New Natural Pigment Fraction Isolated from Saw Palmetto: Potential for Adjuvant Therapy of Hepatocellular Carcinoma
Abstract
:1. Introduction
2. Results
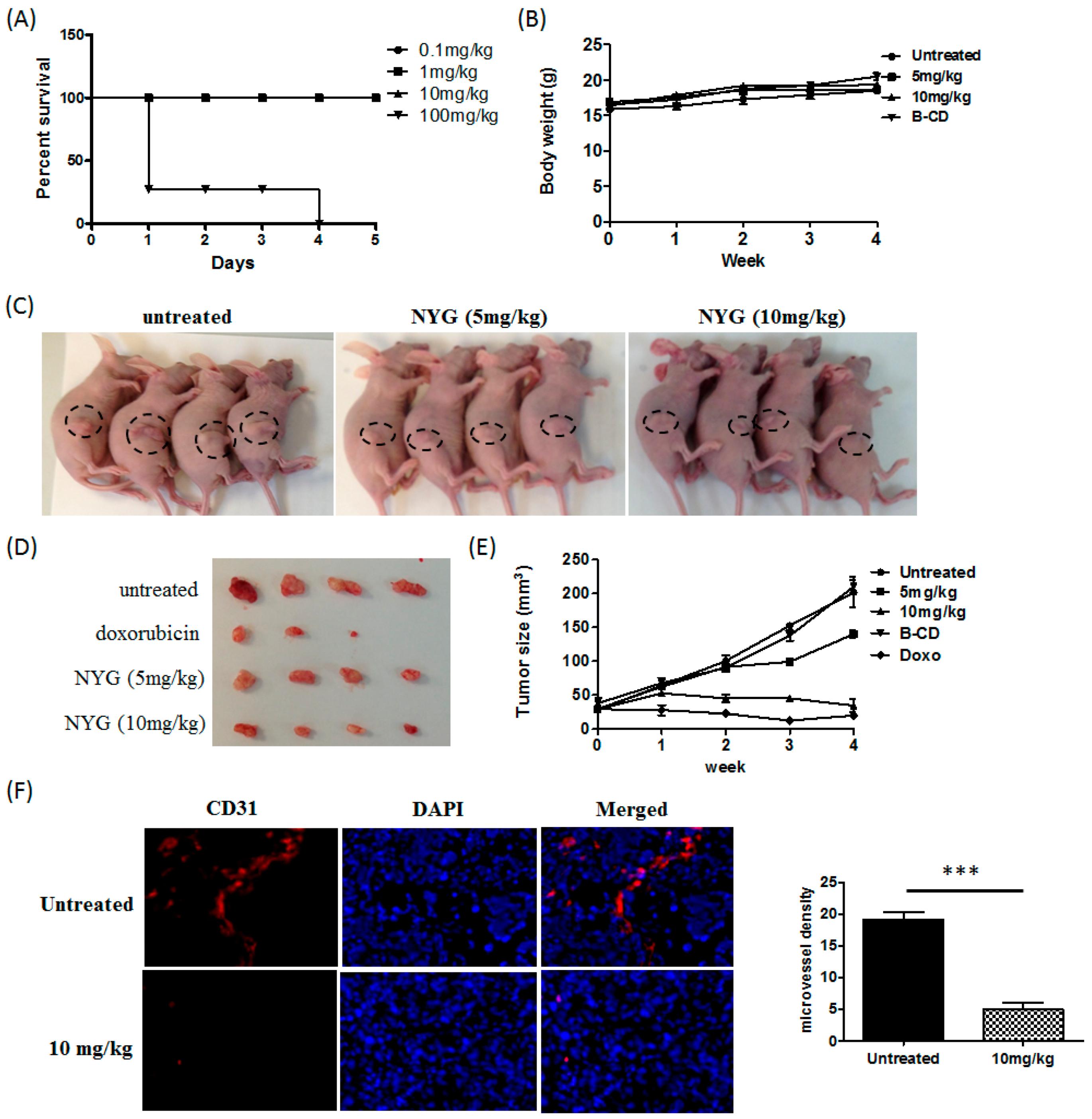
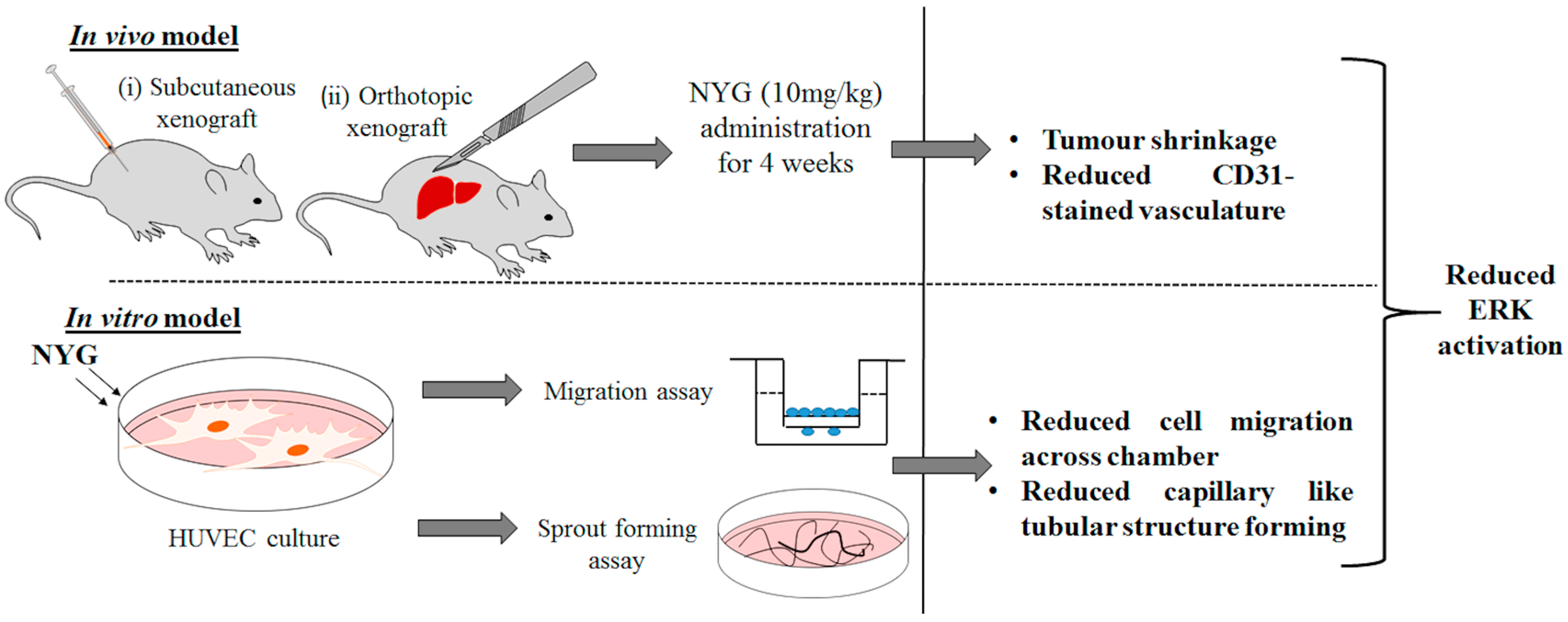
2.1. NYG Suppressed Xenograft Growth of Hepatocellular Carcinoma (HCC) in Vivo
