumar, A.; Rimando, A.M.; Zhang, X.; Levenson, A.S. Resveratrol and pterostilbene epigenetically restore PTEN expression by targeting oncomiRs of the miR-17 family in prostate cancer. Oncotarget 2015, 6, 27214–27226. [Google Scholar] [CrossRef] [PubMed]
- Derynck, R.; Akhurst, R.J. Differentiation plasticity regulated by TGF-beta family proteins in development and disease. Nat. Cell Biol. 2007, 9, 1000–1004. [Google Scholar] [CrossRef] [PubMed]
- Elhefny, R.A.; Shaban, M.M.; Shaker, O.G. Prognostic value of pro-inflammatory cytokine and pro-angiogenesis factor in differentiating malignant from benign exudative effusion. Clin. Respir. J. 2017, 11, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.Y.; Tan, M.S.; Yu, J.T.; Tan, L. Role of pro-inflammatory cytokines released from microglia in Alzheimer’s disease. Ann. Transl. Med. 2015, 3, 136. [Google Scholar] [PubMed]
- Yang, W.L.; Roland, I.H.; Godwin, A.K.; Xu, X.X. Loss of TNF-alpha-regulated COX-2 expression in ovarian cancer cells. Oncogene 2005, 24, 7991–8002. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C. The role of inflammatory cytokines in endothelial dysfunction. Basic Res. Cardiol. 2008, 103, 398–406. [Google Scholar] [CrossRef] [PubMed]
- Huan, W.; Ling, Z.; Lingpeng, W.; Qina, Z.; Hongli, W.; Yuemei, H.; Yitong, M. Pterostilbene suppresses vascular adhesion molecule expression in TNF-α-stimulated vascular muscle cells. Int. J. Clin. Exp. Pathol. 2016, 9, 1432–1438. [Google Scholar]
- Liu, J.; Fan, C.; Yu, L.; Yang, Y.; Jiang, S.; Ma, Z.; Hu, W.; Li, T.; Yang, Z.; Tian, T.; et al. Pterostilbene exerts an anti-inflammatory effect via regulating endoplasmic reticulum stress in endothelial cells. Cytokine 2016, 77, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.T.; Murray, G.I. Current mechanistic insights into the roles of matrix metalloproteinases in tumour invasion and metastasis. J. Pathol. 2015, 237, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.H.; Lee, S.H.; Liao, I.C.; Huang, S.H.; Cheng, H.C.; Liao, P.C. Secretomic analysis identifies alpha-1 antitrypsin (A1AT) as a required protein in cancer cell migration, invasion, and pericellular fibronectin assembly for facilitating lung colonization of lung adenocarcinoma cells. Mol. Cell. Proteomics 2012, 11, 1320–1339. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Willmarth, N.E.; Zhou, J.; Katiyar, S.; Wang, M.; Liu, Y.; McCue, P.A.; Quong, A.A.; Lisanti, M.P.; Pestell, R.G. microRNA 17/20 inhibits cellular invasion and tumor metastasis in breast cancer by heterotypic signaling. Proc. Natl. Acad. Sci. USA 2010, 107, 8231–8236. [Google Scholar] [CrossRef] [PubMed]
- Morishita, Y.; Ookawara, S.; Hirahara, I.; Muto, S.; Nagata, D. HIF-1alpha mediates Hypoxia-induced epithelial-mesenchymal transition in peritoneal mesothelial cells. Ren. Fail. 2016, 38, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Yu, S.; Li, J.; Yin, Y.; Liu, Y.; Zhang, Q.; Guan, H.; Li, Y.; Xiao, H. MiR-20b Displays Tumor-Suppressor Functions in Papillary Thyroid Carcinoma by Regulating the MAPK/ERK Signaling Pathway. Thyroid 2016, 26, 1733–1743. [Google Scholar] [CrossRef] [PubMed]
- Shan, S.W.; Fang, L.; Shatseva, T.; Rutnam, Z.J.; Yang, X.; Du, W.; Lu, W.Y.; Xuan, J.W.; Deng, Z.; Yang, B.B. Mature miR-17-5p and passenger miR-17-3p induce hepatocellular carcinoma by targeting PTEN, GalNT7 and vimentin in different signal pathways. J. Cell Sci. 2013, 126, 1517–1530. [Google Scholar] [CrossRef] [PubMed]
