Melanin-Based Contrast Agents for Biomedical Optoacoustic Imaging and Theranostic Applications
Abstract
1. Introduction
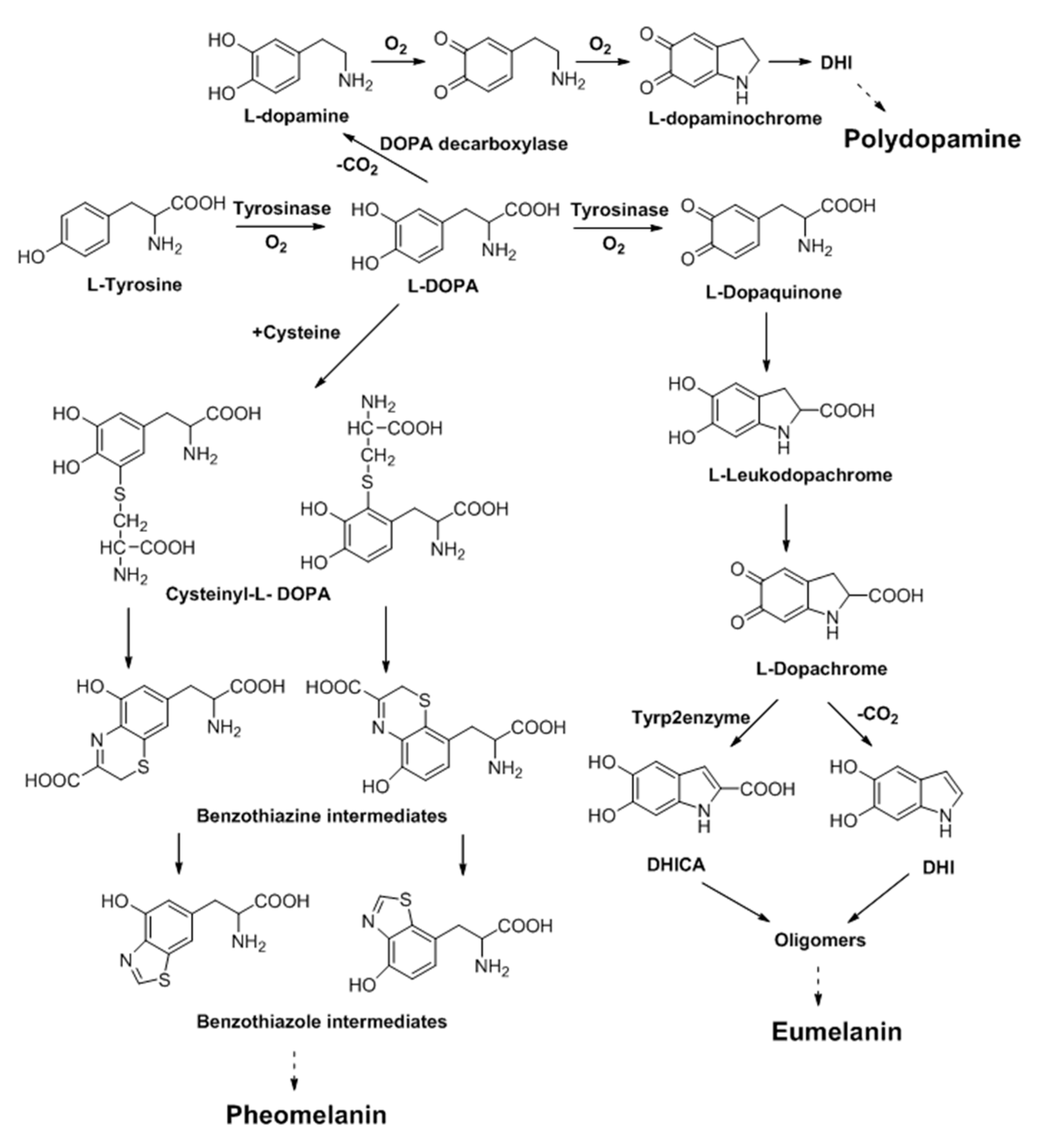
2. Melanin and Synthesis
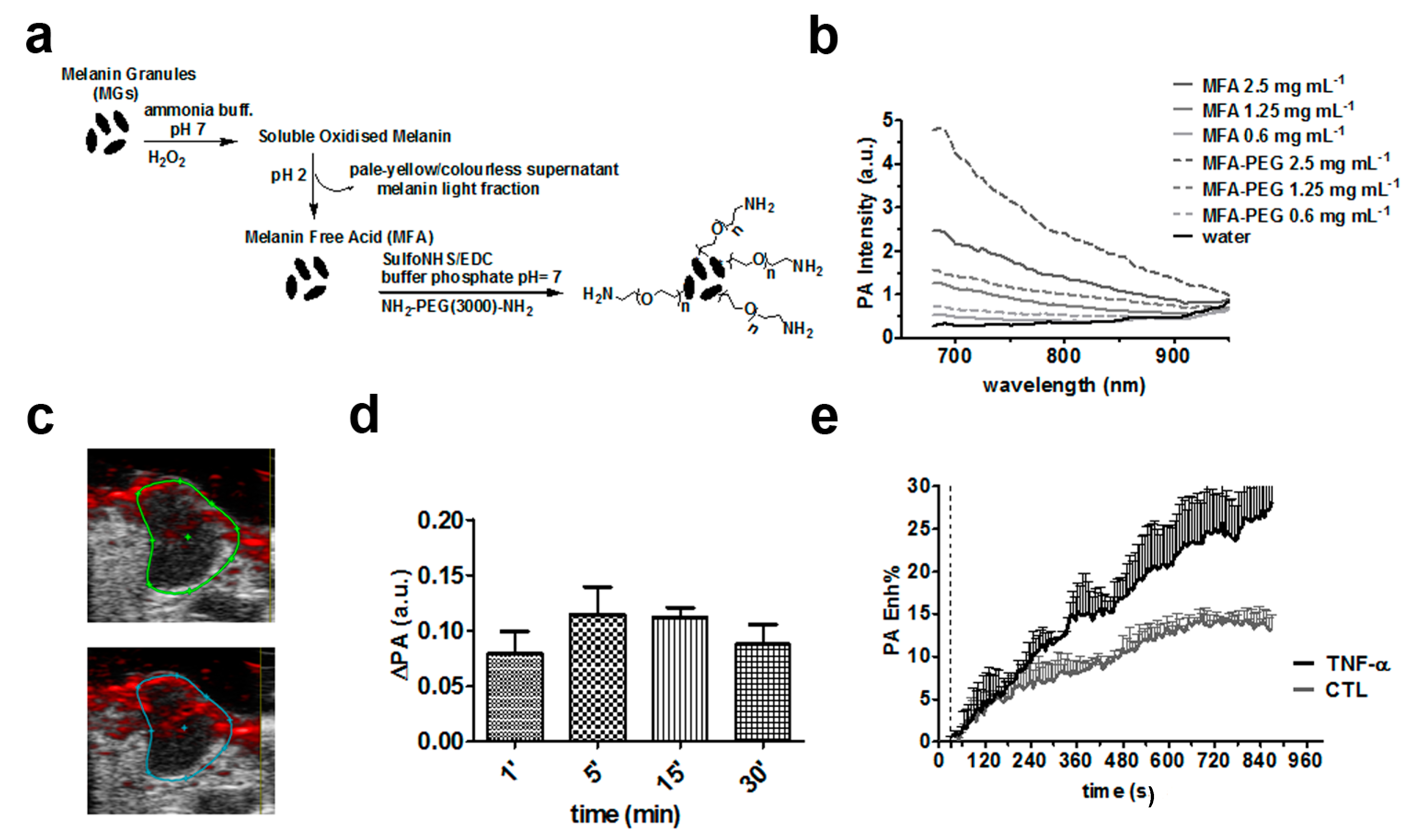
3. Non-Specific Melanin-Based Probes for Tumor Imaging
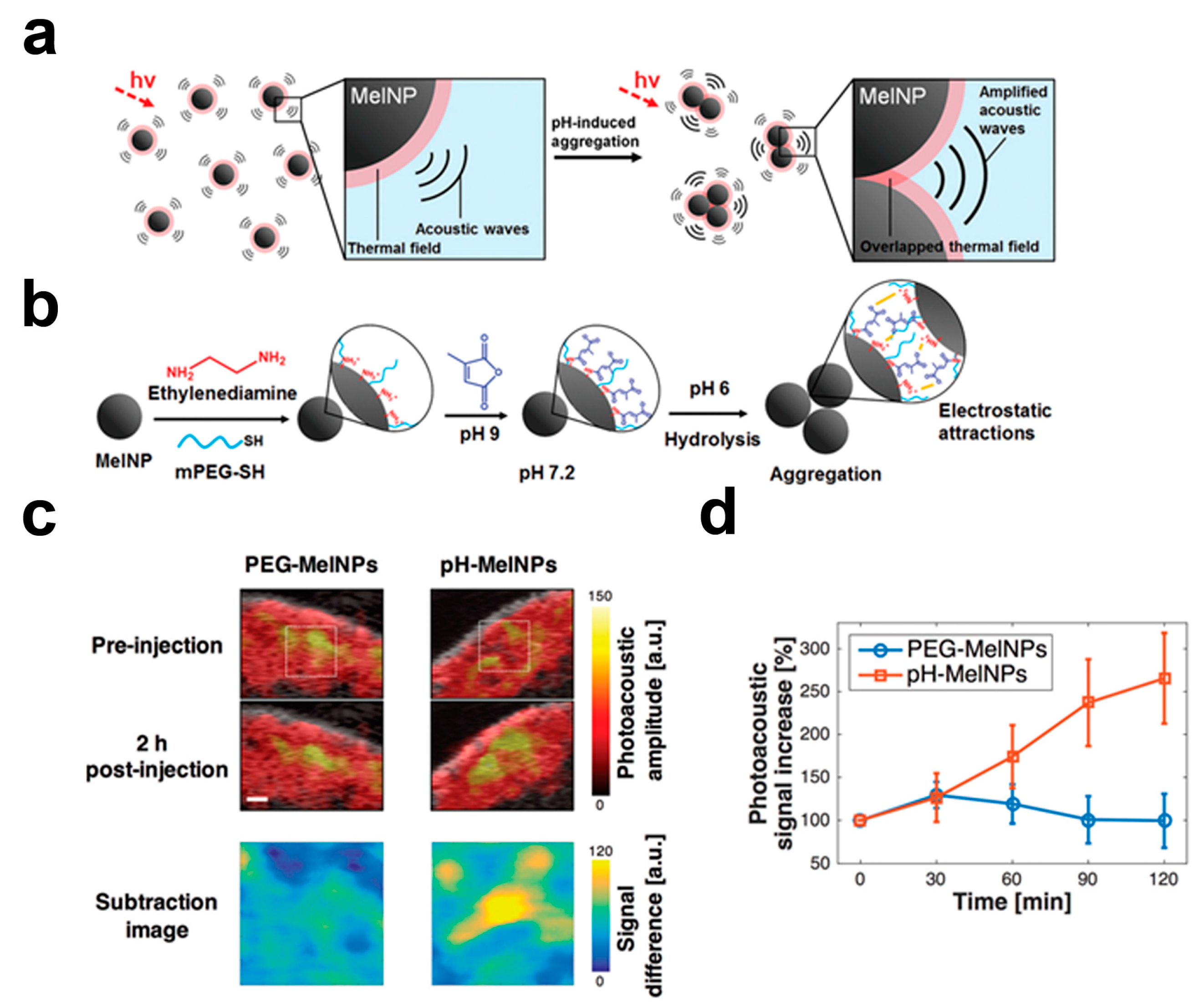
4. Responsive Melanin-Based Probes
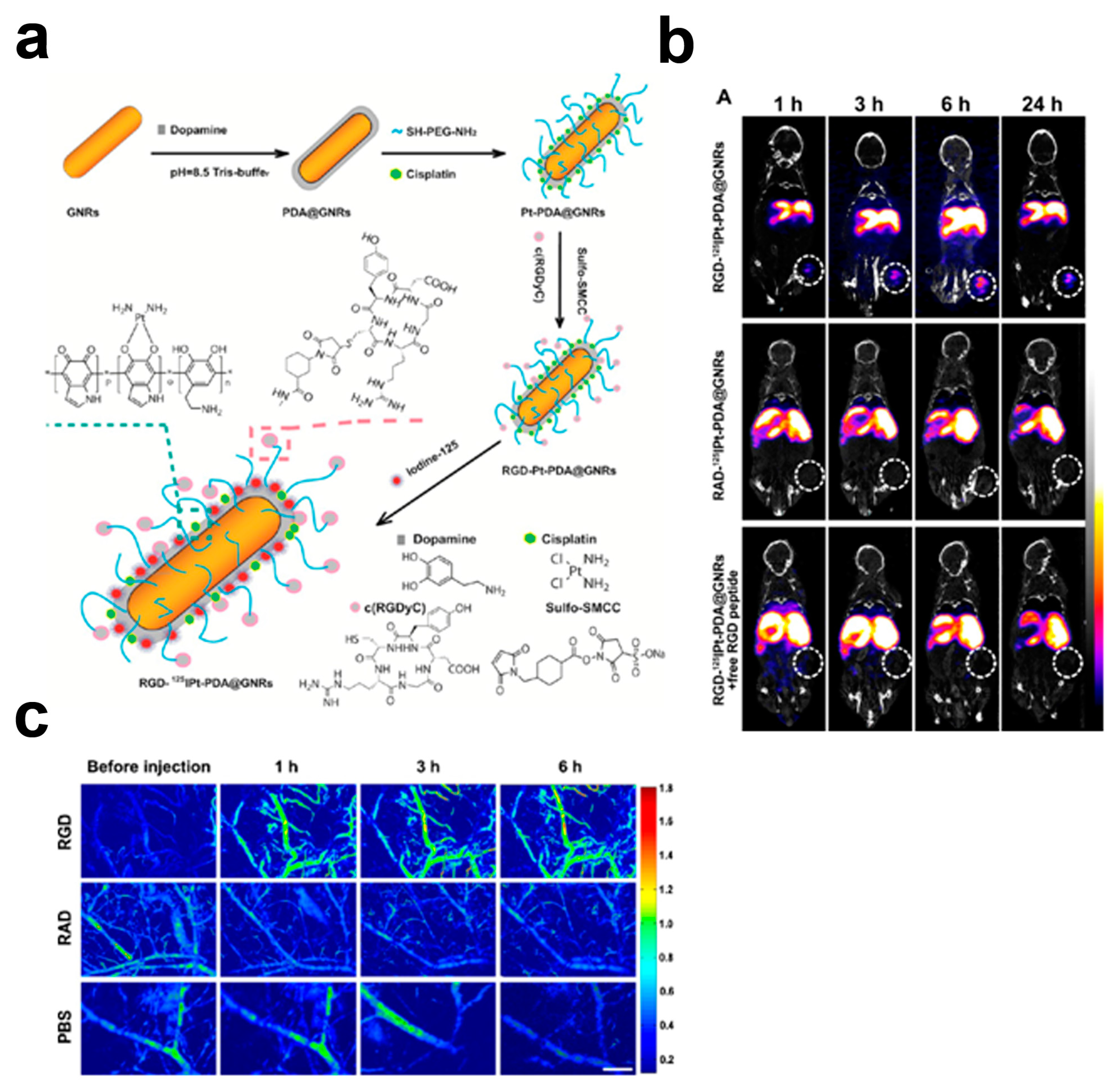
5. Targeted Melanin-Based Probes
6. Multi-Modality Melanin-Based Probes
7. Theranostic Melanin-Based Probes
8. Summary and Outlook
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| PA | Photoacoustic (Optoacoustic) Imaging |
| NIR | Near Infra Red |
| NP | Nanoparticles |
| PDA | Polydopamine |
| MNP | Melanin nanoparticles |
| PEG | Polyethylene glycol |
| PET | Positron Emission Tomography |
| SPECT | Single Photon Emission Computed Tomography |
| MRI | Magnetic Resonance Imaging |
| CT | Computed Tomography |
| OI | Optical Imaging |
| US | Ultrasound |
| b.w. | body weight |
References
- Kruger, R.A.; Liu, P.; Fang, Y.R.; Appledorn, C.R. Photoacoustic ultrasound (PAUS)—Reconstruction tomography. Med. Phys. 1995, 22, 1605–1609. [Google Scholar] [CrossRef] [PubMed]
- Ntziachristos, V.; Razansky, D. Molecular imaging by means of multispectral optoacoustic tomography (MSOT). Chem. Rev. 2010, 110, 2783–2794. [Google Scholar] [CrossRef] [PubMed]
- Oraevsky, A.A.; Jacques, S.L.; Esenaliev, R.O.; Tittel, F.K. Laser-Based Optoacoustic Imaging in Biological Tissues. In Laser-Tissue Interaction V, Proceedings of The Society of Photo-Optical Instrumentation Engineers (SPIE); SPIE: Los Angeles, CA, USA, 1994; Volume 2134, pp. 122–128. [Google Scholar]
- Su, R.; Ermilov, S.A.; Liopo, A.V.; Oraevsky, A.A. Three-dimensional optoacoustic imaging as a new noninvasive technique to study long-term biodistribution of optical contrast agents in small animal models. J. Biomed. Opt. 2012, 17, 101506. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Ermilov, S.A.; Su, R.; Brecht, H.P.; Oraevsky, A.A.; Anastasio, M.A. An imaging model incorporating ultrasonic transducer properties for three-dimensional optoacoustic tomography. IEEE Trans. Med. Imaging 2011, 30, 203–214. [Google Scholar] [CrossRef] [PubMed]
- Razansky, D.; Deliolanis, N.C.; Vinegoni, C.; Ntziachristos, V. Deep tissue optical and optoacoustic molecular imaging technologies for pre-clinical research and drug discovery. Curr. Pharm. Biotechnol. 2012, 13, 504–522. [Google Scholar] [CrossRef] [PubMed]
- Dean-Ben, X.L.; Gottschalk, S.; Mc Larney, B.; Shoham, S.; Razansky, D. Advanced optoacoustic methods for multiscale imaging of in vivo dynamics. Chem. Soc. Rev. 2017, 46, 2158–2198. [Google Scholar] [CrossRef] [PubMed]
- Fronheiser, M.P.; Ermilov, S.A.; Brecht, H.P.; Conjusteau, A.; Su, R.; Mehta, K.; Oraevsky, A.A. Real-time optoacoustic monitoring and three-dimensional mapping of a human arm vasculature. J. Biomed. Opt. 2010, 15, 021305. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, M.; Buehler, A.; Aguirre, J.; Ntziachristos, V. Three-dimensional multispectral optoacoustic mesoscopy reveals melanin and blood oxygenation in human skin in vivo. J. Biophotonics 2016, 9, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Bohndiek, S.E.; Sasportas, L.S.; Machtaler, S.; Jokerst, J.V.; Hori, S.; Gambhir, S.S. Photoacoustic Tomography Detects Early Vessel Regression and Normalization During Ovarian Tumor Response to the Antiangiogenic Therapy Trebananib. J. Nucl. Med. 2015, 56, 1942–1947. [Google Scholar] [CrossRef] [PubMed]
- Heijblom, M.; Piras, D.; Brinkhuis, M.; van Hespen, J.C.; van den Engh, F.M.; van der Schaaf, M.; Klaase, J.M.; van Leeuwen, T.G.; Steenbergen, W.; Manohar, S. Photoacoustic image patterns of breast carcinoma and comparisons with Magnetic Resonance Imaging and vascular stained histopathology. Sci Rep. 2015, 5, 11778. [Google Scholar] [CrossRef] [PubMed]
