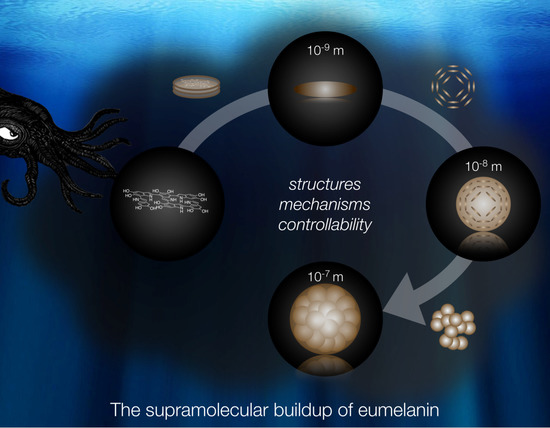
The Supramolecular Buildup of Eumelanin: Structures, Mechanisms, Controllability
Abstract
1. Introduction
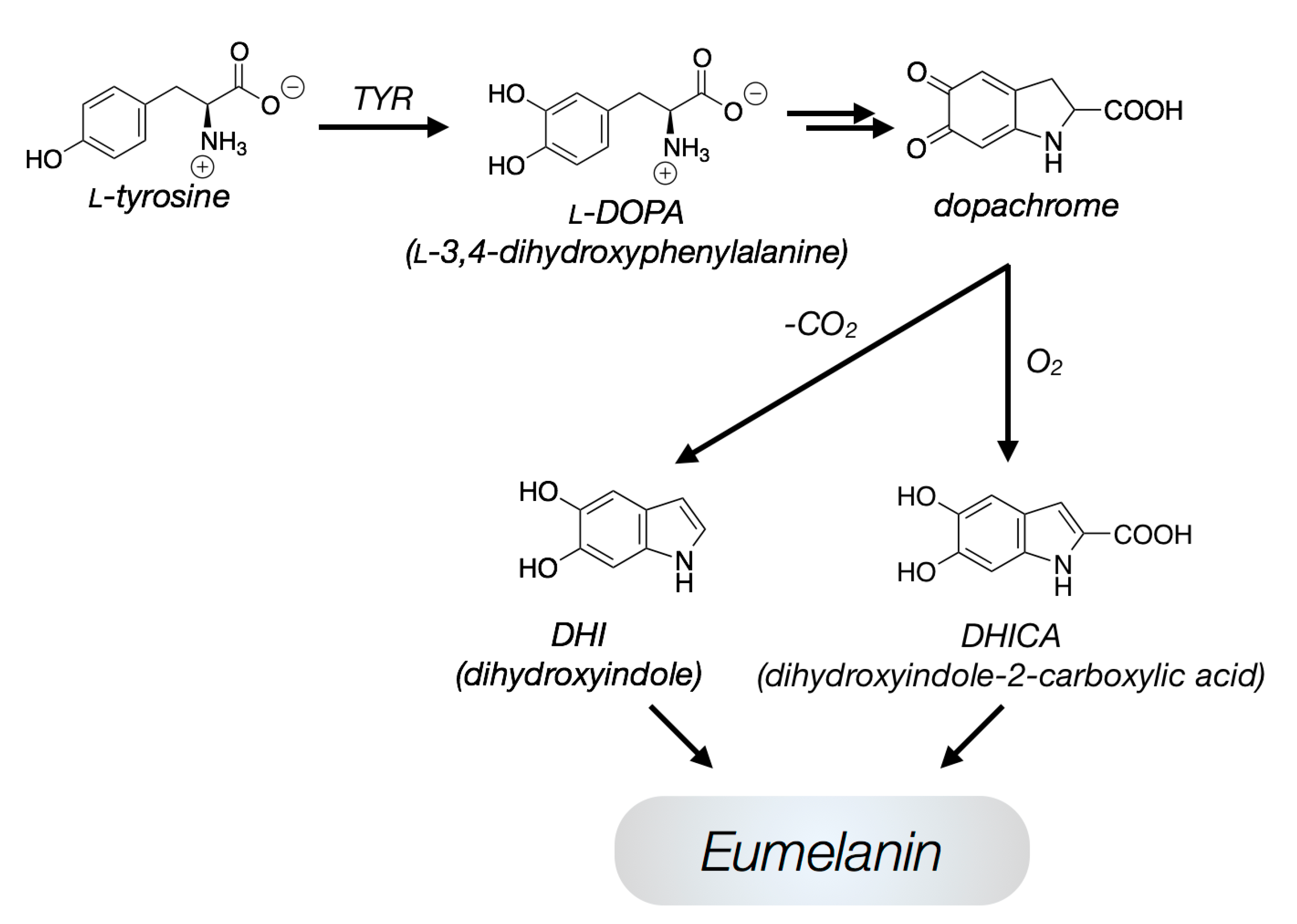
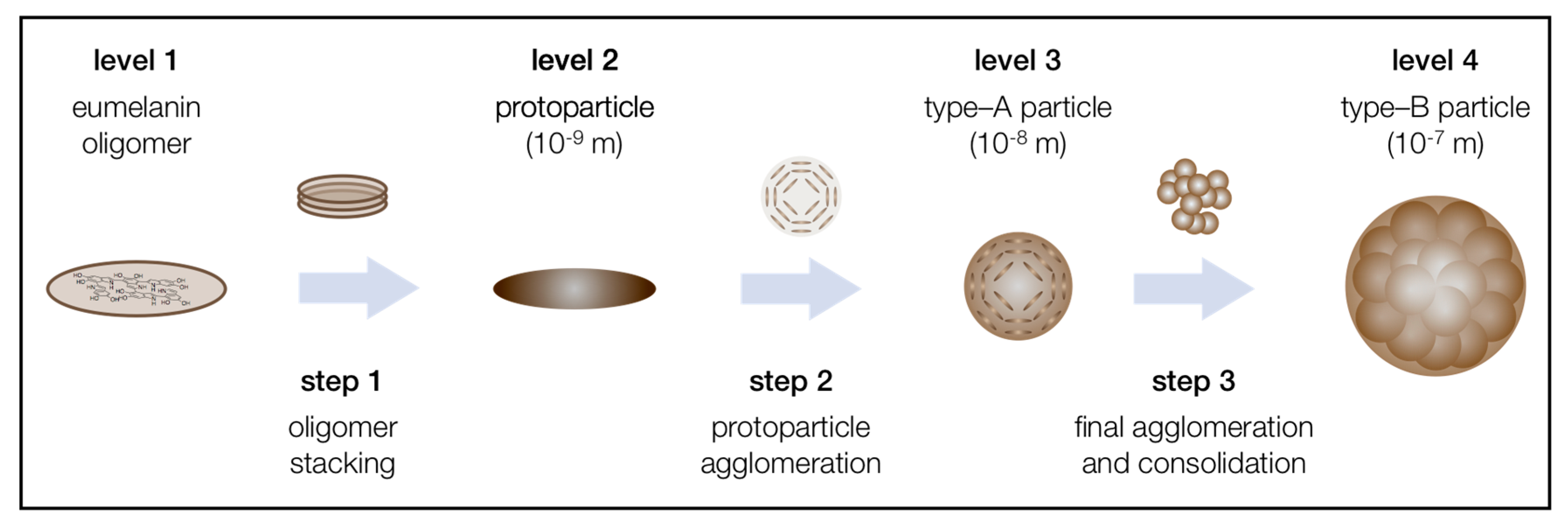
2. Structures
2.1. General Remarks
2.2. Level 1—Oligomeric Sheets
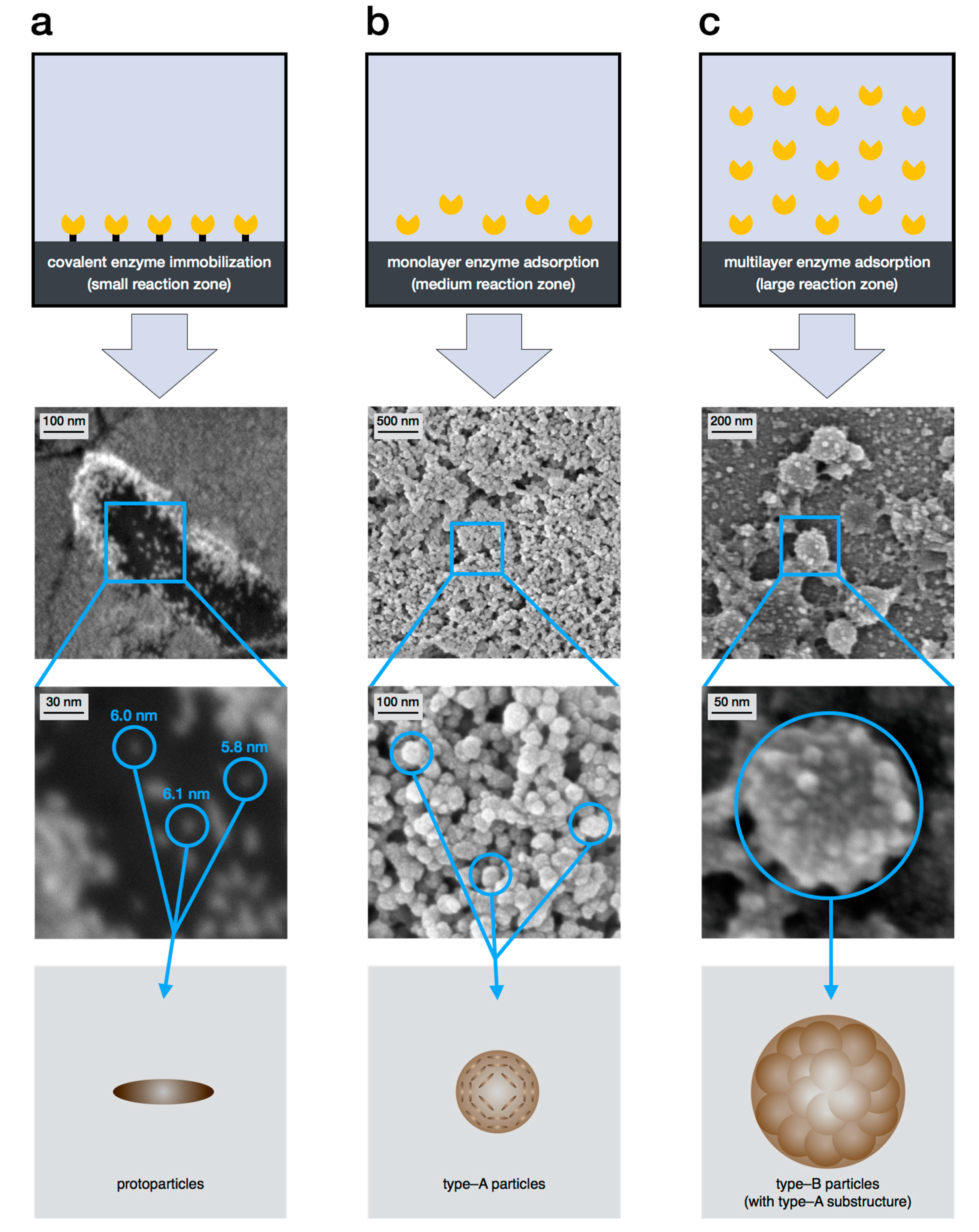
2.3. Level 2—Protoparticles
2.4. Level 3—Type-A Particles
2.5. Level 4—Type-B Particles
3. Mechanisms
3.1. General Remarks
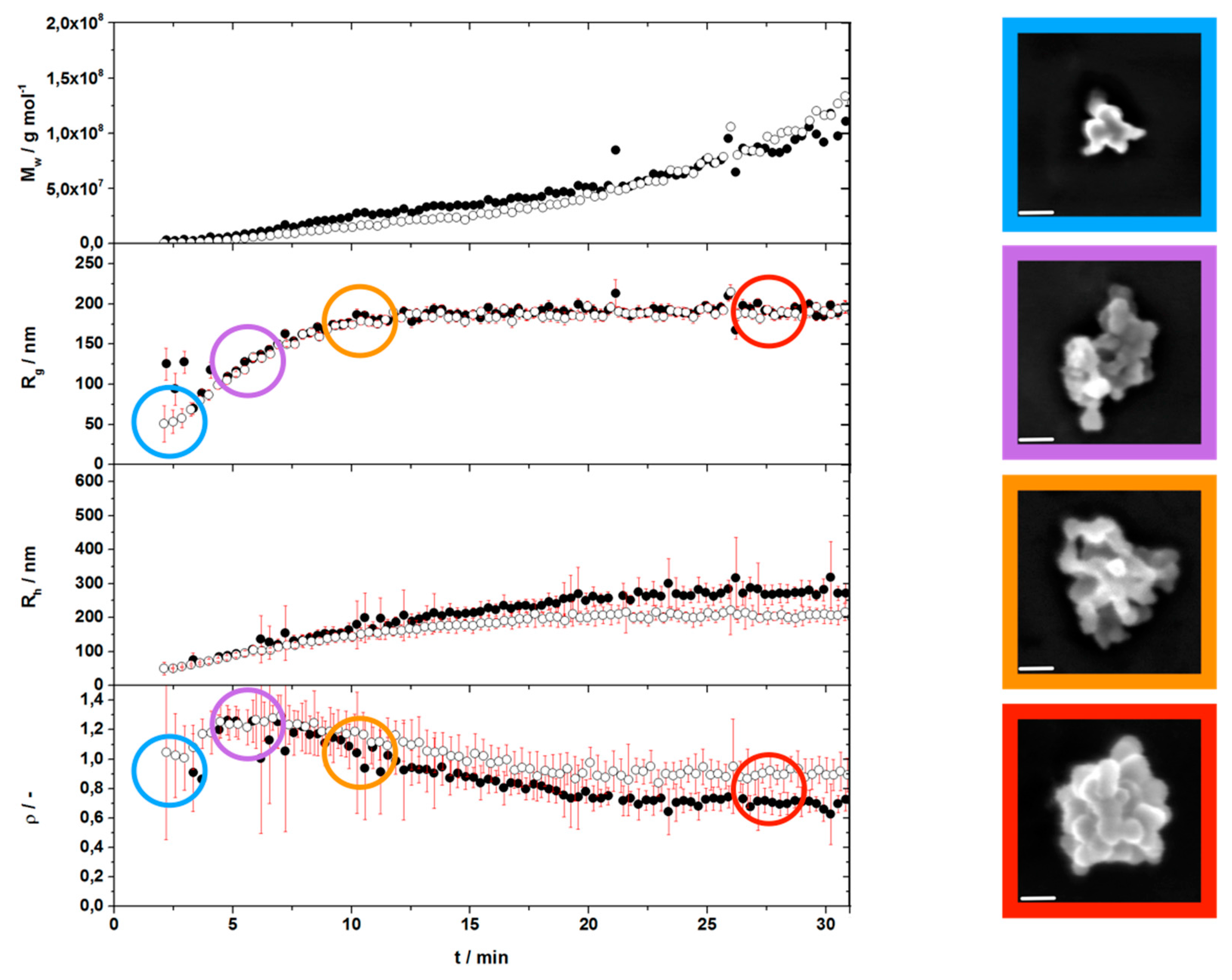
3.2. Agglomeration Step 1—From Level 1 to Level 2
3.3. Agglomeration Step 2—From Level 2 to Level 3
3.4. Agglomeration Step 3—From Level 3 to the Final Level 4
4. Controllability
4.1. Motivation
4.2. Aggregation Breaking and Specific Syntheses in Solution
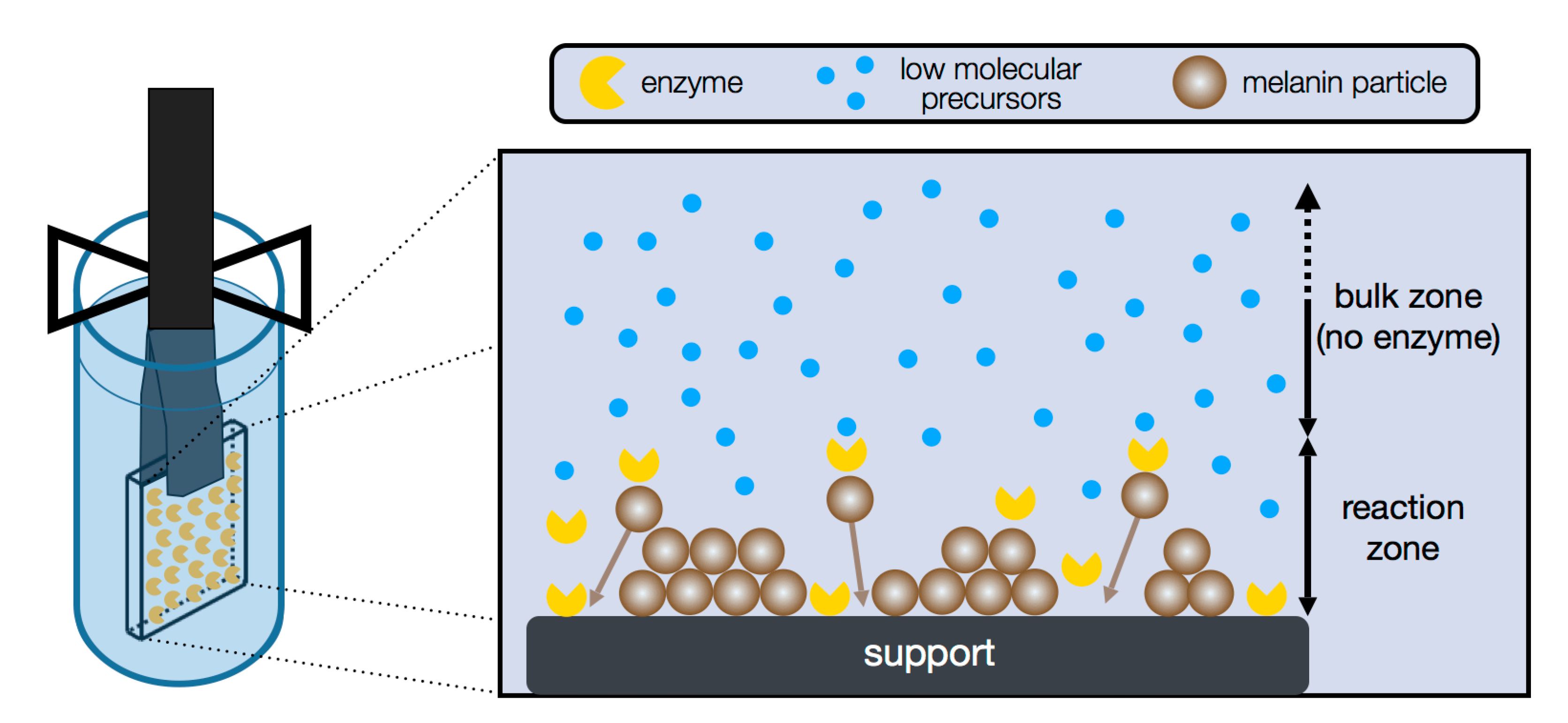
4.3. Specific Synthesis via Enzyme Mediated Autodeposition
5. Résumé and Future Perspectives
Author Contributions
Conflicts of Interest
References
- Schweitzer, A.D.; Revskaya, E.; Chu, P.; Pazo, V.; Friedman, M.; Nosanchuk, J.D.; Cahill, S.; Frases, S.; Casadevall, A.; Dadachova, E. Melanin-coated naonparticles for protection of bone marrow during radiation therapy of cancer. Int. J. Radiat. Oncol. Biol. Phys. 2010, 78, 1494–1502. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ai, K.; Liu, J.; Deng, M.; He, Y.; Lu, L. Dopamine-melanin colloidal nanospheres: An efficient near-infrared photothermal therapeutic agent for in vivo cancer therapy. Adv. Mater. 2013, 25, 1353–1359. [Google Scholar] [CrossRef] [PubMed]
- Meredith, P.; Sarna, T. The physical and chemical properties of eumelanin. Pigment Cell Res. 2006, 19, 572–594. [Google Scholar] [CrossRef] [PubMed]
- D’Ischia, M.; Napolitano, A.; Pezzella, A.; Meredith, P.; Sarna, T. Chemische und strukturelle Vielfalt der Eumelanine−ein kaum erforschtes optoelektronisches Biopolymer. Angew. Chem. 2009, 121, 3972–3979. [Google Scholar] [CrossRef]
