Secondary Metabolites in Ramalina terebrata Detected by UHPLC/ESI/MS/MS and Identification of Parietin as Tau Protein Inhibitor
Abstract
:1. Introduction
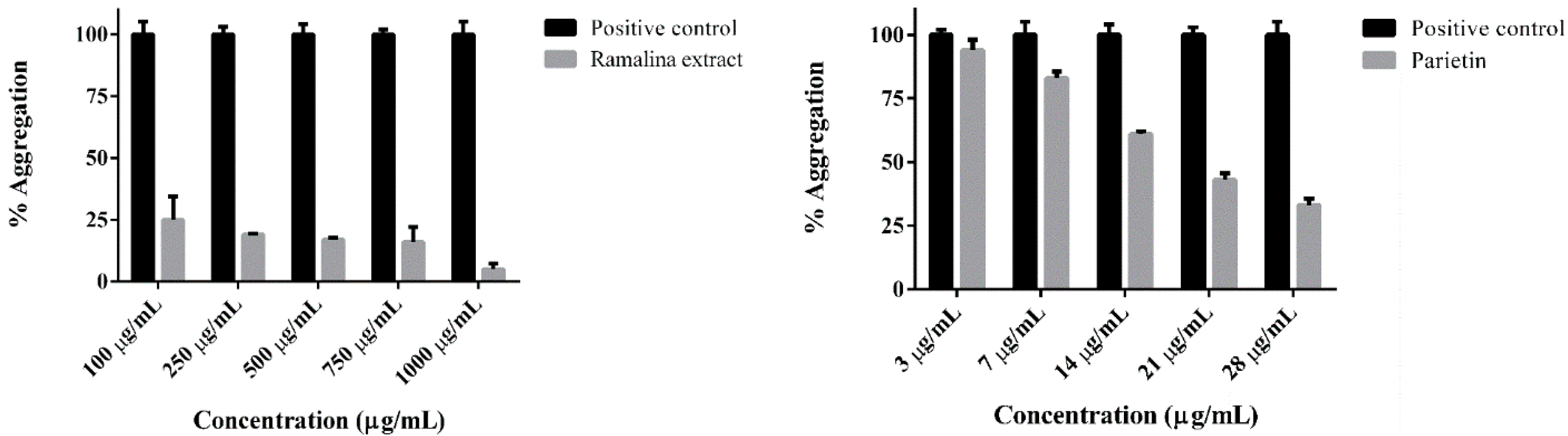
2. Results and Discussion
3. Materials and Methods
3.1. Collection and Identification of Lichen Species
3.2. Extraction
3.3. Tau Protein Production
3.4. Thioflavin T Assay
3.5. UHPLC-Q/Orbitrap/MS/MS
3.5.1. Instrument
3.5.2. LC Parameters
3.5.3. MS Parameters
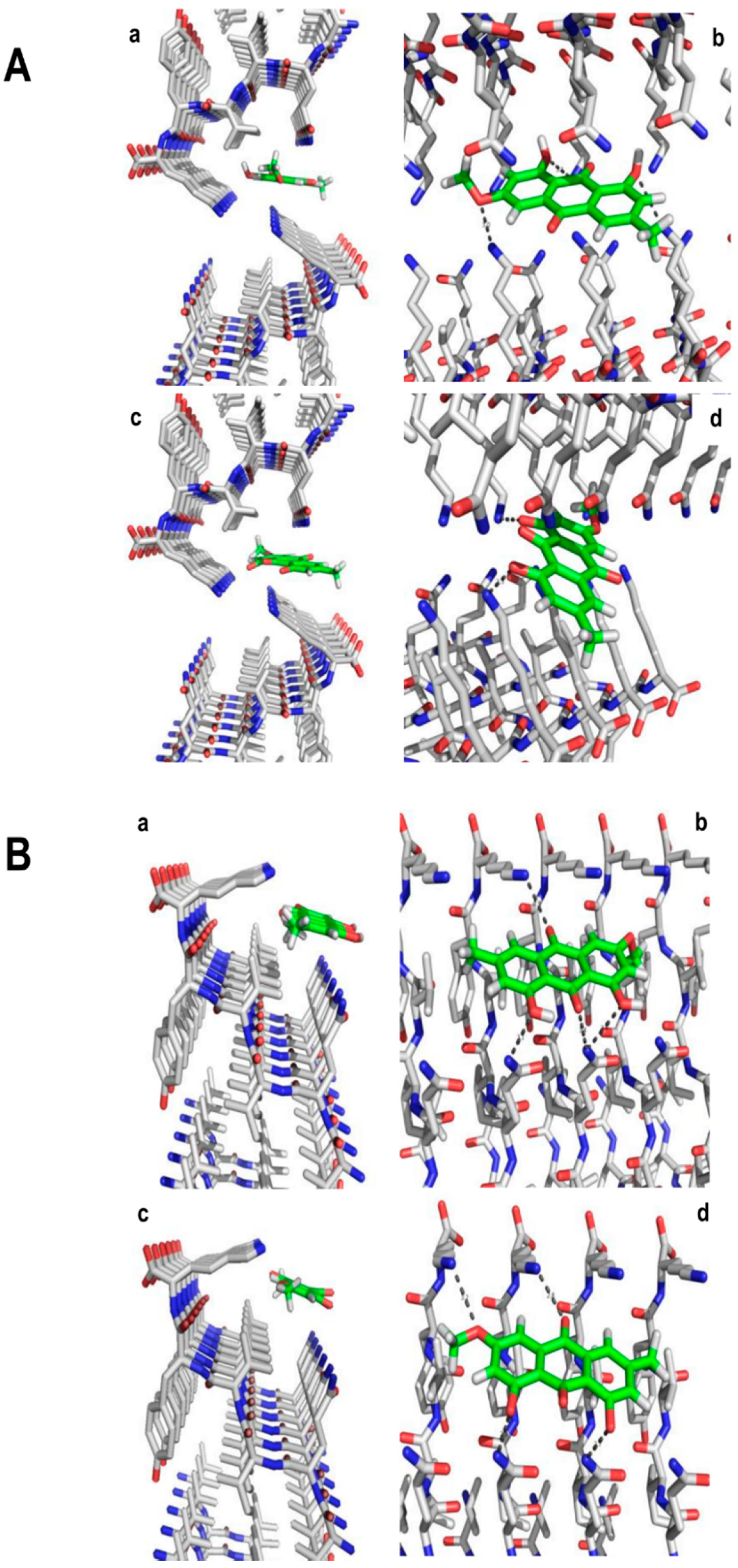
3.6. Molecular Modeling
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Shukla, V.; Joshi, G.; Rawat, M.S.M. Lichens as a potential natural source of bioactive compounds: A review. Phytochem. Rev. 2010, 9, 303–314. [Google Scholar] [CrossRef]
- Molnar, K.; Farkas, E. Current results on biological activities of lichen secondary metabolites: A review. Z. Naturforsch. C 2010, 65, 157–173. [Google Scholar] [CrossRef]
- Basile, A.; Rigano, D.; Loppi, S.; di Santi, A.; Nebbioso, A.; Sorbo, S.; Conte, B.; Paoli, L.; de Ruberto, F.; Molinari, A.M.; et al. Antiproliferative, antibacterial and antifungal activity of the lichen Xanthoria parietina and its secondary metabolite parietin. Int. J. Mol. Sci. 2015, 16, 7861–7875. [Google Scholar] [CrossRef] [PubMed]
- Gauslaa, Y.; Ustvedt, E.M. Is parietin a UV-B or a blue light screening pigment in lichen Xanthoria parietina. Photochem. Photobiol. Sci. 2003, 2, 424–432. [Google Scholar] [CrossRef]
- Boustie, J.; Grube, M. Lichens—A promising source of bioactive secondary metabolites. Plant Genet. Resour. 2005, 3, 273–287. [Google Scholar] [CrossRef]
- Muller, K. Pharmaceutically relevant metabolites from lichens. Appl. Microbiol. Biotechnol. 2001, 56, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Stocker-Worgotter, E. Metabolic diversity of lichen-forming ascomycetous fungi: Culturing, polyketide and shikimate metabolite production, and PKS genes. Nat. Prod. Rep. 2008, 25, 188–200. [Google Scholar] [CrossRef] [PubMed]
- Gotz, J.; Ittner, A.; Ittner, L.M. Tau-targeted treatment strategies in Alzheimer’s disease. Br. J. Pharmacol. 2012, 165, 1246–1259. [Google Scholar] [CrossRef] [PubMed]
