Application of Chromatographic and Spectroscopic Methods towards the Quality Assessment of Ginger (Zingiber officinale) Rhizomes from Ecological Plantations
Abstract
:1. Introduction
2. Results and Discussion
2.1. Qualitative and Quantitative GC-MS and LC-MS Analysis of Extracts
2.1.1. GC-MS Profiling of Diethyl Extracts
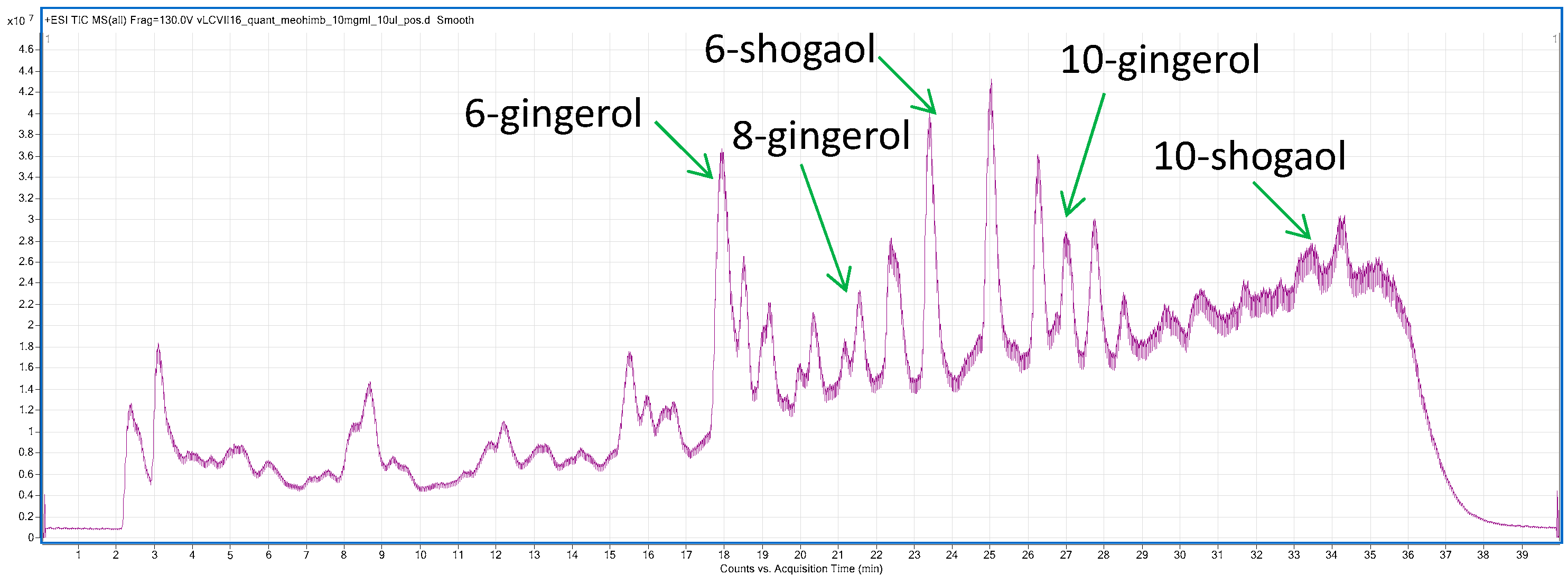
2.1.2. LC-MS Profiling of Ethanolic Extract from Ginger Rhizomes
2.2. Macroelement Content in Ginger Samples
2.3. Trace Element Content of Ginger Rhizomes
2.4. Heavy Metal Content in Ginger Cultivated on Shikoku Island
3. Materials and Methods
3.1. Reagents (Chemicals)
3.2. Investigated Plant Material
3.3. GC-MS Analysis of the Rhizomes
3.3.1. Sample Preparation
3.3.2. The GC-MS-Based Study of Ginger Rhizomes
3.4. LC-MS Determination of Phenolics Present in Ginger Rhizomes
3.4.1. Sample Preparation
3.4.2. Determination of Phenolics in the Extract
3.5. The Elemental Analysis of the Samples
3.5.1. Sample Preparation
3.5.2. Digestion of Samples
3.5.3. Preparation of Standard Solution
3.5.4. Analytical Determination of Metals by FAAS and ETAAS
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Banerjee, S.; Mullick, H.I.; Banerjee, J.; Ghosh, A. Zingiber officinale: “A natural gold”. Int. J. Pharm. Biosci. 2011, 2, 283–294. [Google Scholar]
- Jelled, A.; Fernandes, A.; Barros, L.; Chahdoura, H.; Achour, L.; Ferreira, I.C.F.R.; Ben Cheikh, H. Chemical and antioxidant parameters of dried forms of ginger rhizomes. Ind. Crop. Prod. 2015, 77, 30–35. [Google Scholar] [CrossRef]
- Bartley, J.P.; Jacobs, A.L. Effects of drying on flavour compounds in Australian-grown ginger (Zingiber officinale). J. Sci. Food Agric. 2000, 80, 209–215. [Google Scholar] [CrossRef]
- Jin, Z.; Lee, G.; Kim, S.; Park, C.S.; Park, Y.S.; Jin, Y.H. Ginger and its pungent constituents non-competitively inhibit serotonin currents on visceral afferent neurons. Korean J. Physiol. Pharmacol. 2014, 18, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Bliddal, H.; Rosetzsky, A.; Schlichting, P.; Weidner, M.S.; Andersen, L.A.; Ibfelt, H.H.; Christensen, K.; Jensen, O.N.; Barslev, J. A randomized, placebo-controlled, cross-over study of ginger extracts and ibuprofen in osteoarthritis. Osteoarthr. Cartil. 2000, 8, 9–12. [Google Scholar] [CrossRef] [PubMed]
- Baraniak, J.; Kania, M. Herbal products with analgesic, anti-inflammatory and antirheumatic activity. Postep. Fitoter. 2011, 12, 94–99. [Google Scholar]