- Liu, Y.; Ai, K.; Lu, L. Polydopamine and its derivative materials: Synthesis and promising applications in energy, environmental, and biomedical fields. Chem. Rev. 2014, 114, 5057–5115. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Ren, J.; Han, B.; Xu, L.; Han, L.; Jia, L. Stability of polydopamine and poly(DOPA) melanin-like films on the surface of polymer membranes under strongly acidic and alkaline conditions. Colloids Surf. B Biointerfaces 2013, 110, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Panzella, L.; Gentile, G.; D’Errico, G.; Della Vecchia, N.F.; Errico, M.E.; Napolitano, A.; Carfagna, C.; D’Ischia, M. Atypical structural and π-electron features of a melanin polymer that lead to superior free-radical-scavenging properties. Angew. Chem. Int. Ed. 2013, 52, 12684–12687. [Google Scholar] [CrossRef] [PubMed]
- Fisher, O.Z.; Larson, B.L.; Hill, P.S.; Graupner, D.; Nguyen-Kim, M.T.; Kehr, N.S.; de Cola, L.; Langer, R.; Anderson, D.G. Melanin-like hydrogels derived from gallic macromers. Adv. Mater. 2012, 24, 3032–3036. [Google Scholar] [CrossRef] [PubMed]
- Batagin-neto, A.; Bronze-uhle, S. Electronic structure calculations of ESR. Phys. Chem. Chem. Phys. 2015, 17, 7264–7274. [Google Scholar] [CrossRef] [PubMed]
- Nighswander-Rempel, S.P.; Riesz, J.; Gilmore, J.; Bothma, J.P.; Meredith, P. Quantitative fluorescence excitation spectra of synthetic eumelanin. J. Phys. Chem. B 2005, 109, 20629–20635. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Fan, Q.; Yang, M.; Cheng, K.; Lu, X.; Zhang, L.; Huang, W.; Cheng, Z. Engineering melanin nanoparticles as an efficient drug-delivery system for imaging-guided chemotherapy. Adv. Mater. 2015, 27, 5063–5069. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, J.; Wang, Y.; Wang, C.; Xiao, J.; Zhang, Q.; Cheng, Y. Multi-responsive photothermal-chemotherapy with drug-loaded melanin-like nanoparticles for synergetic tumor ablation. Biomaterials 2016, 81, 114–124. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Jiang, C.; Zhang, D.; Wang, Y.; Ren, X.; Ai, K.; Chen, X.; Lu, L. Targeted polydopamine nanoparticles enable photoacoustic imaging guided chemo-photothermal synergistic therapy of tumor. Acta Biomater. 2017, 47, 124–134. [Google Scholar] [CrossRef] [PubMed]
- Mostert, A.B.; Powell, B.J.; Pratt, F.L.; Hanson, G.R.; Sarna, T.; Gentle, I.R.; Meredith, P. Role of semiconductivity and ion transport in the electrical conduction of melanin. Proc. Natl. Acad. Sci. USA 2012, 109, 8943–8947. [Google Scholar] [CrossRef] [PubMed]
- Clulow, A.J.; Mostert, A.B.; Sheliakina, M.; Nelson, A.; Booth, N.; Burn, P.L.; Gentle, I.R.; Meredith, P. The structural impact of water sorption on device-quality melanin thin films. Soft Matter 2017, 13, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Ito, S. A chemist’s view of melanogenesis. Pigment Cell Res. 2003, 16, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Reale, S.; Crucianelli, M.; Pezzella, A.; d’Ischia, M.; de Angelis, F. Exploring the frontiers of synthetic eumelanin polymers by high-resolution matrix-assisted laser/desorption ionization mass spectrometry. J. Mass Spectrom. 2012, 47, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Panzella, L.; Pezzella, A.; Napolitano, A.; d’Ischia, M. The first 5,6-dihydroxyindole tetramer by oxidation of 5,5′,6,6′-tetrahydroxy-2,4′-biindolyl and an unexpected issue of positional reactivity en route to eumelanin-related polymers. Org. Lett. 2007, 9, 1411–1414. [Google Scholar] [CrossRef] [PubMed]