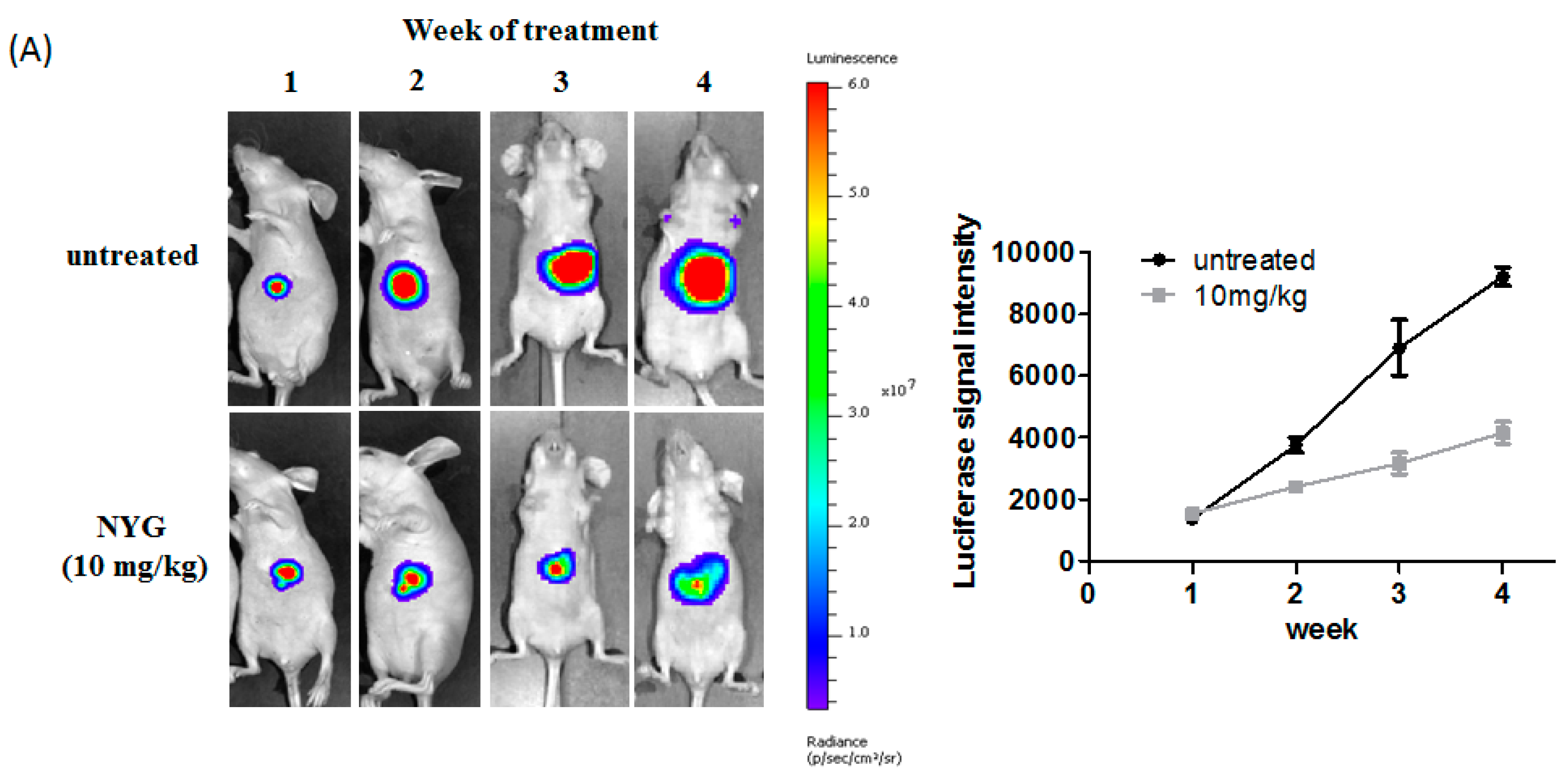
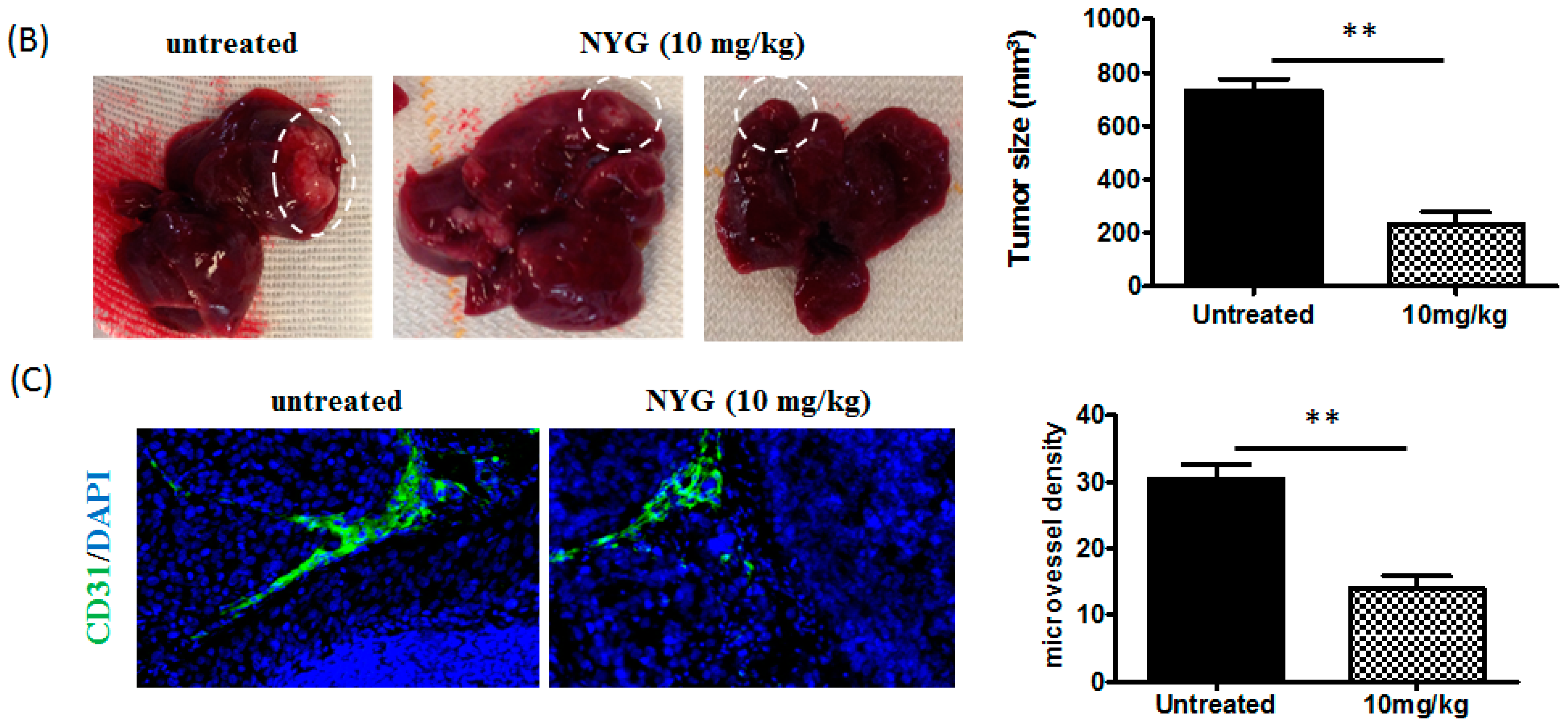
2.2. NYG Inhibited Orthotopic Implanted HCC Growth in Vivo
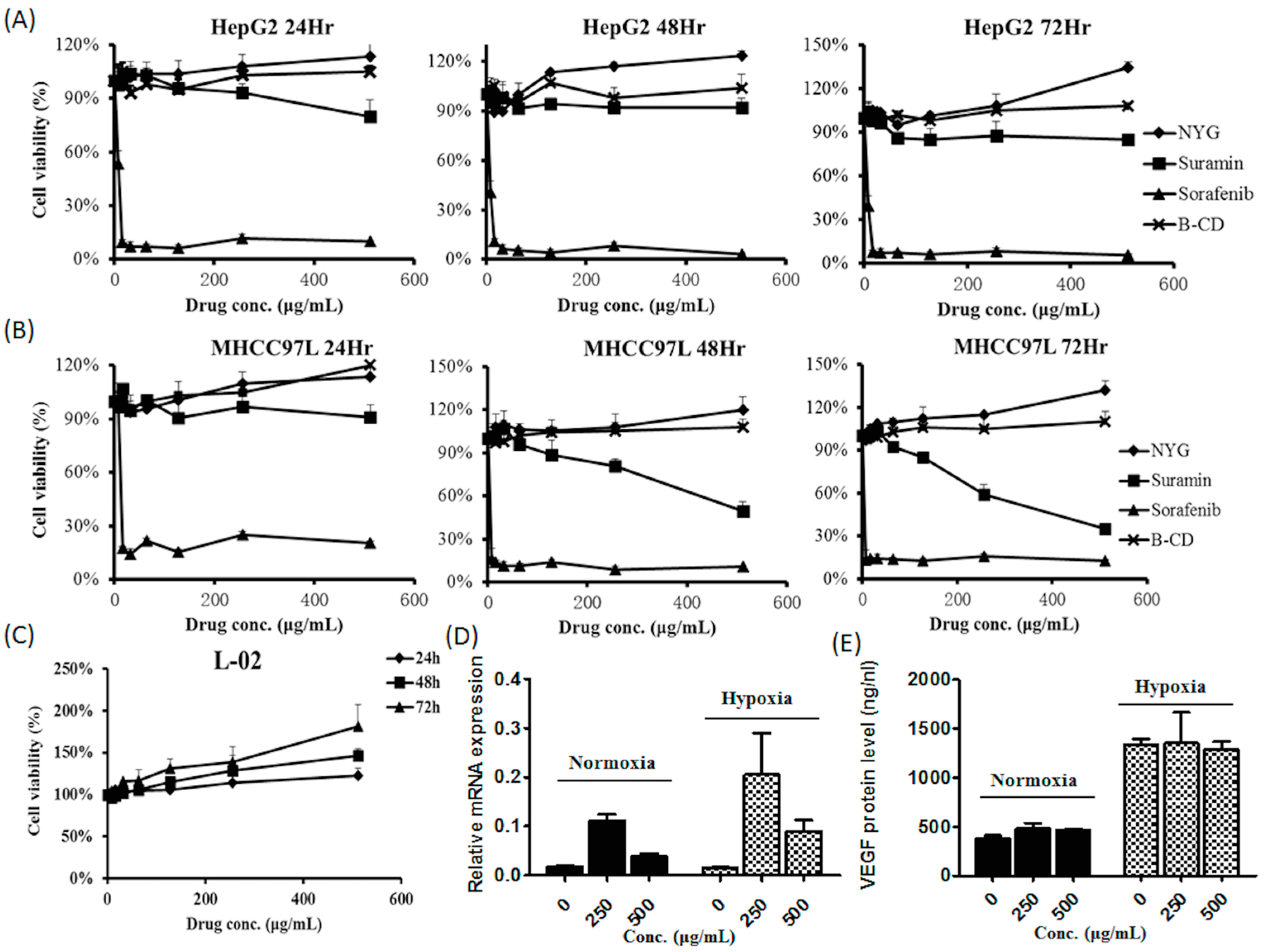
2.3. NYG Exerted Minimal Effect on in Vitro Cultured HCC Cells
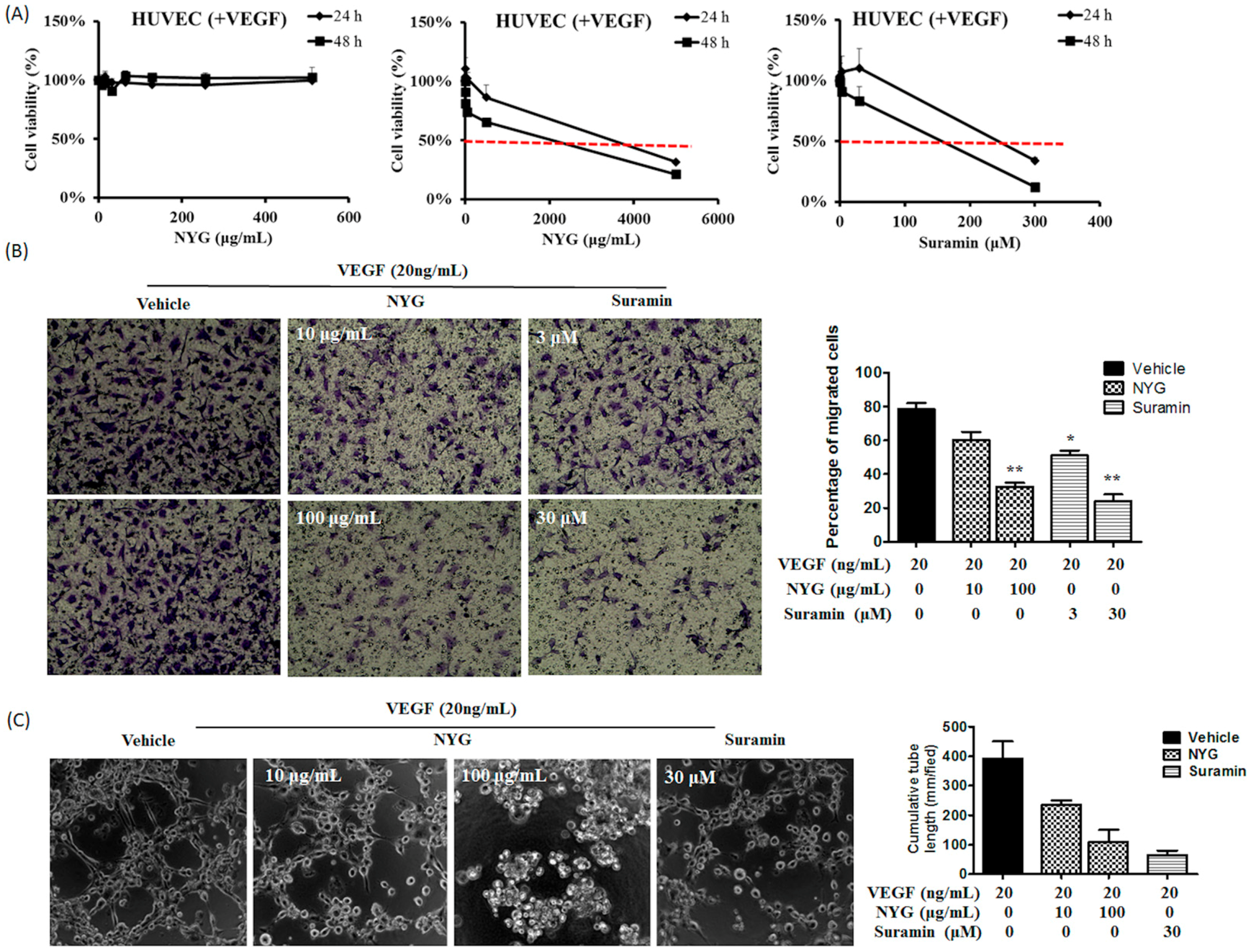
2.4. NYG Reduced Migration and Tube Formation of HUVECs