- Chakraborty, B.; Nath, A.; Saikia, H.; Sengupta, M. Bactericidal activity of selected medicinal plants against multidrug resistant bacterial strains from clinical isolates. Asian Pac. J. Trop. Dis. 2014, 7, 435–441. [Google Scholar] [CrossRef]
- Nurtjahja-Tjendraputra, E.; Ammit, A.J.; Roufogalis, B.D.; Tran, V.H.; Duke, C.C. Effective anti-platelet and COX-1 enzyme inhibitors from pungent constituents of ginger. Thromb. Res. 2003, 111, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Khandouzi, N.; Shidfar, F.; Rajab, A.; Rahideh, T.; Hosseini, P.; Taheri, M.M. The effects of ginger on fasting blood sugar, hemoglobin A1c, apolipoprotein B, apolipoprotein A-I and malondialdehyde in type 2 diabetic patients. Iran. J. Pharm. Res. 2015, 14, 131–140. [Google Scholar] [PubMed]
- Lebda, MA.; Taha, N.M.; Korshom, M.A.; Mandour, A.; El-Morshedy, A.M. Biochemical effect of ginger on some blood and liver parameters in male New Zealand rabbits. Online J. Anim. Feed Res. 2012, 2, 197–202. [Google Scholar]
- Srinivasan, K. Antioxidant potential of spices and their active constituents. Crit. Rev. Food Sci. Nutr. 2014, 54, 352–372. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.J.; Chen, C.Y.; Chung, L.Y.; Yen, C.M. Larvicidal activities of ginger (Zingiber officinale) against Angiostrongylus cantonensis. Acta Trop. 2010, 115, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Bello, M.O.; Ibrahim, A.O.; Ogunwande, I.A.; Olawore, N.O. Heavy trace metals and macronutrients status in herbal plants of Nigeria. Food Chem. 2004, 85, 67–71. [Google Scholar]
- Wohlmuth, H.; Leach, D.N.; Smith, M.K.; Myers, S.P. Gingerol content of diploid and tetraploid clones of ginger (Zingiber officinale Roscoe). J. Agric. Food Chem. 2005, 53, 5772–5778. [Google Scholar] [CrossRef] [PubMed]
- Van Beek, T.A.; Posthumus, M.A.; Lelyvelld, G.P.; Phiet, H.V.; Yen, B.T. Investigation of the essential oil of Vietnamese ginger. Phytochemistry 1987, 26, 3005–3010. [Google Scholar] [CrossRef]
- MacLeod, A.J.; Pieris, N.M. Volatile aroma constituents of Sri Lankan ginger. Phytochemistry 1984, 23, 353–359. [Google Scholar] [CrossRef]
- Sasikumar, B.; Krishnamoorthy, B.; Saji, K.V.; George, J.K.; Peter, K.V.; Ravindran, P.N. Spice diversity and conservation of plants that yield major spices in India. Plant Genet. Resour. Newsl. 1999, 118, 19–26. [Google Scholar]
- Chrubasik, S.; Pittler, M.H.; Roufogalis, B.D. Zingiberis rhizoma: A comprehensive review on the ginger effect and efficacy profiles. Phytomedicine 2005, 12, 684–701. [Google Scholar] [CrossRef] [PubMed]