- Rao, E.; Jiang, C.; Ji, M.; Huang, X.; Iqbal, J.; Lenz, G.; Wright, G.; Staudt, L.M.; Zhao, Y.; McKeithan, T.W.; et al. The miRNA-17 approximately 92 cluster mediates chemoresistance and enhances tumor growth in mantle cell lymphoma via PI3K/AKT pathway activation. Leukemia 2012, 26, 1064–1072. [Google Scholar] [CrossRef] [PubMed]
- Maehama, T.; Dixon, J.E. The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate. J. Biol. Chem. 1998, 273, 13375–13378. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Mitra, D.; Sullivan, R.J.; Wittner, B.S.; Kimura, A.M.; Pan, S.; Hoang, M.P.; Brannigan, B.W.; Lawrence, D.P.; Flaherty, K.T.; et al. Isolation and molecular characterization of circulating melanoma cells. Cell Rep. 2014, 7, 645–653. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.X.; Bai, F. Single-cell analyses of circulating tumor cells. Cancer Biol. Med. 2015, 12, 184–192. [Google Scholar] [PubMed]
- Xing, F.; Liu, Y.; Sharma, S.; Wu, K.; Chan, M.D.; Lo, H.W.; Carpenter, R.L.; Metheny-Barlow, L.J.; Zhou, X.; Qasem, S.A.; et al. Activation of the c-Met Pathway Mobilizes an Inflammatory Network in the Brain Microenvironment to Promote Brain Metastasis of Breast Cancer. Cancer Res. 2016, 76, 4970–4980. [Google Scholar] [CrossRef] [PubMed]
- Dhar, S.; Kumar, A.; Zhang, L.; Rimando, A.M.; Lage, J.M.; Lewin, J.R.; Atfi, A.; Zhang, X.; Levenson, A.S. Dietary pterostilbene is a novel MTA1-targeted chemopreventive and therapeutic agent in prostate cancer. Oncotarget 2016, 7, 18469–18484. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Dhar, S.; Rimando, A.M.; Lage, J.M.; Lewin, J.R.; Zhang, X.; Levenson, A.S. Epigenetic potential of resveratrol and analogs in preclinical models of prostate cancer. Ann. N. Y. Acad. Sci. 2015, 1348, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Butt, N.A.; Kumar, A.; Dhar, S.; Rimando, A.M.; Akhtar, I.; Hancock, J.C.; Lage, J.M.; Pound, C.R.; Lewin, J.R.; Gomez, C.R.; et al. Targeting MTA1/HIF-1alpha signaling by pterostilbene in combination with histone deacetylase inhibitor attenuates prostate cancer progression. Cancer Med. 2017, 6, 2673–2685. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, P.; Asensi, M.; Segarra, R.; Ortega, A.; Benlloch, M.; Obrador, E.; Varea, M.T.; Asensio, G.; Jorda, L.; Estrela, J.M. Association between pterostilbene and quercetin inhibits metastatic activity of B16 melanoma. Neoplasia 2005, 7, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Xie, B.; Xu, Z.; Hu, L.; Chen, G.; Wei, R.; Yang, G.; Li, B.; Chang, G.; Sun, X.; Wu, H.; et al. Pterostilbene Inhibits Human Multiple Myeloma Cells via ERK1/2 and JNK Pathway in vitro and in vivo. Int. J. Mol. Sci. 2016, 17, 1927. [Google Scholar] [CrossRef] [PubMed]
- Chiou, Y.S.; Tsai, M.L.; Wang, Y.J.; Cheng, A.C.; Lai, W.M.; Badmaev, V.; Ho, C.T.; Pan, M.H. Pterostilbene inhibits colorectal aberrant crypt foci (ACF) and colon carcinogenesis via suppression of multiple signal transduction pathways in azoxymethane-treated mice. J. Agric. Food Chem. 2010, 58, 8833–8841. [Google Scholar] [CrossRef] [PubMed]
- Suh, N.; Paul, S.; Hao, X.; Simi, B.; Xiao, H.; Rimando, A.M.; Reddy, B.S. Pterostilbene, an active constituent of blueberries, suppresses aberrant crypt foci formation in the azoxymethane-induced colon carcinogenesis model in rats. Clin. Cancer Res. 2007, 13, 350–355. [Google Scholar] [CrossRef] [PubMed]