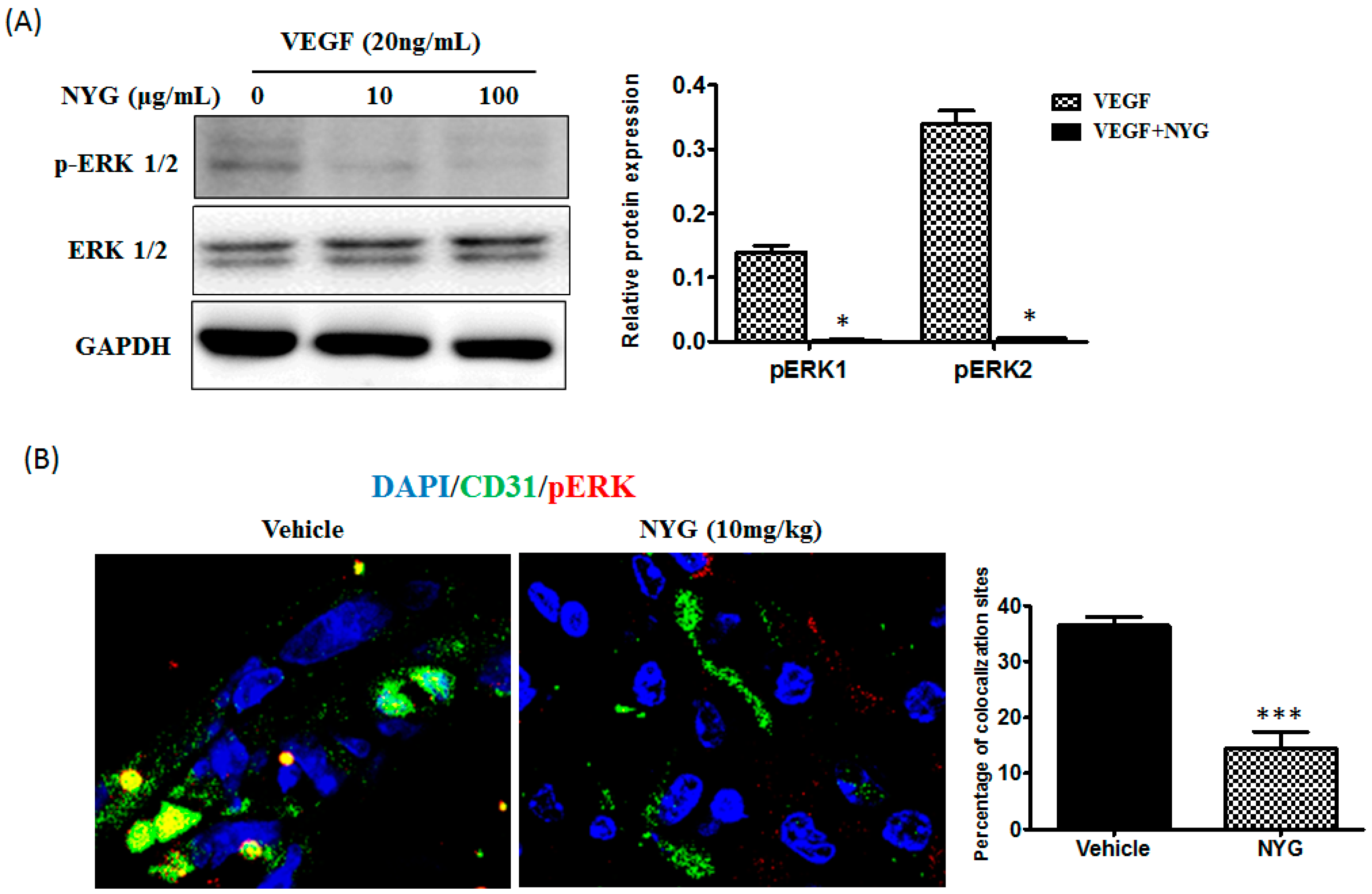
2.5. Inhibition of Tumor Neovascularization by NYG May Be Related to Inactivation of ERK in Endothelial Cells
3. Discussion
4. Materials and Methods
4.1. Preparation of NYG (Red Pigment from Saw Palmetto)
4.2. Cell Line and Cell Culture
4.3. Cell Viability Assay
4.4. Migration and Tube Formation Assay
4.5. Quantitative Real-Time PCR
4.6. Western Blotting
4.7. Immunohistochemistry
4.8. ELISA Assay on VEGF Secretion
4.9. Animal Studies
4.9.1. Subcutaneous Xenograft Model
4.9.2. Orthotopic Implantation Model
4.10. Statistical Analysis
5. Conclusion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Liver Cancer Incidence Statistics. Available online: http://www.cancerresearchuk.org/cancer-info/cancerstats/types/liver/incidence/uk-liver-cancer-incidence-statistics#world (accessed on 12 June 2014).
- IARC, W.H.O. Globocan 2012: Estimated Incidence, Mortality and Prevalence Worldwide in 2012. Available online: http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx (accessed on 10 June 2012).
- American Cancer Society. How is Liver Cancer Treated? Available online: http://www.cancer.org/cancer/livercancer/detailedguide/liver-cancer-treating-general-info (accessed on 10 June 2014).
