Strawberry Achenes Are an Important Source of Bioactive Compounds for Human Health
Abstract
:1. Introduction
2. Results
2.1. Achene Contribution to Total Fruit Weight
2.2. Antioxidant Composition in Non-Digested Raw Fruits and Achenes
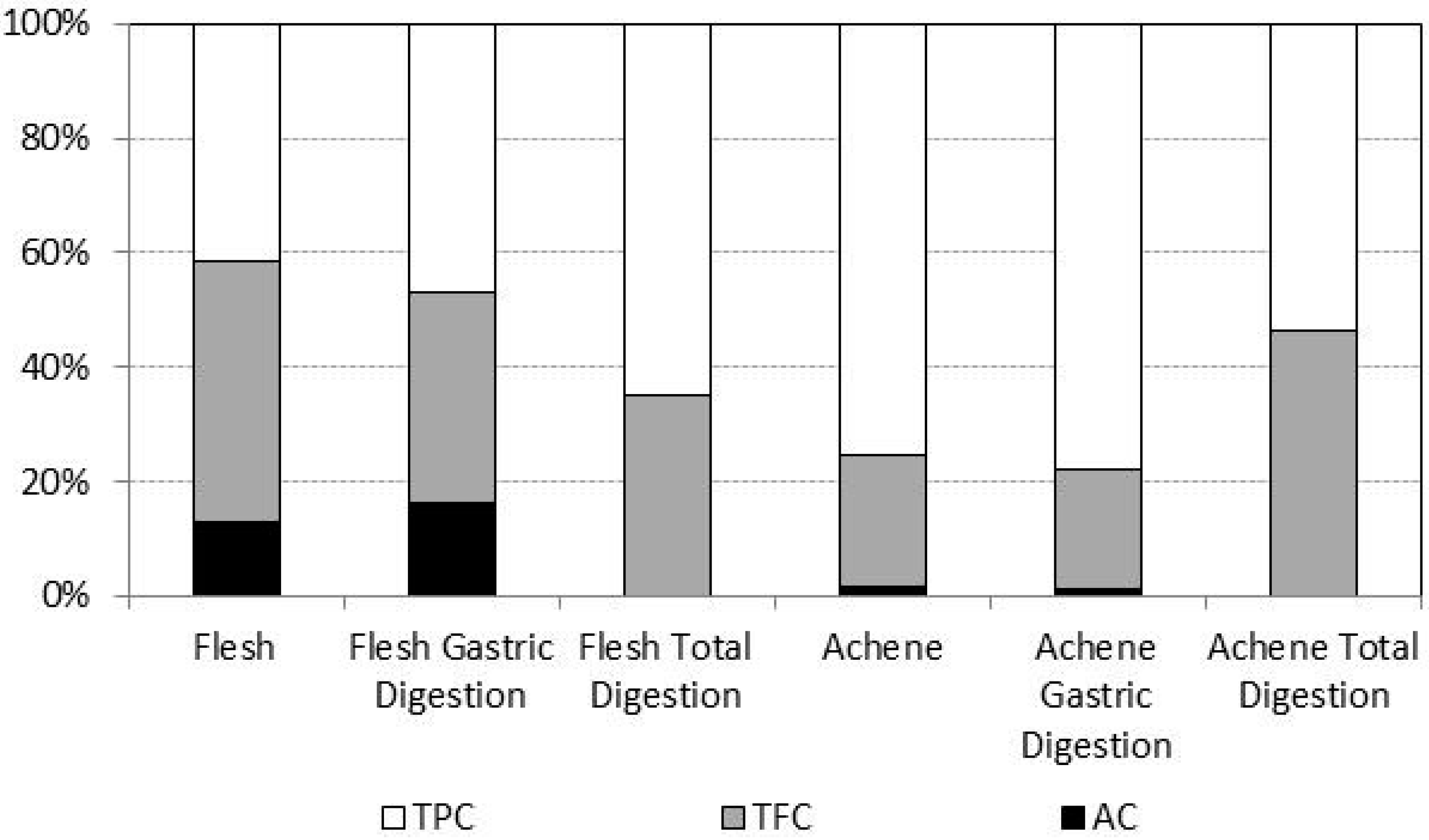
2.3. Bioaccessibility of Antioxidant Compounds after in Vitro Digestion
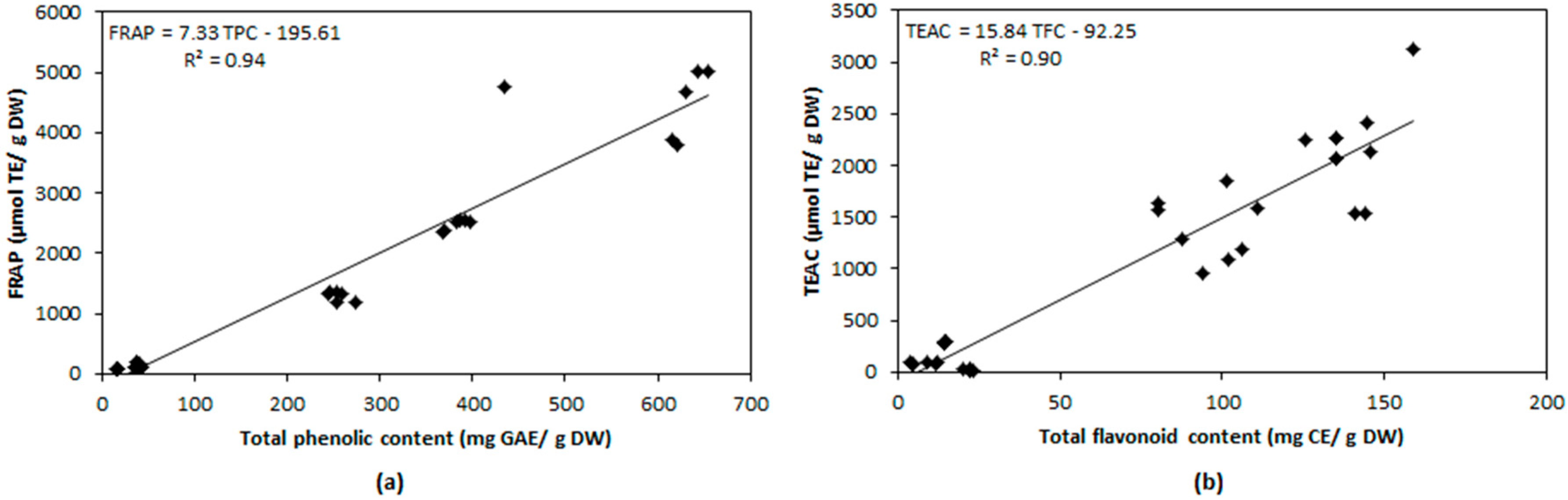
2.4. Relationship between Antioxidant Compounds and Antioxidant Capacity
2.5. Efficiency of Extraction Conditions
3. Discussion
4. Materials and Methods
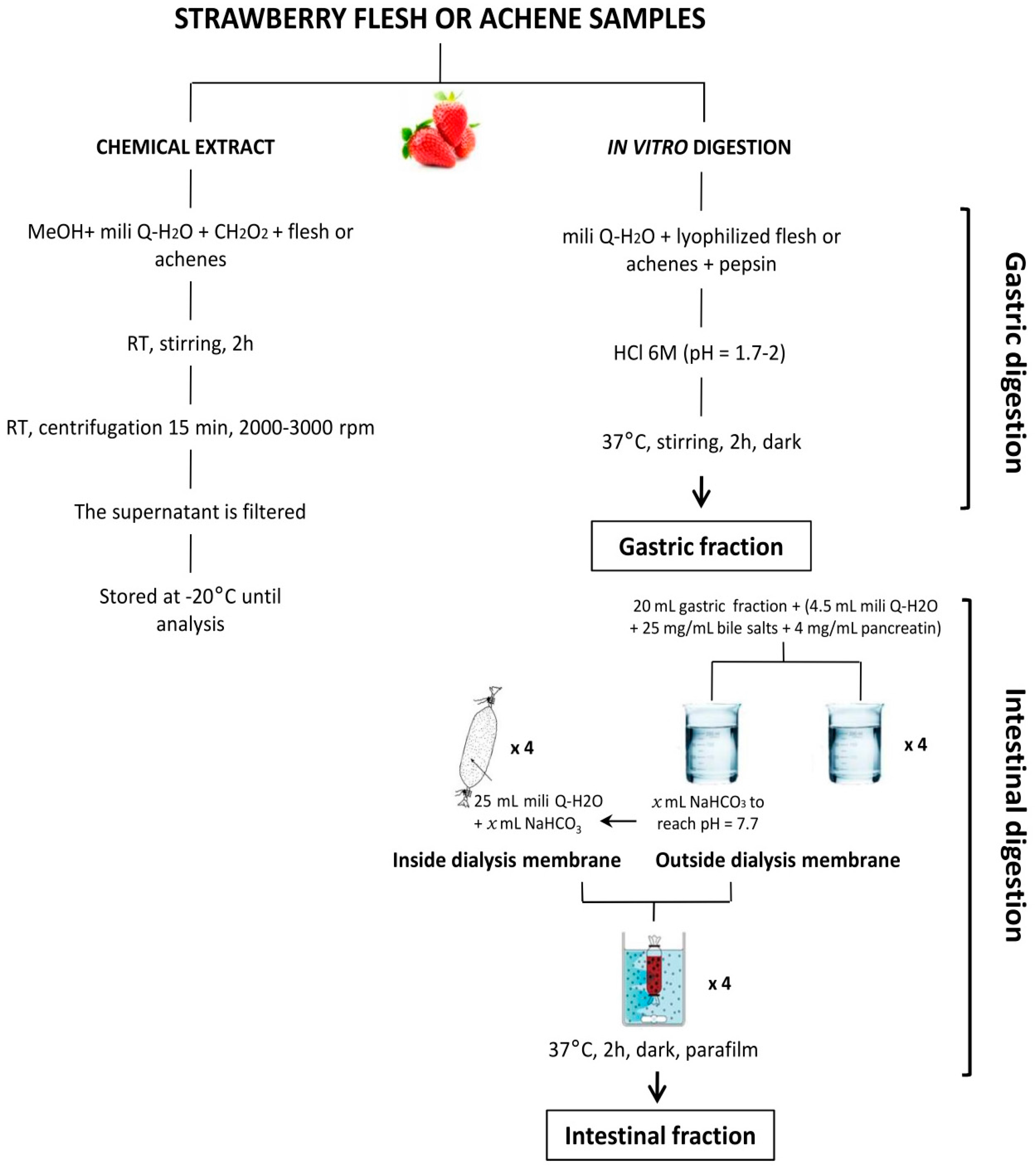
4.1. Strawberry Material and Sample Preparation
4.2. Measurement of Antioxidant Compounds
4.2.1. Total Phenolic, Flavonoid and Anthocyanin Content
4.2.2. Total Antioxidant Capacity
4.3. Determination of Phenolic Acids and Anthocyanins by HPLC
4.4. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Oszmianski, J.; Wojdylo, A. A Comparative study of phenolic content and antioxidant activity of strawberry puree, clear, and cloudy juices. Eur. Food Res. Technol. 2009, 228, 623–631. [Google Scholar] [CrossRef]
