Understanding the Inguinal Sinus in Sheep (Ovis aries)—Morphology, Secretion, and Expression of Progesterone, Estrogens, and Prolactin Receptors
Abstract
1. Introduction
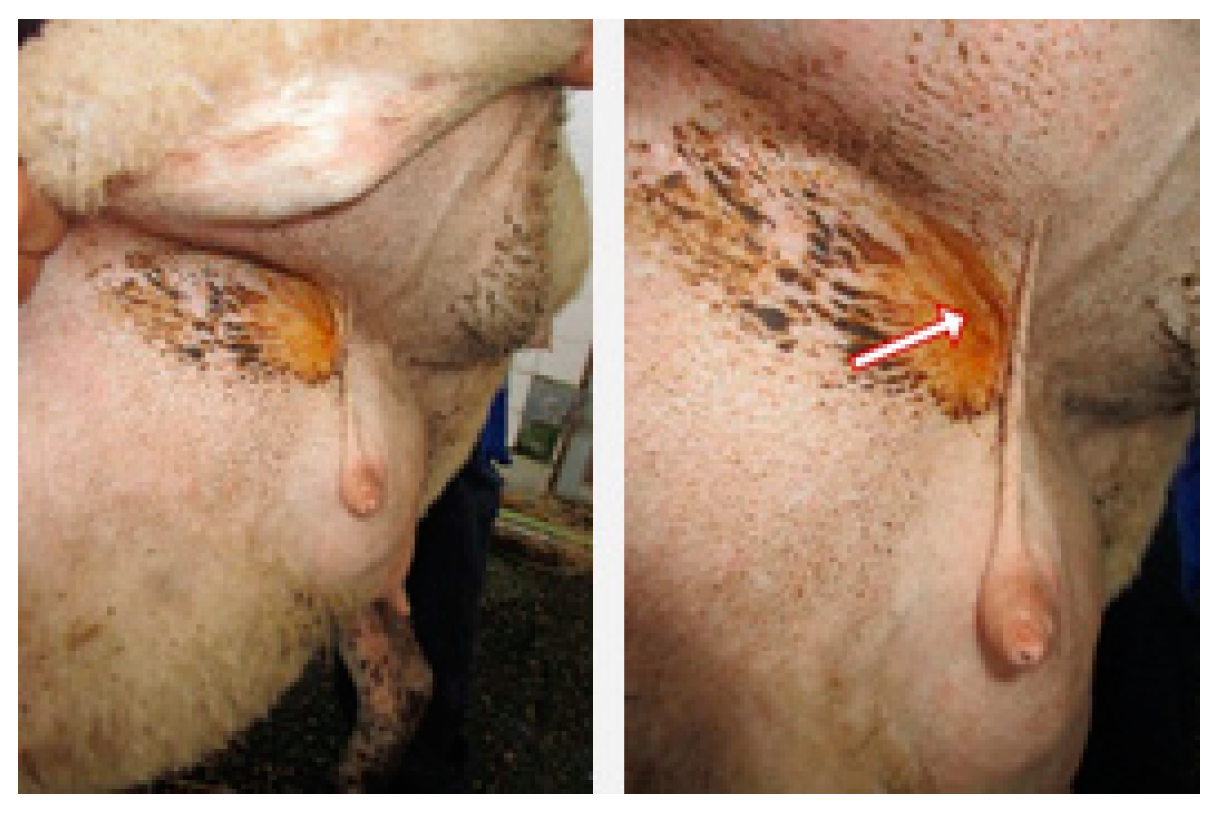
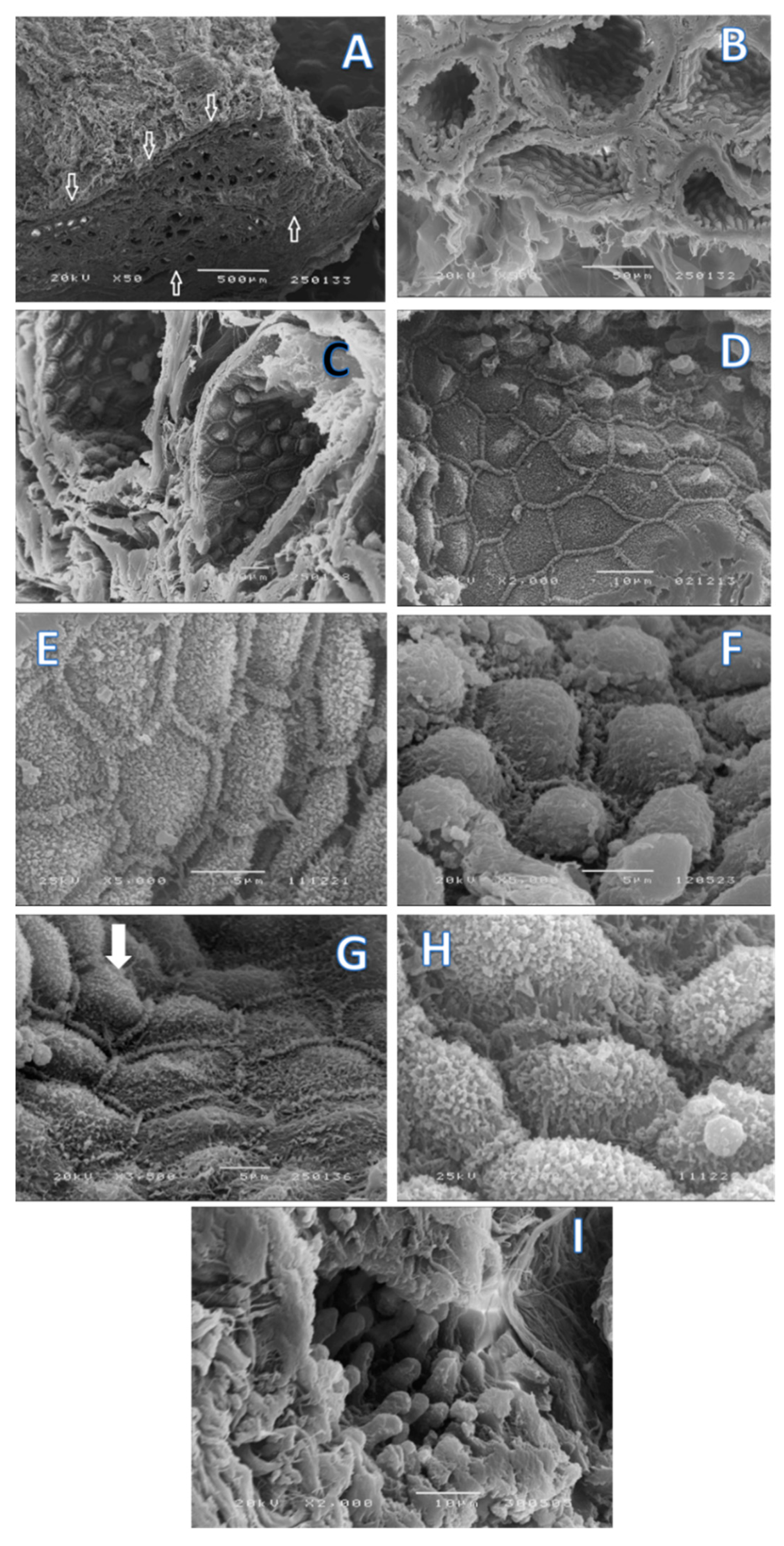
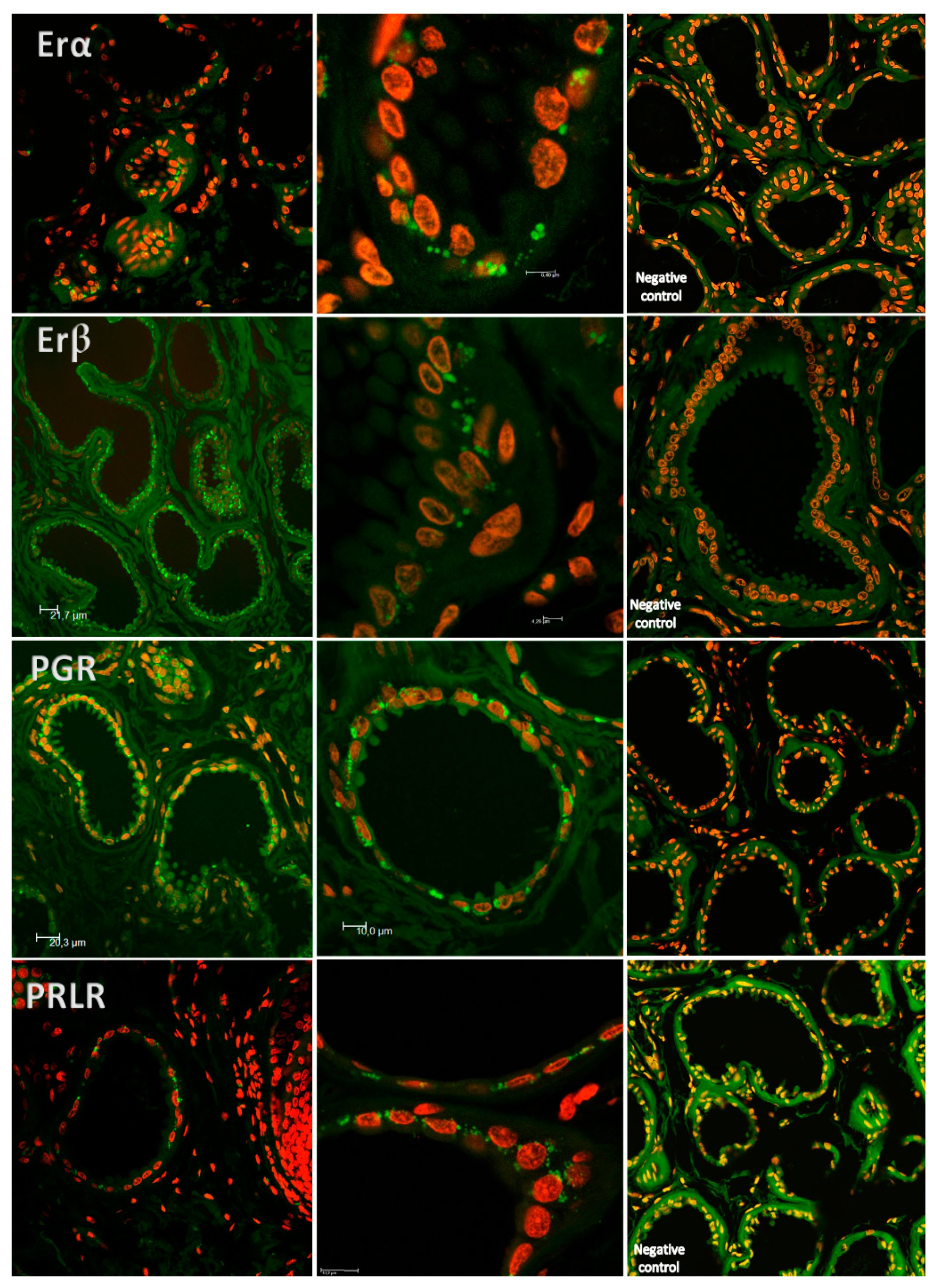
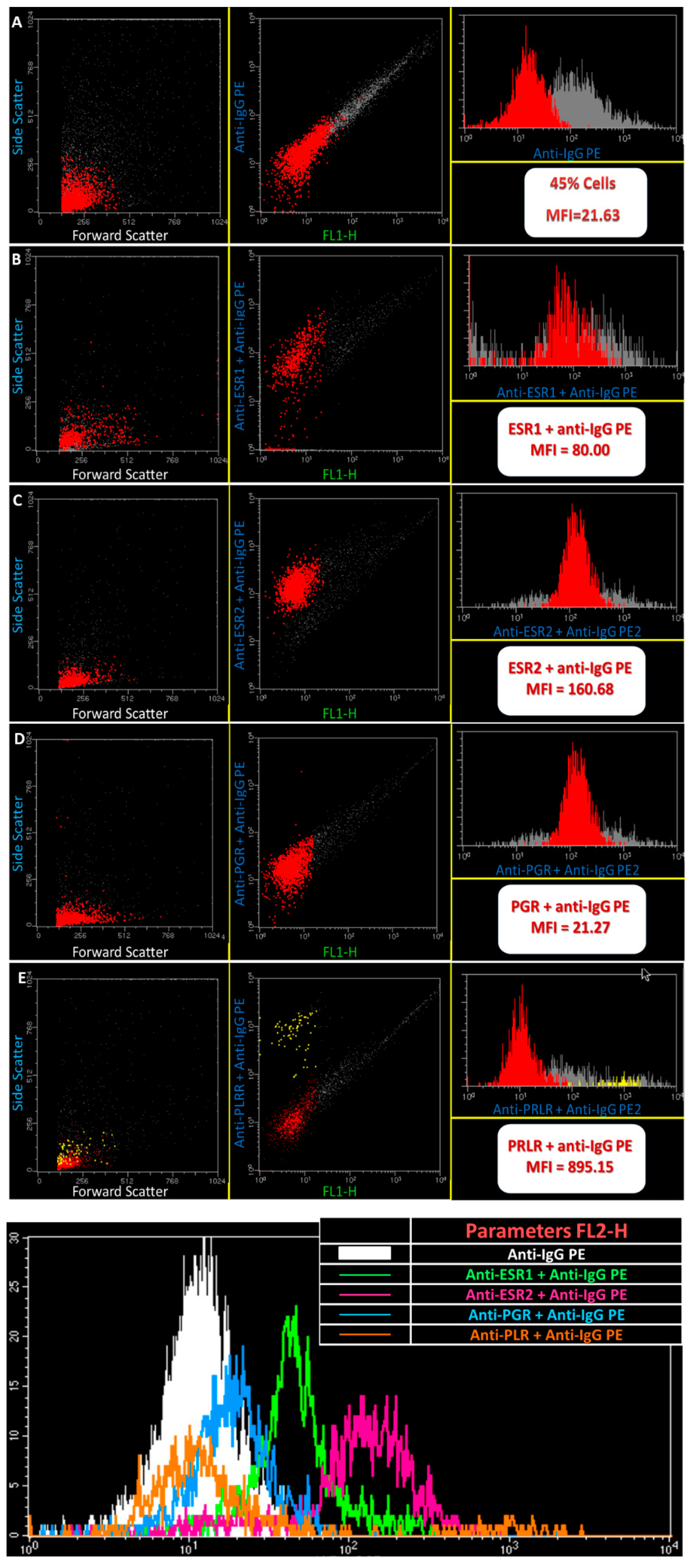
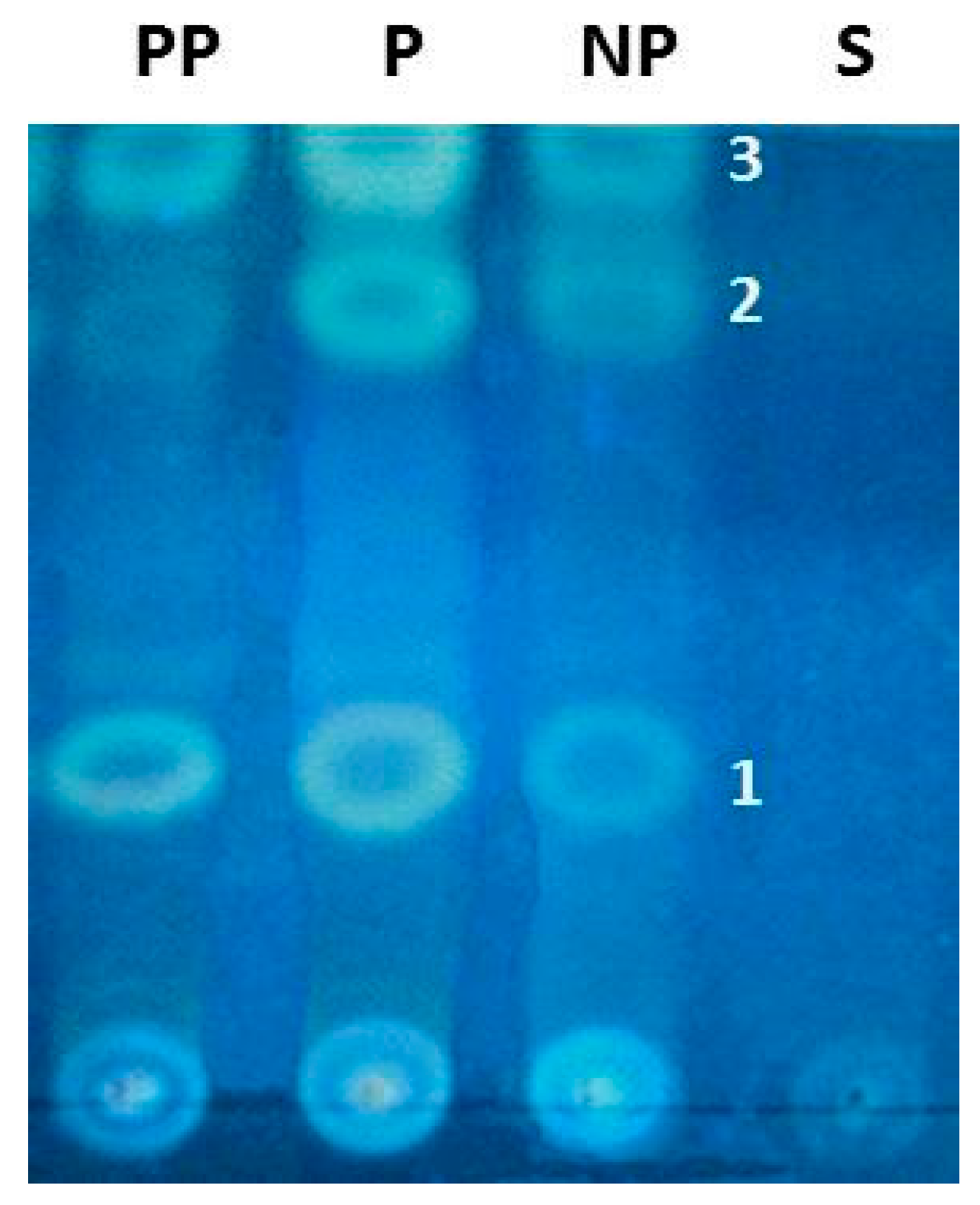
2. Results
3. Discussion
4. Materials and Methods
4.1. Histology Evaluation
4.2. Scanning Electron Microscopy
4.3. Flow Cytometry Analysis
4.4. Laser-Scanning Confocal Microscopy
4.5. Genomic Analysis
4.6. Progesterone Analysis
4.7. Chemical Studies
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Brockman, J.H.; Snowdon, C.; Roper, T.; Naguib, M.; Wynne-Eduards, K. Advances in the Study of Behavior; Elsevier Inc.: San Diego, CA, USA, 2009; Volume 36, ISBN 978-0-12-374474-6. [Google Scholar]
- Fuchs, S. Optimality of parental investment: The influence of nursing on reproductive success of mother and female young house mice. Behav. Ecol. Sociobiol. 1982, 10, 39–51. [Google Scholar] [CrossRef]
- Voloschin, L.M.; Tramezzani, J.H. Milk Ejection Reflex Linked to Slow Wave Sleep in Nursing Rats. Endocrinology 1979, 105, 1202–1207. [Google Scholar] [CrossRef] [PubMed]
- Korhonen, H.; Marnila, P.; Gill, H.S. Milk immunoglobulins and complement factors. Br. J. Nutr. 2000, 84, 75–80. [Google Scholar] [CrossRef]
- Schaal, B. Pheromones for Newborns; Mucignat-Caretta, C., Raton, B., Eds.; Taylor & Francis: Abingdon, UK, 2014; Chapter 17; ISBN-13: 978-1-4665-5341. [Google Scholar]
- Nowak, R. Suckling, Milk, and the Development of Preferences toward Maternal Cues by Neonates: From Early Learning to Filial Attachment? Adv. Study Behav. 2006, 36, 1–58. [Google Scholar] [CrossRef]
- Serra, J.; Ferreira, G.; Mirabito, L.; Lévy, F.; Nowak, R. Post-oral and Perioral Stimulations during Nursing Enhance Appetitive Olfactory Memory in Neonatal rabbits. Chem. Senses 2009, 34, 405–413. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Litwack, G. Vitamines and Hormones in: Pheromones; Elsevier: San Diego, CA, USA, 2010; ISBN 978-0-12-381516-3. [Google Scholar]
- Mykytowycz, R. Skin Glands as Organs of Communication in Mammal. J. Investig. Dermatol. 1974, 62, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Hudson, R.; González-Mariscal, G.; Beyer, C. Chin marking behavior, sexual receptivity, and pheromone emission in steroid-treated, ovariectomized rabbits. Horm. Behav. 1990, 24, 1–13. [Google Scholar] [CrossRef]
