Subtype Diagnosis of Primary Aldosteronism: Is Adrenal Vein Sampling Always Necessary?
Abstract
:1. Introduction
2. Comparison of AVS with Imaging Techniques
3. Patients in Which AVS Can Be Avoided
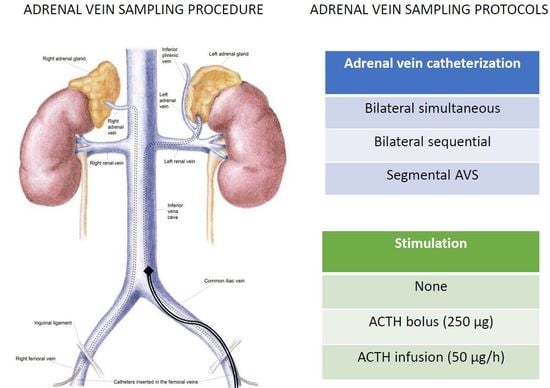
4. AVS Methodology
5. Bilaterally Simultaneous vs. Sequential Cannulation
6. Segmental AVS
7. Cosyntropin Stimulation
8. Improving the Success Rate
9. AVS Interpretation Criteria
10. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Käyser, S.C.; Dekkers, T.; Groenewoud, H.J.; van der Wilt, G.J.; Carel Bakx, J.; van der Wel, M.C.; Hermus, A.R.; Lenders, J.W.; Deinum, J. Study heterogeneity and estimation of prevalence of primary aldosteronism: A systematic review and meta-regression analysis. J. Clin. Endocrinol. Metab. 2016, 101, 2826–2835. [Google Scholar] [CrossRef] [PubMed]
- Monticone, S.; Burrello, J.; Tizzani, D.; Bertello, C.; Viola, A.; Buffolo, F.; Gabetti, L.; Mengozzi, G.; Williams, T.A.; Rabbia, F.; Veglio, F.; Mulatero, P. Prevalence and Clinical Manifestations of Primary Aldosteronism Encountered in Primary Care Practice. J. Am. Coll. Cardiol. 2017, 69, 1811–1820. [Google Scholar] [CrossRef] [PubMed]
- Mulatero, P.; Tizzani, D.; Viola, A.; Bertello, C.; Monticone, S.; Mengozzi, G.; Schiavone, D.; Williams, T.A.; Einaudi, S.; La Grotta, A.; et al. Prevalence and characteristics of familial hyperaldosteronism: The PATOGEN study (Primary Aldosteronism in TOrino-GENetic forms). Hypertension 2011, 58, 797–803. [Google Scholar] [CrossRef] [PubMed]
- Milliez, P.; Girerd, X.; Plouin, P.-F.; Blacher, J.; Safar, M.E.; Mourad, J.-J. Evidence for an increased rate of cardiovascular events in patients with primary aldosteronism. J. Am. Coll. Cardiol. 2005, 45, 1243–1248. [Google Scholar] [CrossRef] [PubMed]
- Mulatero, P.; Monticone, S.; Bertello, C.; Viola, A.; Tizzani, D.; Iannaccone, A.; Crudo, V.; Burrello, J.; Milan, A.; Rabbia, F.; et al. Long-term cardio- and cerebrovascular events in patients with primary aldosteronism. J. Clin. Endocrinol. Metab. 2013, 98, 4826–4833. [Google Scholar] [CrossRef] [PubMed]
- Funder, J.W.; Carey, R.M.; Mantero, F.; Murad, M.H.; Reincke, M.; Shibata, H.; Stowasser, M.; Young, W.F. The Management of Primary Aldosteronism: Case Detection, Diagnosis, and Treatment: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2016, 101, 1889–1916. [Google Scholar] [CrossRef] [PubMed]
- Rossi, G.P.; Auchus, R.J.; Brown, M.; Lenders, J.W.M.; Naruse, M.; Plouin, P.F.; Satoh, F.; Young, W.F. An expert consensus statement on use of adrenal vein sampling for the subtyping of primary aldosteronism. Hypertension 2014, 63, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Monticone, S.; Viola, A.; Rossato, D.; Veglio, F.; Reincke, M.; Gomez-Sanchez, C.; Mulatero, P. Adrenal vein sampling in primary aldosteronism: Towards a standardised protocol. Lancet Diabetes Endocrinol. 2015, 3, 296–303. [Google Scholar] [CrossRef]
- Bookstein, J.J. The roles of angiography in adrenal disease. In Abrams Angiography, 2nd ed.; Little Brown: Boston, MA, USA, 1983; pp. 1395–1424. [Google Scholar]
- Walters, N.A.; Thomson, K.R. Urogenital Venography. In Practical Interventional Uroradiology; Edward Arnold: Edinburgh, Scotland, 1993. [Google Scholar]
- Mulatero, P.; Bertello, C.; Rossato, D.; Mengozzi, G.; Milan, A.; Garrone, C.; Giraudo, G.; Passarino, G.; Garabello, D.; Verhovez, A.; et al. Roles of clinical criteria, computed tomography scan, and adrenal vein sampling in differential diagnosis of primary aldosteronism subtypes. J. Clin. Endocrinol. Metab. 2008, 93, 1366–1371. [Google Scholar] [CrossRef] [PubMed]
