New Therapeutic Agent against Arterial Thrombosis: An Iridium(III)-Derived Organometallic Compound
Abstract
:1. Introduction
2. Results
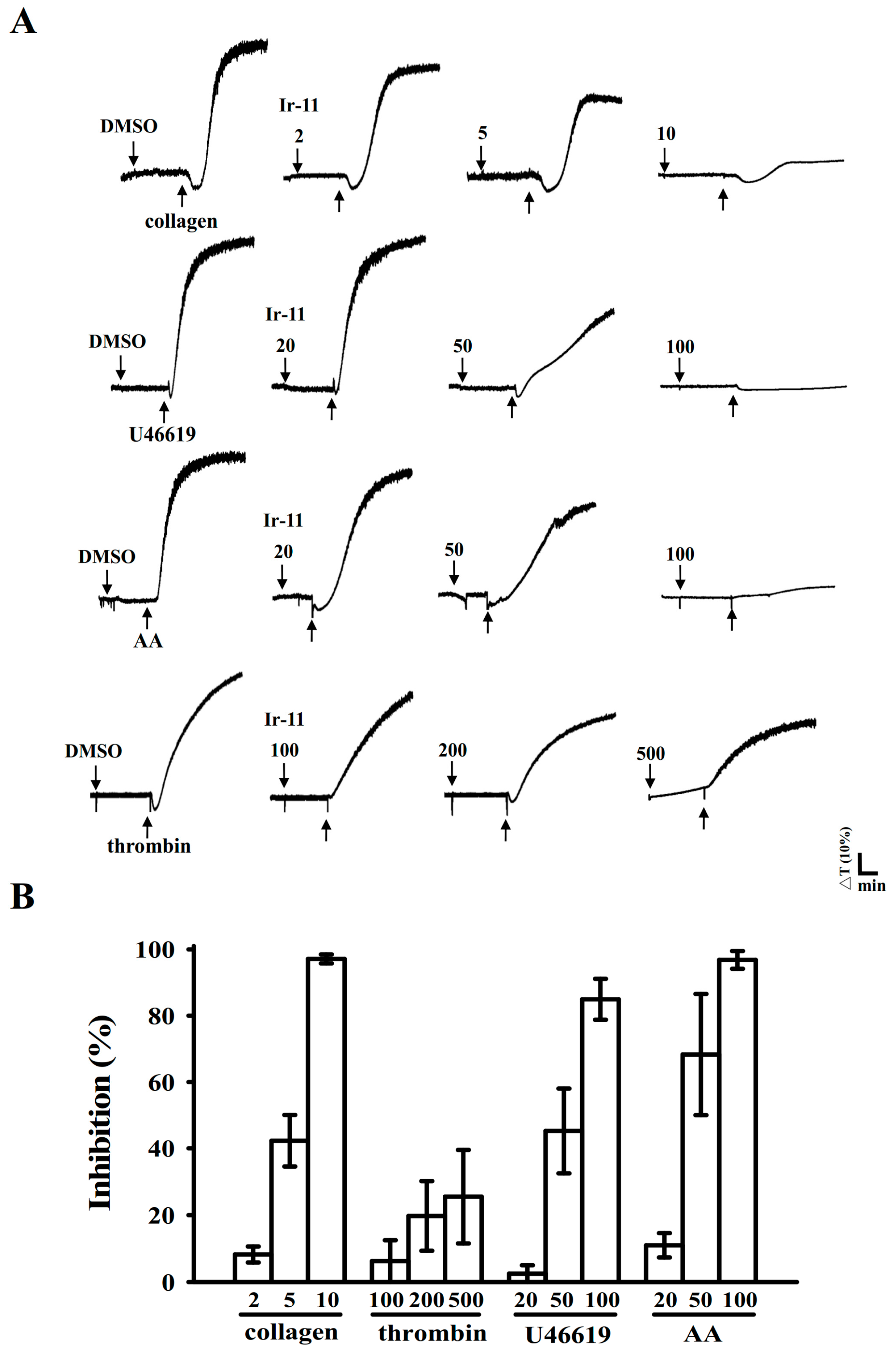
2.1. Inhibitory Effects of Ir-11 on Aggregation of Washed Human Platelets
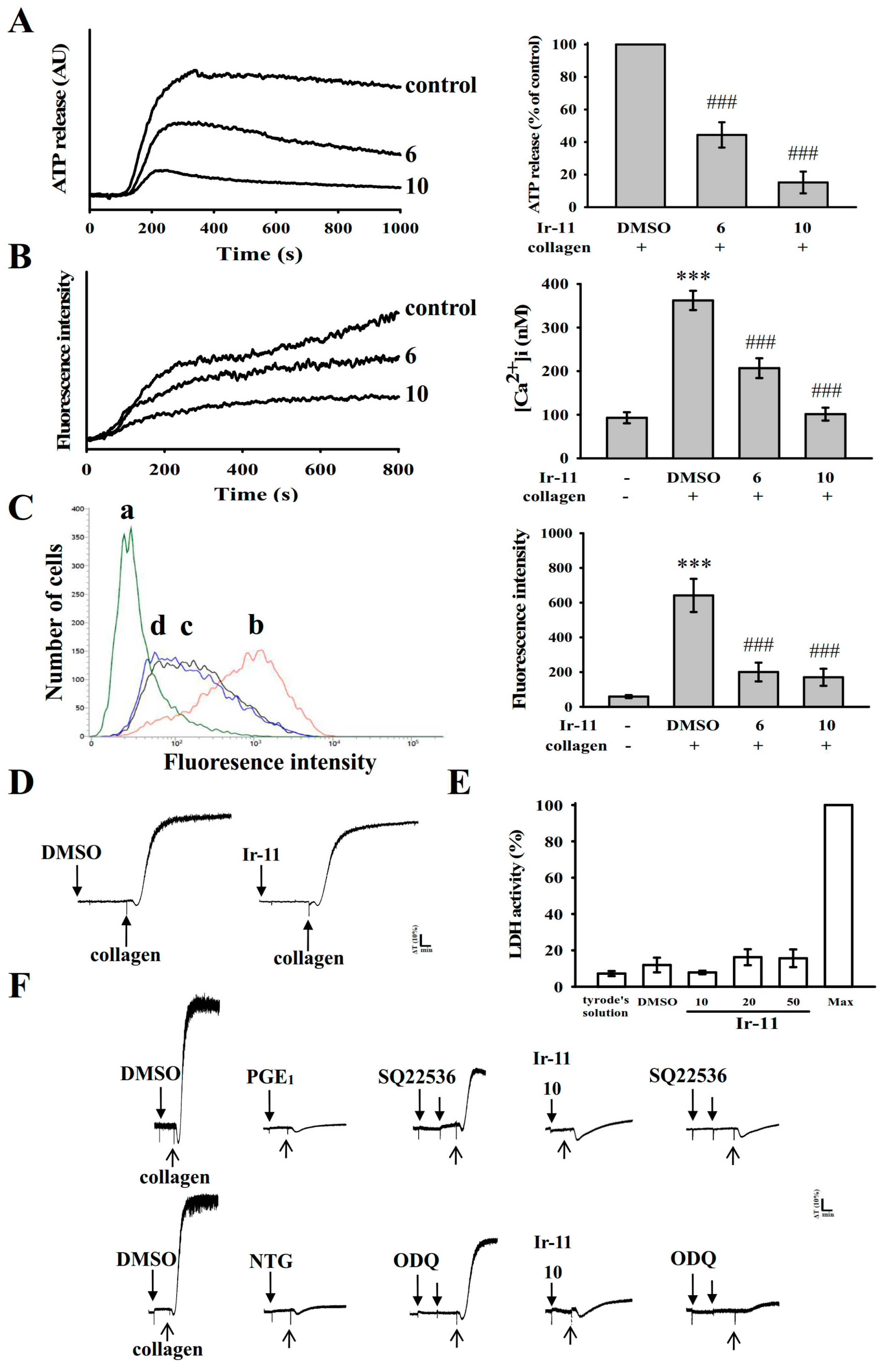
2.2. Regulatory Role of Ir-11 in Adenosine Triphosphate (ATP) Release, Relative [Ca2+]i Mobilization, Surface P-Selectin Expression, Cytotoxicity, and Cyclic Nucleotide Formation in Washed Human Platelets
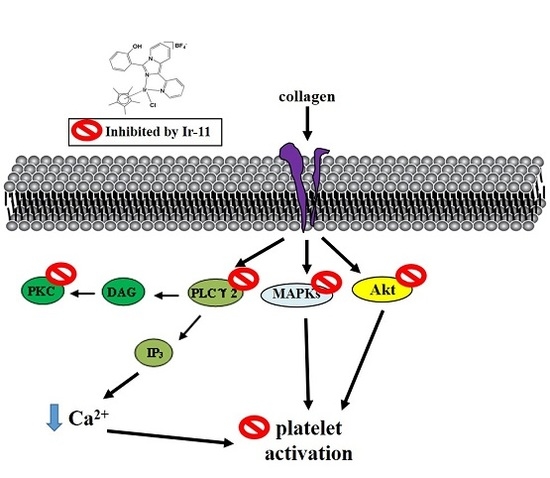
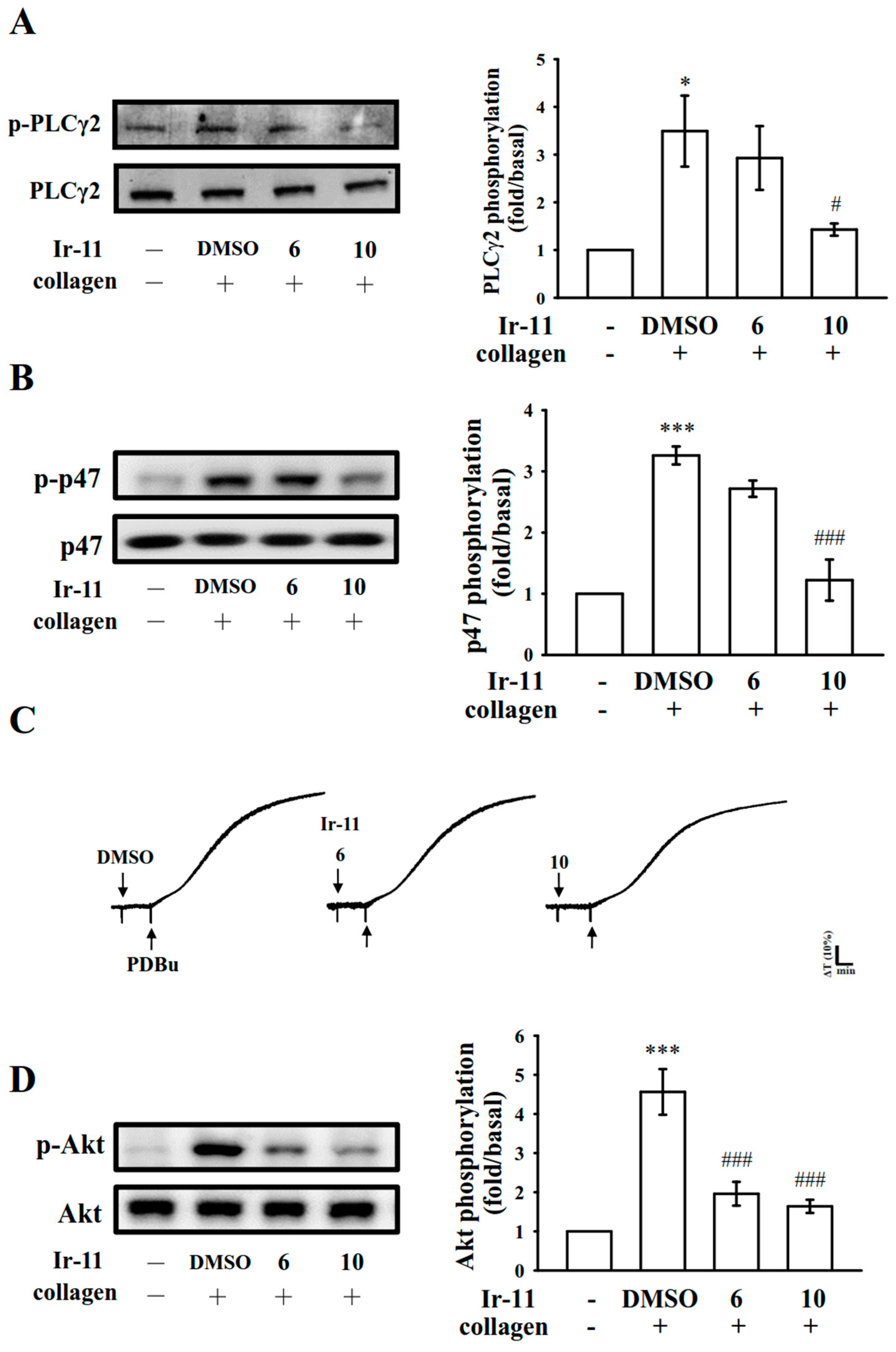
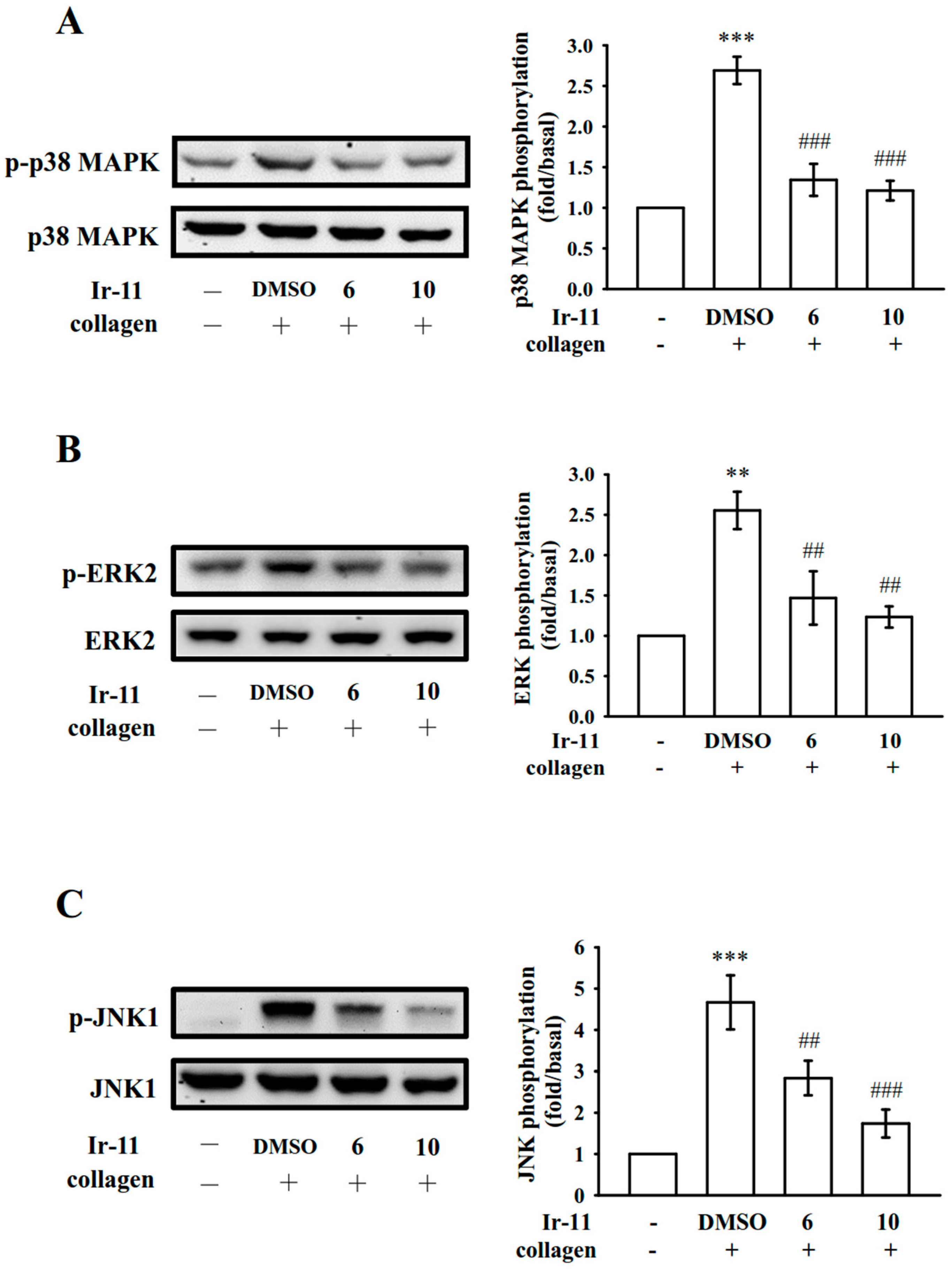
2.3. Effect of Ir-11 on the Phospholipase Cγ2/Protein Kinase C (PLCγ2/PKC) Cascade and Mitogen-Activated Protein Kinase (MAPK) and Akt Activation
2.4. Assessment of OH·-Scavenging Activity of Ir-11 through ESR Spectrometry
2.5. Crucial Roles of Ir-11 in Bleeding Time and Adenosine Diphosphate (ADP)-Induced Acute Pulmonary Thromboembolism In Vivo
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
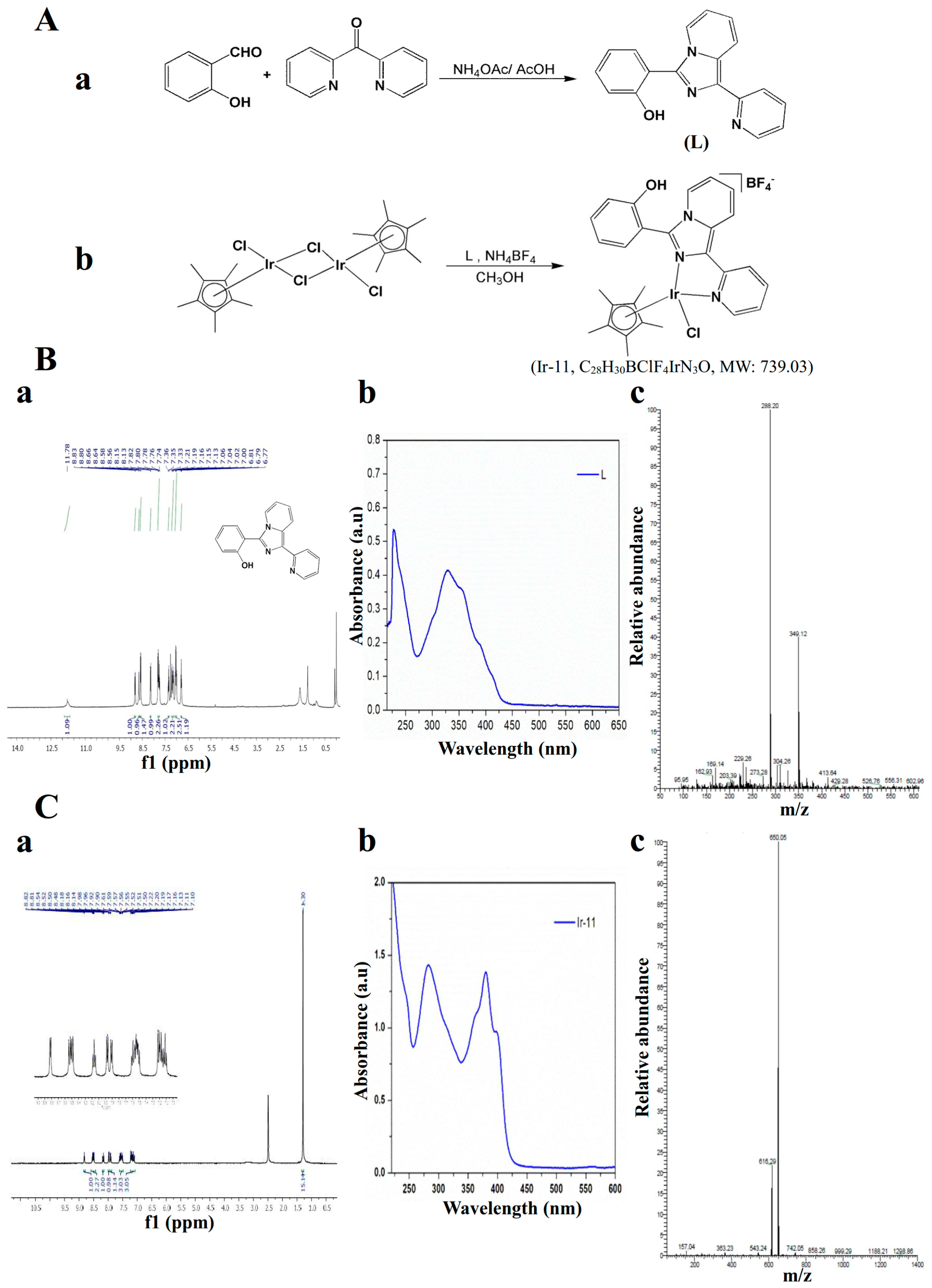
4.2. Synthesis of 1-(2-Pyridyl)-3-(2-hydroxyphenyl)imidazo[1,5-a]pyridine (L)
4.3. Synthesis of [Ir(Cp*)(L)Cl]BF4] (Ir-11)
4.4. Platelet Aggregation
4.5. Measurement of Relative [Ca2+]i Mobilization by Using Fura-2AM Fluorescence
4.6. Detection of Lactate Dehydrogenase
4.7. Flow Cytometric Analysis of Surface P-Selectin Expression
4.8. Immunoblotting of Protein Phosphorylation