- Johnson, P.J. Non-surgical treatment of hepatocellular carcinoma. HPB 2005, 7, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Pang, R.; Poon, R.T. Angiogenesis and antiangiogenic therapy in hepatocellular carcinoma. Cancer Lett. 2006, 242, 151–167. [Google Scholar] [CrossRef] [PubMed]
- National Cancer Institute. Fda Approval for Sorafenib Tosylate. Available online: http://www.cancer.gov/cancertopics/druginfo/fda-sorafenib-tosylate#Anchor-Live-50484 (accessed on 10 June 2014).
- Childs, M. Do Drugs Really Have to Be So Expensive? Available online: http://www.bbc.com/news/health-21834442 (accessed on 13 December 2015).
- Bent, S.; Kane, C.; Shinohara, K.; Neuhaus, J.; Hudes, E.S.; Goldberg, H.; Avins, A.L. Saw palmetto for benign prostatic hyperplasia. N. Engl. J. Med. 2006, 354, 557–566. [Google Scholar] [CrossRef] [PubMed]
- Sirab, N.; Robert, G.; Fasolo, V.; Descazeaud, A.; Vacherot, F.; Taille Ade, L.; Terry, S. Lipidosterolic extract of serenoa repens modulates the expression of inflammation related-genes in benign prostatic hyperplasia epithelial and stromal cells. Int. J. Mol. Sci. 2013, 14, 14301–14320. [Google Scholar] [CrossRef] [PubMed]
- Villaverde, N.; Galvis, A.; Marcano, A.; Priestap, H.A.; Bennett, B.C.; Barbieri, M.A. Saw palmetto ethanol extract inhibits adipocyte differentiation. J. Nat. Med. 2013, 67, 619–625. [Google Scholar] [CrossRef] [PubMed]
- Hostanska, K.; Suter, A.; Melzer, J.; Saller, R. Evaluation of cell death caused by an ethanolic extract of serenoae repentis fructus (prostasan) on human carcinoma cell lines. Anticancer Res. 2007, 27, 873–881. [Google Scholar] [PubMed]
- Wadsworth, T.L.; Worstell, T.R.; Greenberg, N.M.; Roselli, C.E. Effects of dietary saw palmetto on the prostate of transgenic adenocarcinoma of the mouse prostate model (tramp). Prostate 2007, 67, 661–673. [Google Scholar] [CrossRef] [PubMed]
- Bonnar-Pizzorno, R.M.; Littman, A.J.; Kestin, M.; White, E. Saw palmetto supplement use and prostate cancer risk. Nutr. Cancer 2006, 55, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Andriole, G.L.; McCullum-Hill, C.; Sandhu, G.S.; Crawford, E.D.; Barry, M.J.; Cantor, A. The effect of increasing doses of saw palmetto fruit extract on serum prostate specific antigen: Analysis of the camus randomized trial. J. Urol. 2013, 189, 486–492. [Google Scholar] [CrossRef] [PubMed]
- Abe, M.; Ito, Y.; Suzuki, A.; Onoue, S.; Noguchi, H.; Yamada, S. Isolation and pharmacological characterization of fatty acids from saw palmetto extract. Anal. Sci.: Int. J. Jpn. Soc. Anal. Chem. 2009, 25, 553–557. [Google Scholar] [CrossRef]
- Raman, P.; Dewitt, D.L.; Nair, M.G. Lipid peroxidation and cyclooxygenase enzyme inhibitory activities of acidic aqueous extracts of some dietary supplements. Phytother. Res.: PTR 2008, 22, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Goldmann, W.H.; Sharma, A.L.; Currier, S.J.; Johnston, P.D.; Rana, A.; Sharma, C.P. Saw palmetto berry extract inhibits cell growth and cox-2 expression in prostatic cancer cells. Cell Biol. Int. 2001, 25, 1117–1124. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.Y.; San-Marina, S.; Wang, N.; Hong, M.; Li, S.; Li, L.; Cheung, F.; Wen, X.Y.; Feng, Y. Preclinical models for investigation of herbal medicines in liver diseases: Update and perspective. Evid. Based Complement. Altern. Med. 2016, 2016, 4750163. [Google Scholar] [CrossRef] [PubMed]
- Eilken, H.M.; Adams, R.H. Dynamics of endothelial cell behavior in sprouting angiogenesis. Curr. Opin. Cell Biol. 2010, 22, 617–625. [Google Scholar] [CrossRef] [PubMed]
