Azithromycin and Chloramphenicol Diminish Neutrophil Extracellular Traps (NETs) Release
Abstract
1. Introduction
2. Results
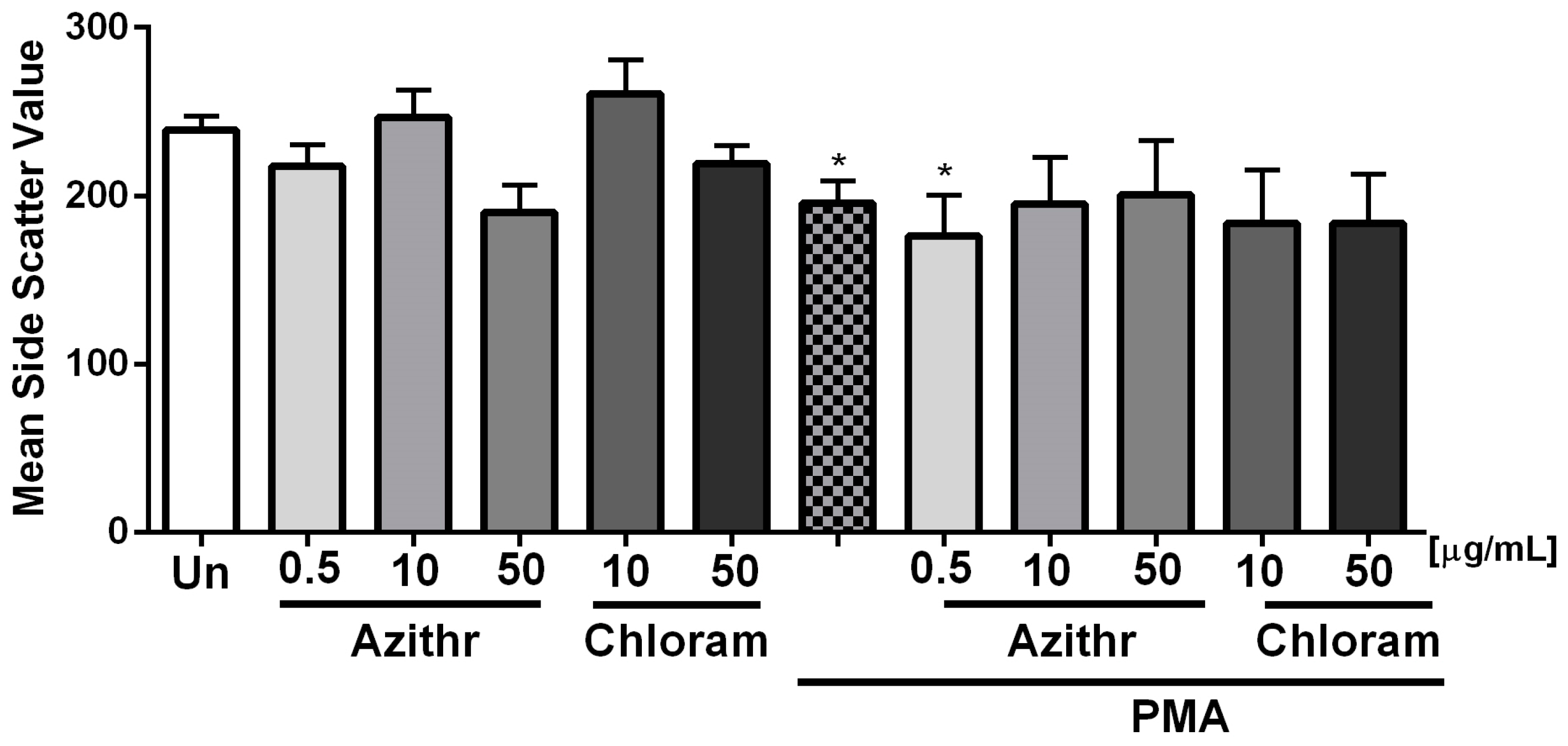
2.1. Degranulation
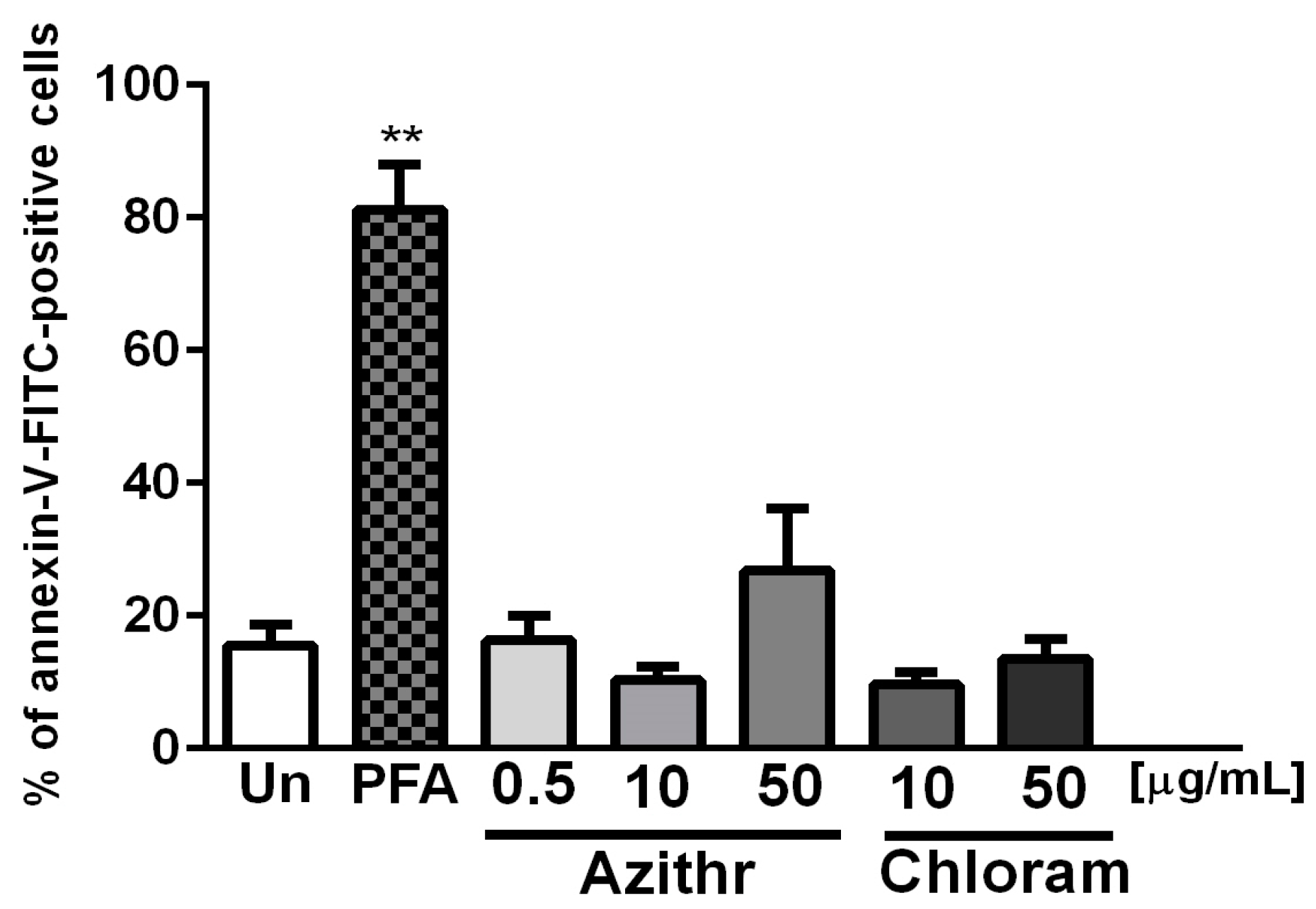
2.2. Apoptosis
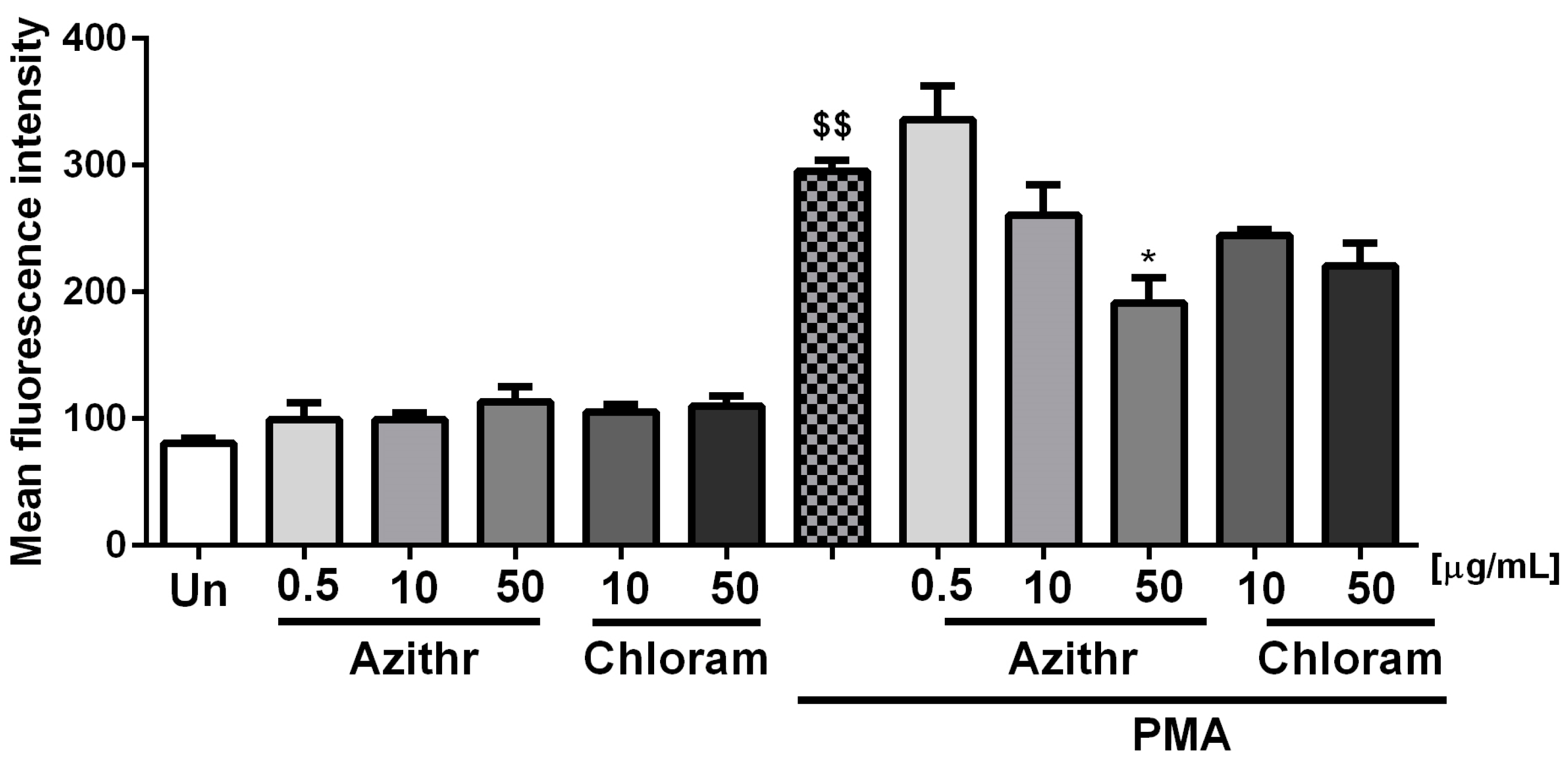
2.3. Oxidative Burst
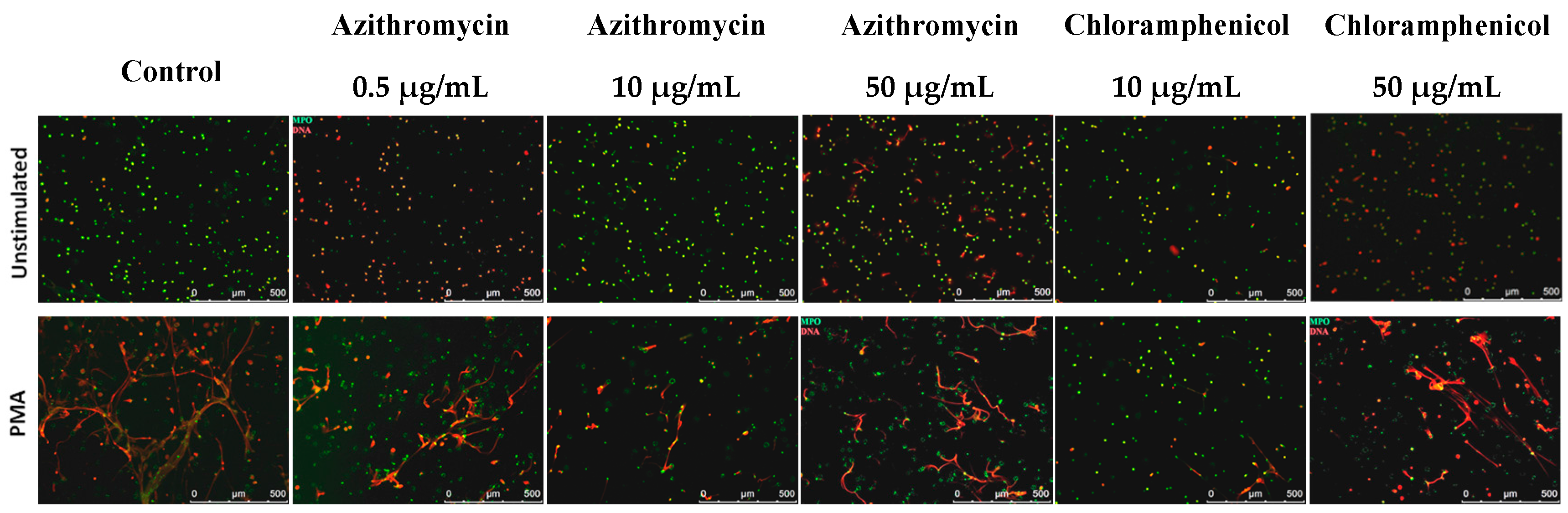
2.4. NETosis
3. Discussion
4. Materials and Methods
4.1. Antibiotics
4.2. Neutrophil Isolation and Preparation
4.3. Degranulation
4.4. Apoptosis
4.5. Respiratory Burst
4.6. NET Quantification
4.7. NET Visualization
4.8. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| ROS | Reactive oxygen species |
| NETs | Neutrophil extracellular traps |
| MPO | Myeloperoxidase |
| PMA | Phorbol 12-myristate 13-acetate |
| PFA | Paraformaldehyde |
| SSC | Side scatter channel |
| f.c. | Final concentration |
References
- Manda, A.; Pruchniak, M.P.; Arazna, M.; Demkow, U.A. Neutrophil extracellular traps in physiology and pathology. Cent.-Eur. J. Immunol. 2014, 39, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Branzk, N.; Papayannopoulos, V. Molecular mechanisms regulating NETosis in infection and disease. Semin. Immunopathol. 2013, 35, 513–530. [Google Scholar] [CrossRef] [PubMed]
- Munoz, J.; Geister, R. Inhibition of phagocytosis by aureomycin. Proc. Soc. Exp. Biol. Med. 1950, 75, 367–370. [Google Scholar] [CrossRef] [PubMed]