- Grundke-Iqbal, I.; Iqbal, K.; Tung, Y.C.; Quinian, M.; Wisniewski, H.M.; Binder, L.I. Abnormal phosphorylation of the microtubule-associated protein tau in Alzheimer cytoskeletal pathology. Proc. Natl. Acad. Sci. USA 1986, 83, 4913–4917. [Google Scholar] [CrossRef]
- Von Bergen, M.; Friedhoff, P.; Biernat, J.; Heberle, J.; Mandelkow, E.M.; Mandelkow, E. Assembly of τ protein into Alzheimer paired helical filaments depends on a local sequence motif (306VQIVYK311) forming β structure. Proc. Natl. Acad. Sci. USA 2000, 97, 5129–5134. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Luo, Y.; Dinkel, P.; Zheng, J.; Wei, G.; Margittai, M.; Nussinov, R.; Ma, B. Cross-seeding and conformational selection between three- and four-repeat human tau proteins. J. Biol. Chem. 2012, 287, 14950–14959. [Google Scholar] [CrossRef] [PubMed]
- Mukrasch, M.D.; Biernat, J.; von Bergen, M.; Griesinger, C.; Mandelkow, E.; Zweckstetter, M. Sites of tau important for aggregation populate β-structure and bind to microtubules and polyanions. J. Biol. Chem. 2005, 280, 24978–24986. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.L.; Fernandez, C.; Fan, J.B.; Shewmaker, F.; Chen, J.; Minton, A.P.; Liang, Y. Quantitative characterization of heparin binding to tau protein implication for inducer-mediated tau filament formation. J. Biol. Chem. 2010, 285, 3592–3599. [Google Scholar] [CrossRef] [PubMed]
- Nelson, R.; Eisenberg, D. Recent atomic models of amyloid fibril structure. Curr. Opin. Struct. Biol. 2006, 16, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Kirschner, D.A.; Abraham, C.; Selkoe, D.J. X-ray diffraction from intraneuronal paired helical filaments and extraneuronal amyloid fibers in Alzheimer disease indicates cross-β conformation. Proc. Natl. Acad. Sci. USA 1986, 83, 503–507. [Google Scholar] [CrossRef] [PubMed]
- Nelson, R.; Sawaya, M.R.; Balbirnie, M.; Madsen, A.; Riekel, C.; Grothe, R.; Eisenberg, D. Structure of the cross-β spine of amyloid-like fibrils. Nature 2005, 435, 773–778. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Dominguez, G.; Romero-González, R.; Garrido Frenich, A. Multi-class methodology to determine pesticides and mycotoxins in green tea and royal jelly supplements by liquid chromatography coupled to Orbitrap high resolution mass spectrometry. Food Chem. 2016, 197, 907–915. [Google Scholar] [CrossRef] [PubMed]