- Gonzalez-Mariscal, G.; Chirino, R.; Hudson, R. Prolactin Stimulates Emission of Nipple Pheromone in Ovariectomized New Zealand White Rabbits. Biol. Reprod. 1994, 50, 373–376. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Brennan, P.; Keverne, E.B. Biological complexity and adaptability of simple mammalian olfactory memory systems. Neurosci. Biobehav. Rev. 2015, 50, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Oboti, L.; Ibarra-Soria, X.; Pérez-Gómez, A.; Schmid, A.; Pyrski, M.; Paschek, N.; Kircher, S.; Logan, D.W.; Leinders-Zufall, T.; Zufall, F.; et al. Pregnancy and estrogen enhance neural progenitor-cell proliferation in the vomeronasal sensory epithelium. BMC Biol. 2015, 13, 104. [Google Scholar] [CrossRef] [PubMed]
- Rekwot, P.I.; Ogwu, D.; Oyedipe, E.O.; Sekoni, V.O. The role of pheromones and biostimulation in animal reproduction. Anim. Reprod. Sci. 2001, 65, 157–170. [Google Scholar] [CrossRef]
- Parillo, F.; Diverio, S. Glycocomposition of the apocrine interdigital gland secretions in the fallow deer (Dama dama). Res. Vet. Sci. 2009, 86, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Nowak, R. Neonatal survival: Contributions from behavioural studies in sheep. Appl. Anim. Behav. Sci. 1996, 49, 61–72. [Google Scholar] [CrossRef]
- Nowak, R.; Porter, R.H.; Lévy, F.; Orgeur, P.; Schaal, B. Role of mother–young interactions in the survival of offspring in domestic mammals. Rev. Reprod. 2000, 5, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Vince, M.A.; Ward, T.M. The responsiveness of newly born Clun forest lambs to odour sources in the ewe. Behaviour 1984, 89, 117–127. [Google Scholar] [CrossRef]
- Schaal, B.; Orgeur, P.; Arnould, C. Olfactory preferences in newborn lambs: Possible influence of prenatal experience. Behaviour 1995, 132, 5351–5365. [Google Scholar] [CrossRef]
- Vince, M.A.; Lynch, J.J.; Mottershead, B.E.; Green, G.C. Interactions between normal ewes and newly born lambs deprived of visual, olfactory and tactile sensory information. Appl. Anim. Behav. Sci. 1987, 19, 119–136. [Google Scholar] [CrossRef]
- Vince, M.A.; Billing, A.E. Infancy in the sheep: The part played by sensory stimulation in bounding between ewe and lamb. In Advances in Infancy Research; Lipsitt, L.P., Rovee-Collier, C., Eds.; Ablex Noorwood: New York, NY, USA, 1986; Volume 4, pp. 1–37. [Google Scholar]
- Logan, D.W.; Brunet, L.J.; Webb, W.R.; Cutforth, T.; Ngai, J.; Stowers, L. Learned Recognition of Maternal Signature Odors Mediates the First Suckling Episode in Mice. Curr. Biol. 2012, 22, 1998–2007. [Google Scholar] [CrossRef] [PubMed]