4.9. Measurement of OH· Formation in Either Platelet Suspensions or the Fenton Reaction Solution through Electron Spin Resonance Spectrometry
4.10. Measurement of Bleeding Time in Mouse Tail Vein
4.11. ADP-Induced Acute Pulmonary Thromboembolism in Mice
4.12. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jayakumar, T.; Yang, C.H.; Geraldine, P.; Yen, T.L.; Sheu, J.R. The pharmacodynamics of antiplatelet compounds in thrombosis treatment. Expert Opin. Drug Metab. Toxicol. 2016, 12, 615–632. [Google Scholar] [CrossRef] [PubMed]
- Shah, B.H.; Lashari, I.; Rana, S.; Saeed, O.; Rasheed, H.; Arshad Saeed, S. Synergistic interaction of adrenaline and histamine in human platelet aggregation is mediated through activation of phospholipase, map kinase and cyclo-oxygenase pathways. Pharmacol. Res. 2000, 42, 479–483. [Google Scholar] [CrossRef] [PubMed]
- Belloc, C.; Lu, H.; Fridman, C.R.; Legrand, Y.; Menashi, S. The effect of platelets on invasiveness and protease production of human mammary tumor cells. Int. J. Cancer 1995, 60, 413–417. [Google Scholar] [CrossRef] [PubMed]
- Felding-Habermann, B.; Ooole, T.E.; Smith, J.W.; Fransvea, E.; Ruggeri, Z.M.; Ginsberg, M.H.; Hughes, P.E.; Pampori, N.; Shattil, S.J.; Saven, A.; et al. Integrin activation controls metastasis in human breast cancer. Proc. Natl. Acad. Sci. USA 2001, 98, 1853–1858. [Google Scholar] [CrossRef] [PubMed]