- Koch, W.; Kukula-Koch, W.; Dziedzic, M.; Głowniak, K.; Asakawa, Y. Influence of thermal processing and in vitro digestion on the antioxidant potential of ginger and ginger containing products. Nat. Prod. Comm. 2016, 11, 1153–1156. [Google Scholar]
- Gupta, S.; Pandotra, P.; Ram, G.; Anand, R.; Gupta, A.P.; Husain, K.; Bedi, Y.S.; Mallavarapu, G.R. Composition of a monoterpenoid-rich essential oil from the rhizome of Zingiber officinale from north western Himalayas. Nat. Prod. Comm. 2011, 6, 93–96. [Google Scholar]
- Raina, V.K.; Kumar, A.; Aggarwal, K.K. Essential oil composition of ginger (Zingiber officinale Roscoe) rhizomes from different places in India. J. Essent. Oil Bear. Plant. 2005, 8, 187–191. [Google Scholar] [CrossRef]
- Nirmala Menon, A.; Padmakumari, K.P.; Sankari Kutty, B.; Sumathikutty, M.A.; Sreekumar, M.M.; Arumugham, C. Effects of processing on the flavor compounds of Indian fresh ginger (Zingiber officinale Rosc.). J. Essent. Oil Res. 2007, 19, 105–109. [Google Scholar] [CrossRef]
- Owolabi, M.S.; Oladimeji, M.O.; Labunmi, L.; Singh, G.; Marimuthu, P.; Isidorov, V.A. Compositions and biological potentials of the essential oil of Zingiber officinale oil (Roscoe) from Nigeria. Bull. Pure Appl. Sci. 2007, 26, 113–119. [Google Scholar]
- Yang, Z.; Yang, W.; Peng, Q.; He, Q.; Feng, Y.; Luo, S.; Yu, Z. Volatile phytochemical composition of rhizome of ginger after extraction by headspace solid-phase microextraction, petroleum ether extraction and steam distillation extraction. Bangladesh J. Pharmacol. 2009, 4, 136–143. [Google Scholar] [CrossRef]
- Lima, I.P.; Resende, J.T.V.; Oliveira, J.R.F.; Faria, M.V.; Dias, D.M.; Resende, N.C.V. Selection of tomato genotypes for processing with high zingiberene content, resistant to pests. Hortic. Bras. 2016, 34, 387–391. [Google Scholar] [CrossRef]
- Liao, P.-C.; Yang, T.-S.; Chou, J.-C.; Ho, C.-L.; Chao, L.K.P. Anti-inflammatory activity of neral and geranial isolated from fruits of Litsea cubeba Lour. J. Funct. Foods 2015, 19, 248–258. [Google Scholar] [CrossRef]
- Çelik, K.; Toǧar, B.; Türkez, H.; Taşpinar, N. In vitro cytotoxic, genotoxic, and oxidative effects of acyclic sesquiterpene farnesene. Turk. J. Biol. 2014, 38, 253–259. [Google Scholar] [CrossRef]
- Bartley, J.P. A new method for the determination of pungent compounds in ginger (Zingiber officinale). J. Sci. Food Agric. 1995, 68, 215–222. [Google Scholar] [CrossRef]