- Pezzella, A.; Panzella, L.; Natangelo, A.; Arzillo, M.; Napolitano, A.; d’Ischia, M. 5,6-Dihydroxyindole tetramers with “anomalous” interunit bonding patterns by oxidative coupling of 5,5′,6,6′-tetrahydroxy-2,7′-biindolyl: Emerging complexities on the way toward an improved model of eumelanin buildup. J. Org. Chem. 2007, 72, 9225–9230. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Suzuki, N.; Takebayashi, S.; Commo, S.; Wakamatsu, K. Neutral pH and copper ions promote eumelanogenesis after the dopachrome stage. Pigment Cell Melanoma Res. 2013, 26, 817–825. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, A.; d’Ischia, M.; Misuraca, G.; Prota, G. Effect of metal ions on the rearrangement of dopachrome. BBA Gen. Subj. 1987, 925, 203–209. [Google Scholar] [CrossRef]
- Panzella, L.; Napolitano, A.; d’Ischia, M. Is DHICA the key to dopachrome tautomerase and melanocyte functions? Pigment Cell Melanoma Res. 2011, 24, 248–249. [Google Scholar] [CrossRef] [PubMed]
- Micillo, R.; Panzella, L.; Koike, K.; Monfrecola, G.; Napolitano, A.; d’Ischia, M. “Fifty shades” of black and red or how carboxyl groups fine tune eumelanin and pheomelanin properties. Int. J. Mol. Sci. 2016, 17, 746. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Simon, J.D. Metal-ion interactions and the structural organization of Sepia eumelanin. Pigment Cell Res. 2005, 18, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Simon, J.D. Isolation and biophysical studies of natural eumelanins: Applications of imaging technologies and ultrafast spectroscopy. Pigment Cell Res. 2003, 16, 606–618. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.J.; Ju, K.Y.; Lee, J.K. The synthetic melanin nanoparticles having an excellent binding capacity of heavy metal ions. Bull. Korean Chem. Soc. 2012, 33, 3788–3792. [Google Scholar] [CrossRef]
- Watt, A.A.R.; Bothma, J.P.; Meredith, P. The supramolecular structure of melanin. Soft Matter 2009, 5, 3754. [Google Scholar] [CrossRef]