- Boucharaba, A.; Serre, C.M.; Grès, S.; Saulnier-Blache, J.S.; Bordet, J.C.; Guglielmi, J.; Clézardin, P.; Peyruchaud, O. Platelet-derived lysophosphatidic acid supports the progression of osteolytic bone metastases in breast cancer. J. Clin. Investig. 2004, 114, 1714–1725. [Google Scholar] [CrossRef] [PubMed]
- Iavicoli, I.; Cufino, V.; Corbi, M.; Goracci, M.; Caredda, E.; Cittadini, A.; Bergamaschi, A.; Sgambato, A. Rhodium and iridium salts inhibit proliferation and induce DNA damage in rat fibroblasts in vitro. Toxicol. In Vitro 2012, 26, 963–969. [Google Scholar] [CrossRef] [PubMed]
- Iavicoli, I.; Fontana, L.; Marinaccio, A.; Alimonti, A.; Pino, A.; Bergamaschi, A.; Calabrese, E.J. The effects of iridium on the renal function of female Wistar rats. Ecotoxicol. Environ. Saf. 2011, 74, 1795–1799. [Google Scholar] [CrossRef] [PubMed]
- Yellol, J.; Pérez, S.A.; Buceta, A.; Yellol, G.; Donaire, A.; Szumlas, P.; Bednarski, P.J.; Makhloufi, G.; Janiak, C.; Espinosa, A.; et al. Novel C,N-cyclometalated benzimidazole ruthenium(II) and iridium(III) complexes as antitumor and antiangiogenic agents: A structure−activity relationship study. J. Med. Chem. 2015, 58, 7310–7327. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, F.; Donnelly, K.; Muenzner, J.K.; Rehm, T.; Novohradsky, V.; Brabec, V.; Kasparkova, J.; Albrecht, M.; Schobert, R.; Mueller, T. Effects of histidin-2-ylidene vs. imidazol-2-ylidene ligands on the anticancer and antivascular activity of complexes of ruthenium, iridium, platinum, and gold. J. Inorg. Biochem. 2016, 163, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Harrison, P.; Cramer, E.M. Platelet α-granules. Blood Rev. 1993, 7, 52–62. [Google Scholar] [CrossRef]