- Schaal, B.; Coureaud, G.; Doucet, S.; Delaunay, E.l.; Allam, M.; Moncomble, A.-S.; Montigny, D.; Patris, B.; Holley, A. Mammary olfactory signalisation in females and odour pocessing in neonates: Ways evolved by rabbits and humans. Behav. Brain Res. 2009, 200, 346–358. [Google Scholar] [CrossRef] [PubMed]
- Morrow-Tesch, J.; McGlone, J.J. Sources of maternal odors and the development of odor preferences in baby pigs. J. Anim. Sci. 1990, 68, 3563–3571. [Google Scholar] [CrossRef] [PubMed]
- Schaal, B. Mammary Odor Cues and Pheromones: Mammalian Infant-Directed Communication about Maternal State, Mammae, and Milk. In Vitamins and Hormones; Litwack, G., Ed.; Elsevier Inc.: San Diego, CA, USA, 2009; Chapter 4; Volume 83, pp. 83–136. [Google Scholar]
- Vaglio, S.; Minicozzi, P.; Bonometti, E.; Mello, G.; Chiarelli, B. Volatile signals during pregnancy: A possible chemical basis for mother-infant recognition. J. Chem. Ecol. 2009, 53, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Doucet, S.; Sooussignan, R.; Sagot, P.; Schaal, B. The secretion of areolar (Montgomery’s) glands from lactating women elicits selective, unconditional responses in neonates. PLoS ONE 2009, 4, e7579. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Legendre, A.; Faure, P.; Tiesset, H.; Potin, C.; Jakob, I.; Sicard, G.; Schaal, B.; Artur, Y.; Coureaud, G.; Heydel, J.M. When the Nose Must Remain Responsive: Glutathione Conjugation of the Mammary Pheromone in the Newborn Rabbit. Chem. Sens. 2014, 39, 425–437. [Google Scholar] [CrossRef] [PubMed]
- Hudson, R.; Rojas, C.; Carolina Rojas, L.; Martínez-Gómez, M.; Distel, H. Rabbit Nipple-Search Pheromone versus Rabbit Mammary Pheromone Revisited. In Chemical Signals in Vertebrates; Muller-Schwarze, D., Ed.; Springer Link: London, UK, 2008; pp. 315–324. [Google Scholar]
- Coureaud, G.; Charra, R.; Datiche, F.; Sinding, C.; Thomas-Danguin, T.; Languille, S.; Hars, B.; Schaal, B. A pheromone to behave, a pheromone to learn: The rabbit mammary pheromone. J. Comp. Physiol. A Neuroethol. Sens. Neural. Behav. Physiol. 2010, 196, 779–790. [Google Scholar] [CrossRef] [PubMed]
- Brus, M.; Meurisse, M.; Franceschini, I.; Keller, M.; Lévy, F. Evidence for cell proliferation in the sheep brain and its down-regulation by parturition and interactions with the young. Horm. Behav. 2010, 58, 737–746. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, D. Breeding the Flock: Modern Research and Reproduction in Sheep; Inkata Pr.: Berlin, Germany, 1988; ISBN 0909605459. [Google Scholar]
- Vince, M.A. Newborn lambs and their dams: The interaction that leads to suckling. Adv. Study Behav. 1993, 22, 239–268. [Google Scholar]