- White, E.A.; Schambelan, M.; Rost, C.R.; Biglieri, E.G.; Moss, A.A.; Korobkin, M. Use of computed tomography in diagnosing the cause of primary aldosteronism. N. Engl. J. Med. 1980, 303, 1503–1507. [Google Scholar] [CrossRef] [PubMed]
- Young, W.F.; Stanson, A.W.; Thompson, G.B.; Grant, C.S.; Farley, D.R.; van Heerden, J.A. Role for adrenal venous sampling in primary aldosteronism. Surgery 2004, 136, 1227–1235. [Google Scholar] [CrossRef] [PubMed]
- Nwariaku, F.E.; Miller, B.S.; Auchus, R.; Holt, S.; Watumull, L.; Dolmatch, B.; Nesbitt, S.; Vongpatanasin, W.; Victor, R.; Wians, F.; et al. Primary hyperaldosteronism: Effect of adrenal vein sampling on surgical outcome. Arch. Surg. 2006, 141, 497–502. [Google Scholar] [CrossRef] [PubMed]
- Kempers, M.J.; Lenders, J.W.; van Outheusden, L.; van der Wilt, G.J.; Schultze Kool, L.J.; Hermus, A.R.; Deinum, J. Systematic review: Diagnostic procedures to differentiate unilateral from bilateral adrenal abnormality in primary aldosteronism. Ann. Intern. Med. 2009, 151, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Dekkers, T.; Prejbisz, A.; Kool, L.J.S.; Groenewoud, H.J.M.M.; Velema, M.; Spiering, W.; Kołodziejczyk-Kruk, S.; Arntz, M.; Kądziela, J.; Langenhuijsen, J.F.; et al. SPARTACUS Investigators Adrenal vein sampling versus CT scan to determine treatment in primary aldosteronism: An outcome-based randomised diagnostic trial. Lancet Diabetes Endocrinol. 2016, 4, 739–746. [Google Scholar] [CrossRef]
- Puar, T.H.; Khoo, J.J.; Ng, K.S.; Kam, J.W.; Wang, K.W. Adrenal vein sampling versus CT scanning in primary aldosteronism. Lancet Diabetes Endocrinol 2016, 4, 885–886. [Google Scholar] [CrossRef]
- Rossi, G.P.; Funder, J.W. Adrenal Venous Sampling Versus Computed Tomographic Scan to Determine Treatment in Primary Aldosteronism (The SPARTACUS Trial): A Critique. Hypertension 2017, 69, 396–397. [Google Scholar] [CrossRef] [PubMed]
- Sukor, N.; Gordon, R.D.; Ku, Y.K.; Jones, M.; Stowasser, M. Role of unilateral adrenalectomy in bilateral primary aldosteronism: A 22-year single center experience. J. Clin. Endocrinol. Metab. 2009, 94, 2437–2445. [Google Scholar] [CrossRef] [PubMed]
- Rossi, G.P.; Barisa, M.; Allolio, B.; Auchus, R.J.; Amar, L.; Cohen, D.; Degenhart, C.; Deinum, J.; Fischer, E.; Gordon, R.; et al. The Adrenal Vein Sampling International Study (AVIS) for Identifying the Major Subtypes of Primary Aldosteronism. J. Clin. Endocrinol. Metab. 2012, 97, 1606–1614. [Google Scholar] [CrossRef] [PubMed]
- Riester, A.; Fischer, E.; Degenhart, C.; Reiser, M.F.; Bidlingmaier, M.; Beuschlein, F.; Reincke, M.; Quinkler, M. Age below 40 or a recently proposed clinical prediction score cannot bypass adrenal venous sampling in primary aldosteronism. J. Clin. Endocrinol. Metab. 2014, 99, E1035–E1039. [Google Scholar] [CrossRef] [PubMed]
- Lim, V.; Guo, Q.; Grant, C.S.; Thompson, G.B.; Richards, M.L.; Farley, D.R.; Young, W.F. Accuracy of adrenal imaging and adrenal venous sampling in predicting surgical cure of primary aldosteronism. J. Clin. Endocrinol. Metab. 2014, 99, 2712–2719. [Google Scholar] [CrossRef] [PubMed]
- Küpers, E.M.; Amar, L.; Raynaud, A.; Plouin, P.-F.; Steichen, O. A clinical prediction score to diagnose unilateral primary aldosteronism. J. Clin. Endocrinol. Metab. 2012, 97, 3530–3537. [Google Scholar] [CrossRef] [PubMed]