- Singer, W.D.; Brown, H.A.; Sternweis, P.C. Regulation of eukaryotic phosphatidylinositol-specific phospholipase C and phospholipase D. Annu. Rev. Biochem. 1997, 6, 475–509. [Google Scholar] [CrossRef] [PubMed]
- Woulfe, D.S. Akt signaling in platelet and thrombosis. Expert Rev. Hematol. 2010, 3, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Bugaud, F.; Nadal-Wollbold, F.; Levy-Toledano, S.; Rosa, J.P.; Bryckaert, M. Regulation of c-jun-NH2 terminal kinase and extracellular-signal regulated kinase in human platelets. Blood 1999, 94, 3800–3805. [Google Scholar] [PubMed]
- Tesfamariam, B. Involvement of platelets in tumor cell metastasis. Pharmacol. Ther. 2016, 157, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Lip, G.Y.; Chin, B.S.; Blann, A. Cancer and the prothrombotic state. Lancet Oncol. 2002, 3, 27–34. [Google Scholar] [CrossRef]
- Ferroni, P.; Della-Morte, D.; Palmirotta, R.; McClendon, M.; Testa, G.; Abete, P.; Rengo, F.; Rundek, T.; Guadagni, F.; Roselli, M. Platinum-based compounds and risk for cardiovascular toxicity in the elderly: Role of the antioxidants in chemoprevention. Rejuvenation Res. 2011, 14, 293–308. [Google Scholar] [CrossRef] [PubMed]
- Jafri, M.; Protheroe, A. Cisplatin-associated thrombosis. Anticancer Drugs 2008, 19, 927–929. [Google Scholar] [CrossRef] [PubMed]
