Phellinus linteus Grown on Germinated Brown Rice Increases Cetuximab Sensitivity of KRAS-Mutated Colon Cancer
Abstract
:1. Introduction
2. Results
2.1. Phellinus Linteus on Germinated Brown Rice (PBR) Inhibits Kirsten Rat Sarcoma Viral Oncogene Homolog (KRAS)-Mutated Colon Cancer Cell Proliferation
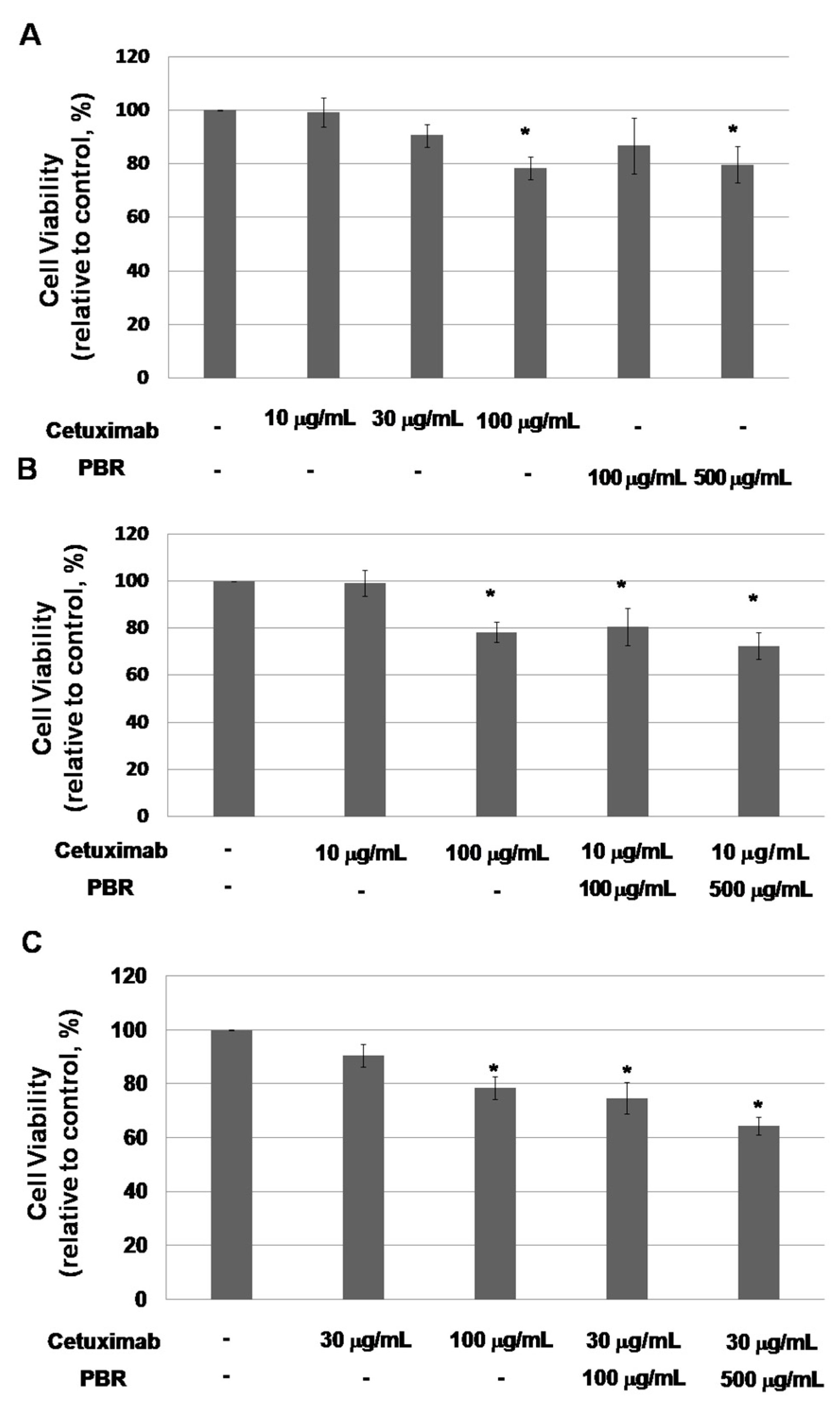
2.1.1. PBR Extract Increases the Sensitivity of a KRAS-Mutated Colon Cancer Cell Line to Cetuximab
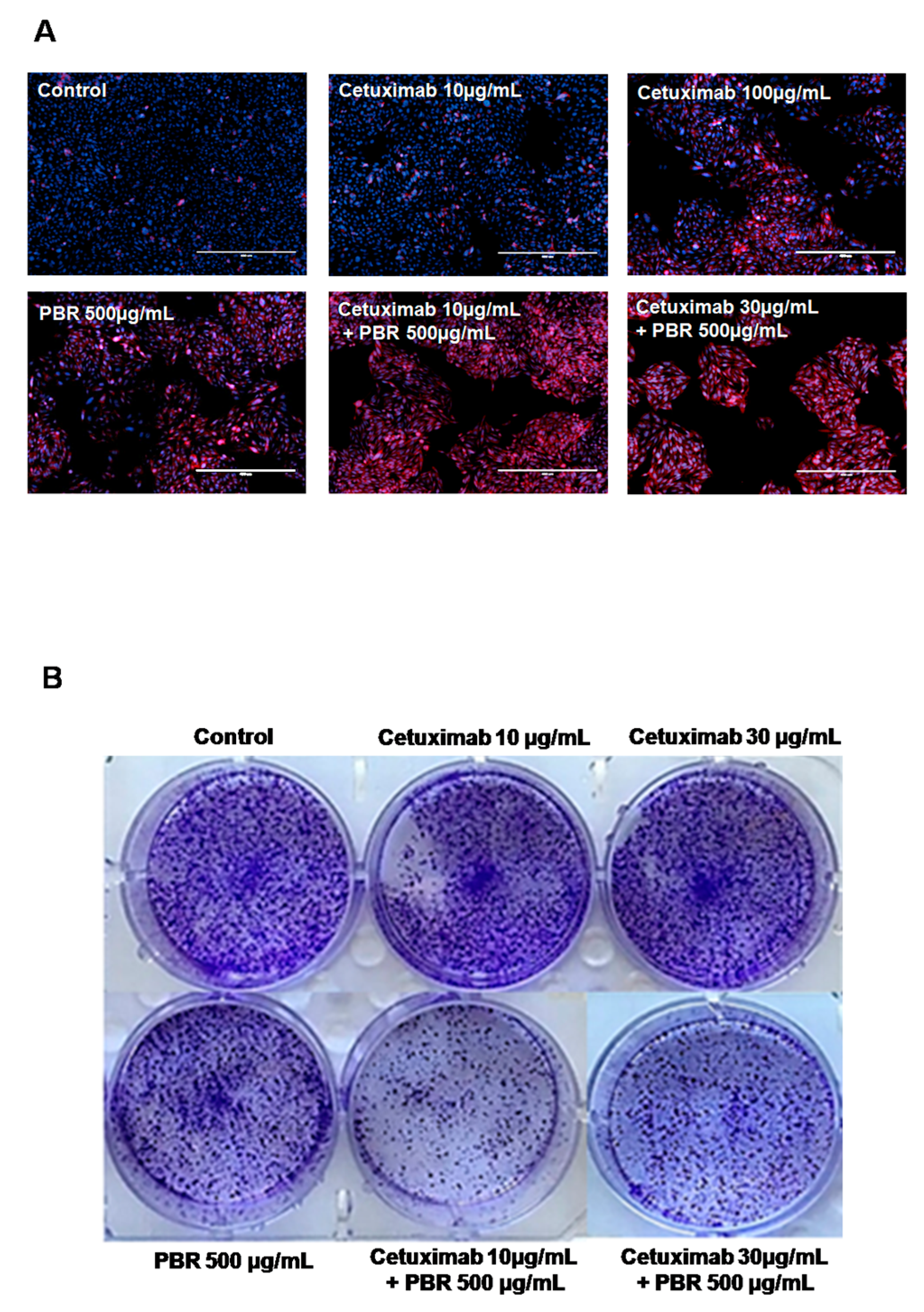
2.1.2. Cetuximab and PBR Extract Co-Treatment Alters Cell Morphology and Clonogenic Characteristics
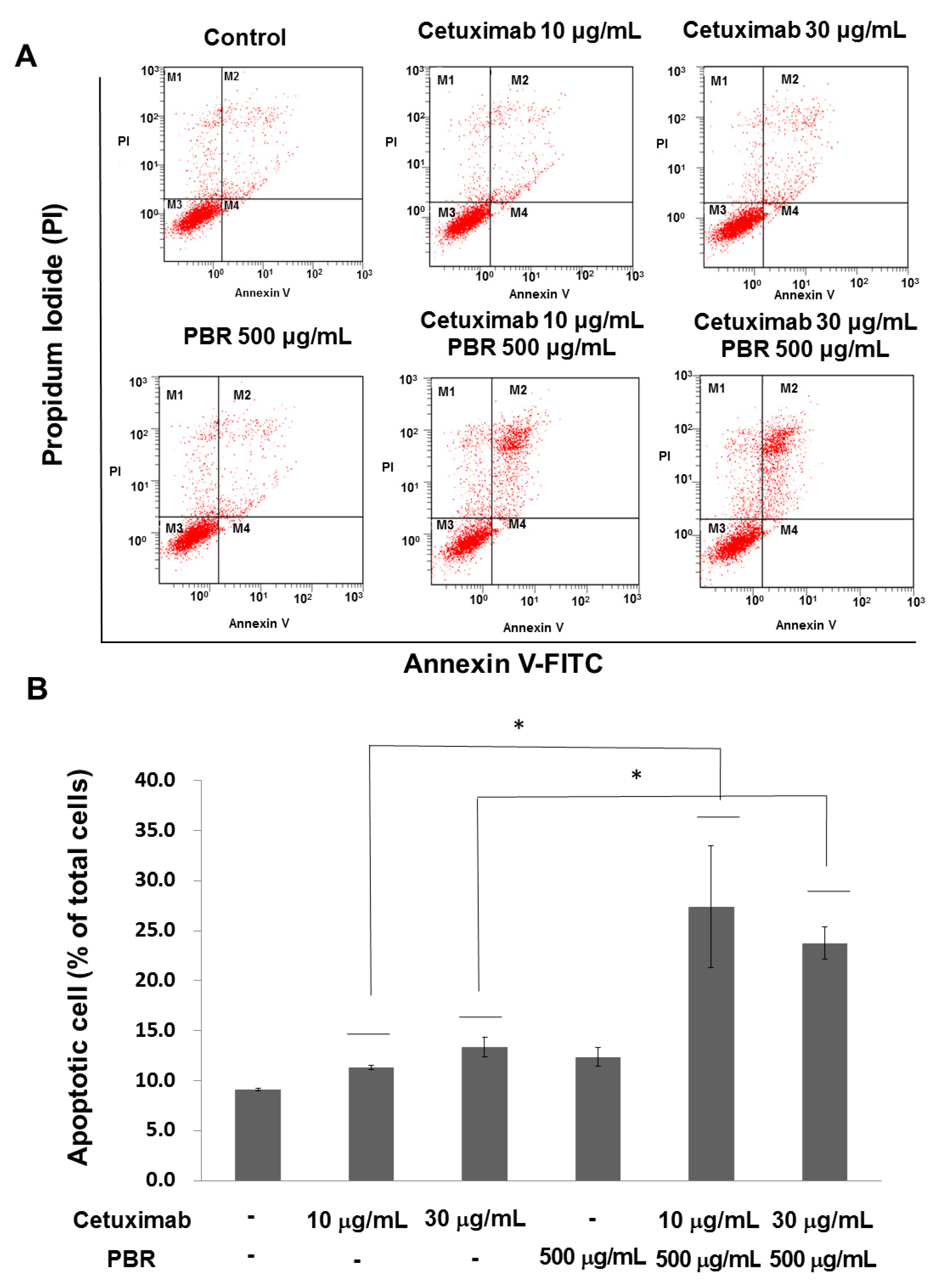
2.1.3. PBR ExtractInduced KRAS-Mutated Colon Cancer Cell Apoptosis
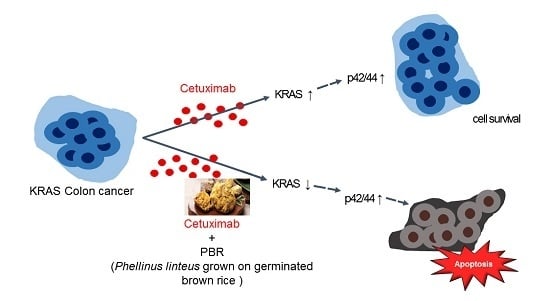
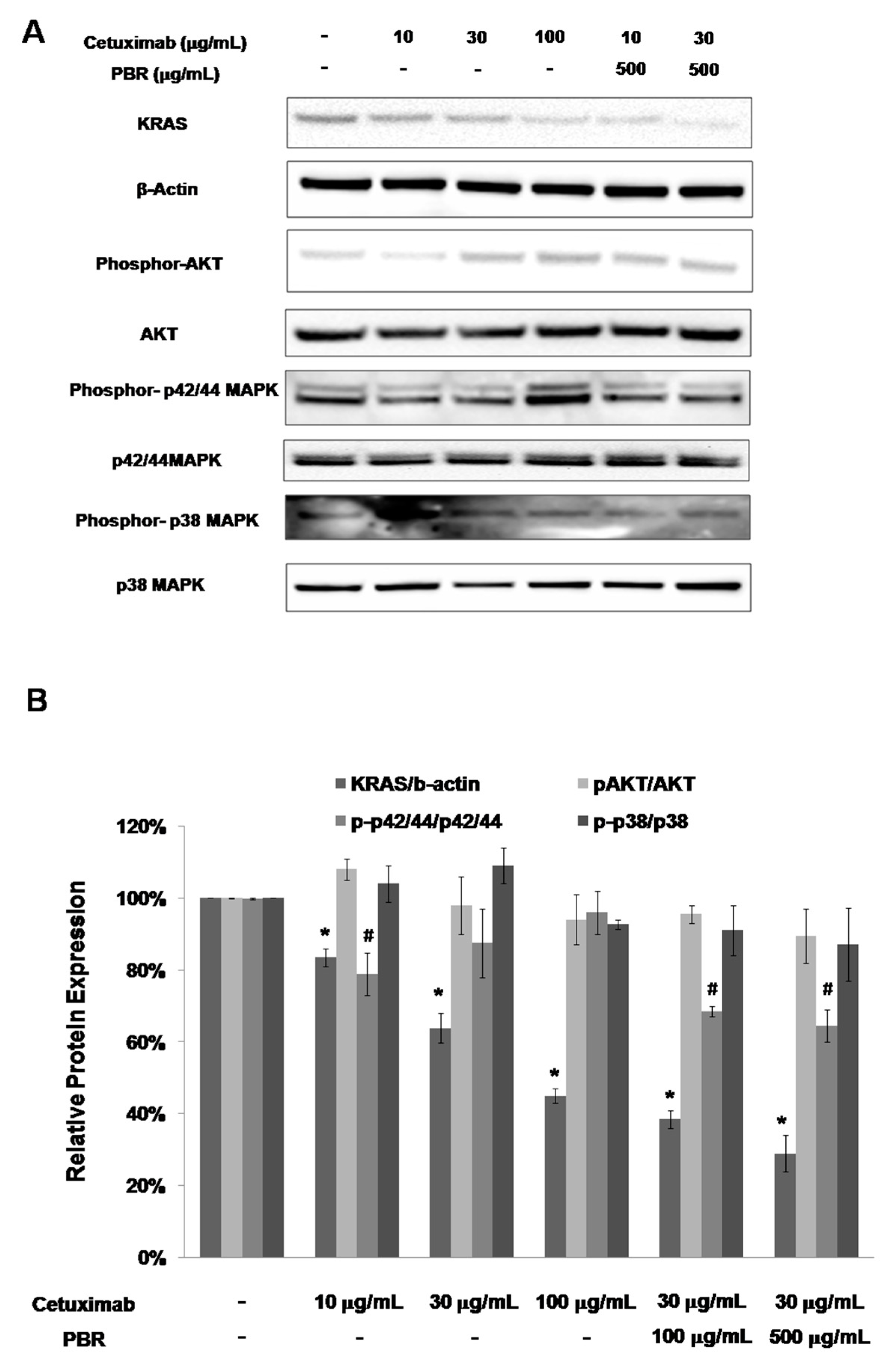
2.2. Effect of the Combination Treatment of PBR Extract and Cetuximab on KRAS and Mitogen-Activated Protein Kinase (MAPK) Signaling Pathways
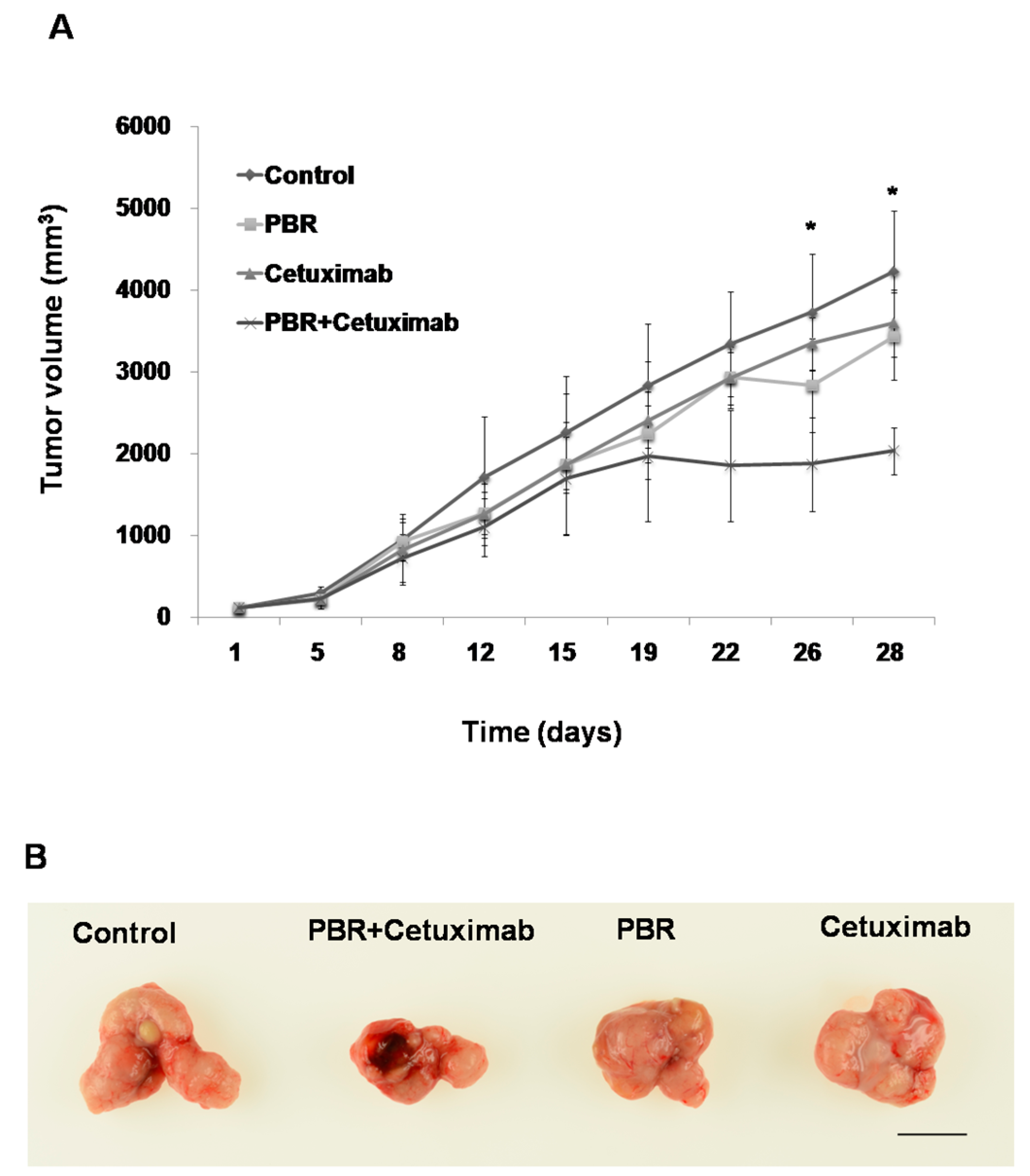
2.3. PBR Extract Inhibits Xenografted Colon Cancer Growth In Vivo
3. Discussion
4. Materials and Methods
4.1. Preparation of PBR Extract
4.2. Cell Viability by MTS Proliferation Assay and DAPI/PI Staining
4.3. Live/Dead Staining
4.4. Clonogenic Assay
4.5. Flow Cytometry
4.6. Western Blotting
4.7. Animal Experiment
4.8. Statistical Analysis