- Sell, C. A Fragrant Introduction to Terpenoid Chemistry; The Royal Society of Chemistry: Cambridge, UK, 2003; ISBN 0-85404-681-X. [Google Scholar]
- Poindron, P.; Lévy, F. Physiological, sensory and experiential determinants of maternal behaviour in sheep. In Mammalian Parenting: Biochemical, Neurobiological and Behavioral Determinants; Krasnegor, N.A., Bridges, R.S., Eds.; Oxford University Press: New York, NY, USA, 1990; pp. 133–156, 485–488. ISBN1 0195056000. ISBN2 9780195056006. [Google Scholar]
- Poindron, P.; Nowak, R.; Lévy, F.; Porter, R.H.; Schaal, B. Development of exclusive mother-young bonding in sheep and goats. In Oxford Reviews of Reproductive Biology; Michigan, S.R., Ed.; Oxford University Press: Oxford, UK, 1993; Volume 15, pp. 311–364. [Google Scholar]
- Foitzik, K.; Krause, K.; Conrad, F.; Nakamura, M.; Funk, W.; Paus, R. Human scalp hair follicles are both a target and a source of prolactin, which serves as an autocrine and/or paracrine promoter of apoptosis-driven hair follicle regression. Am. J. Pathol. 2006, 168, 748–756. [Google Scholar] [CrossRef] [PubMed]
- Egli, M.; Leeners, B.; Kruge, TH. Prolactin secretion patterns: Basic mechanisms and clinical implications for reproduction. Reproduction 2010, 140, 643–654. [Google Scholar] [CrossRef] [PubMed]
- Foitzik, K.; Krause, K.; Nixon, A.J.; Ford, C.A.; Ohnemus, U.; Pearson, A.J.; Paus, R. Prolactin and its receptor are expressed in murine hair follicle epithelium, show hair cycle-dependent expression, and induce catagen. Am. J. Pathol. 2003, 162, 1611–1621. [Google Scholar] [CrossRef]
- Foitzik, K.; Langan, E.A.; Paus, R. Prolactin and the skin: A dermatological perspective on an ancient pleiotropic peptide hormone. J. Investig. Dermatol. 2009, 129, 1071–1087. [Google Scholar] [CrossRef] [PubMed]
- Freeman, M.E.; Kanyicska, B.; Lerant, A.; Nagy, G. Prolactin: Structure, function, and regulation of secretion. Physiol. Rev. 2000, 80, 1523–1631. [Google Scholar] [PubMed]
- Kikuyama, S.; Yamamoto, K.; Iwata, T.; Toyoda, F. Peptide and protein pheromones in amphibians. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2002, 132, 69–74. [Google Scholar] [CrossRef]
- González-Mariscal, G. Neuroendocrinology of Maternal Behaviour in the Rabbit. Horm. Behav. 2001, 40, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Shaaban, A.M.; Green, A.R.; Karthik, S.; Alizadeh, Y.; Hughes, T.A.; Harkins, L.; Ellis, I.O.; Robertson, J.F.; Paish, E.C.; Saunders, P.T.; et al. Nuclear and cytoplasmic expression of ERbeta1, ERbeta2, and ERbeta5 identifies distinct prognostic outcome for breast cancer patients. Clin. Cancer. Res. 2008, 14, 5228–5235. [Google Scholar] [CrossRef] [PubMed]