- Chitescu, C.L.; Kaklamanos, G.; Nicolau, A.I.; Stolker, A.A.M. High sensitive multiresidue analysis of pharmaceuticals and antifungals in surface water using U-HPLC-Q-Exactive Orbitrap HRMS. Application to the Danube river basin on the Romanian territory. Sci. Total Environ. 2015, 532, 501–511. [Google Scholar] [CrossRef] [PubMed]
- Simirgiotis, M.J.; Borquez, J.; Neves-Vieira, M.; Brito, I.; Alfaro-Lira, S.; Winterhalter, P.; Echiburu-Chau, C.; Jerz, G.; Cardenas, A. Fast isolation of cytotoxic compounds from the native Chilean species Gypothamnium pinifolium Phil. collected in the Atacama Desert, northern Chile. Ind. Crops Prod. 2015, 76, 69–76. [Google Scholar] [CrossRef]
- Simirgiotis, M.J.; Quispe, C.; Areche, C.; Sepulveda, B. Phenolic compounds in Chilean Mistletoe (Quintral, Tristerix tetrandus) analyzed by UHPLC–Q/Orbitrap/MS/MS and its antioxidant properties. Molecules 2016, 21, 245–260. [Google Scholar] [CrossRef] [PubMed]
- Simirgiotis, M.J.; Quispe, C.; Borquez, J.; Areche, C.; Sepulveda, B. Fast detection of phenolic compounds in extracts of Easter Pears (Pyrus communis) from the Atacama Desert by ultrahigh-performance liquid chromatography and mass spectrometry (UHPLC–Q/Orbitrap/MS/MS). Molecules 2016, 21, 92–106. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, D.; Caballero, J. Is it reliable to use common molecular docking methods for comparing the binding affinities of enantiomers pairs for their protein target? Int. J. Mol. Sci. 2016, 17, 525–540. [Google Scholar] [CrossRef] [PubMed]
- Kaur, J.; Sundar, S.; Singh, N. Molecular docking, structure-activity relationship and biological evaluation of the anticancer drug monastrol as a pteridine reductase inhibitor in a clinical isolate of Leishmania donovani. J. Antimicrob. Chemother. 2010, 65, 1742–1748. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Fan, J.; Lu, H.; Wan, C.; Li, X.; Li, H.; Yang, D.; Zhang, Y.; Xiao, Y.; Qin, Z. Synthesis, resolution and biological evaluation of cyclopropyl analogs of abscisic acid. Bioorg. Med. Chem. 2015, 23, 6210–6217. [Google Scholar] [CrossRef] [PubMed]