- Sze, W.C.C.; Soh, L.M.; Lau, J.H.; Reznek, R.; Sahdev, A.; Matson, M.; Riddoch, F.; Carpenter, R.; Berney, D.; Grossman, A.B.; et al. Diagnosing unilateral primary aldosteronism—comparison of a clinical prediction score, computed tomography and adrenal venous sampling. Clin. Endocrinol. 2014, 81, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Haketa, A.; Ueno, T.; Ikeda, Y.; Hatanaka, Y.; Tanaka, S.; Otsuka, H.; Abe, M.; Fukuda, N.; Soma, M. Scoring system for the diagnosis of bilateral primary aldosteronism in the outpatient setting before adrenal venous sampling. Clin. Endocrinol. 2016, 86, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Young, W.F., Jr.; Klee, G.G. Primary aldosteronism. Diagnostic evaluation. Endocrinol. Metab. Clin. North Am. 1988, 17, 367–395. [Google Scholar] [PubMed]
- Lau, J.H.G.; Sze, W.C.C.; Reznek, R.H.; Matson, M.; Sahdev, A.; Carpenter, R.; Berney, D.M.; Akker, S.A.; Chew, S.L.; Grossman, A.B.; et al. A prospective evaluation of postural stimulation testing, computed tomography and adrenal vein sampling in the differential diagnosis of primary aldosteronism. Clin. Endocrinol. 2012, 76, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Mulatero, P.; Stowasser, M.; Loh, K.-C.; Fardella, C.E.; Gordon, R.D.; Mosso, L.; Gomez-Sanchez, C.E.; Veglio, F.; Young, W.F., Jr. Increased diagnosis of primary aldosteronism, including surgically correctable forms, in centers from five continents. J. Clin. Endocrinol. Metab. 2004, 89, 1045–1050. [Google Scholar] [CrossRef] [PubMed]
- Phillips, J.L.; Walther, M.M.; Pezzullo, J.C.; Rayford, W.; Choyke, P.L.; Berman, A.A.; Linehan, W.M.; Doppman, J.L.; Gill, J.R., Jr. Predictive value of preoperative tests in discriminating bilateral adrenal hyperplasia from an aldosterone-producing adrenal adenoma. J. Clin. Endocrinol. Metab. 2000, 85, 4526–4533. [Google Scholar] [CrossRef] [PubMed]
- Sonoyama, T.; Sone, M.; Miyashita, K.; Tamura, N.; Yamahara, K.; Park, K.; Oyamada, N.; Taura, D.; Inuzuka, M.; Kojima, K.; et al. Significance of adrenocorticotropin stimulation test in the diagnosis of an aldosterone-producing adenoma. J. Clin. Endocrinol. Metab. 2011, 96, 2771–2778. [Google Scholar] [CrossRef] [PubMed]
- Moriya, A.; Yamamoto, M.; Kobayashi, S.; Nagamine, T.; Takeichi-Hattori, N.; Nagao, M.; Harada, T.; Tanimura-Inagaki, K.; Onozawa, S.; Murata, S.; et al. ACTH stimulation test and computed tomography are useful for differentiating the subtype of primary aldosteronism. Endocr. J. 2017, 64, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Eisenhofer, G.; Dekkers, T.; Peitzsch, M.; Dietz, A.S.; Bidlingmaier, M.; Treitl, M.; Williams, T.A.; Bornstein, S.R.; Haase, M.; Rump, L.C.; Willenberg, H.S.; Beuschlein, F.; Deinum, J.; Lenders, J.W.M.; Reincke, M. Mass Spectrometry-Based Adrenal and Peripheral Venous Steroid Profiling for Subtyping Primary Aldosteronism. Clin. Chem. 2016, 62, 514–524. [Google Scholar] [CrossRef] [PubMed]
- Mulatero, P.; di Cella, S.M.; Monticone, S.; Schiavone, D.; Manzo, M.; Mengozzi, G.; Rabbia, F.; Terzolo, M.; Gomez-Sanchez, E.P.; Gomez-Sanchez, C.E.; et al. 18-Hydroxycorticosterone, 18-hydroxycortisol, and 18-oxocortisol in the diagnosis of primary aldosteronism and its subtypes. J. Clin. Endocrinol. Metab. 2012, 97, 881–889. [Google Scholar] [CrossRef] [PubMed]
- Satoh, F.; Morimoto, R.; Ono, Y.; Iwakura, Y.; Omata, K.; Kudo, M.; Takase, K.; Seiji, K.; Sasamoto, H.; Honma, S.; et al. Measurement of peripheral plasma 18-oxocortisol can discriminate unilateral adenoma from bilateral diseases in patients with primary aldosteronism. Hypertension 2015, 65, 1096–1102. [Google Scholar] [CrossRef] [PubMed]