- Barni, S.; Labianca, R.; Agnelli, G.; Bonizzoni, E.; Verso, M.; Mandalà, M.; Brighenti, M.; Petrelli, F.; Bianchini, C.; Perrone, T.; et al. Chemotherapy-associated thromboembolic risk in cancer outpatients and effect of nadroparin thromboprophylaxis: Results of a retrospective analysis of the PROTECHT study. J. Transl. Med. 2011, 9, 179. [Google Scholar] [CrossRef] [PubMed]
- Dasanu, C.A. Gemcitabine: Vascular toxicity and prothrombotic potential. Expert Opin. Drug Saf. 2008, 7, 703–716. [Google Scholar] [CrossRef] [PubMed]
- Borsig, L.; Wong, R.; Feramisco, J.; Nadeau, D.R.; Varki, N.M.; Varki, A. Heparin and cancer revisited: Mechanistic connections involving platelets, P-selectin, carcinoma mucins, and tumor metastasis. Proc. Natl. Acad. Sci. USA 2001, 98, 3352–3357. [Google Scholar] [CrossRef] [PubMed]
- Kaibuchi, K.; Sano, K.; Hoshijima, M.; Takai, Y.; Nishizuka, Y. Phosphatidylinositol turnover in platelet activation; calcium mobilization and protein phosphorylation. Cell Calcium 1982, 3, 323–335. [Google Scholar] [CrossRef]
- Ragab, A.; Séverin, S.; Gratacap, M.P.; Aguado, E.; Malissen, M.; Jandrot-Perrus, M.; Malissen, B.; Ragab-Thomas, J.; Payrastre, B. Roles of the C-terminal tyrosine residues of LAT in GP VI-induced platelet activation: Insights into the mechanism of PLC gamma 2 activation. Blood 2007, 110, 2466–2474. [Google Scholar] [CrossRef] [PubMed]