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Siegel, R.; Desantis, C.; Jemal, A. Colorectal cancer statistics, 2014. CA Cancer J. Clin. 2014, 64, 104–117. [Google Scholar] [CrossRef] [PubMed]
- Fearon, E.R.; Vogelstein, B. A genetic model for colorectal tumorigenesis. Cell 1990, 61, 759–767. [Google Scholar] [CrossRef]
- Okumura, S.; Janne, P.A. Molecular pathways: The basis for rational combination using MEK inhibitors in KRAS-mutant cancers. Clin. Cancer Res. 2014, 20, 4193–4199. [Google Scholar] [CrossRef] [PubMed]
- De Roock, W.; Claes, B.; Bernasconi, D.; De Schutter, J.; Biesmans, B.; Fountzilas, G.; Kalogeras, K.T.; Kotoula, V.; Papamichael, D.; Laurent-Puig, P.; et al. Effects of KRAS, BRAF, NRAS, and PIK3CA mutations on the efficacy of cetuximab plus chemotherapy in chemotherapy-refractory metastatic colorectal cancer: A retrospective consortium analysis. Lancet Oncol. 2010, 11, 753–762. [Google Scholar] [CrossRef]
- Bardelli, A.; Siena, S. Molecular mechanisms of resistance to cetuximab and panitumumab in colorectal cancer. J. Clin. Oncol. 2010, 28, 1254–1261. [Google Scholar] [CrossRef] [PubMed]
- Van Cutsem, E.; Kohne, C.H.; Hitre, E.; Zaluski, J.; Chang Chien, C.R.; Makhson, A.; D'Haens, G.; Pinter, T.; Lim, R.; Bodoky, G.; et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N. Engl.J.Med. 2009, 360, 1408–1417. [Google Scholar] [CrossRef] [PubMed]