- Rossi, G.P.; Ragazzo, F.; Seccia, T.M.; Maniero, C.; Barisa, M.; Calò, L.A.; Frigo, A.C.; Fassina, A.; Pessina, A.C. Hyperparathyroidism can be useful in the identification of primary aldosteronism due to aldosterone-producing adenoma. Hypertension 2012, 60, 431–436. [Google Scholar] [CrossRef] [PubMed]
- Burton, T.J.; Mackenzie, I.S.; Balan, K.; Koo, B.; Bird, N.; Soloviev, D.V.; Azizan, E.A.B.; Aigbirhio, F.; Gurnell, M.; Brown, M.J. Evaluation of the sensitivity and specificity of (11)C-metomidate positron emission tomography (PET)-CT for lateralizing aldosterone secretion by Conn’s adenomas. J. Clin. Endocrinol. Metab. 2012, 97, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Sanchez, C.E.; Montgomery, M.; Ganguly, A.; Holland, O.B.; Gomez-Sanchez, E.P.; Grim, C.E.; Weinberger, M.H. Elevated urinary excretion of 18-oxocortisol in glucocorticoid-suppressible aldosteronism. J. Clin. Endocrinol. Metab. 1984, 59, 1022–1024. [Google Scholar] [CrossRef] [PubMed]
- Geller, D.S.; Zhang, J.; Wisgerhof, M.V.; Shackleton, C.; Kashgarian, M.; Lifton, R.P. A novel form of human mendelian hypertension featuring nonglucocorticoid-remediable aldosteronism. J. Clin. Endocrinol. Metab. 2008, 93, 3117–3123. [Google Scholar] [CrossRef] [PubMed]
- Williams, T.A.; Peitzsch, M.; Dietz, A.S.; Dekkers, T.; Bidlingmaier, M.; Riester, A.; Treitl, M.; Rhayem, Y.; Beuschlein, F.; Lenders, J.W.M.; et al. Genotype-Specific Steroid Profiles Associated With Aldosterone-Producing Adenomas. Hypertension 2016, 67, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Azizan, E.A.B.; Lam, B.Y.H.; Newhouse, S.J.; Zhou, J.; Kuc, R.E.; Clarke, J.; Happerfield, L.; Marker, A.; Hoffman, G.J.; Brown, M.J. Microarray, qPCR, and KCNJ5 sequencing of aldosterone-producing adenomas reveal differences in genotype and phenotype between zona glomerulosa- and zona fasciculata-like tumors. J. Clin. Endocrinol. Metab. 2012, 97, E819–E829. [Google Scholar] [CrossRef] [PubMed]
- Monticone, S.; Castellano, I.; Versace, K.; Lucatello, B.; Veglio, F.; Gomez-Sanchez, C.E.; Williams, T.A.; Mulatero, P. Immunohistochemical, genetic and clinical characterization of sporadic aldosterone-producing adenomas. Mol. Cell. Endocrinol. 2015, 411, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.; Scholl, U.I.; Yue, P.; Björklund, P.; Zhao, B.; Nelson-Williams, C.; Ji, W.; Cho, Y.; Patel, A.; Men, C.J.; et al. K+ channel mutations in adrenal aldosterone-producing adenomas and hereditary hypertension. Science 2011, 331, 768–772. [Google Scholar] [CrossRef] [PubMed]
- Mulatero, P.; Monticone, S.; Rainey, W.E.; Veglio, F.; Williams, T.A. Role of KCNJ5 in familial and sporadic primary aldosteronism. Nat. Rev. Endocrinol. 2013, 9, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Williams, T.A.; Lenders, J.W.M.; Burrello, J.; Beuschlein, F.; Reincke, M. KCNJ5 Mutations: Sex, Salt and Selection. Horm. Metab. Res. 2015, 47, 953–958. [Google Scholar] [CrossRef] [PubMed]
- Hennings, J.; Sundin, A.; Hägg, A.; Hellman, P. 11C-metomidate positron emission tomography after dexamethasone suppression for detection of small adrenocortical adenomas in primary aldosteronism. Langenbecks Arch. Surg. 2010, 395, 963–967. [Google Scholar] [CrossRef] [PubMed]
- Mendichovszky, I.A.; Powlson, A.S.; Manavaki, R.; Aigbirhio, F.I.; Cheow, H.; Buscombe, J.R.; Gurnell, M.; Gilbert, F.J. Targeted Molecular Imaging in Adrenal Disease-An Emerging Role for Metomidate PET-CT. Diagnostics 2016, 6, 42. [Google Scholar] [CrossRef] [PubMed]
- Abe, T.; Naruse, M.; Young, W.F.; Kobashi, N.; Doi, Y.; Izawa, A.; Akama, K.; Okumura, Y.; Ikenaga, M.; Kimura, H. A Novel CYP11B2-Specific Imaging Agent for Detection of Unilateral Subtypes of Primary Aldosteronism. J. Clin. Endocrinol. Metab. 2016, 101, 1008–1015. [Google Scholar] [CrossRef] [PubMed]