- Healy, D.P.; Silverman, P.A.; Neely, A.N.; Holder, I.A.; Babcock, G.E. Effect of antibiotics on polymorphonuclear neutrophil apoptosis. Pharmacotherapy 2002, 22, 578–585. [Google Scholar] [CrossRef] [PubMed]
- Seklecki, M.M.; Quintiliani, R.; Maderazo, E.G. Aminoglycoside antibiotics moderately impair granulocyte function. Antimicrob. Agents Chemother. 1978, 13, 552–554. [Google Scholar] [CrossRef] [PubMed]
- Forsgren, A.; Schmeling, D. Effect of antibiotics of chemotaxis of human leukocytes. Antimicrob. Agents Chemother. 1977, 11, 580–584. [Google Scholar] [CrossRef] [PubMed]
- Hoeben, D.; Dosogne, H.; Heyneman, R.; Burvenich, C. Effect of antibiotics on the phagocytotic and respiratory burst activity of bovine granulocytes. Eur. J. Pharmacol. 1997, 332, 289–297. [Google Scholar] [CrossRef]
- Lai, P.C.; Schibler, M.R.; Walters, J.D. Azithromycin enhances phagocytic killing of Aggregatibacter actinomycetemcomitans Y4 by human neutrophils. J. Periodontol. 2015, 86, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Jerjomiceva, N.; Seri, H.; Vollger, L.; Wang, Y.; Zeitouni, N.; Naim, H.Y.; von Kockritz-Blickwede, M. Enrofloxacin enhances the formation of neutrophil extracellular traps in bovine granulocytes. J. Innate Immun. 2014, 6, 706–712. [Google Scholar] [CrossRef] [PubMed]
- Schilcher, K.; Andreoni, F.; Uchiyama, S.; Ogawa, T.; Schuepbach, R.A.; Zinkernagel, A.S. Increased neutrophil extracellular trap-mediated Staphylococcus aureus clearance through inhibition of nuclease activity by clindamycin and