- Walter, U.; Eigenthaler, M.; Geiger, J.; Reinhard, M. Role of cyclic nucleotide-dependent protein kinases and their common substrate VASP in the regulation of human platelets. Adv. Exp. Med. Biol. 1993, 344, 237–249. [Google Scholar] [PubMed]
- Chang, L.; Karin, M. Mammalian MAP kinase signaling cascades. Nature 2001, 410, 37–40. [Google Scholar] [CrossRef] [PubMed]
- Adam, F.; Kauskot, A.; Rosa, J.P.; Bryckaert, M. Mitogen-activated protein kinases in hemostasis and thrombosis. J. Thromb. Haemost. 2008, 6, 2007–2016. [Google Scholar] [CrossRef] [PubMed]
- Canobbio, I.; Reineri, S.; Sinigaglia, F.; Balduini, C.; Torti, M. A role for p38 MAP kinase in platelet activation by von Willebrand factor. Thromb. Haemost. 2004, 91, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Flevaris, P.; Li, Z.; Zhang, G.; Zheng, Y.; Liu, J.; Du, X. Two distinct roles of mitogen-activated protein kinases in platelets and a novel Rac1-MAPK-dependent integrin outside-in retractile signaling pathway. Blood 2009, 113, 893–901. [Google Scholar] [CrossRef] [PubMed]
- Adam, F.; Kauskot, A.; Nurden, P.; Sulpice, E.; Hoylaerts, M.F.; Davis, R.J.; Rosa, J.P.; Bryckaert, M. Platelet JNK1 is involved in secretion and thrombus formation. Blood 2010, 115, 4083–4092. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; De, S.; Damron, D.S.; Chen, W.S.; Hay, N.; Byzova, T.V. Impaired platelet responses to thrombin and collagen in AKT-1-deficient mice. Blood 2004, 104, 1703–1710. [Google Scholar] [CrossRef] [PubMed]