- Revankar, C.M.; Cimino, D.F.; Sklar, L.A.; Arterburn, J.B.; Prossnitz, E.R. A Transmembrane Intracellular Estrogen Receptor Mediates Rapid Cell Signaling. Science 2005, 307, 1625–1630. [Google Scholar] [CrossRef] [PubMed]
- Robert, X.-D.; Song, R.X.-D.; Zhang, Z.; Santen, R.J. Estrogen Rapid Action via Protein Complex Formation Involving ERα and Src; Cell Press: Cambridge, MA, USA, 2005; Volume 16, pp. 347–353. [Google Scholar]
- Simoncini, T.; Rabkin, E.; Liao, J.K. Membrane estrogen receptor interaction with ph osphatidylinositol 3-kinase in endothelial cells. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Hammes, S.R. The further redefining of steroid-mediated signalling. Proc. Nat. Acad. Sci. USA 2003, 100, 2168–2170. [Google Scholar] [CrossRef] [PubMed]
- Cato, A.; Nestl, A.; Mink, S. Rapid actions of steroid receptors in cellular signaling pathways. Science’s STKE 2002. [Google Scholar] [CrossRef] [PubMed]
- Levin, E.R. Cell localization, physiology and nongenomic actions of estrogen receptors. J. Appl. Physiol. 2001, 91, 1860–1867. [Google Scholar] [PubMed]
- Wittliff, J.L.; Hilf, R.; Brooks, W.F., Jr.; Savlov, E.D.; Hall, T.; Robert, A.O. Specific estrogen-binding capacity of the cytoplasmic receptor in normal and neoplastic breast tissues of humans. J. Cancer Res. 1972, 11, 1–73. [Google Scholar]
- Welsh, A.W.; Lannin, D.R.; Gregory, S.; Young, G.S.; Mark, E.; Sherman, M.E.; Jonine, D.; Figueroa, J.D.; Lynn, N.; Henry, N.L.; et al. Cytoplasmic Estrogen Receptor in breast cancer. Clin. Cancer Res. 2012, 18, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Klinge, C.M. Estrogen receptor interaction with estrogen response elements. Nucleic Acids Res. 2001, 29, 2905–2919. [Google Scholar] [CrossRef] [PubMed]
- McKenna, N.J.; O’Malley, B.W. Combinatorial control of gene expression by nuclear receptors and coregulators. Cell 2002, 108, 465–474. [Google Scholar] [CrossRef]
- Stossi, F.; Likhite, V.S.; Katzenellenbogen, J.A.; Katzenellenbogen, B.S. Estrogen-occupied estrogen receptor represses cyclin G2 gene expression and recruits a repressor complex at the cyclin G2 promoter. J. Biol. Chem. 2006, 281, 16272–16278. [Google Scholar] [CrossRef] [PubMed]
- Farach-Carson, M.C.; Davis, P.J. Steroid hormone interactions with target cells: Cross talk between membrane and nuclear pathways. J. Pharmacol. Exper. Therap. 2003, 30, 839–845. [Google Scholar] [CrossRef] [PubMed]