- Clancy, C.M.R.; Simon, J.D. Ultrastructural organization of eumelanin from Sepia officinalis measured by atomic force microscopy. Biochemistry 2001, 40, 13353–13360. [Google Scholar] [CrossRef] [PubMed]
- D’Ischia, M.; Wakamatsu, K.; Napolitano, A.; Briganti, S.; Garcia-Borron, J.C.; Kovacs, D.; Meredith, P.; Pezzella, A.; Picardo, M.; Sarna, T.; et al. Melanins and melanogenesis: Methods, standards, protocols. Pigment Cell Melanoma Res. 2013, 26, 616–633. [Google Scholar] [CrossRef] [PubMed]
- Clancy, C.M.R.; Nofsinger, J.B.; Hanks, R.K.; Simon, J.D. A hierarchical self-assembly of eumelanin. J. Phys. Chem. B 2000, 104, 7871–7873. [Google Scholar] [CrossRef]
- Büngeler, A.; Hämisch, B.; Huber, K.; Bremser, W.; Strube, O.I. Insight into the final step of the supramolecular buildup of eumelanin. Langmuir 2017. [Google Scholar] [CrossRef] [PubMed]
- Nofsinger, J.B.; Forest, S.E.; Eibest, L.M.; Gold, K.A.; Simon, J.D. Probing the building blocks of eumelanins using scanning electron microscopy. Pigment Cell Res. 2000, 13, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Arzillo, M.; Mangiapia, G.; Pezzella, A.; Heenan, R.K.; Radulescu, A.; Paduano, L.; d’Ischia, M. Eumelanin buildup on the nanoscale: Aggregate growth/assembly and visible absorption development in biomimetic 5,6-dihydroxyindole polymerization. Biomacromolecules 2012, 13, 2379–2390. [Google Scholar] [CrossRef] [PubMed]
- Lampel, A.; Mcphee, S.A.; Park, H.; Scott, G.G.; Humagain, S.; Hekstra, D.R.; Yoo, B.; Frederix, P.W.; Li, T.; Abzalimov, R.R.; et al. Polymeric peptide pigments with sequence-encoded properties. Science 2017, 356, 1064–1068. [Google Scholar] [CrossRef] [PubMed]
- D’Ischia, M.; Messersmith, P.B. From sequence to color. Science 2017, 356, 1011–1012. [Google Scholar] [CrossRef] [PubMed]
- Donato, L.; Algieri, C.; Rizzi, A.; Giorno, L. Kinetic study of tyrosinase immobilized on polymeric membrane. J. Memb. Sci. 2014, 454, 346–350. [Google Scholar] [CrossRef]