- Lievre, A.; Bachet, J.B.; Le Corre, D.; Boige, V.; Landi, B.; Emile, J.F.; Cote, J.F.; Tomasic, G.; Penna, C.; Ducreux, M.; et al. KRAS mutation status is predictive of response to cetuximab therapy in colorectal cancer. Cancer Res. 2006, 66, 3992–3995. [Google Scholar] [CrossRef] [PubMed]
- Misale, S.; Yaeger, R.; Hobor, S.; Scala, E.; Janakiraman, M.; Liska, D.; Valtorta, E.; Schiavo, R.; Buscarino, M.; Siravegna, G.; et al. Emergence of KRAS mutations and acquired resistance to anti-EGFR therapy in colorectal cancer. Nature 2012, 486, 532–536. [Google Scholar] [CrossRef] [PubMed]
- Normanno, N.; Morabito, A.; De Luca, A.; Piccirillo, M.C.; Gallo, M.; Maiello, M.R.; Perrone, F. Target-based therapies in breast cancer: Current status and future perspectives. Endocr. Relat. Cancer 2009, 16, 675–702. [Google Scholar] [CrossRef] [PubMed]
- McCormick, F. KRAS as a Therapeutic Target. Clin. Cancer Res. 2015, 21, 1797–1801. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Choi, S.Y.; Hong, S.M.; Hwang, S.G.; Park, D.K. The ethyl acetate extract of Phellinus linteus grown on germinated brown rice induces G0/G1 cell cycle arrest and apoptosis in human colon carcinoma HT29 cells. Phytother. Res. 2010, 24, 1019–1026. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Park, H.J. Anti-inflammatory effect of Phellinus linteus grown on germinated brown rice on dextran sodium sulfate-induced acute colitis in mice and LPS-activated macrophages. J. Ethnopharmacol. 2014, 154, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.K.; Hwang, S.L.; Yun, I.J.; Do, E.J.; Lee, W.H.; Jung, Y.M.; Hong, S.C.; Park, D.C. Antitumor Effects and Immunomodulating Activities of Phellinus linteus Extract in a CT-26 Cell-Injected Colon Cancer Mouse Model. Mycobiology 2009, 37, 128–132. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.G.; Ji, D.F.; Zhong, S.; Zhu, J.X.; Chen, S.; Hu, G.Y. Anti-tumor effects of proteoglycan from Phellinus linteus by immunomodulating and inhibiting Reg IV/EGFR/Akt signaling pathway in colorectal carcinoma. Int. J. Biol. Macromol. 2011, 48, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Song, K.S.; Li, G.; Kim, J.S.; Jing, K.; Kim, T.D.; Kim, J.P.; Seo, S.B.; Yoo, J.K.; Park, H.D.; Hwang, B.D.; et al. Protein-bound polysaccharide from Phellinus linteus inhibits tumor growth, invasion, and angiogenesis and alters Wnt/beta-catenin in SW480 human colon cancer cells. BMC Cancer 2011, 11, 307. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.Y.; Han, M.G.; Song, Y.S.; Shin, B.C.; Shin, Y.I.; Lee, H.J.; Moon, D.O.; Lee, C.M.; Kwak, J.Y.; Bae, Y.S.; et al. Proteoglycan isolated from Phellinus linteus induces toll-like receptors 2- and 4-mediated maturation of murine dendritic cells via activation of ERK, p38, and NF-kappaB. Biol. Pharm. Bull. 2004, 27, 1656–1662. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Han, E.S.; Park, D.K.; Lee, C.; Lee, K.W. An extract of Phellinus linteus grown on germinated brown rice inhibits inflammation markers in RAW264.7 macrophages by suppressing inflammatory cytokines, chemokines, and mediators and up-regulating antioxidant activity. J. Med. Food 2010, 13, 1468–1477. [Google Scholar] [CrossRef] [PubMed]
- Downward, J. Targeting RAS signalling pathways in cancer therapy. Nat. Rev. Cancer 2003, 3, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Gomes, S.E.; Simoes, A.E.; Pereira, D.M.; Castro, R.E.; Rodrigues, C.M.; Borralho, P.M. miR-143 or miR-145 overexpression increases cetuximab-mediated antibody-dependent cellular cytotoxicity in human colon cancer cells. Oncotarget 2016, 7, 9368–9387. [Google Scholar] [CrossRef] [PubMed]