immunoglobulin. J. Infect. Dis. 2014, 210, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Konstantinidis, T.; Kambas, K.; Mitsios, A.; Panopoulou, M.; Tsironidou, V.; Dellaporta, E.; Kouklakis, G.; Arampatzioglou, A.; Angelidou, I.; Mitroulis, I.; et al. Immunomodulatory Role of Clarithromycin in Acinetobacter Baumannii Infection via Formation of Neutrophil Extracellular Traps. Antimicrob. Agents Chemother. 2016, 60, 1040–1048. [Google Scholar] [CrossRef] [PubMed]
- Hand, W.L.; Hand, D.L. Characteristics and mechanisms of azithromycin accumulation and efflux in human polymorphonuclear leukocytes. Int. J. Antimicrob. Agents 2001, 18, 419–425. [Google Scholar] [CrossRef]
- Retsema, J.; Girard, A.; Schelkly, W.; Manousos, M.; Anderson, M.; Bright, G.; Borovoy, R.; Brennan, L.; Mason, R. Spectrum and mode of action of azithromycin (CP-62,993), a new 15-membered-ring macrolide with improved potency against gram-negative organisms. Antimicrob. Agents Chemother. 1987, 31, 1939–1947. [Google Scholar] [CrossRef] [PubMed]
- Bosnar, M.; Kelneric, Z.; Munic, V.; Erakovic, V.; Parnham, M.J. Cellular uptake and efflux of azithromycin, erythromycin, clarithromycin, telithromycin, and cethromycin. Antimicrob. Agents Chemother. 2005, 49, 2372–2377. [Google Scholar] [CrossRef] [PubMed]
- Keshari, R.S.; Verma, A.; Barthwal, M.K.; Dikshit, M. Reactive oxygen species-induced activation of ERK and p38 MAPK mediates PMA-induced NETs release from human neutrophils. J. Cell. Biochem. 2013, 114, 532–540. [Google Scholar] [CrossRef] [PubMed]