- Xiao, M.; Li, Y.; Allen, M.C.; Deheyn, D.D.; Yue, X.; Zhao, J.; Gianneschi, N.C.; Shawkey, M.D.; Dhinojwala, A. Bio-inspired structural colors produced via self-assembly of synthetic melanin nanoparticles. ACS Nano 2015, 9, 5454–5460. [Google Scholar] [CrossRef] [PubMed]
- Xiao, M.; Li, Y.; Zhao, J.; Wang, Z.; Gao, M.; Gianneschi, N.C.; Dhinojwala, A.; Shawkey, M.D. Stimuli-responsive structurally colored films from bioinspired synthetic melanin nanoparticles. Chem. Mater. 2016, 28, 5516–5521. [Google Scholar] [CrossRef]
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Mussel-inspired surface chemistry for multifunctional coatings. Science 2007, 318, 426–430. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.M.; Hwang, N.S.; Yeom, J.; Park, S.Y.; Messersmith, P.B.; Choi, I.S.; Langer, R.; Anderson, D.G.; Lee, H. One-step multipurpose surface functionalization by adhesive catecholamine. Adv. Funct. Mater. 2012, 22, 2949–2955. [Google Scholar] [CrossRef] [PubMed]
- Kasemset, S.; Lee, A.; Miller, D.J.; Freeman, B.D.; Sharma, M.M. Effect of polydopamine deposition conditions on fouling resistance, physical properties, and permeation properties of reverse osmosis membranes in oil/water separation. J. Membr. Sci. 2013, 208–216, 425–426. [Google Scholar] [CrossRef]
- Strube, O.I.; Büngeler, A.; Bremser, W. Site-specific in situ synthesis of eumelanin nanoparticles by an enzymatic autodeposition-like process. Biomacromolecules 2015, 16, 1608–1613. [Google Scholar] [CrossRef] [PubMed]
- Strube, O.I.; Büngeler, A.; Bremser, W. Enzyme mediated in situ synthesis and deposition of nonaggregated melanin protoparticles. Macromol. Mater. Eng. 2016, 301, 801–804. [Google Scholar] [CrossRef]