- Jayakumar, T.; Chen, W.F.; Lu, W.J.; Chou, D.S.; Hsiao, G.; Hsu, C.Y.; Sheu, J.R.; Hsieh, C.Y. A novel antithrombotic effect of sulforaphane via activation of platelet adenylate cyclase: Ex vivo and in vivo studies. J. Nutr. Biochem. 2013, 24, 1086–1095. [Google Scholar] [CrossRef] [PubMed]
- Wachowicz, B.; Olas, B.; Zbikowska, H.M.; Buczyński, A. Generation of reactive oxygen species in blood platelets. Platelets 2002, 13, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Sheu, J.R.; Chao, S.H.; Yen, M.H.; Huang, T.F. In vivo antithrombotic effect of triflavin, an Arg-Gly-Asp containing peptide on platelet plug formation in mesenteric microvessels of mice. Thromb. Haemost. 1994, 72, 617–621. [Google Scholar] [PubMed]
- Lind, S.E. The bleeding time does not predict surgical bleeding. Blood 1991, 77, 2547–2552. [Google Scholar] [PubMed]
- Sheu, J.R.; Lee, C.R.; Lin, C.H.; Hsiao, G.; Ko, W.C.; Chen, Y.C.; Yen, M.H. Mechanisms involved in the antiplatelet activity of Staphylococcus aureus lipoteichoic acid in human platelets. Thromb. Haemost. 2000, 83, 777–784. [Google Scholar] [PubMed]
- Chou, D.S.; Hsiao, G.; Shen, M.Y.; Tsai, Y.J.; Chen, T.F.; Sheu, J.R. ESR spin trapping of a carbon-centered free radical from agonist-stimulated human platelets. Free Radic. Biol. Med. 2005, 39, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Sheu, J.R.; Hung, W.C.; Wu, C.H.; Lee, Y.M.; Yen, M.H. Antithrombotic effect of rutaecarpine, an alkaloid isolated from Evodia rutaecarpa, on platelet plug formation in in vivo experiments. Br. J. Haematol. 