- Sood, S. Chloramphenicol—A Potent Armament Against Multi-Drug Resistant (MDR) Gram Negative Bacilli? J. Clin. Diagn. Res. JCDR 2016, 10, DC01–DC03. [Google Scholar] [CrossRef] [PubMed]
- Yipp, B.G.; Kubes, P. NETosis: How vital is it? Blood 2013, 122, 2784–2794. [Google Scholar] [CrossRef] [PubMed]
- Van Vlem, B.; Vanholder, R.; De Paepe, P.; Vogelaers, D.; Ringoir, S. Immunomodulating effects of antibiotics: Literature review. Infection 1996, 24, 275–291. [Google Scholar] [CrossRef] [PubMed]
- Hauser, W.E., Jr.; Remington, J.S. Effect of antibiotics on the immune response. Am. J. Med. 1982, 72, 711–716. [Google Scholar] [CrossRef]
- Kovaleva, A.; Remmelts, H.H.; Rijkers, G.T.; Hoepelman, A.I.; Biesma, D.H.; Oosterheert, J.J. Immunomodulatory effects of macrolides during community-acquired pneumonia: A literature review. J. Antimicrob. Chemother. 2012, 67, 530–540. [Google Scholar] [CrossRef] [PubMed]
- Zarogoulidis, P.; Papanas, N.; Kioumis, I.; Chatzaki, E.; Maltezos, E.; Zarogoulidis, K. Macrolides: From in vitro anti-inflammatory and immunomodulatory properties to clinical practice in respiratory diseases. Eur. J. Clin. Pharmacol. 2012, 68, 479–503. [Google Scholar] [CrossRef] [PubMed]
- Manda-Handzlik, A.; Bystrzycka, W.; Sieczkowska, S.; Demkow, U.; Ciepiela, O. Antibiotics Modulate the Ability of Neutrophils to Release Neutrophil Extracellular Traps. Adv. Exp. Med. Biol. 2016, 944, 47–52. [Google Scholar] [CrossRef]