- Grulich, M.; Brezovsky, J.; Stepanek, V.; Palyzova, A.; Kyslikova, E.; Kyslik, P. Resolution of α/β-amino acids by enantioselective penicillin G acylase from Achromobacter sp. J. Mol. Catal. B Enzym. 2015, 122, 240–247. [Google Scholar] [CrossRef]
- Ibrahim, M.A.; Abou-Seri, S.M.; Hanna, M.M.; Abdalla, M.M.; El Sayed, N.A. Design, synthesis and biological evaluation of novel condensed pyrrolo[1,2–c] pyrimidines featuring morpholine moiety as PI3Kα inhibitors. Eur. J. Med. Chem. 2015, 99, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Uysal, A.; Zengin, G.; Mollica, A.; Gunes, E.; Locatelli, M.; Yilmaz, T.; Aktumsek, A. Chemical and biological insights on Cotoneaster integerrimus: A new (−)-epicatechin source of food and medicinal applications. Phytomedicine 2016, 23, 979–988. [Google Scholar] [CrossRef] [PubMed]
- Mocan, A.; Zengin, G.; Uysal, A.; Gunes, E.; Mollica, A.; Degirmenci, N.S.; Alpsoy, L.; Aktumsek, A. Biological and chemical insights of Morina persica L.: A source of bioactive compounds with multifunctional properties. J. Funct. Foods 2016, 25, 94–109. [Google Scholar] [CrossRef]
- Cornejo, A.; Jimenez, J.M.; Caballero, L.; Melo, F.; Maccioni, R.B. Fulvic acid inhibits aggregation and promotes disassembly of tau fibrils associated with Alzheimer’s disease. J. Alzheimers. Dis. 2011, 27, 143–153. [Google Scholar] [PubMed]
- Huneck, S.; Yoshimura, I. Data of lichen substances. In Identification of Lichen Substances; Springer Verlag: Berlin, Germany, 1996; Volume 3, pp. 125–446. [Google Scholar]
- Naiki, H.; Higuchi, K.; Hosokawa, M.; Takeda, T. Fluorometric determination of amyloid fibrils in vitro using the fluorescent dye, thioflavine T. Anal. Biochem. 1989, 177, 244–249. [Google Scholar] [CrossRef]