- Daniil, G.; Fernandes-Rosa, F.L.; Chemin, J.; Blesneac, I.; Beltrand, J.; Polak, M.; Jeunemaitre, X.; Boulkroun, S.; Amar, L.; Strom, T.M.; et al. CACNA1H Mutations Are Associated With Different Forms of Primary Aldosteronism. EBioMedicine 2016, 13, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Daunt, N. Adrenal vein sampling: How to make it quick, easy, and successful. Radiographics 2005, 25 (Suppl. S1), S143–S158. [Google Scholar] [CrossRef] [PubMed]
- Monticone, S.; Satoh, F.; Dietz, A.S.; Goupil, R.; Lang, K.; Pizzolo, F.; Gordon, R.D.; Morimoto, R.; Reincke, M.; Stowasser, M.; Mulatero, P. Clinical Management and Outcomes of Adrenal Hemorrhage Following Adrenal Vein Sampling in Primary Aldosteronism. Hypertension 2016, 67, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Seccia, T.M.; Miotto, D.; Battistel, M.; Motta, R.; Barisa, M.; Maniero, C.; Pessina, A.C.; Rossi, G.P. A stress reaction affects assessment of selectivity of adrenal venous sampling and of lateralization of aldosterone excess in primary aldosteronism. Eur. J. Endocrinol. 2012, 166, 869–875. [Google Scholar] [CrossRef] [PubMed]
- Almarzooqi, M.-K.; Chagnon, M.; Soulez, G.; Giroux, M.-F.; Gilbert, P.; Oliva, V.L.; Perreault, P.; Bouchard, L.; Bourdeau, I.; Lacroix, A.; et al. Adrenal vein sampling in primary aldosteronism: Concordance of simultaneous vs sequential sampling. Eur. J. Endocrinol. 2017, 176, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Satoh, F.; Morimoto, R.; Seiji, K.; Satani, N.; Ota, H.; Iwakura, Y.; Ono, Y.; Kudo, M.; Nezu, M.; Omata, K.; et al. Is there a role for segmental adrenal venous sampling and adrenal sparing surgery in patients with primary aldosteronism? Eur. J. Endocrinol. 2015, 173, 465–477. [Google Scholar] [CrossRef] [PubMed]
- Satani, N.; Ota, H.; Seiji, K.; Morimoto, R.; Kudo, M.; Iwakura, Y.; Ono, Y.; Nezu, M.; Omata, K.; Ito, S.; et al. Intra-adrenal Aldosterone Secretion: Segmental Adrenal Venous Sampling for Localization. Radiology 2016, 278, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Melby, J.C.; Spark, R.F.; Dale, S.L.; Egdahl, R.H.; Kahn, P.C. Diagnosis and localization of aldosterone-producing adenomas by adrenal-vein cateterization. N. Engl. J. Med. 1967, 277, 1050–1056. [Google Scholar] [CrossRef] [PubMed]
- El Ghorayeb, N.; Mazzuco, T.L.; Bourdeau, I.; Mailhot, J.-P.; Zhu, P.S.; Thérasse, E.; Lacroix, A. Basal and Post-ACTH Aldosterone and Its Ratios Are Useful During Adrenal Vein Sampling in Primary Aldosteronism. J. Clin. Endocrinol. Metab. 2016, 101, 1826–1835. [Google Scholar] [CrossRef] [PubMed]
- Wolley, M.J.; Ahmed, A.H.; Gordon, R.D.; Stowasser, M. Does ACTH improve the diagnostic performance of adrenal vein sampling for subtyping primary aldosteronism? Clin. Endocrinol. 2016, 85, 703–709. [Google Scholar] [CrossRef] [PubMed]
- Monticone, S.; Satoh, F.; Giacchetti, G.; Viola, A.; Morimoto, R.; Kudo, M.; Iwakura, Y.; Ono, Y.; Turchi, F.; Paci, E.; et al. Effect of adrenocorticotropic hormone stimulation during adrenal vein sampling in primary aldosteronism. Hypertension 2012, 59, 840–846. [Google Scholar] [CrossRef] [PubMed]
- Seccia, T.M.; Miotto, D.; de Toni, R.; Pitter, G.; Mantero, F.; Pessina, A.C.; Rossi, G.P. Adrenocorticotropic hormone stimulation during adrenal vein sampling for identifying surgically curable subtypes of primary aldosteronism: Comparison of 3 different protocols. Hypertension 2009, 53, 761–766. [Google Scholar] [CrossRef] [PubMed]