- Stoffels, I.; Morscher, S.; Helfrich, I.; Hillen, U.; Leyh, J.; Burton, N.C.; Sardella, T.C.; Claussen, J.; Poeppel, T.D.; Bachmann, H.S.; et al. Metastatic status of sentinel lymph nodes in melanoma determined noninvasively with multispectral optoacoustic imaging. Sci. Transl. Med. 2015, 7, 317ra199. [Google Scholar] [CrossRef] [PubMed]
- Heijblom, M.; Klaase, J.M.; van den Engh, F.M.; van Leeuwen, T.G.; Steenbergen, W.; Manohar, S. Imaging tumor vascularization for detection and diagnosis of breast cancer. Technol. Cancer Res. Treat. 2011, 10, 607–623. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Huang, L.; Jiang, M.S.; Jiang, H. Contrast agents for photoacoustic and thermoacoustic imaging: A review. Int. J. Mol. Sci. 2014, 15, 23616–23639. [Google Scholar] [CrossRef] [PubMed]
- Luke, G.P.; Yeager, D.; Emelianov, S.Y. Biomedical applications of photoacoustic imaging with exogenous contrast agents. Ann. Biomed. Eng. 2012, 40, 422–437. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, S.; Wang, S.; Reinecke, D.; Kiser, W.J.; Kruger, R.A.; DeGrado, T.R. Synthesis and evaluation of near-infrared (NIR) dye-herceptin conjugates as photoacoustic computed tomography (PCT) probes for HER2 expression in breast cancer. Bioconj. Chem. 2008, 19, 1186–1193. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.; Huang, S.W.; Day, K.C.; O’Donnell, M.; Agayan, R.R.; Day, M.A.; Kopelman, R.; Ashkenazi, S. Indocyanine-green-embedded PEBBLEs as a contrast agent for photoacoustic imaging. J. Biomed. Opt. 2007, 12, 044020. [Google Scholar] [CrossRef] [PubMed]
- Pramanik, M.; Swierczewska, M.; Green, D.; Sitharaman, B.; Wang, L.V. Single-walled carbon nanotubes as a multimodal-thermoacoustic and photoacoustic-contrast agent. J. Biomed. Opt. 2009, 14, 034018. [Google Scholar] [CrossRef] [PubMed]
- Xiang, L.; Yuan, Y.; Xing, D.; Ou, Z.; Yang, S.; Zhou, F. Photoacoustic molecular imaging with antibody-functionalized single-walled carbon nanotubes for early diagnosis of tumor. J. Biomed. Opt. 2009, 14, 021008. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Huang, Q.; Ku, G.; Wen, X.; Zhou, M.; Guzatov, D.; Brecht, P.; Su, R.; Oraevsky, A.; Wang, L.V.; et al. Photoacoustic imaging of living mouse brain vasculature using hollow gold nanospheres. Biomaterials 2010, 31, 2617–2626. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Chen, X. Gold nanoparticles for photoacoustic imaging. Nanomedicine (Lond) 2015, 10, 299–320. [Google Scholar] [CrossRef] [PubMed]
- Song, K.H.; Kim, C.; Cobley, C.M.; Xia, Y.; Wang, L.V. Near-infrared gold nanocages as a new class of tracers for photoacoustic sentinel lymph node mapping on a rat model. Nano Lett. 2009, 9, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Nie, L.; Chen, X. Structural and functional photoacoustic molecular tomography aided by emerging contrast agents. Chem. Soc. Rev. 2014, 43, 7132–7170. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Stein, E.W.; Ashkenazi, S.; Wang, L.V. Nanoparticles for photoacoustic imaging. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2009, 1, 360–368. [Google Scholar] [CrossRef] [PubMed]
- Jokerst, J.V.; Van de Sompel, D.; Bohndiek, S.E.; Gambhir, S.S. Cellulose Nanoparticles are a Biodegradable Photoacoustic Contrast Agent for Use in Living Mice. Photoacoustics 2014, 2, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Weber, J.; Beard, P.C.; Bohndiek, S.E. Contrast agents for molecular photoacoustic imaging. Nat. Methods 2016, 13, 639–650. [Google Scholar] [CrossRef] [PubMed]