- Bystrzycka, W.; Moskalik, A.; Sieczkowska, S.; Manda-Handzlik, A.; Demkow, U.; Ciepiela, O. The effect of clindamycin and amoxicillin on neutrophil extracellular trap (NET) release. Cent.-Eur. J. Immunol. 2016, 41, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Konig, M.F.; Andrade, F. A Critical Reappraisal of Neutrophil Extracellular Traps and NETosis Mimics Based on Differential Requirements for Protein Citrullination. Front. Immunol. 2016, 7, 461. [Google Scholar] [CrossRef] [PubMed]
- Skrzeczynska-Moncznik, J.; Wlodarczyk, A.; Zabieglo, K.; Kapinska-Mrowiecka, M.; Marewicz, E.; Dubin, A.; Potempa, J.; Cichy, J. Secretory leukocyte proteinase inhibitor-competent DNA deposits are potent stimulators of plasmacytoid dendritic cells: Implication for psoriasis. J. Immunol. 2012, 189, 1611–1617. [Google Scholar] [CrossRef] [PubMed]
- Maueroder, C.; Mahajan, A.; Paulus, S.; Gosswein, S.; Hahn, J.; Kienhofer, D.; Biermann, M.H.; Tripal, P.; Friedrich, R.P.; Munoz, L.E.; et al. Menage-a-Trois: The Ratio of Bicarbonate to CO2 and the pH Regulate the Capacity of Neutrophils to Form NETs. Front. Immunol. 2016, 7, 583. [Google Scholar] [CrossRef] [PubMed]
- Koup, J.R.; Lau, A.H.; Brodsky, B.; Slaughter, R.L. Chloramphenicol pharmacokinetics in hospitalized patients. Antimicrob. Agents Chemother. 1979, 15, 651–657. [Google Scholar] [CrossRef] [PubMed]