- Govindarajan, V.S. Ginger—Chemistry, technology, and quality evaluation. Part 1. Crit. Rev. Food Sci. Nutr. 1982, 17, 1–96. [Google Scholar] [CrossRef] [PubMed]
- Yogeshwer, S.; Madhulika, S. Cancer preventive properties of ginger: A brief review. Food Chem. Toxicol. 2007, 45, 683–690. [Google Scholar]
- Cheng, X.-L.; Liu, Q.; Peng, Y.-B.; Qi, L.-W.; Li, P. Steamed ginger (Zingiber officinale): Changed chemical profile and increased anticancer potential. Food Chem. 2011, 129, 1785–1792. [Google Scholar] [CrossRef]
- Otunola, G.A.; Oloyede, O.B.; Oladiji, A.T.; Afolayan, A.J. Comparative analysis of the chemical composition of three spices—Allium sativum L., Zingiber officinale Rosc. and Capsicum frutescens L. commonly consumed in Nigeria. Afr. J. Biotechnol. 2010, 9, 6927–6931. [Google Scholar]
- Pandotra, P.; Viz, B.; Ram, G.; Gupta, A.P.; Gupta, S. Multi-elemental profiling and chemo-metric validation revealed nutritional qualities of Zingiber officinale. Ecotoxicol. Environ. Saf. 2015, 114, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Wagesho, Y.; Chandravanshi, B.S. Levels of essential and non-essential metals in ginger (Zingiber officinale) cultivated in Ethiopia. Springerplus 2015, 4, 107–120. [Google Scholar] [CrossRef] [PubMed]
- Nkwenkeu, S.F.; Kennedy, G.; Philippe, S.; Zayed, J. Oral manganese intake estimated with dietary records and with direct chemical analysis. Sci. Total Environ. 2002, 287, 147–153. [Google Scholar] [CrossRef]
- Food and Nutrition Board. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc; National Academy Press: Washington, DC, USA, 2002. [Google Scholar]
- Lamari, Z.; Larbi, R.; Yagoubi, B. Trace element content of ginger and sage medicinal plants from Algeria. Health 2011, 3, 542–544. [Google Scholar] [CrossRef]
- Nazir, R.; Khan, M.; Masab, M.; Rehman, H.U.; Rauf, N.U.; Shahab, S.; Ameer, N.; Sajed, M.; Ullah, M.; Rafeeq, M.; et al. Accumulation of Heavy Metals (Ni, Cu, Cd, Cr, Pb, Zn, Fe) in the soil, water and plants and analysis of physico-chemical parameters of soil and water collected from Tanda Dam kohat. J. Pharm. Sci. Res. 2015, 7, 89–97. [Google Scholar]
- Krejpcio, Z.; Król, E.; Sionkowski, S. Evaluation of heavy metals content in spices and herbs available on the Polish market. Pol. J. Environ. Stud. 2007, 16, 97–100. [Google Scholar]
- European Commission Directive No 629/2008 Adopted on 2 July 2008. Available online: http://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX%3A32008R0629 (accessed on 10 December 2016).