- Iyer, A.K.; Khaled, G.; Fang, J.; Maeda, H. Exploiting the enhanced permeability and retention effect for tumor targeting. Drug Discov. Today 2006, 11, 812–818. [Google Scholar] [CrossRef] [PubMed]
- Monaco, I.; Arena, F.; Biffi, S.; Locatelli, E.; Bortot, B.; La Cava, F.; Marini, G.M.; Severini, G.M.; Terreno, E.; Comes Franchini, M. Synthesis of Lipophilic Core-Shell Fe3O4@SiO2@Au Nanoparticles and Polymeric Entrapment into Nanomicelles: A Novel Nanosystem for in Vivo Active Targeting and Magnetic Resonance-Photoacoustic Dual Imaging. Bioconj. Chem. 2017, 28, 1382–1390. [Google Scholar] [CrossRef] [PubMed]
- Liopo, A.; Su, R.; Oraevsky, A.A. Melanin nanoparticles as a novel contrast agent for optoacoustic tomography. Photoacoustics 2015, 3, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Repenko, T.; Fokong, S.; De Laporte, L.; Go, D.; Kiessling, F.; Lammers, T.; Kuehne, A.J. Water-soluble dopamine-based polymers for photoacoustic imaging. Chem. Commun. 2015, 51, 6084–6087. [Google Scholar] [CrossRef] [PubMed]
- Longo, D.L.; Stefania, R.; Callari, C.; De Rose, F.; Rolle, R.; Conti, L.; Consolino, L.; Arena, F.; Aime, S. Water Soluble Melanin Derivatives for Dynamic Contrast Enhanced Photoacoustic Imaging of Tumor Vasculature and Response to Antiangiogenic Therapy. Adv. Healthc. Mater. 2017, 6. [Google Scholar] [CrossRef] [PubMed]
- Ju, K.Y.; Kang, J.; Pyo, J.; Lim, J.; Chang, J.H.; Lee, J.K. pH-Induced aggregated melanin nanoparticles for photoacoustic signal amplification. Nanoscale 2016, 8, 14448–14456. [Google Scholar] [CrossRef] [PubMed]
- Fan, Q.; Cheng, K.; Hu, X.; Ma, X.; Zhang, R.; Yang, M.; Lu, X.; Xing, L.; Huang, W.; Gambhir, S.S.; et al. Transferring biomarker into molecular probe: Melanin nanoparticle as a naturally active platform for multimodality imaging. J. Am. Chem. Soc. 2014, 136, 15185–15194. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Jiang, C.; Zhang, D.; Wang, Y.; Ren, X.; Ai, K.; Chen, X.; Lu, L. Targeted polydopamine nanoparticles enable photoacoustic imaging guided chemo-photothermal synergistic therapy of tumor. Acta Biomater. 2017, 47, 124–134. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Su, H.; Cai, J.; Cheng, D.; Ma, Y.; Zhang, J.; Zhou, C.; Liu, S.; Shi, H.; Zhang, Y.; et al. A Multifunctional Platform for Tumor Angiogenesis-Targeted Chemo-Thermal Therapy Using Polydopamine-Coated Gold Nanorods. ACS Nano 2016, 10, 10404–10417. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Liu, C.; Song, L.; Cui, H.; Gao, G.; Liu, P.; Sheng, Z.; Cai, L. Indocyanine green-loaded polydopamine-iron ions coordination nanoparticles for photoacoustic/magnetic resonance dual-modal imaging-guided cancer photothermal therapy. Nanoscale 2016, 8, 17150–17158. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.P.; Fan, Q.L.; Yang, M.; Cheng, K.; Lu, X.M.; Zhang, L.; Huang, W.; Cheng, Z. Engineering Melanin Nanoparticles as an Efficient Drug-Delivery System for Imaging-Guided Chemotherapy. Adv. Mater. 2015, 27, 5063–5069. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.H.; Sun, Y.; Tang, C.; Cheng, K.; Zhang, R.; Fan, Q.; Xu, L.; Huang, D.; Zhao, A.; Cheng, Z. Chelator-Free and Biocompatible Melanin Nanoplatform with Facile-Loading Gadolinium and Copper-64 for Bioimaging. Bioconj. Chem. 2017, 28, 1925–1930. [Google Scholar] [CrossRef] [PubMed]
- Ju, K.Y.; Lee, J.W.; Im, G.H.; Lee, S.; Pyo, J.; Park, S.B.; Lee, J.H.; Lee, J.K. Bio-inspired, melanin-like nanoparticles as a highly efficient contrast agent for T1-weighted magnetic resonance imaging. Biomacromolecules 2013, 14, 3491–3497. [Google Scholar] [CrossRef] [PubMed]
- Zacharakis, G.; Ripoll, J.; Weissleder, R.; Ntziachristos, V. Fluorescent protein tomography scanner for small animal imaging. IEEE Trans. Med. Imaging 2005, 24, 878–885. [Google Scholar] [CrossRef] [PubMed]