- Alosi, J.A.; McDonald, D.E.; Schneider, J.S.; Privette, A.R.; McFadden, D.W. Pterostilbene inhibits breast cancer in vitro through mitochondrial depolarization and induction of caspase-dependent apoptosis. J. Surg. Res. 2010, 161, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Mannal, P.; McDonald, D.; McFadden, D. Pterostilbene and tamoxifen show an additive effect against breast cancer in vitro. Am. J. Surg. 2010, 200, 577–580. [Google Scholar] [CrossRef] [PubMed]
- Kai, L.; Wang, J.; Ivanovic, M.; Chung, Y.T.; Laskin, W.B.; Schulze-Hoepfner, F.; Mirochnik, Y.; Satcher, R.L., Jr.; Levenson, A.S. Targeting prostate cancer angiogenesis through metastasis-associated protein 1 (MTA1). Prostate 2011, 71, 268–280. [Google Scholar] [CrossRef] [PubMed]
- Bao, Q.Y.; Zhang, N.; Geng, D.D.; Xue, J.W.; Merritt, M.; Zhang, C.; Ding, Y. The enhanced longevity and liver targetability of Paclitaxel by hybrid liposomes encapsulating Paclitaxel-conjugated gold nanoparticles. Int. J. Pharm. 2014, 477, 408–415. [Google Scholar] [CrossRef] [PubMed]
- Gentile, P.; Chiono, V.; Carmagnola, I.; Hatton, P.V. An overview of poly(lactic-co-glycolic) acid (PLGA)-based biomaterials for bone tissue engineering. Int. J. Mol. Sci. 2014, 15, 3640–3659. [Google Scholar] [CrossRef] [PubMed]
- Makadia, H.K.; Siegel, S.J. Poly Lactic-co-Glycolic Acid (PLGA) as Biodegradable Controlled Drug Delivery Carrier. Polymers (Basel) 2011, 3, 1377–1397. [Google Scholar] [CrossRef] [PubMed]
- Mura, S.; Nicolas, J.; Couvreur, P. Stimuli-responsive nanocarriers for drug delivery. Nat. Mater. 2013, 12, 991–1003. [Google Scholar] [CrossRef] [PubMed]
- Probst, C.E.; Zrazhevskiy, P.; Bagalkot, V.; Gao, X. Quantum dots as a platform for nanoparticle drug delivery vehicle design. Adv. Drug. Deliv. Rev. 2013, 65, 703–718. [Google Scholar] [CrossRef] [PubMed]
- Yong, K.T.; Wang, Y.; Roy, I.; Rui, H.; Swihart, M.T.; Law, W.C.; Kwak, S.K.; Ye, L.; Liu, J.; Mahajan, S.D.; et al. Preparation of quantum dot/drug nanoparticle formulations for traceable targeted delivery and therapy. Theranostics 2012, 2, 681–694. [Google Scholar] [CrossRef] [PubMed]
- Viraka Nellore, B.P.; Kanchanapally, R.; Pramanik, A.; Sinha, S.S.; Chavva, S.R.; Hamme, A., 2nd; Ray, P.C. Aptamer-conjugated graphene oxide membranes for highly efficient capture and accurate identification of multiple types of circulating tumor cells. Bioconjug. Chem. 2015, 26, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Ha, D.; Yang, N.; Nadithe, V. Exosomes as therapeutic drug carriers and delivery vehicles across biological membranes: Current perspectives and future challenges. Acta Pharm. Sin. B 2016, 6, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.P.; Hielscher, A. Fibronectin: How Its Aberrant Expression in Tumors May Improve Therapeutic Targeting. J. Cancer 2017, 8, 674–682. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Lu, Z.-R. Targeting fibronectin for cancer imaging and therapy. J. Mater. Chem. B 2017, 5, 639–654. [Google Scholar] [CrossRef] [PubMed]
- Pulakazhi Venu, V.K.; Uboldi, P.; Dhyani, A.; Patrini, A.; Baetta, R.; Ferri, N.; Corsini, A.; Muro, A.F.; Catapano, A.L.; Norata, G.D. Fibronectin extra domain A stabilises atherosclerotic plaques in apolipoprotein E and in LDL-receptor-deficient mice. Thromb. Haemost. 2015, 114, 186–197. [Google Scholar] [CrossRef] [PubMed]
- Kumra, H.; Reinhardt, D.P. Fibronectin-targeted drug delivery in cancer. Adv. Drug. Deliv. Rev. 2016, 97, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, L.; Velcheti, V. Checkpoint immunotherapy: Good for cancer therapy, bad for rheumatic diseases. Ann. Rheum. Dis. 2017, 76. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.N.; Trinite, B.; Levy, D.N. Potent Inhibition of HIV-1 Replication in Resting CD4 T Cells by Resveratrol and Pterostilbene. Antimicrob. Agents Chemother. 2017, 61. [Google Scholar] [CrossRef] [PubMed]