- Levine, H. Thioflavine T interaction with synthetic Alzheimer’s disease β-amyloid peptides: Detection of amyloid aggregation in solution. Protein Sci. 1993, 2, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, S.; Suzuki, N.; Masuda, M.; Hisanaga, S.; Iwatsubo, T.; Goedert, M.; Hasegawa, M. Inhibition of heparin-induced tau filament formation by phenothiazines, polyphenols, and porphyrins. J. Biol. Chem. 2005, 280, 7614–7623. [Google Scholar] [CrossRef] [PubMed]
- Porat, Y.; Abramowitz, A.; Gazit, E. Inhibition of amyloid fibril formation by polyphenols: Structural similarity and aromatic interactions as a common inhibition mechanism. Chem. Biol. Drug Des. 2006, 67, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Crowe, A.; Ballatore, C.; Hyde, E.; Trojanowski, J.Q.; Lee, V.M. High throughput screening for small molecule inhibitors of heparin-induced tau fibril formation. Biochem. Biophys. Res. Commun. 2007, 358, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Pickhardt, M.; Gazova, Z.; von Bergen, M.; Khlistunova, I.; Wang, Y.; Hascher, A.; Mandelkow, E.M.; Biernat, J.; Mandelkow, E. Anthraquinones inhibit tau aggregation and dissolve Alzheimer’s paired helical filaments in vitro and in cells. J. Biol. Chem. 2005, 280, 3628–3635. [Google Scholar] [CrossRef] [PubMed]
- Landau, M.; Sawaya, M.R.; Faull, K.F.; Laganowsky, A.; Jiang, L.; Sievers, S.A.; Liu, J.; Barrio, J.R.; Eisenberg, D. Towards a pharmacophore for amyloid. PLoS Biol. 2011, 9, e1001080. [Google Scholar] [CrossRef] [PubMed]
- Le Pogam, P.; Schinkovitz, A.; Legouin, B.; Le lamer, A.; Boustie, J.; Richomme, P. Matrix-free UV-laser desorption ionization mass spectrometry as a versatile approach for accelerating dereplication studies on lichens. Anal. Chem. 2015, 87, 10421–10428. [Google Scholar] [CrossRef] [PubMed]
- Musharraf, S.G.; Kanwal, N.; Thadhani, V.M.; Choudhary, M.I. Rapid identification of lichen compounds based on the structure-fragmentation relationship using ESI-MS/MS analysis. Anal. Methods 2015, 7, 6066–6076. [Google Scholar] [CrossRef]
- Moreira, A.S.N.; Braz-Filho, R.; Mussi-Dias, V.; Vieira, I.J.C. Chemistry and biological activity of Ramalina lichenized fungi. Molecules 2015, 20, 8952–8987. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.G.; Koh, H.Y.; Oh, H.; Han, S.J.; Kim, I.C.; Lee, H.K.; Yim, J.H. Human dermal fibroblast proliferation activity of usimine-C from Antarctic lichen Ramalina terebrata. Biotechnol. Lett. 2010, 32, 471–475. [Google Scholar] [CrossRef] [PubMed]