- Meredith, P.; Sarna, T. The physical and chemical properties of eumelanin. Pigment Cell Res. 2006, 19, 572–594. [Google Scholar] [CrossRef] [PubMed]
- D’ISCHIA, M.; Napolitano, A.; Ball, V.; Chen, C.T.; Buehler, M.J. Polydopamine and eumelanin: From structure-property relationships to a unified tailoring strategy. Acc. Chem. Res. 2014, 47, 3541–3550. [Google Scholar] [CrossRef] [PubMed]
- Arzillo, M.; Mangiapia, G.; Pezzella, A.; Heenan, R.K.; Radulescu, A.; Paduano, L.; d’Ischia, M. Eumelanin buildup on the nanoscale: Aggregate growth/assembly and visible absorption development in biomimetic 5,6-dihydroxyindole polymerization. Biomacromolecules 2012, 13, 2379–2390. [Google Scholar] [CrossRef] [PubMed]
- Aime, S.; Fasano, M.; Terreno, E.; Groombridge, C.J. NMR studies of melanins: Characterization of a soluble melanin free acid from Sepia ink. Pigment Cell Res. 1991, 4, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Mussel-inspired surface chemistry for multifunctional coatings. Science 2007, 318, 426–430. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Rho, J.; Messersmith, P.B. Facile Conjugation of Biomolecules onto Surfaces via Mussel Adhesive Protein Inspired Coatings. Adv. Mater. 2009, 21, 431–434. [Google Scholar] [CrossRef] [PubMed]
- D’ISCHIA, M.; Wakamatsu, K.; Cicoira, F.; Di Mauro, E.; Garcia-Borron, J.C.; Commo, S.; Galvan, I.; Ghanem, G.; Kenzo, K.; Meredith, P.; et al. Melanins and melanogenesis: From pigment cells to human health and technological applications. Pigment Cell Melanoma Res. 2015, 28, 520–544. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Turkbey, B.; Watanabe, R.; Choyke, P.L. Cancer drug delivery: Considerations in the rational design of nanosized bioconjugates. Bioconj. Chem. 2014, 25, 2093–2100. [Google Scholar] [CrossRef] [PubMed]
- Brecht, H.P.; Su, R.; Fronheiser, M.; Ermilov, S.A.; Conjusteau, A.; Oraevsky, A.A. Whole-body three-dimensional optoacoustic tomography system for small animals. J. Biomed. Opt. 2009, 14, 064007. [Google Scholar] [CrossRef] [PubMed]
- Micillo, R.; Panzella, L.; Koike, K.; Monfrecola, G.; Napolitano, A.; d’Ischia, M. “Fifty Shades” of Black and Red or How Carboxyl Groups Fine Tune Eumelanin and Pheomelanin Properties. Int. J. Mol. Sci. 2016, 17, 746. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.S.; Frey, W.; Aglyamov, S.; Emelianov, S. Environment-dependent generation of photoacoustic waves from plasmonic nanoparticles. Small 2012, 8, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Kiessling, F.; Morgenstern, B.; Zhang, C. Contrast agents and applications to assess tumor angiogenesis In Vivo by magnetic resonance imaging. Curr. Med. Chem. 2007, 14, 77–91. [Google Scholar] [CrossRef] [PubMed]
- Longo, D.L.; Arena, F.; Consolino, L.; Minazzi, P.; Geninatti-Crich, S.; Giovenzana, G.B.; Aime, S. Gd-AAZTA-MADEC, an improved blood pool agent for DCE-MRI studies on mice on 1 T scanners. Biomaterials 2016, 75, 47–57. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, J.P.; Jackson, A.; Parker, G.J.; Jayson, G.C. DCE-MRI biomarkers in the clinical evaluation of antiangiogenic and vascular disrupting agents. Br. J. Cancer 2007, 96, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Consolino, L.; Longo, D.L.; Dastru, W.; Cutrin, J.C.; Dettori, D.; Lanzardo, S.; Oliviero, S.; Cavallo, F.; Aime, S. Functional imaging of the angiogenic switch in a transgenic mouse model of human breast cancer by dynamic contrast enhanced magnetic resonance imaging. Int. J. Cancer 2016, 139, 404–413. [Google Scholar] [CrossRef] [PubMed]