- George, S.; Miao, D.; Demetri, G.D.; Adeegbe, D.; Rodig, S.J.; Shukla, S.; Lipschitz, M.; Amin-Mansour, A.; Raut, C.P.; Carter, S.L.; et al. Loss of PTEN Is Associated with Resistance to Anti-PD-1 Checkpoint Blockade Therapy in Metastatic Uterine Leiomyosarcoma. Immunity 2017, 46, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Nikhil, K.; Sharan, S.; Wishard, R.; Palla, S.R.; Krishna Peddinti, R.; Roy, P. Pterostilbene carboxaldehyde thiosemicarbazone, a resveratrol derivative inhibits 17beta-Estradiol induced cell migration and proliferation in HUVECs. Steroids 2016, 108, 17–30. [Google Scholar] [CrossRef] [PubMed]
- Riche, D.M.; McEwen, C.L.; Riche, K.D.; Sherman, J.J.; Wofford, M.R.; Deschamp, D.; Griswold, M. Analysis of safety from a human clinical trial with pterostilbene. J. Toxicol. 2013. [Google Scholar] [CrossRef] [PubMed]
- Riche, D.M.; Riche, K.D.; Blackshear, C.T.; McEwen, C.L.; Sherman, J.J.; Wofford, M.R.; Griswold, M.E. Pterostilbene on metabolic parameters: A randomized, double-blind, and placebo-controlled trial. Evid. Based Complement. Altern. Med. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]

| Gene/Phenomena | Effect/Regulation | Pathway | Tumor Types/Model * | Reference |
|---|---|---|---|---|
| Enhancement of endoplasmic reticulum stress (ERS) signaling | ||||
| IRE-1, p53, ATF4, p-PERK, CHOP9 | up | ERS | NSCLC | [29] |
| Bcl-2 | down | ERS | HEC | [100] |
| PUMA, caspases 9, caspases12 | up | ERS | HEC | [100] |
| cell proliferation | down | ERS | bladder cancer | [101] |
| ROS generation | up | ERS-ROS | breast cancer prostate cancer | [91,102,103,104] |
| antioxidant activity | up | ERS-ROS | pancreatic cancere sophageal cancer colon cancer | [91,102,103,104] |
| Induction of autophagy | ||||
| chemotherapeutic effect | up | autophagy | lung cancer | [105] |
| enhance apoptosis | up | apoptosis | bladder cancer | [101] |
| cancer cell growth | down | apoptosis | A375, A549, MCF7, HT29 | [106] |
| autophagic cell death | up | autophagy | human oral cancer | [107] |
| Regulation of miRNA profiles | ||||
| miR-17, miR-20a, miR-106a, miR-106b | down | apoptosis | prostate cancer | [108] |
| PTEN | up | apoptosis | prostate cancer | [108] |
| miR663b | down | apoptosis/Bcl-2 | EC | [109] |
| Inhibiting the function of growth receptors | ||||
| AKT, ERK, AR | down | AKT/ERK | prostate cancer | [110] |
| ER-α36 | down | MAPK/ERK, PI3K/AKT | breast cancer | [111] |
| EGFR | down | apoptosis, autophagy | murine lung tumors | [112] |
| Affecting epigenetic pathway | ||||
| SIRT1 | down | DNA damage response, apoptosis | breast cancer | [113] |
| MAML2 | down | DNA damage response, apoptosis | breast cancer | [114] |
| MTA1 | down | p53 acetylation | prostate cancer | [115] |
| Gene/Phenomena | Effect/Regulation | Pathway | Tumor Types/Model * | Reference |
|---|---|---|---|---|
| Effects in senescence induction | ||||
| hTERT | down | senescence | lung cancer | [119] |
| ATM/Chk2 | up | senescence | lung cancer | [119] |
| p53 | up | senescence | lung cancer | [119] |
| Effects against EMT | ||||
| Fas-induced EMT | down | ERK1/2, GSK3β/β-catenin | TNBC | [123] |
| Fas-associated death domain | down | FAK signaling | TNBC | [108,124] |