- Alexandre-Pires, G.; Martins, C.; Miguel Galvão, A.; Correia, M.; Ramilo, D.; Quaresma, M.; Ligeiro, D.; Nunes, T.; Caldeira, R.M.; Ferreira-Dias, G. Morphological Aspects and Expression of Estrogen and Progesterone Receptors in the Interdigital Sinus in Cyclic Ewes. Microsc. Res. Tech. 2014, 77, 313–325. [Google Scholar] [CrossRef] [PubMed]
- Zaviacic, M.; Zajickova, M.; Blazekova, J.; Donarov, L.; Stvrtina, S.; Mikuleck, M.; Zaviacic, T.; Holom, A.N.K.; Breza, J. Size, macroanatomy and histology of the normal prostate in the adult human female: A minireview. J. Histotechnol. 2000, 21, 61–69. [Google Scholar] [CrossRef]
- Gesase, A.P.; Satoh, Y.; Ono, K. Secretagogue-induced apocrine secretion in the rat Harderian gland of the rat. Cell Tissue Res. 1996, 285, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Gesase, A.P.; Satoh, Y. Apocrine secretory mechanism: Recent findings and unresolved problems. Histol. Histopathol. 2003, 18, 597–608. [Google Scholar] [PubMed]
- Jarret, A. Physiology and Pathophysiology of the Skin: The Sweat Gland, Skin Permeation, Lymphatics, the Nails; Academic Press: Salt Lake, UT, USA, 1978; ISBN1 0123806054. ISBN2 9780123806055. [Google Scholar]
- Pourlis, A.F. Functional morphological characteristics of the interdigital sinus in the sheep. Folia Morphol. 2010, 69, 107–111. [Google Scholar]
- Cristofoletti, P.T.; Ribeiro, A.F.; Terra, W.R. Apocrine secretion of amylase and exocytosis of trypsin along the midgut of Tenebrio molitor larvae. J. Insect. Physiol. 2001, 47, 143–155. [Google Scholar] [CrossRef]
- Craven, A.J.; Ormandy, C.J.; Robertson, F.G.; Wilkins, R.J.; Kelly, P.A.; Nixon, A.J.; Pearson, A.J.; Craven, T. Prolactin signaling influences the timing mechanism of the hair follicle: Analysis of hair growth cycles in prolactin receptor knockout mice. Endocrinology 2001, 142, 2533–2539. [Google Scholar] [CrossRef] [PubMed]
- Craven, A.J.; Nixon, A.J.; Ashby, M.G.; Ormandy, C.J.; Blazek, K.; Wilkins, R.J.; Pearson, A.J. Prolactin delays hair regrowth in mice. J. Endocrinol. 2006, 19, 1415–1425. [Google Scholar] [CrossRef] [PubMed]
- Goffin, V.; Bernichtein, S.; Touraine, P.; Kelly, P.A. Development and potential clinical uses of human prolactin receptor antagonists. Endocr. Rev. 2005, 26, 400–422. [Google Scholar] [CrossRef] [PubMed]
- Horseman, N.D.; Gregerson, K.A. Prolactin actions. Soc. Endocrinol. 2014, 52, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Oftedal, O.T. The origin of lactation as a water source for parchment-shelled eggs. J. Mammary Gland Biol. Neoplasia 2002, 7, 253–266. [Google Scholar] [CrossRef] [PubMed]