- Longo, D.L.; Dastru, W.; Consolino, L.; Espak, M.; Arigoni, M.; Cavallo, F.; Aime, S. Cluster analysis of quantitative parametric maps from DCE-MRI: Application in evaluating heterogeneity of tumor response to antiangiogenic treatment. Magn. Reson. Imaging 2015, 33, 725–736. [Google Scholar] [CrossRef] [PubMed]
- Consolino, L.; Longo, D.L.; Sciortino, M.; Dastru, W.; Cabodi, S.; Giovenzana, G.B.; Aime, S. Assessing tumor vascularization as a potential biomarker of imatinib resistance in gastrointestinal stromal tumors by dynamic contrast-enhanced magnetic resonance imaging. Gastric Cancer 2017, 20, 629–639. [Google Scholar] [CrossRef] [PubMed]
- Miao, Q.Q.; Pu, K.Y. Emerging Designs of Activatable Photoacoustic Probes for Molecular Imaging. Bioconj. Chem. 2016, 27, 2808–2823. [Google Scholar] [CrossRef] [PubMed]
- Miao, Q.; Lyu, Y.; Ding, D.; Pu, K. Semiconducting Oligomer Nanoparticles as an Activatable Photoacoustic Probe with Amplified Brightness for In Vivo Imaging of pH. Adv. Mater. 2016, 28, 3662–3668. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Liu, X.; Chen, J.; Zeng, J.; Cheng, Z.; Liu, Z. A Self-Assembled Albumin-Based Nanoprobe for In Vivo Ratiometric Photoacoustic pH Imaging. Adv. Mater. 2015, 27, 6820–6827. [Google Scholar] [CrossRef] [PubMed]
- Yin, C.; Zhen, X.; Fan, Q.; Huang, W.; Pu, K. Degradable Semiconducting Oligomer Amphiphile for Ratiometric Photoacoustic Imaging of Hypochlorite. ACS Nano 2017, 11, 4174–4182. [Google Scholar] [CrossRef] [PubMed]
- Pu, K.; Shuhendler, A.J.; Jokerst, J.V.; Mei, J.; Gambhir, S.S.; Bao, Z.; Rao, J. Semiconducting polymer nanoparticles as photoacoustic molecular imaging probes in living mice. Nat. Nanotechnol. 2014, 9, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhang, P.; Smaga, L.P.; Hoffman, R.A.; Chan, J. Photoacoustic Probes for Ratiometric Imaging of Copper(II). J. Am. Chem. Soc. 2015, 137, 15628–15631. [Google Scholar] [CrossRef] [PubMed]
- Cash, K.J.; Li, C.; Xia, J.; Wang, L.V.; Clark, H.A. Optical drug monitoring: Photoacoustic imaging of nanosensors to monitor therapeutic lithium In Vivo. ACS Nano 2015, 9, 1692–1698. [Google Scholar] [CrossRef] [PubMed]
- Dragulescu-Andrasi, A.; Kothapalli, S.R.; Tikhomirov, G.A.; Rao, J.; Gambhir, S.S. Activatable oligomerizable imaging agents for photoacoustic imaging of furin-like activity in living subjects. J. Am. Chem. Soc. 2013, 135, 11015–11022. [Google Scholar] [CrossRef] [PubMed]
- Razansky, D.; Harlaar, N.J.; Hillebrands, J.L.; Taruttis, A.; Herzog, E.; Zeebregts, C.J.; van Dam, G.M.; Ntziachristos, V. Multispectral optoacoustic tomography of matrix metalloproteinase activity in vulnerable human carotid plaques. Mol. Imaging Biol. 2012, 14, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Zhu, L.; Nie, L.; Sun, X.; Cheng, L.; Wu, C.; Niu, G.; Chen, X.; Liu, Z. Visualization of protease activity In Vivo using an activatable photo-acoustic imaging probe based on CuS nanoparticles. Theranostics 2014, 4, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Estrella, V.; Chen, T.; Lloyd, M.; Wojtkowiak, J.; Cornnell, H.H.; Ibrahim-Hashim, A.; Bailey, K.; Balagurunathan, Y.; Rothberg, J.M.; Sloane, B.F.; et al. Acidity generated by the tumor microenvironment drives local invasion. Cancer Res. 2013, 73, 1524–1535. [Google Scholar] [CrossRef] [PubMed]
- Longo, D.L.; Bartoli, A.; Consolino, L.; Bardini, P.; Arena, F.; Schwaiger, M.; Aime, S. In Vivo Imaging of Tumor Metabolism and Acidosis by Combining PET and MRI-CEST pH Imaging. Cancer Res. 2016, 76, 6463–6470. [Google Scholar] [CrossRef] [PubMed]
- Rouleau, L.; Berti, R.; Ng, V.W.; Matteau-Pelletier, C.; Lam, T.; Saboural, P.; Kakkar, A.K.; Lesage, F.; Rheaume, E.; Tardif, J.C. VCAM-1-targeting gold nanoshell probe for photoacoustic imaging of atherosclerotic plaque in mice. Contrast Media Mol. Imaging 2013, 8, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.W.; Galanzha, E.I.; Shashkov, E.V.; Moon, H.M.; Zharov, V.P. Golden carbon nanotubes as multimodal photoacoustic and photothermal high-contrast molecular agents. Nat. Nanotechnol. 2009, 4, 688–694. [Google Scholar] [CrossRef] [PubMed]