- Kabata-Pendias, A.; Pendias, H. Biogeochemia Pierwiastków Śladowych; PWN: Warsaw, Poland, 1993. [Google Scholar]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectroscopy, 4th ed.; Allured: Carol Stream, IL, USA, 1995. [Google Scholar]
- Govender, A.; Kindness, A.; Jonnalagadda, S.B. Impact of soil quality on elemental uptake by Zingiber officinal (ginger rhizome). Int. J. Environ. Anal. Chem. 2009, 89, 367–382. [Google Scholar] [CrossRef]
| Compound | Retention Time | Retention Index | Content (%) | Compound | Retention Time | Retention Index | Content (%) |
|---|---|---|---|---|---|---|---|
| α-Pinene | 9.04 | 1009 | 3.8 | Citronellyl acetate | 22.29 | 1535 | 0.13 |
| Camphene | 9.61 | 1025 | 1.04 | α-Copaene | 22.96 | 1566 | 0.47 |
| Myrcene | 11.20 | 1086 | 0.35 | Geranyl acetate | 23.13 | 1575 | 0.29 |
| α-Phellandren | 11.65 | 1102 | 0.37 | cis-β-Elemene | 23.38 | 1586 | 0.83 |
| γ-Terpinen | 12.48 | 1133 | 5.12 | Sesquithujene | 23.71 | 1601 | 0.27 |
| Linalool | 14.89 | 1224 | 0.12 | β-Ylangene | 24.09 | 1620 | 0.17 |
| Citronellal | 16.55 | 1291 | 0.23 | β-Copaene | 24.36 | 1632 | 0.09 |
| Borneol | 16.94 | 1306 | 1.25 | γ-Elemene | 24.44 | 1637 | 0.79 |
| Terpinen-4-ol | 17.31 | 1328 | 0.12 | (E)-β-Farnesene | 24.99 | 1662 | 0.68 |
| Isogeraniol | 17.48 | 1328 | 0.10 | allo-Aromadendrene | 25.17 | 1671 | 0.23 |
| α-Terpineol | 17.72 | 1337 | 0.52 | ar-Curcumene | 25.70 | 1696 | 6.29 |
| n-Decanal | 18.20 | 1356 | 0.41 | α-Zingiberene | 26.10 | 1716 | 37.87 |
| Citronellol | 18.85 | 1384 | 0.26 | (E,E)-α-Farnesene | 26.34 | 1731 | 9.62 |
| Neral | 19.20 | 1399 | 1.13 | γ-Cadinene | 26.40 | 1745 | 0.70 |
| Geraniol | 19.70 | 1420 | 0.26 | β-Sesquiphellandrene | 26.76 | 1750 | 11.38 |
| Geranial | 20.10 | 1438 | 8.24 | (E)-γ-Bisabolene | 26.89 | 1759 | 0.23 |
| Isobornyl acetate | 20.49 | 1455 | 0.14 | Elemol | 27.31 | 1779 | 0.63 |
| Tridecane | 20.69 | 1464 | 0.25 | Zingiberenol | 28.25 | 1829 | 0.44 |
| δ-Elemene | 21.92 | 1518 | 0.18 | ||||
| Total content | ~95% |
| Compound | (6)-Gingerol | (8)-Gingerol | (10)-Gingerol | (6)-Shogaol | (10)-Shogaols |
|---|---|---|---|---|---|
| Mean content (mg/kg) | 268.3 | 68.8 | 75.2 | 133.2 | 16.0 |
| SD | 25.2 | 6.44 | 6.52 | 9.74 | 0.28 |
| RSD (%) | 9.40 | 9.36 | 8.67 | 7.31 | 1.74 |
| Macroelements | Trace Elements | Heavy Metals | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Elements | Ca | Mg | K | Na | Zn | Cu | Fe | Mn | Cr | Ni | Pb | Cd |
| Fresh rhizome | 159.0 ± 31.3 | 239.8 ± 21.8 | 4052 ± 443 | 115.6 ± 31.4 | 2.54 ± 0.44 | 1.03 ± 0.06 | 12.5 ± 2.67 | 69.9 ± 10.0 | 0.064 ± 0.01 | 0.163 ± 0.02 | 0.021 ± 0.006 | 0.002 ± 0.0008 |
| Dry weight | 1587 ± 337 | 2572 ± 485 | 40,963 ± 9359 | 1261 ± 427 | 25.0 ± 6.90 | 11.1 ± 1.76 | 140.5 ± 49.4 | 758.4 ± 150.3 | 0.795 ± 0.054 | 1.67 ± 0.28 | 0.218 ± 0.032 | 0.02 ± 0.004 |
| Macro- and Trace Elements | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Parameter | Ca | Mg | K | Na | Zn | Cu | Fe | Mn | Cr |