- Dunn, E.F.; Iida, M.; Myers, R.A.; Campbell, D.A.; Hintz, K.A.; Armstrong, E.A.; Li, C.; Wheeler, D.L. Dasatinib sensitizes KRAS mutant colorectal tumors to cetuximab. Oncogene 2011, 30, 561–574. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Lee, I.; Han, B.; Park, J.O.; Jang, J.; Park, C.; Kang, W.K. Effect of simvastatin on cetuximab resistance in human colorectal cancer with KRAS mutations. J. Natl. Cancer Inst. 2011, 103, 674–688. [Google Scholar] [CrossRef] [PubMed]
- Weng, W.H.; Leung, W.H.; Pang, Y.J.; Hsu, H.H. Lauric acid can improve the sensitization of Cetuximab in KRAS/BRAF mutated colorectal cancer cells by retrievable microRNA-378 expression. Oncol.Rep. 2016, 35, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.A.; Lee, D.H.; Moon, J.H.; Hong, S.W.; Shin, J.S.; Hwang, I.Y.; Shin, Y.J.; Kim, J.H.; Gong, E.Y.; Kim, S.M.; et al. L-Ascorbic acid can abrogate SVCT-2-dependent cetuximab resistance mediated by mutant KRAS in human colon cancer cells. Free Radic. Biol. Med. 2016, 95, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Ma, C.M.; Park, D.K.; Yoshimi, Y.; Hatanaka, M.; Hattori, M. Transformation of ergosterol peroxide to cytotoxic substances by rat intestinal bacteria. Biol. Pharm. Bull. 2008, 31, 949–954. [Google Scholar] [CrossRef] [PubMed]
- Ortega, A. A new role for GABA: Inhibition of tumor cell migration. Trends Pharmacol. Sci. 2003, 24, 151–154. [Google Scholar] [CrossRef]