- De la Zerda, A.; Liu, Z.; Bodapati, S.; Teed, R.; Vaithilingam, S.; Khuri-Yakub, B.T.; Chen, X.; Dai, H.; Gambhir, S.S. Ultrahigh sensitivity carbon nanotube agents for photoacoustic molecular imaging in living mice. Nano Lett. 2010, 10, 2168–2172. [Google Scholar] [CrossRef] [PubMed]
- De la Zerda, A.; Bodapati, S.; Teed, R.; May, S.Y.; Tabakman, S.M.; Liu, Z.; Khuri-Yakub, B.T.; Chen, X.; Dai, H.; Gambhir, S.S. Family of enhanced photoacoustic imaging agents for high-sensitivity and multiplexing studies in living mice. ACS Nano 2012, 6, 4694–4701. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liu, C.; Gong, X.; Hu, D.; Lin, R.; Sheng, Z.; Zheng, C.; Yan, M.; Chen, J.; Cai, L.; et al. In vivo photoacoustic molecular imaging of breast carcinoma with folate receptor-targeted indocyanine green nanoprobes. Nanoscale 2014, 6, 14270–14279. [Google Scholar] [CrossRef] [PubMed]
- Galanzha, E.I.; Shashkov, E.V.; Kelly, T.; Kim, J.W.; Yang, L.; Zharov, V.P. In vivo magnetic enrichment and multiplex photoacoustic detection of circulating tumour cells. Nat. Nanotechnol. 2009, 4, 855–860. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.K. Molecular regulation of vessel maturation. Nat. Med. 2003, 9, 685–693. [Google Scholar] [CrossRef] [PubMed]
- Neeman, M.; Gilad, A.A.; Dafni, H.; Cohen, B. Molecular imaging of angiogenesis. J. Magn. Reson. Imaging 2007, 25, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Pysz, M.A.; Gambhir, S.S.; Willmann, J.K. Molecular imaging: Current status and emerging strategies. Clin. Radiol. 2010, 65, 500–516. [Google Scholar] [CrossRef] [PubMed]
- Cunha, L.; Horvath, I.; Ferreira, S.; Lemos, J.; Costa, P.; Vieira, D.; Veres, D.S.; Szigeti, K.; Summavielle, T.; Mathe, D.; et al. Preclinical imaging: An essential ally in modern biosciences. Mol. Diagn. Ther. 2014, 18, 153–173. [Google Scholar] [CrossRef] [PubMed]
- O’Farrell, A.C.; Shnyder, S.D.; Marston, G.; Coletta, P.L.; Gill, J.H. Non-invasive molecular imaging for preclinical cancer therapeutic development. Br. J. Pharmacol. 2013, 169, 719–735. [Google Scholar] [CrossRef] [PubMed]
- Weissleder, R.; Pittet, M.J. Imaging in the era of molecular oncology. Nature 2008, 452, 580–589. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Jia, C.; Huang, S.W.; O’Donnell, M.; Gao, X. Multifunctional nanoparticles as coupled contrast agents. Nat. Commun. 2010, 1, 41. [Google Scholar] [CrossRef] [PubMed]
- Gong, H.; Peng, R.; Liu, Z. Carbon nanotubes for biomedical imaging: The recent advances. Adv. Drug Deliv. Rev. 2013, 65, 1951–1963. [Google Scholar] [CrossRef] [PubMed]
- Cheheltani, R.; Ezzibdeh, R.M.; Chhour, P.; Pulaparthi, K.; Kim, J.; Jurcova, M.; Hsu, J.C.; Blundell, C.; Litt, H.I.; Ferrari, V.A.; et al. Tunable, biodegradable gold nanoparticles as contrast agents for computed tomography and photoacoustic imaging. Biomaterials 2016, 102, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Jing, L.; Liang, X.; Deng, Z.; Feng, S.; Li, X.; Huang, M.; Li, C.; Dai, Z. Prussian blue coated gold nanoparticles for simultaneous photoacoustic/CT bimodal imaging and photothermal ablation of cancer. Biomaterials 2014, 35, 5814–5821. [Google Scholar] [CrossRef] [PubMed]
- Lv, G.; Guo, W.; Zhang, W.; Zhang, T.; Li, S.; Chen, S.; Eltahan, A.S.; Wang, D.; Wang, Y.; Zhang, J.; et al. Near-Infrared Emission CuInS/ZnS Quantum Dots: All-in-One Theranostic Nanomedicines with Intrinsic Fluorescence/Photoacoustic Imaging for Tumor Phototherapy. ACS Nano 2016, 10, 9637–9645. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.H.; Liao, A.H.; Chen, J.H.; Wang, C.R.; Li, P.C. Photoacoustic/ultrasound dual-modality contrast agent and its application to thermotherapy. J. Biomed. Opt. 2012, 17, 045001. [Google Scholar] [CrossRef] [PubMed]