- Young, W.F.; Stanson, A.W. What are the keys to successful adrenal venous sampling (AVS) in patients with primary aldosteronism? Clin. Endocrinol. 2009, 70, 14–17. [Google Scholar] [CrossRef] [PubMed]
- Stowasser, M.; Gordon, R.D.; Gunasekera, T.G.; Cowley, D.C.; Ward, G.; Archibald, C.; Smithers, B.M. High rate of detection of primary aldosteronism, including surgically treatable forms, after “non-selective” screening of hypertensive patients. J. Hypertens. 2003, 21, 2149–2157. [Google Scholar] [CrossRef] [PubMed]
- Vonend, O.; Ockenfels, N.; Gao, X.; Allolio, B.; Lang, K.; Mai, K.; Quack, I.; Saleh, A.; Degenhart, C.; Seufert, J.; et al. Adrenal venous sampling: Evaluation of the German Conn’s registry. Hypertension 2011, 57, 990–995. [Google Scholar] [CrossRef] [PubMed]
- Betz, M.J.; Degenhart, C.; Fischer, E.; Pallauf, A.; Brand, V.; Linsenmaier, U.; Beuschlein, F.; Bidlingmaier, M.; Reincke, M. Adrenal vein sampling using rapid cortisol assays in primary aldosteronism is useful in centers with low success rates. Eur. J. Endocrinol. 2011, 165, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Auchus, R.J.; Michaelis, C.; Wians, F.H., Jr.; Dolmatch, B.L.; Josephs, S.C.; Trimmer, C.K.; Anderson, M.E.; Nwariaku, F.E. Rapid cortisol assays improve the success rate of adrenal vein sampling for primary aldosteronism. Ann. Surg. 2009, 249, 318–321. [Google Scholar] [CrossRef] [PubMed]
- Mengozzi, G.; Rossato, D.; Bertello, C.; Garrone, C.; Milan, A.; Pagni, R.; Veglio, F.; Mulatero, P. Rapid cortisol assay during adrenal vein sampling in patients with primary aldosteronism. Clin. Chem. 2007, 53, 1968–1971. [Google Scholar] [CrossRef] [PubMed]
- Yoneda, T.; Karashima, S.; Kometani, M.; Usukura, M.; Demura, M.; Sanada, J.; Minami, T.; Koda, W.; Gabata, T.; Matsui, O.; et al. Impact of New Quick Gold Nanoparticle-Based Cortisol Assay During Adrenal Vein Sampling for Primary Aldosteronism. J. Clin. Endocrinol. Metab. 2016, 101, 2554–2561. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-C.; Lee, B.-C.; Liu, K.-L.; Chang, Y.-C.; Wu, V.-C.; Huang, K.-H. Non-stimulated adrenal venous sampling using Dyna computed tomography in patients with primary aldosteronism. Sci. Rep. 2016, 6, 37143. [Google Scholar] [CrossRef] [PubMed]
- Mulatero, P.; Bertello, C.; Sukor, N.; Gordon, R.; Rossato, D.; Daunt, N.; Leggett, D.; Mengozzi, G.; Veglio, F.; Stowasser, M. Impact of different diagnostic criteria during adrenal vein sampling on reproducibility of subtype diagnosis in patients with primary aldosteronism. Hypertension 2010, 55, 667–673. [Google Scholar] [CrossRef] [PubMed]
- Mailhot, J.-P.; Traistaru, M.; Soulez, G.; Ladouceur, M.; Giroux, M.-F.; Gilbert, P.; Zhu, P.S.; Bourdeau, I.; Oliva, V.L.; Lacroix, A.; et al. Adrenal Vein Sampling in Primary Aldosteronism: Sensitivity and Specificity of Basal Adrenal Vein to Peripheral Vein Cortisol and Aldosterone Ratios to Confirm Catheterization of the Adrenal Vein. Radiology 2015, 277, 887–894. [Google Scholar] [CrossRef] [PubMed]
- Umakoshi, H.; Naruse, M.; Wada, N.; Ichijo, T.; Kamemura, K.; Matsuda, Y.; Fujii, Y.; Kai, T.; Fukuoka, T.; Sakamoto, R.; et al. WAVES-J Study Group Adrenal Venous Sampling in Patients With Positive Screening but Negative Confirmatory Testing for Primary Aldosteronism. Hypertension 2016, 67, 1014–1019. [Google Scholar] [CrossRef] [PubMed]
- Stowasser, M. Adrenal venous sampling for differentiating unilateral from bilateral primary aldosteronism: Still the best, but could be better. Hypertension 2015, 65, 704–706. [Google Scholar] [CrossRef] [PubMed]