- Oftedal, O.T. The mammary gland and its origin during synapsid evolution. J. Mammary Gland Biol. Neoplasia 2002, 7, 225–252. [Google Scholar] [CrossRef] [PubMed]
- Oftedal, O.T. The evolution of milk secretion and its ancient origins. Animal 2012, 6, 355–368. [Google Scholar] [CrossRef] [PubMed]
- Nicoll, C.S. Prolactin: Ontogeny and evolution of prolactin’s functions. Fed. Proc. 1980, 39, 2561–2566. [Google Scholar]
- Srivastava, S.; Matsuda, M.; Hou, Z.; Bailey, J.P.; Kitazawa, R.; Herbst, M.P.; Horseman, N.D. Receptor activator of NF-κB ligand induction via Jak2 and Stat5a in mammary epithelial cells. J. Biol. Chem. 2003, 278, 46171–46178. [Google Scholar] [CrossRef] [PubMed]
- Bole-Feysot, C.; Goffin, V.; Edery, M.; Binart, N.; Kelly, P.A. Prolactin (PRL) and its receptor: Actions, signal transduction pathways and phenotypes observed in PRL receptor knockout mice. Endocr. Rev. 1998, 19, 225–268. [Google Scholar] [CrossRef] [PubMed]
- Horseman, N.D.; Yu-Lee, L.Y. Transcriptional regulation by the helix bundle peptide hormones: Growth hormone, prolactin, and hematopoietic cytokines. Endocr. Rev. 1994, 15, 627–649. [Google Scholar] [CrossRef] [PubMed]
- Brooks, C.L. Molecular mechanisms of prolactin and its receptor. Endocr. Rev. 2012, 33, 504–525. [Google Scholar] [CrossRef] [PubMed]
- Sidis, Y.; Horseman, N.D. Prolactin induces rapid p95/p70 tyrosine phosphorylation, and protein binding to GAS-like sites in the anx Icp35 and c-fos genes. Endocrinology 1994, 134, 1979–1985. [Google Scholar] [CrossRef] [PubMed]
- Standke, G.J.R.; Meier, V.S.; Groner, B. Mammary gland factor activated by prolactin in mammary epithelial cells and acute-phase response factor activated by interleukin-6 in liver cells share DNA binding and transactivation potential. Molecul. Endocrinol. 1994, 8, 469–477. [Google Scholar] [CrossRef]
- Schramek, D.A.; Sigl, V.; Kenner, L.; Pospisilik, J.A.; Lee, H.J.; Hanada, R.; Joshi, P.A.; Aliprantis Glimcher, L.; Pasparakis, M.; Khokha, R.; et al. Osteoclast differentiation factor RANKL controls development Leibbrandt of progestin-driven mammary cancer. Nature 2010, 468, 98–102. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Bonizzi, G.; Seagroves, T.N.; Greten, F.R.; Johnson, R.; Schmidt, E.V.; Karin, M. IKKα provides an essential link between RANK signaling and cyclin D1 expression during mammary gland development. Cell 2001, 107, 763–775. [Google Scholar] [CrossRef]
- Baxter, F.O.; Came, P.J.; Abell, K.; Kedjouar, B.; Huth, M.; Rajewsky, K.; Pasparakis, M.; Watson, C.J. IKKβ/2 induces TWEAK and apoptosis in mammary epithelial cells. Development 2006, 133, 3485–3494. [Google Scholar] [CrossRef] [PubMed]