2000, 110, 110–115. [Google Scholar] [CrossRef] [PubMed]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsia, C.-W.; Velusamy, M.; Tsao, J.-T.; Hsia, C.-H.; Chou, D.-S.; Jayakumar, T.; Lee, L.-W.; Li, J.-Y.; Sheu, J.-R. New Therapeutic Agent against Arterial Thrombosis: An Iridium(III)-Derived Organometallic Compound. Int. J. Mol. Sci. 2017, 18, 2616. https://doi.org/10.3390/ijms18122616
Hsia C-W, Velusamy M, Tsao J-T, Hsia C-H, Chou D-S, Jayakumar T, Lee L-W, Li J-Y, Sheu J-R. New Therapeutic Agent against Arterial Thrombosis: An Iridium(III)-Derived Organometallic Compound. International Journal of Molecular Sciences. 2017; 18(12):2616. https://doi.org/10.3390/ijms18122616
Chicago/Turabian StyleHsia, Chih-Wei, Marappan Velusamy, Jeng-Ting Tsao, Chih-Hsuan Hsia, Duen-Suey Chou, Thanasekaran Jayakumar, Lin-Wen Lee, Jiun-Yi Li, and Joen-Rong Sheu. 2017. "New Therapeutic Agent against Arterial Thrombosis: An Iridium(III)-Derived Organometallic Compound" International Journal of Molecular Sciences 18, no. 12: 2616. https://doi.org/10.3390/ijms18122616
APA StyleHsia, C.-W., Velusamy, M., Tsao, J.-T., Hsia, C.-H., Chou, D.-S., Jayakumar, T., Lee, L.-W., Li, J.-Y., & Sheu, J.-R. (2017). New Therapeutic Agent against Arterial Thrombosis: An Iridium(III)-Derived Organometallic Compound. International Journal of Molecular Sciences, 18(12), 2616. https://doi.org/10.3390/ijms18122616