- Monticone, S.; Satoh, F.; Viola, A.; Fischer, E.; Vonend, O.; Bernini, G.; Lucatello, B.; Quinkler, M.; Ronconi, V.; Morimoto, R.; et al. Aldosterone suppression on contralateral adrenal during adrenal vein sampling does not predict blood pressure response after adrenalectomy. J. Clin. Endocrinol. Metab. 2014, 99, 4158–4166. [Google Scholar] [CrossRef] [PubMed]
- Umakoshi, H.; Tanase-Nakao, K.; Wada, N.; Ichijo, T.; Sone, M.; Inagaki, N.; Katabami, T.; Kamemura, K.; Matsuda, Y.; Fujii, Y.; et al. Importance of contralateral aldosterone suppression during adrenal vein sampling in the subtype evaluation of primary aldosteronism. Clin. Endocrinol. 2015, 83, 462–467. [Google Scholar] [CrossRef] [PubMed]
- Wolley, M.J.; Gordon, R.D.; Ahmed, A.H.; Stowasser, M. Does contralateral suppression at adrenal venous sampling predict outcome following unilateral adrenalectomy for primary aldosteronism? A retrospective study. J. Clin. Endocrinol. Metab. 2015, 100, 1477–1484. [Google Scholar] [CrossRef] [PubMed]
- Auchus, R.J.; Wians, F.H.; Anderson, M.E.; Dolmatch, B.L.; Trimmer, C.K.; Josephs, S.C.; Chan, D.; Toomay, S.; Nwariaku, F.E. What we still do not know about adrenal vein sampling for primary aldosteronism. Horm. Metab. Res. 2010, 42, 411–415. [Google Scholar] [CrossRef] [PubMed]
- Espiner, E.A.; Ross, D.G.; Yandle, T.G.; Richards, A.M.; Hunt, P.J. Predicting surgically remedial primary aldosteronism: Role of adrenal scanning, posture testing, and adrenal vein sampling. J. Clin. Endocrinol. Metab. 2003, 88, 3637–3644. [Google Scholar] [CrossRef] [PubMed]
- Dekkers, T.; Deinum, J.; Schultzekool, L.J.; Blondin, D.; Vonend, O.; Hermus, A.R.R.M.; Peitzsch, M.; Rump, L.C.; Antoch, G.; Sweep, F.C.G.J.; et al. Plasma metanephrine for assessing the selectivity of adrenal venous sampling. Hypertension 2013, 62, 1152–1157. [Google Scholar] [CrossRef] [PubMed]
- Eisenhofer, G.; Keiser, H.; Friberg, P.; Mezey, E.; Huynh, T.T.; Hiremagalur, B.; Ellingson, T.; Duddempudi, S.; Eijsbouts, A.; Lenders, J.W. Plasma metanephrines are markers of pheochromocytoma produced by catechol-O-methyltransferase within tumors. J. Clin. Endocrinol. Metab. 1998, 83, 2175–2185. [Google Scholar] [CrossRef] [PubMed]
- Goupil, R.; Wolley, M.; Ungerer, J.; McWhinney, B.; Mukai, K.; Naruse, M.; Gordon, R.D.; Stowasser, M. Use of plasma metanephrine to aid adrenal venous sampling in combined aldosterone and cortisol over-secretion. Endocrinol. Diabet. Metab. Case Rep. 2015, 2015, 150075. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, T.; Omura, M.; Satoh, F.; Shibata, H.; Takahashi, K.; Tamura, N.; Tanabe, A. Guidelines for the diagnosis and treatment of primary aldosteronism—The Japan Endocrine Society 2009. Endocr. J. 2011, 58, 711–721. [Google Scholar] [CrossRef] [PubMed]
| Test/Clinical Score | Sensitivity | Specificity | Accuracy | Reference |
|---|---|---|---|---|
| Age < 40 and unilateral adrenal nodule > 1 cm with normal contralateral adrenal gland [13] | n.a. | 100% | n.a. | [11] |
| 68% | 83% | 71% | [21] | |
| 71% | n.a. | n.a. | [22] | |
| 18% | 100% | 54% | [23] | |
| Age < 35 and unilateral adrenal nodule > 1 cm with normal contralateral adrenal gland | 100% | n.a. | n.a. | [22] |
| Typical Conn’s adenoma, serum K+ < 3.5 mmol/L and/or eGFR ≥ 100 mL/min/1.73 m2 | 53% | 100% | 74% | [23] |
| 46% | 80% | 58% | [21] | |
| 39% | 89% | 56% | [24] | |
| Typical Conn’s adenoma, serum K+ < 3.5 mmol/L and/or eGFR ≥ 100 mL/min/1.73 m2 and age < 40 years | 59% | 100% | 68% | [21] |
| Serum K+ ≤ 3 mmol/L and PAC ≥ 25 ng/dL and/or urinary aldosterone greater ≥ 30 µg/24 h (+stage III hypertension) | 32% (23%) | 95% (97%) | 67% (64%) | [11] |