| TPA | down | PKC-dependent signaling | HCC | [32,125,126] |
| Effects against inflammation | ||||
| IL-1β, TNFα, iNOS, and COX-2 | down | inflammatory microenvironments | HT-29 | [127] |
| COX-2, iNOS, IL-6, and IL-1β | down | inflammatory | 3T3-L1 | [128] |
| Effects against cell migration and invasion | ||||
| cell migration | down | cell migration ability | human lung adenocarcinoma | [29] |
| p38 kinase | down | MMP-9 activity | MCF-7 | [129] |
| MMP-9 | down | migratory and invasive | MDA-MB-231, Hs578t | [108] |
| cell migration and invasion | down | Rac1/WAVE/Arp2/3 pathway | MDA-MB-231 | [130] |
| TPA-induced PI3K/Akt and protein kinase C | down | EMT- and MMP-9-mediated invasion, migration and metastasis | human hepatoma cell | [126] |
| IAP | down | NF-κB activation | malignant pancreas cell | [71,108] |
| Effects against cancer stemness | ||||
| CD133 | down | Wnt/β-catenin | lung cancer, glioblastoma, HCC, breast cancer | [39,131,132], [133], [134], [135] |
| M2 macrophage | down | EMT-associated NF-κB/miR488 | breast cancer | [135] |
| MUC1, NF-κB, β-catenin | down | macrophage polarization and lung cancer cell stemness | lung cancer | [133] |
| Effects against polyFN assembly on CTCs | ||||
| ERK | down | PI3K/AKT signaling | LLC | [6,49,136] |
| Effects against the diminished circulating miR-17-92 cluster | ||||
| miR-17-92 | up | PTEN | prostate cancer | [137] |
| Administration | Doses | Animal Models | Reference |
|---|---|---|---|
| Lung cancer | |||
| oral | 5 mg/kg | C57BL6 mice | [49] |
| oral | 50 or 250 mg/kg | A/J mice | [112] |
| intraperitoneally | 50 mg/kg | nude mice | [29] |
| Breast cancer | |||
| oral | 56 mg/kg | nude mice | [111] |
| intraperitoneally | 30 mg/kg | nude mice | [155] |
| intraperitoneally | 5 mg/kg | NOD/SCID mice | [131] |
| Prostate cancer | |||
| oral | 100 mg/kg diet | PTEN-heterozygous mice | [156,157] |
| intraperitoneally | 50 mg/kg | orthotopic PCa xenograft | [115] |
| oral | 10 mg/kg | PTEN-null mice | [158] |
| Hepatocellular melanoma and myeloma cancer | |||
| Intravenous | 20 mg/kg | [159] | |
| intraperitoneal | 50 mg/kg | NOD/SCID mice | [160] |
| Colon cancer and pancreatic cancer | |||
| oral | 40 ppm | F344 rats | [132] |
| oral | 50 ppm | BALB/c mice | [91,161] |
| oral | 40 ppm | F344 rats | [162] |
| oral | 100 μg/kg/day or 500 μg/kg/day | [104] | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, R.-J.; Kuo, H.-C.; Cheng, L.-H.; Lee, Y.-H.; Chang, W.-T.; Wang, B.-J.; Wang, Y.-J.; Cheng, H.-C. Apoptotic and Nonapoptotic Activities of Pterostilbene against Cancer. Int. J. Mol. Sci. 2018, 19, 287. https://doi.org/10.3390/ijms19010287
Chen R-J, Kuo H-C, Cheng L-H, Lee Y-H, Chang W-T, Wang B-J, Wang Y-J, Cheng H-C. Apoptotic and Nonapoptotic Activities of Pterostilbene against Cancer. International Journal of Molecular Sciences. 2018; 19(1):287. https://doi.org/10.3390/ijms19010287
Chicago/Turabian StyleChen, Rong-Jane, Hsiao-Che Kuo, Li-Hsin Cheng, Yu-Hsuan Lee, Wen-Tsan Chang, Bour-Jr Wang, Ying-Jan Wang, and Hung-Chi Cheng. 2018. "Apoptotic and Nonapoptotic Activities of Pterostilbene against Cancer" International Journal of Molecular Sciences 19, no. 1: 287. https://doi.org/10.3390/ijms19010287
APA StyleChen, R.-J., Kuo, H.-C., Cheng, L.-H., Lee, Y.-H., Chang, W.-T., Wang, B.-J., Wang, Y.-J., & Cheng, H.-C. (2018). Apoptotic and Nonapoptotic Activities of Pterostilbene against Cancer. International Journal of Molecular Sciences, 19(1), 287. https://doi.org/10.3390/ijms19010287