- Mavria, G.; Vercoulen, Y.; Yeo, M.; Paterson, H.; Karasarides, M.; Marais, R.; Bird, D.; Marshall, C.J. Erk-mapk signaling opposes rho-kinase to promote endothelial cell survival and sprouting during angiogenesis. Cancer Cell 2006, 9, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Prasadam, I.; Zhou, Y.; Du, Z.; Chen, J.; Crawford, R.; Xiao, Y. Osteocyte-induced angiogenesis via vegf-mapk-dependent pathways in endothelial cells. Mol. Cell. Biochem. 2014, 386, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Weidner, N.; Semple, J.P.; Welch, W.R.; Folkman, J. Tumor angiogenesis and metastasis–correlation in invasive breast carcinoma. N. Engl. J Med. 1991, 324, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Goel, H.L.; Mercurio, A.M. Vegf targets the tumour cell. Nature Rev. Cancer 2013, 13, 871–882. [Google Scholar] [CrossRef] [PubMed]
- Hoeben, A.; Landuyt, B.; Highley, M.S.; Wildiers, H.; Van Oosterom, A.T.; De Bruijn, E.A. Vascular endothelial growth factor and angiogenesis. Pharmacol. Rev. 2004, 56, 549–580. [Google Scholar] [CrossRef] [PubMed]
- Narasimhan, P.; Liu, J.; Song, Y.S.; Massengale, J.L.; Chan, P.H. Vegf stimulates the erk 1/2 signaling pathway and apoptosis in cerebral endothelial cells after ischemic conditions. Stroke 2009, 40, 1467–1473. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, R.; Zabuawala, T.; Huang, H.; Zhang, J.; Gulati, P.; Fernandez, S.; Karlo, J.C.; Landreth, G.E.; Leone, G.; Ostrowski, M.C. Erk1 and erk2 regulate endothelial cell proliferation and migration during mouse embryonic angiogenesis. PLoS ONE 2009, 4, e8283. [Google Scholar] [CrossRef] [PubMed]
- Gidwani, B.; Vyas, A. A comprehensive review on cyclodextrin-based carriers for delivery of chemotherapeutic cytotoxic anticancer drugs. BioMed Res. Int. 2015, 2015, 198–268. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, G.; Tiwari, R.; Rai, A.K. Cyclodextrins in delivery systems: Applications. J. Pharmacy Bioallied Sci. 2010, 2, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Irie, T.; Uekama, K. Pharmaceutical applications of cyclodextrins. Iii. Toxicological issues and safety evaluation. J. Pharm. Sci. 1997, 86, 147–162. [Google Scholar] [CrossRef] [PubMed]
- Schonfelder, U.; Radestock, A.; Elsner, P.; Hipler, U.C. Cyclodextrin-induced apoptosis in human keratinocytes is caspase-8 dependent and accompanied by mitochondrial cytochrome c release. Exp. Dermatol. 2006, 15, 883–890. [Google Scholar] [CrossRef] [PubMed]
- Leroy-Lechat, F.; Wouessidjewe, D.; Andreux, J.-P.; Puisieux, F.; Duchêne, D. Evaluation of the cytotoxicity of cyclodextrins and hydroxypropylated derivatives. Int. J. Pharm. 1994, 101, 97–103. [Google Scholar] [CrossRef]
- Kwok, C. Management of Side Effects from Chemotherapy. Available online: https://www.hkacs.org.hk/ufiles/Chemotherapy.pdf (accessed on 13 January 2016).
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Method 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Tan, H.Y.; Wang, N.; Man, K.; Tsao, S.W.; Che, C.M.; Feng, Y. Autophagy-induced relb/p52 activation mediates tumour-associated macrophage repolarisation and suppression of hepatocellular carcinoma by natural compound baicalin. Cell Death Dis. 2015, 6, e1942. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, H.-Y.; Wang, N.; Takahashi, M.; Feng, Y.; Li, H.; Feng, Y. New Natural Pigment Fraction Isolated from Saw Palmetto: Potential for Adjuvant Therapy of Hepatocellular Carcinoma. Int. J. Mol. Sci. 2016, 17, 1277. https://doi.org/10.3390/ijms17081277
Tan H-Y, Wang N, Takahashi M, Feng Y, Li H, Feng Y. New Natural Pigment Fraction Isolated from Saw Palmetto: Potential for Adjuvant Therapy of Hepatocellular Carcinoma. International Journal of Molecular Sciences. 2016; 17(8):1277. https://doi.org/10.3390/ijms17081277
Chicago/Turabian StyleTan, Hor-Yue, Ning Wang, Masao Takahashi, Yigang Feng, Hongyun Li, and Yibin Feng. 2016. "New Natural Pigment Fraction Isolated from Saw Palmetto: Potential for Adjuvant Therapy of Hepatocellular Carcinoma" International Journal of Molecular Sciences 17, no. 8: 1277. https://doi.org/10.3390/ijms17081277
APA StyleTan, H.-Y., Wang, N., Takahashi, M., Feng, Y., Li, H., & Feng, Y. (2016). New Natural Pigment Fraction Isolated from Saw Palmetto: Potential for Adjuvant Therapy of Hepatocellular Carcinoma. International Journal of Molecular Sciences, 17(8), 1277. https://doi.org/10.3390/ijms17081277