- Oh, C.H.; Oh, S.H. Effects of germinated brown rice extracts with enhanced levels of GABA on cancer cell proliferation and apoptosis. J. Med. Food 2004, 7, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Nam, K.S.; Jo, Y.S.; Kim, Y.H.; Hyun, J.W.; Kim, H.W. Cytotoxic activities of acetoxyscirpenediol and ergosterol peroxide from Paecilomyces tenuipes. Life Sci. 2001, 69, 229–237. [Google Scholar] [CrossRef]
- Hong, F.; Yan, J.; Baran, J.T.; Allendorf, D.J.; Hansen, R.D.; Ostroff, G.R.; Xing, P.X.; Cheung, N.K.; Ross, G.D. Mechanism by which orally administered β-1,3-glucans enhance the tumoricidal activity of antitumor monoclonal antibodies in murine tumor models. J. Immunol. 2004, 173, 797–806. [Google Scholar] [CrossRef] [PubMed]
- Cheung, N.K.; Modak, S. Oral (1-->3),(1-->4)-beta-D-glucan synergizes with antiganglioside GD2 monoclonal antibody 3F8 in the therapy of neuroblastoma. Clin. Cancer Res. 2002, 8, 1217–1223. [Google Scholar] [PubMed]
- Cheung, N.K.; Modak, S.; Vickers, A.; Knuckles, B. Orally administered beta-glucans enhance anti-tumor effects of monoclonal antibodies. Cancer Immunol. Immunother. 2002, 51, 557–564. [Google Scholar] [PubMed]