- Byeon, H.E.; Park, B.K.; Yim, J.H.; Lee, H.K.; Moon, E.Y.; Rhee, D.K.; Pyo, S. Stereocalpin A inhibits the expression of adhesion molecules in activated vascular smooth muscle cells. Int. Immunopharmacol. 2012, 12, 315–325. [Google Scholar] [CrossRef] [PubMed]
- Paudel, B.; Bhattarai, H.D.; Koh, H.Y.; Lee, S.G.; Han, S.J.; Lee, H.K.; Oh, H.; Shin, H.W.; Yim, J.H. Ramalin, a novel nontoxic antioxidant compound from the Antarctic lichen Ramalina terebrata. Phytomedicine 2011, 18, 1285–1290. [Google Scholar] [CrossRef] [PubMed]
- Cansaran, D.; Atakol, O.; Halici, M.G.; Aksoy, A. HPLC analysis of usnic acid in some Ramalina species from Anatolia and investigation of their antimicrobial activities. Pharm. Biol. 2007, 45, 77–81. [Google Scholar] [CrossRef]
- Vinayaka, K.S.; Kekuda, T.R.P.; Swathi, D.; Kumar, S.V. Studies on chemical composition and in vitro antibacterial activity od solvent extracts of the lichen Ramalina hossei. BioTechnol. Indian J. 2009, 3, 309–311. [Google Scholar]
- Paudel, B.; Bhattarai, H.D.; Lee, H.K.; Oh, H.; Shin, H.W.; Han, J.; Yim, J.H. Antibacterial activities of ramalin, usnic acid, and its three derivatives isolates from the Antarctic lichen Ramalina terebrata. Z. Naturforschung. C 2010, 65, 34–38. [Google Scholar]
- Alzate-Morales, J.H.; Vergara-Jaque, A.; Caballero, J. Computational study on the interaction of N1 substituted pyrazole derivatives with B-Raf kinase: An unusual water wire hydrogen-bond network and novel interactions at the entrance of the active site. J. Chem. Inf. Model. 2010, 50, 1101–1112. [Google Scholar] [CrossRef] [PubMed]
- Friesner, R.A.; Banks, J.L.; Murphy, R.B.; Halgren, T.A.; Klicic, J.J.; Mainz, D.T.; Repasky, M.P.; knoll, E.H.; Shelley, M.; Perry, J.K.; et al. Glide: A new approach for rapid, accurate docking and scoring 1. Method and assessment of docking accuracy. J. Med. Chem. 2004, 47, 1739–1749. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, W.L.; Maxwell, D.S.; Tirado-Rives, J. Development and testing of the OPLS all-atom force field on conformational energetics and properties of organic liquids. J. Am. Chem. Soc. 1996, 118, 11225–11236. [Google Scholar] [CrossRef]