- Hannah, A.; Luke, G.; Wilson, K.; Homan, K.; Emelianov, S. Indocyanine green-loaded photoacoustic nanodroplets: Dual contrast nanoconstructs for enhanced photoacoustic and ultrasound imaging. ACS Nano 2014, 8, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Hong, L.; Liu, Y.; Simon, J.D. Binding of metal ions to melanin and their effects on the aerobic reactivity. Photochem. Photobiol. 2004, 80, 477–481. [Google Scholar] [CrossRef]
- Li, Y.; Xie, Y.; Wang, Z.; Zang, N.; Carniato, F.; Huang, Y.; Andolina, C.M.; Parent, L.R.; Ditri, T.B.; Walter, E.D.; et al. Structure and Function of Iron-Loaded Synthetic Melanin. ACS Nano 2016, 10, 10186–10194. [Google Scholar] [CrossRef] [PubMed]
- Pierre, V.C.; Allen, M.J.; Caravan, P. Contrast agents for MRI: 30+ years and where are we going? J. Biol. Inorg. Chem. 2014, 19, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Aime, S.; Botta, M.; Terreno, E. Gd(III)-based contrast agents for MRI. In Advances in Inorganic Chemistry—Including Bioinorganic Studies; Elsevier B.V.: Amsterdam, The Netherlands, 2005; Volume 57, pp. 173–237. [Google Scholar]
- Botta, M.; Tei, L. Relaxivity Enhancement in Macromolecular and Nanosized GdIII-Based MRI Contrast Agents. Eur. J. Inorg. Chem. 2012, 1945–1960. [Google Scholar] [CrossRef]
- Sherry, A.D.; Caravan, P.; Lenkinski, R.E. Primer on gadolinium chemistry. J. Magn. Reson. Imaging 2009, 30, 1240–1248. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.; Li, Y.; Hu, C.; Huang, Y.; He, Q.; Gao, H. Melanin-originated carbonaceous dots for triple negative breast cancer diagnosis by fluorescence and photoacoustic dual-mode imaging. J. Colloid Interface Sci. 2017, 497, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Liu, Y.; Chen, J.; He, Q.; Gao, H. A simple one-step synthesis of melanin-originated red shift emissive carbonaceous dots for bioimaging. J. Colloid Interface Sci. 2016, 480, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Lammers, T.; Aime, S.; Hennink, W.E.; Storm, G.; Kiessling, F. Theranostic nanomedicine. Acc. Chem. Res. 2011, 44, 1029–1038. [Google Scholar] [CrossRef] [PubMed]
- Wolfram, J.; Zhu, M.; Yang, Y.; Shen, J.; Gentile, E.; Paolino, D.; Fresta, M.; Nie, G.; Chen, C.; Shen, H.; et al. Safety of Nanoparticles in Medicine. Curr. Drug Targets 2015, 16, 1671–1681. [Google Scholar] [CrossRef] [PubMed]
- Ings, R.M. The melanin binding of drugs and its implications. Drug Metab. Rev. 1984, 15, 1183–1212. [Google Scholar] [CrossRef] [PubMed]
- Llovet, J.M.; Ricci, S.; Mazzaferro, V.; Hilgard, P.; Gane, E.; Blanc, J.F.; de Oliveira, A.C.; Santoro, A.; Raoul, J.L.; Forner, A.; et al. Sorafenib in advanced hepatocellular carcinoma. N. Engl. J. Med. 2008, 359, 378–390. [Google Scholar] [CrossRef] [PubMed]
- De Melo-Diogo, D.; Pais-Silva, C.; Dias, D.R.; Moreira, A.F.; Correia, I.J. Strategies to Improve Cancer Photothermal Therapy Mediated by Nanomaterials. Adv. Healthc. Mater. 2017, 6. [Google Scholar] [CrossRef] [PubMed]
- ANSI Z136.3 American National Standard for Safe Use of Lasers in Health Care, 2011th ed.; Laser Institute of America: Orlando, FL, USA, 2011.
- Liu, Y.; Ai, K.; Liu, J.; Deng, M.; He, Y.; Lu, L. Dopamine-melanin colloidal nanospheres: An efficient near-infrared photothermal therapeutic agent for In Vivo cancer therapy. Adv. Mater. 2013, 25, 1353–1359. [Google Scholar] [CrossRef] [PubMed]