- Zajac, G.W.; Gallas, J.M.; Cheng, J.; Eisner, M.; Moss, S.C.; Alvarado-Swaisgood, A.E. The fundamental unit of synthetic melanin: A verification by tunneling microscopy of X-ray scattering results. Biochim. Biophys. Acta 1994, 1199, 271–278. [Google Scholar] [CrossRef]
- Gracio, J.D.A.; Singh, Â.M.K.; Ruch, D.; Buehler, M.J.; Al, C.E.T. Self-assembly of tetramers of eumelanin: Experiment, simulation. ACS Nano 2013, 1524–1532. [Google Scholar]
- Ancans, J.; Tobin, D.J.; Hoogduijn, M.J.; Smit, N.P.; Wakamatsu, K.; Thody, A.J. Melanosomal pH controls rate of melanogenesis, eumelanin/phaeomelanin ratio and melanosome maturation in melanocytes and melanoma cells. Exp. Cell Res. 2001, 268, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Pezzella, A.; Napolitano, A.; d’Ischia, M.; Prota, G.; Seraglia, R.; Traldi, P. Identification of partially degraded oligomers of 5,6-dihydroxyindole-2-carboxylic acid in Sepia melanin by matrix-assisted laser desorption/ionization mass spectrometry. Rapid Commun. Mass Spectrom. 1997, 11, 368–372. [Google Scholar] [CrossRef]
- Ju, K.; Lee, Y.; Lee, S.; Park, S.B.; Lee, J. Bioinspired polymerization of dopamine to generate melanin-like nanoparticles having an excellent free-radical-scavenging property. Macromolecules 2011, 12, 625–632. [Google Scholar] [CrossRef] [PubMed]
- D’Ischia, M.; Napolitano, A.; Ball, V.; Chen, C.-T.; Buehler, M.J. Polydopamine and eumelanin: From structure–property relationships to a unified tailoring strategy. Acc. Chem. Res. 2014, 47, 3541–3550. [Google Scholar] [CrossRef] [PubMed]
- Burchard, W.; Schmidt, M.; Stockmayer, W.H. Influence of hydrodynamic preaveraging on Quasi-Elastic scattering from flexible linear and star-branched macromolecules. Macromolecules 1980, 13, 580–587. [Google Scholar] [CrossRef]
- Burchard, W. Static and dynamic light scattering from branched polymers and biopolymers. Adv. Polym. Sci. 1983, 48, 1–124. [Google Scholar]