| No adrenal nodule, serum K+ ≥ 3.5 mmol/L, ARR post-captopril < 490 # | 50%–67% (7 points–5 points) | 100%–94% (7 points–5 points) | 75%–80% (7 points–5 points) | [25] |
| Posture stimulation test | n.a. | n.a. | 85% | [26] |
| 64% | 70% | 67% | [11] | |
| 44%–56% (1 and 4 h respectively) | 71%–75% (1 and 4 h respectively) | 52%–62% | [27] | |
| 70% | 79% | 75% | [28] (Torino) | |
| 51% | 78% | 69% | [28] (Brisbane) | |
| 71% | 100% | 41% | [29] | |
| 35% | 100% | 46% | [29] * | |
| ACTH stimulating test | 91% | 81% | 90% | [30] |
| 83% (to predict BAH) | 88% (to predict BAH) | 84% | [31] | |
| Steroid profiling | 83% | 76% | 80% | [32] |
| Urinary 18OHF > 510 µg/24 h | 35% | 100% | 84% | [33] |
| Plasma 18oxoF > 4.7 ng/dL | 83% | 99% | 92% | [34] |
| Serum 18OHB > 100 ng/dL | n.a. | n.a. | 82% | [26] |
| Serum PTH > 80 ng/L | 74% | 82% | 76% | [35] |
| NP-59 scintigraphy scan | n.a. | n.a. | 72% | [26] |
| 11C-metomidate PET-CT | 76% | 87% | 80% | [36] |
| Index | Measurement | Clinical Significance | Suggested Cut-Off |
|---|---|---|---|
| Selectivity index (SI) | Cortisoladrenal vein/Cortisolperipheral vein | Successful adrenal vein cannulation | SI > 3 for basal studies SI > 5 for ACTH (1-24) stimulated AVS |
| Lateralization index (LI) | (Aldosterone/Cortisol)dominant adrenal vein/(Aldosterone/Cortisol)non dominant adrenal vein | Lateralization of aldosterone production | LI > 4 for unilateral PA LI < 3 for bilateral PA 3 < LI < 4 grey zone |
| Ipsilateral ratio (ILR) | (Aldosterone/Cortisol)dominant adrenal vein/(Aldosterone/Cortisol)peripheral vein | Gradient of aldosterone production between the adrenal vein and a peripheral vein | ILR > 2 is required in some centers together with CLR < 1 to define unilateral PA |
| Contralateral ratio (CLR) | (Aldosterone/Cortisol)non dominant adrenal vein/(Aldosterone/Cortisol)peripheral vein | Suppression of aldosterone production in the non-dominant side | CLR < 1 can be used as additional criteria for the interpretation of suboptimal studies |
| Absolute contralateral aldosterone ratio [56] | Aldosteronenon dominant adrenal vein/Aldosteroneperipheral vein | Absolute suppression of aldosterone production in the non-dominant side | <1.5 predicts outcomes after adrenalectomy |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buffolo, F.; Monticone, S.; Williams, T.A.; Rossato, D.; Burrello, J.; Tetti, M.; Veglio, F.; Mulatero, P. Subtype Diagnosis of Primary Aldosteronism: Is Adrenal Vein Sampling Always Necessary? Int. J. Mol. Sci. 2017, 18, 848. https://doi.org/10.3390/ijms18040848
Buffolo F, Monticone S, Williams TA, Rossato D, Burrello J, Tetti M, Veglio F, Mulatero P. Subtype Diagnosis of Primary Aldosteronism: Is Adrenal Vein Sampling Always Necessary? International Journal of Molecular Sciences. 2017; 18(4):848. https://doi.org/10.3390/ijms18040848
Chicago/Turabian StyleBuffolo, Fabrizio, Silvia Monticone, Tracy A. Williams, Denis Rossato, Jacopo Burrello, Martina Tetti, Franco Veglio, and Paolo Mulatero. 2017. "Subtype Diagnosis of Primary Aldosteronism: Is Adrenal Vein Sampling Always Necessary?" International Journal of Molecular Sciences 18, no. 4: 848. https://doi.org/10.3390/ijms18040848
APA StyleBuffolo, F., Monticone, S., Williams, T. A., Rossato, D., Burrello, J., Tetti, M., Veglio, F., & Mulatero, P. (2017). Subtype Diagnosis of Primary Aldosteronism: Is Adrenal Vein Sampling Always Necessary? International Journal of Molecular Sciences, 18(4), 848. https://doi.org/10.3390/ijms18040848