- Eldridge, M.D.; Murray, C.W.; Auton, T.R.; Paolini, G.V.; Mee, R.P. Empirical scoring functions: The development of a fast empirical scoring function to estimate the binding affinity of ligands in receptor complexes. J. Comput. Aided Mol. Des. 1997, 11, 425–445. [Google Scholar] [CrossRef] [PubMed]
| Peak | Tentative Identification | [M–H]− | Retention Time | Theoretical Mass | Measured Mass | Accuracy | MSn Ions |
|---|---|---|---|---|---|---|---|
| 1 | 9,10,12,13-tetrahydroxyheptadecanoic acid | C17H33O6 | 14.53 | 333.2283 | 333.2267 | 4.8 | – |
| 2 | 9,10,12,13-tetrahydroxyoctadecanoic acid | C18H35O6 | 15.54 | 347.2439 | 347.2423 | 4.6 | – |
| 3 | 9,10,12,13-tetrahydroxynonadecanoic acid | C19H37O6 | 17.45 | 361.2596 | 361.2577 | 5.2 | 343.2472 |
| 4 | 9,10,11,12,13-pentahydroxydocosanoic acid | C22H43O7 | 18.54 | 419.3014 | 419.2995 | 4.5 | – |
| 5 | 9,10,12,13-tetrahydroxyeicosanoic acid | C20H39O6 | 18.61 | 375.2752 | 375.2736 | 4.2 | 357.2628; 187.0962 |
| 6 | 9,10,11,12,13-pentahydroxytricosanoic acid | C23H45O7 | 19.21 | 433.3165 | 433.3150 | 3.5 | – |
| 7 | 9,10,12,13-tetrahydroxyheneicosanoic acid | C21H41O6 | 19.29 | 389.2909 | 389.2890 | 4.8 | 371.2782 |
| 8 | 4-O-dimethylbaemycesic acid | C18H15O8 | 19.58 | 359.0767 | 359.0756 | 3.0 | – |
| 9 | 9,10,12,13-tetrahydroxyeicosanoic acid | C20H39O6 | 19.64 | 375.2747 | 375.2735 | 3.2 | – |
| 10 | 9,10,12,13-tetrahydroxydocosanoic acid | C22H43O6 | 19.80 | 403.3065 | 403.3047 | 4.4 | 385.2940; 215.1274 |
| 11 | 9,10,12,13-tetrahydroxyheneicosanoic acid | C21H41O6 | 19.95 | 389.2909 | 389.2892 | 4.3 | 371.2782 |
| 12 | 9,10,11,12,13-pentahydroxytetracosanoic acid | C24H47O7 | 20.20 | 447.3327 | 447.3306 | 4.7 | – |
| 13 | 9,10,12,13-tetrahydroxydocosanoic acid | C22H43O6 | 20.37 | 403.3065 | 403.3043 | 5.4 | 385.2938; 187.0961 |
| 14 | 9,10,12,13-tetrahidroxytricosanoic acid | C23H45O6 | 20.79 | 417.3222 | 417.3198 | 5.7 | 399.3094 |