| Theoretical value (mg/kg) | 3522 | 752.3 | 10,260 | 6300 | 24 | 2.94 | 22.9 | 9.02 | 0.15 |
| Found value [mg/kg] | 3452 | 808 | 11,191 | 5743 | 25.7 | 3.55 | 19.7 | 10.6 | 0.15 |
| 3609 | 808 | 12,105 | 5969 | 25.8 | 3.30 | 21.1 | 9.36 | 0.14 | |
| 3181 | 725 | 10,184 | 4965 | 22.9 | 3.41 | 21.1 | 9.75 | 0.14 | |
| 3625 | 731 | 11,441 | 6181 | 24.1 | 3.41 | 20.8 | 9.86 | 0.16 | |
| 3741 | 703 | 12,043 | 5281 | 22.8 | 3.47 | 21.8 | 9.54 | 0.17 | |
| 3618 | 765 | 11,673 | 5917 | 24.2 | 3.39 | 24.1 | 10.4 | 0.17 | |
| Average | 3538 | 756.7 | 11,440 | 5676 | 24.3 | 3.42 | 21.4 | 9.93 | 0.16 |
| SD | 197.5 | 44.5 | 707.1 | 461.5 | 1.29 | 0.0861 | 1.46 | 0.50 | 0.01 |
| RSD (%) | 5.58 | 5.88 | 6.18 | 8.13 | 5.30 | 2.51 | 6.79 | 5.00 | 6.25 |
| Recovery (%) | 100.5 | 100.6 | 111.5 | 90.1 | 101.0 | 116.4 | 93.6 | 110.1 | 107 |
| LOD (µg/kg) | 180 | 30 | 60 | 78 | 41 | 187 | 154 | 174 | 0.57 |
| LOQ (µg/kg) | 600 | 110 | 250 | 281 | 152 | 655 | 513 | 618 | 2.20 |
| Parameter | Ni | Pb | Cd |
|---|---|---|---|
| Theoretical value (mg/kg) | 0.5 | 0.5 | 0.3 |
| Found value (mg/kg) | 0.51 | 0.52 | 0.34 |
| 0.54 | 0.54 | 0.33 | |
| 0.49 | 0.56 | 0.35 | |
| 0.61 | 0.48 | 0.31 | |
| 0.56 | 0.55 | 0.34 | |
| 0.47 | 0.51 | 0.31 | |
| Average | 0.53 | 0.53 | 0.33 |
| SD | 0.05 | 0.03 | 0.02 |
| RSD (%) | 9.63 | 5.66 | 6.1 |
| Recovery (%) | 106 | 106 | 110 |
| LOD (µg/kg) | 0.39 | 1.37 | 0.08 |
| LOQ (µg/kg) | 1.57 | 5.01 | 0.30 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koch, W.; Kukula-Koch, W.; Marzec, Z.; Kasperek, E.; Wyszogrodzka-Koma, L.; Szwerc, W.; Asakawa, Y. Application of Chromatographic and Spectroscopic Methods towards the Quality Assessment of Ginger (Zingiber officinale) Rhizomes from Ecological Plantations. Int. J. Mol. Sci. 2017, 18, 452. https://doi.org/10.3390/ijms18020452
Koch W, Kukula-Koch W, Marzec Z, Kasperek E, Wyszogrodzka-Koma L, Szwerc W, Asakawa Y. Application of Chromatographic and Spectroscopic Methods towards the Quality Assessment of Ginger (Zingiber officinale) Rhizomes from Ecological Plantations. International Journal of Molecular Sciences. 2017; 18(2):452. https://doi.org/10.3390/ijms18020452
Chicago/Turabian StyleKoch, Wojciech, Wirginia Kukula-Koch, Zbigniew Marzec, Elwira Kasperek, Lucyna Wyszogrodzka-Koma, Wojciech Szwerc, and Yoshinori Asakawa. 2017. "Application of Chromatographic and Spectroscopic Methods towards the Quality Assessment of Ginger (Zingiber officinale) Rhizomes from Ecological Plantations" International Journal of Molecular Sciences 18, no. 2: 452. https://doi.org/10.3390/ijms18020452
APA StyleKoch, W., Kukula-Koch, W., Marzec, Z., Kasperek, E., Wyszogrodzka-Koma, L., Szwerc, W., & Asakawa, Y. (2017). Application of Chromatographic and Spectroscopic Methods towards the Quality Assessment of Ginger (Zingiber officinale) Rhizomes from Ecological Plantations. International Journal of Molecular Sciences, 18(2), 452. https://doi.org/10.3390/ijms18020452