- Müller-Schwarze, D. The chemical ecology of ungulates. Appl. Anim. Sci. 1991, 29, 389–402. [Google Scholar] [CrossRef]
- Luna, LG. Histopathologic Methods and Color Atlas of Special Stains and Tissue Artifacts, 1st ed.; Johnson Printers: Downers Grove, IL, USA, 1992. [Google Scholar]
- Bruno, T.J.; Svoronos, P.D.N. Handbook of Basic Tables for Chemical Analysis, 2nd ed.; Taylor & Francis Group: New York, NY, USA, 2004; Chapter 3; pp. 177–212. [Google Scholar]

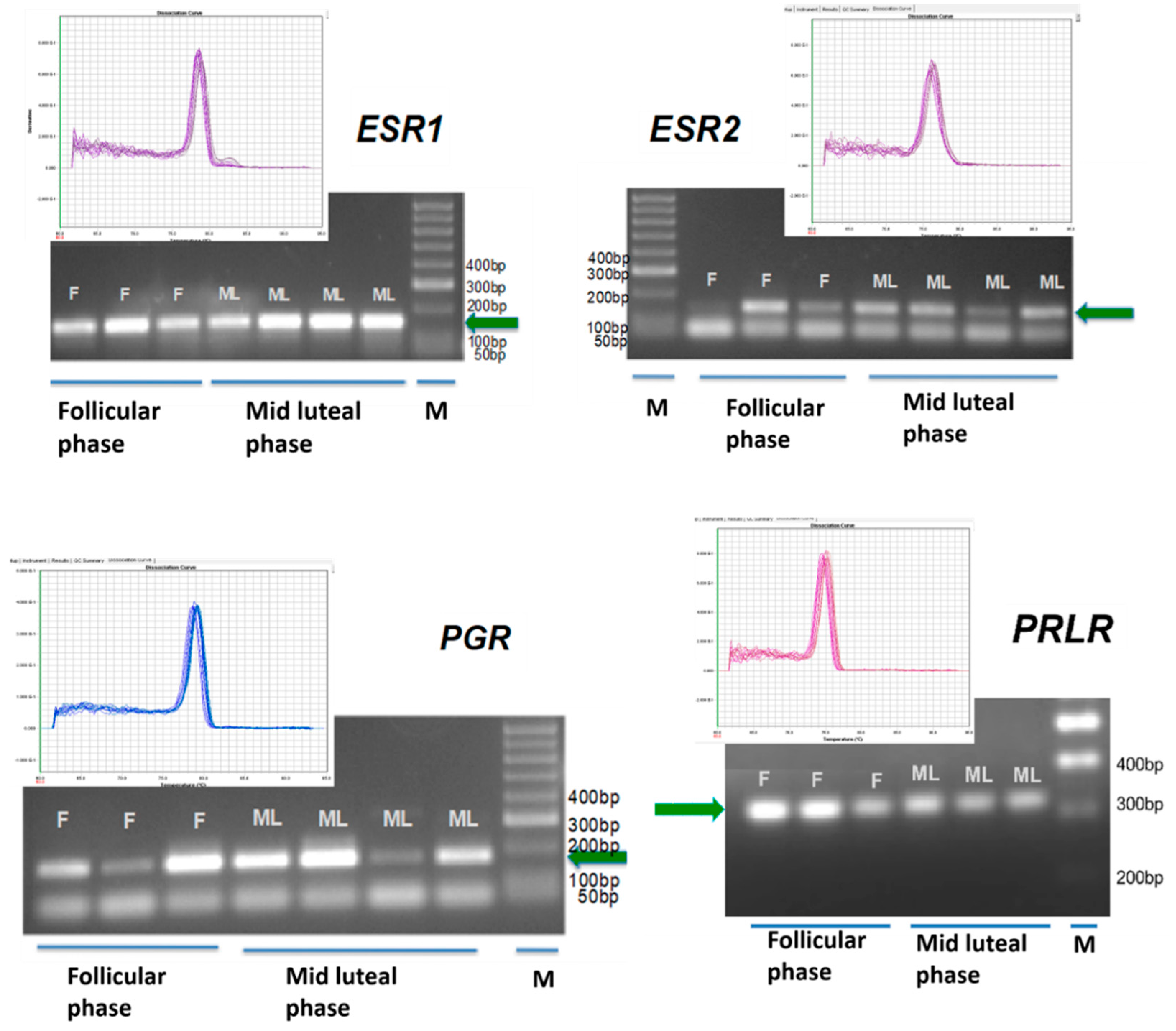
| Gene (Acession Number) | Sequence 5′–3′ Amplicon (Base Pairs) |
|---|---|
| ESR1 (XM_015097472.1) | Forward: CCATGGAATCTGCCAAGGAG (167 bp) Reverse: ATCAATTGTGCACTGGTTGGT |
| ESR2 (NM_001009737.1) | Forward: TGGAGTCTGGTCATGTGAAGGA (150 bp) Reverse: TCATAGCACTTCCGCAGTCG |
| PGR (XM_015100878.1) | Forward: CAGCCAGAGCCCACAGTACA (176 bp) Reverse: TGCAATCGTTTCTTCCAGCA |
| PRLR (NM_001009204.1) | Forward: GTCTCCACCCACCCTGACTG (320 bp) Reverse: AAGCCACTGCCCAGACCATA |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alexandre-Pires, G.; Martins, C.; Galvão, A.M.; Miranda, M.; Silva, O.; Ligeiro, D.; Nunes, T.; Ferreira-Dias, G. Understanding the Inguinal Sinus in Sheep (Ovis aries)—Morphology, Secretion, and Expression of Progesterone, Estrogens, and Prolactin Receptors. Int. J. Mol. Sci. 2017, 18, 1516. https://doi.org/10.3390/ijms18071516
Alexandre-Pires G, Martins C, Galvão AM, Miranda M, Silva O, Ligeiro D, Nunes T, Ferreira-Dias G. Understanding the Inguinal Sinus in Sheep (Ovis aries)—Morphology, Secretion, and Expression of Progesterone, Estrogens, and Prolactin Receptors. International Journal of Molecular Sciences. 2017; 18(7):1516. https://doi.org/10.3390/ijms18071516
Chicago/Turabian StyleAlexandre-Pires, Graça, Catarina Martins, António M. Galvão, Margarida Miranda, Olga Silva, Dário Ligeiro, Telmo Nunes, and Graça Ferreira-Dias. 2017. "Understanding the Inguinal Sinus in Sheep (Ovis aries)—Morphology, Secretion, and Expression of Progesterone, Estrogens, and Prolactin Receptors" International Journal of Molecular Sciences 18, no. 7: 1516. https://doi.org/10.3390/ijms18071516
APA StyleAlexandre-Pires, G., Martins, C., Galvão, A. M., Miranda, M., Silva, O., Ligeiro, D., Nunes, T., & Ferreira-Dias, G. (2017). Understanding the Inguinal Sinus in Sheep (Ovis aries)—Morphology, Secretion, and Expression of Progesterone, Estrogens, and Prolactin Receptors. International Journal of Molecular Sciences, 18(7), 1516. https://doi.org/10.3390/ijms18071516