- Pasparakis, G.; Manouras, T.; Vamvakaki, M.; Argitis, P. Harnessing photochemical internalization with dual degradable nanoparticles for combinatorial photo-chemotherapy. Nat. Commun. 2014, 5, 3623. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, J.; Wang, Y.; Wang, C.; Xiao, J.; Zhang, Q.; Cheng, Y. Multi-responsive photothermal-chemotherapy with drug-loaded melanin-like nanoparticles for synergetic tumor ablation. Biomaterials 2016, 81, 114–124. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Shin, D.W.; Chen, K.; Gheysens, O.; Cao, Q.; Wang, S.X.; Gambhir, S.S.; Chen, X. Peptide-labeled near-infrared quantum dots for imaging tumor vasculature in living subjects. Nano Lett. 2006, 6, 669–676. [Google Scholar] [CrossRef] [PubMed]
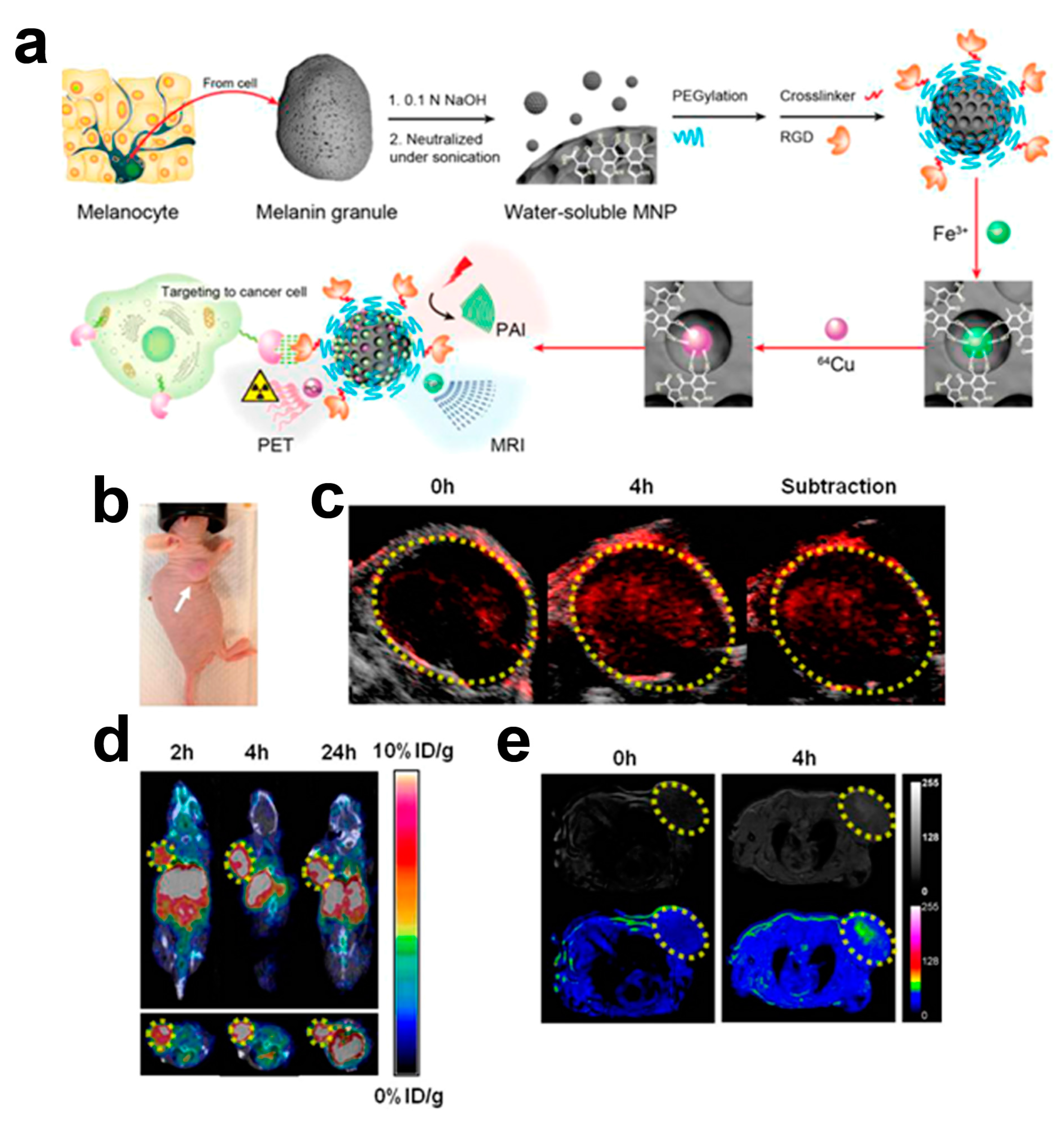
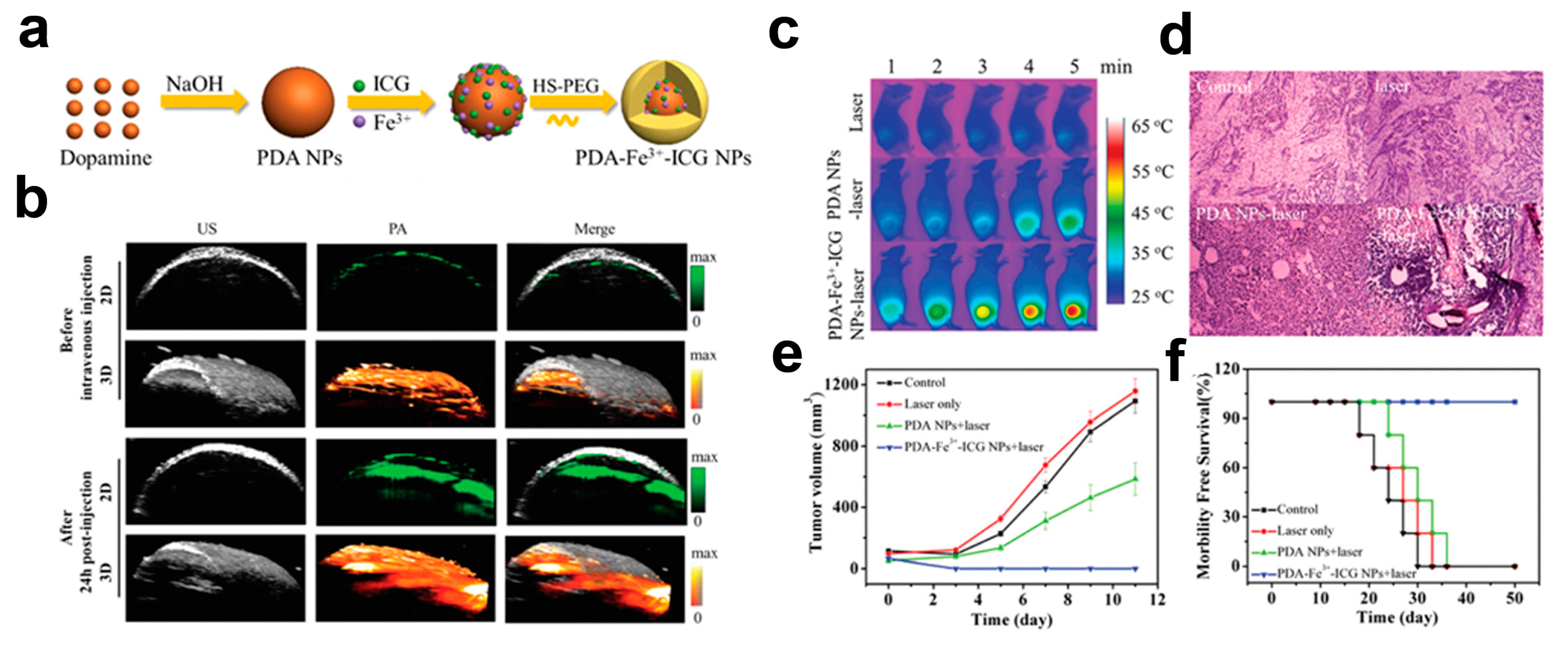
| Contrast Agent | Description | Size (nm) | Target | Principal Applications | Ref. |
|---|---|---|---|---|---|
| PEG-MNP | Pegylated polydopamine nanoparticles | 50 | 3D OA vascular imaging | [29] | |
| PEG-MNP | Linear polydopamine nanoparticles | - | OA vascular imaging | [30] | |
| PEG-MNP | Melanin nanoparticle | 7–10 | Assessing tumor vasculature and response to antiangiogenic therapy | [31] | |
| PEG-MNP-CA | Pegylated polydopamine nanoparticles decorated with citraconic amide | 130 | Tumor acidic microenvironment | OA cancer imaging | [32] |
| 64Cu-Fe-cRGDfC-PEG-MNP | Pegylated polydopamine nanoparticles conjugated to cyclic RGD peptide and loaded with 64Cu2+ and Fe3+ | 5–10 | αvβ3 integrins | OA cancer imaging of integrins and multimodality imaging (OA/PET/MRI) | [33] |
| DOX-RGDC-PEG-MNP | Pegylated polydopamine nanoparticles conjugated to cyclic RGD peptide and loaded with doxorubicin | 120 | αvβ3 integrins | OA cancer imaging of integrins and chemo-photothermal therapy of tumors | [34] |
| 125I-Pt-cRGDyC-PEG-MNP-GNR | Gold nanorods decorated with pegylated polydopamine nanoparticles conjugated to cyclic RGD peptide and loaded with cisplatin and 125I | 54 | αvβ3 integrins | OA cancer imaging of integrins, chemo-photothermal therapy of tumors and multimodality imaging (OA, SPECT) | [35] |
| ICG-Fe-PEG-MNP | Pegylated polydopamine nanoparticles loaded with indocyanine green and Fe3+ | 146 | Photothermal therapy of tumors and multimodality imaging (OA, MRI) | [36] | |
| SRF-PEG-MNP | Pegylated polydopamine nanoparticles loaded with sorafenib | 60 | Imaging-guided chemotherapy | [37] | |
| Gd-64Cu-PEG-MNP | Pegylated polydopamine nanoparticles loaded with Gd3+ and 64Cu2+ | 15 | Multimodality imaging (OA/MRI/PET) | [38] | |
| Fe-MNP | Polydopamine nanoparticles loaded with Fe3+ | 98 | Multimodality imaging (OA/MRI) | [39] | |
| MNP-CD | Melanin-decorated carbonaceous dots | 40 | Multimodality imaging (OA/OI) | [40] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Longo, D.L.; Stefania, R.; Aime, S.; Oraevsky, A. Melanin-Based Contrast Agents for Biomedical Optoacoustic Imaging and Theranostic Applications. Int. J. Mol. Sci. 2017, 18, 1719. https://doi.org/10.3390/ijms18081719
Longo DL, Stefania R, Aime S, Oraevsky A. Melanin-Based Contrast Agents for Biomedical Optoacoustic Imaging and Theranostic Applications. International Journal of Molecular Sciences. 2017; 18(8):1719. https://doi.org/10.3390/ijms18081719
Chicago/Turabian StyleLongo, Dario Livio, Rachele Stefania, Silvio Aime, and Alexander Oraevsky. 2017. "Melanin-Based Contrast Agents for Biomedical Optoacoustic Imaging and Theranostic Applications" International Journal of Molecular Sciences 18, no. 8: 1719. https://doi.org/10.3390/ijms18081719
APA StyleLongo, D. L., Stefania, R., Aime, S., & Oraevsky, A. (2017). Melanin-Based Contrast Agents for Biomedical Optoacoustic Imaging and Theranostic Applications. International Journal of Molecular Sciences, 18(8), 1719. https://doi.org/10.3390/ijms18081719