- Ascione, L.; Pezzella, A.; Ambrogi, V.; Carfagna, C.; d’Ischia, M. Intermolecular π-electron perturbations generate extrinsic visible contributions to eumelanin black chromophore in model polymers with interrupted interring conjugation. Photochem. Photobiol. 2013, 89, 314–318. [Google Scholar] [CrossRef] [PubMed]
- Martin, M.; Salazar, P.; Villalonga, R.; Campuzano, S.; Pingarron, J.M.; Gonzalez-Mora, J.L. Preparation of core-shell Fe3O4@poly(dopamine) magnetic nanoparticles for biosensor construction. J. Mater. Chem. B 2014, 2, 739–746. [Google Scholar] [CrossRef]
- Caruso, F. Immobilization and intracellular delivery of an anticancer drug using mussel-inspired polydopamine capsules. Biomacromolecules, 2012, 13, 2225–2228. [Google Scholar]
- Kim, J.H.; Lee, M.; Park, C.B. Polydopamine as a biomimetic electron gate for artificial photosynthesis. Angew. Chem. Int. Ed. 2014, 53, 6364–6368. [Google Scholar] [CrossRef] [PubMed]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Büngeler, A.; Hämisch, B.; Strube, O.I. The Supramolecular Buildup of Eumelanin: Structures, Mechanisms, Controllability. Int. J. Mol. Sci. 2017, 18, 1901. https://doi.org/10.3390/ijms18091901
Büngeler A, Hämisch B, Strube OI. The Supramolecular Buildup of Eumelanin: Structures, Mechanisms, Controllability. International Journal of Molecular Sciences. 2017; 18(9):1901. https://doi.org/10.3390/ijms18091901
Chicago/Turabian StyleBüngeler, Anne, Benjamin Hämisch, and Oliver I. Strube. 2017. "The Supramolecular Buildup of Eumelanin: Structures, Mechanisms, Controllability" International Journal of Molecular Sciences 18, no. 9: 1901. https://doi.org/10.3390/ijms18091901
APA StyleBüngeler, A., Hämisch, B., & Strube, O. I. (2017). The Supramolecular Buildup of Eumelanin: Structures, Mechanisms, Controllability. International Journal of Molecular Sciences, 18(9), 1901. https://doi.org/10.3390/ijms18091901