- Matzneller, P.; Krasniqi, S.; Kinzig, M.; Sorgel, F.; Huttner, S.; Lackner, E.; Muller, M.; Zeitlinger, M. Blood, tissue, and intracellular concentrations of azithromycin during and after end of therapy. Antimicrob. Agents Chemother. 2013, 57, 1736–1742. [Google Scholar] [CrossRef] [PubMed]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bystrzycka, W.; Manda-Handzlik, A.; Sieczkowska, S.; Moskalik, A.; Demkow, U.; Ciepiela, O. Azithromycin and Chloramphenicol Diminish Neutrophil Extracellular Traps (NETs) Release. Int. J. Mol. Sci. 2017, 18, 2666. https://doi.org/10.3390/ijms18122666
Bystrzycka W, Manda-Handzlik A, Sieczkowska S, Moskalik A, Demkow U, Ciepiela O. Azithromycin and Chloramphenicol Diminish Neutrophil Extracellular Traps (NETs) Release. International Journal of Molecular Sciences. 2017; 18(12):2666. https://doi.org/10.3390/ijms18122666
Chicago/Turabian StyleBystrzycka, Weronika, Aneta Manda-Handzlik, Sandra Sieczkowska, Aneta Moskalik, Urszula Demkow, and Olga Ciepiela. 2017. "Azithromycin and Chloramphenicol Diminish Neutrophil Extracellular Traps (NETs) Release" International Journal of Molecular Sciences 18, no. 12: 2666. https://doi.org/10.3390/ijms18122666
APA StyleBystrzycka, W., Manda-Handzlik, A., Sieczkowska, S., Moskalik, A., Demkow, U., & Ciepiela, O. (2017). Azithromycin and Chloramphenicol Diminish Neutrophil Extracellular Traps (NETs) Release. International Journal of Molecular Sciences, 18(12), 2666. https://doi.org/10.3390/ijms18122666