| 15 | 3-hydroxyumbilicaric acid | C25H21O11 | 21.25 | 497.1089 | 497.1065 | 4.8 | 317.0652; 167.0336 |
| 16 | Gyrophoric acid * | C24H19O10 | 21.27 | 467.0978 | 467.0962 | 3.4 | 317.0647; 167.0336; 149.0230; 123.0438 |
| 17 | Placodiolic acid or Pseudoplacodiolic acid | C19H19O8 | 22.04 | 375.1079 | 375.1070 | 2.4 | 343.0807; 259.0598; 231.0648 |
| 18 | Arthoniaic acid | C29H36O9 | 22.78 | 527.2281 | 527.2290 | −1.7 | – |
| 19 | Pseudoplacodiolic acid or Placodiolic acid | C19H19O8 | 23.65 | 375.1079 | 375.1068 | 2.9 | 343.0805; 259.0597; 231.0647 |
| 20 | Lobaric acid * | C25H27O8 | 24.82 | 455.1711 | 455.1712 | −0.2 | 411.1808; 367.1909; 352.1675; 296.1049 |
| 21 | Usnic acid * | C18H15O7 | 26.17 | 343.0818 | 343.0803 | 4.3 | 328.0583; 259.0612; 231.0663 |
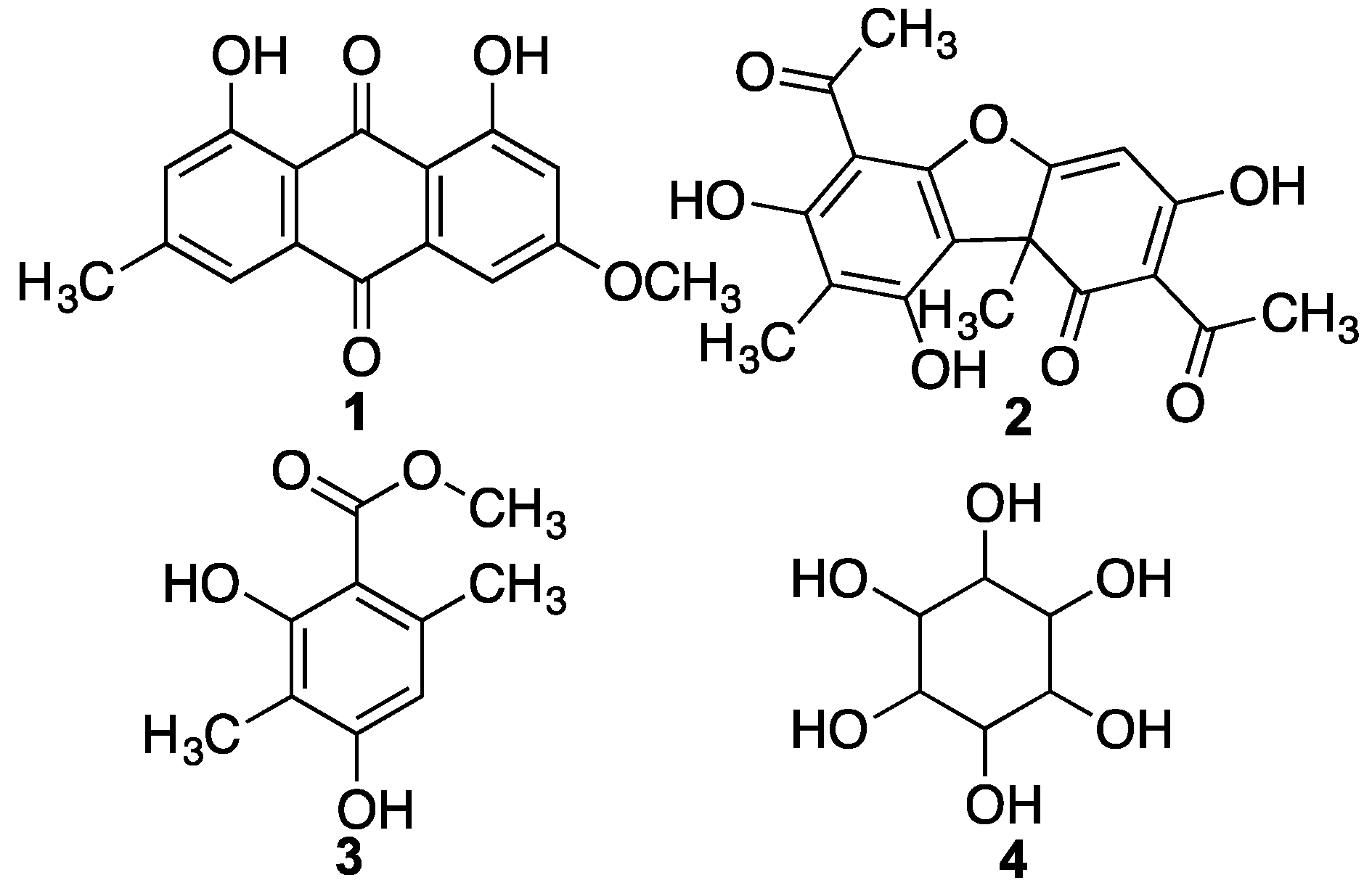
| 22 | Parietin | C16H11O5 | 27.21 | 283.0612 | 283.0601 | 3.9 | – |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cornejo, A.; Salgado, F.; Caballero, J.; Vargas, R.; Simirgiotis, M.; Areche, C. Secondary Metabolites in Ramalina terebrata Detected by UHPLC/ESI/MS/MS and Identification of Parietin as Tau Protein Inhibitor. Int. J. Mol. Sci. 2016, 17, 1303. https://doi.org/10.3390/ijms17081303
Cornejo A, Salgado F, Caballero J, Vargas R, Simirgiotis M, Areche C. Secondary Metabolites in Ramalina terebrata Detected by UHPLC/ESI/MS/MS and Identification of Parietin as Tau Protein Inhibitor. International Journal of Molecular Sciences. 2016; 17(8):1303. https://doi.org/10.3390/ijms17081303
Chicago/Turabian StyleCornejo, Alberto, Francisco Salgado, Julio Caballero, Reinaldo Vargas, Mario Simirgiotis, and Carlos Areche. 2016. "Secondary Metabolites in Ramalina terebrata Detected by UHPLC/ESI/MS/MS and Identification of Parietin as Tau Protein Inhibitor" International Journal of Molecular Sciences 17, no. 8: 1303. https://doi.org/10.3390/ijms17081303
APA StyleCornejo, A., Salgado, F., Caballero, J., Vargas, R., Simirgiotis, M., & Areche, C. (2016). Secondary Metabolites in Ramalina terebrata Detected by UHPLC/ESI/MS/MS and Identification of Parietin as Tau Protein Inhibitor. International Journal of Molecular Sciences, 17(8), 1303. https://doi.org/10.3390/ijms17081303