- Han, E.S.; Oh, J.Y.; Park, H.J. Cordyceps militaris extract suppresses dextran sodium sulfate-induced acute colitis in mice and production of inflammatory mediators from macrophages and mast cells. J. Ethnopharmacol. 2011, 134, 703–710. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, H.-J.; Park, J.-B.; Lee, S.-J.; Song, M. Phellinus linteus Grown on Germinated Brown Rice Increases Cetuximab Sensitivity of KRAS-Mutated Colon Cancer. Int. J. Mol. Sci. 2017, 18, 1746. https://doi.org/10.3390/ijms18081746
Park H-J, Park J-B, Lee S-J, Song M. Phellinus linteus Grown on Germinated Brown Rice Increases Cetuximab Sensitivity of KRAS-Mutated Colon Cancer. International Journal of Molecular Sciences. 2017; 18(8):1746. https://doi.org/10.3390/ijms18081746
Chicago/Turabian StylePark, Hye-Jin, Jeong-Bin Park, Sang-Jae Lee, and Minjung Song. 2017. "Phellinus linteus Grown on Germinated Brown Rice Increases Cetuximab Sensitivity of KRAS-Mutated Colon Cancer" International Journal of Molecular Sciences 18, no. 8: 1746. https://doi.org/10.3390/ijms18081746
APA StylePark, H.-J., Park, J.-B., Lee, S.-J., & Song, M. (2017). Phellinus linteus Grown on Germinated Brown Rice Increases Cetuximab Sensitivity of KRAS-Mutated Colon Cancer. International Journal of Molecular Sciences, 18(8), 1746. https://doi.org/10.3390/ijms18081746