- Podsedek, A. Natural antioxidants and antioxidant capacity of Brassica vegetables: A review. LWT-Food Sci. Technol. 2007, 40, 1–11. [Google Scholar] [CrossRef]
- Atmani, D.; Chaher, N.; Atmani, D.; Berbouc, M.; Debbache, N.; Boudaoud, H. Flavonoids in human health: from structure to biological activity. Curr. Nutr. Food Sci. 2009, 5, 225–237. [Google Scholar] [CrossRef]
- Forbes-Hernández, T.Y.; Giampieri, F.; Gasparrini, M.; Mazzoni, L.; Quiles, J.L.; Alvarez-Suarez, J.M.; Battino, M. The effects of bioactive compounds from plant foods on mitochondrial function: A focus on apoptotic mechanisms. Food Chem. Toxicol. 2014, 68, 154–182. [Google Scholar]
- Forbes-Hernández, T.Y.; Gasparrini, M.; Afrin, S.; Bompadre, S.; Mezzetti, B.; Quiles, J.L.; Giampieri, F.; Battino, M. The healthy effects of strawberry polyphenols: Which strategy behind antioxidant capacity? Crit. Rev. Food Sci. Nutr. 2016, 56, 1–14. [Google Scholar]
- Giampieri, F.; Alvarez-Suarez, J.M.; Battino, M. Strawberry and human health: Effects beyond antioxidant activity. J. Agric. Food Chem. 2014, 62, 3867–3876. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.J.; Lin, J.Y. Anti-inflammatory effects of phenolic extracts from strawberry and mulberry fruits on cytokine secretion profiles using mouse primary splenocytes and peritoneal macrophages. Int. Immunopharmacol. 2013, 16, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Giampieri, F.; Tulipani, S.; Alvarez-Suarez, J.M.; Quiles, J.L.; Mezzetti, B.; Battino, M. The strawberry: Composition, nutritional quality, and impact on human health. Nutrition 2012, 28, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Giampieri, F.; Alvarez-Suarez, J.M.; Mazzoni, L.; Forbes-Hernandez, T.Y.; Gasparrini, M.; Gonzàlez-Paramàs, A.M.; Santos-Buelga, C.; Quiles, J.L.; Bompadre, S.; Mezzetti, B.; et al. An anthocyanin-rich strawberry extract protects against oxidative stress damage and improves mitochondrial functionality in human dermal fibroblasts exposed to an oxidizing agent. Food Funct. 2014, 5, 1939–1948. [Google Scholar] [CrossRef] [PubMed]
- Hannum, S.M. Potential impact of strawberries on human health: A review of the science. Crit. Rev. Food Sci. Nutr. 2004, 44, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Suarez, J.M.; Giampieri, F.; Tulipani, S.; Casoli, T.; di Stefano, G.; González-Paramás, A.M.; Santos-Buelga, C.; Busco, F.; Quiles, J.L.; Cordero, M.D.; et al. One-month strawberry-rich anthocyanin supplementation ameliorates cardiovascular risk, oxidative stress markers and platelet activation in humans. J. Nutr. Biochem. 2014, 25, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Da Silva Pinto, M.; de Carvalho, J.E.; Lajolo, F.M.; Genovese, M.I.; Shetty, K. Evaluation of antiproliferative, anti-type 2 diabetes, and antihypertension potentials of ellagitannins from strawberries (Fragaria × ananassa Duch.) using in vitro models. J. Med. Food 2010, 13, 1027–1035. [Google Scholar] [CrossRef] [PubMed]
- Larrosa, M.; Tomás-Barberán, F.A.; Espin, J.C. The dietary hydrolysable tannin punicalagin releases ellagic acid that induces apoptosis in human colon adenocarcinoma Caco-2 cells by using the mitochondrial pathway. J. Nutr. Biochem. 2006, 17, 611–625. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.S.; Li, J.H. Chemopreventive effect of punicalagin, a novel tannin component isolated from Terminalia catappa, on H-Ras-transformed NIH3T3 cells. Toxicol. Lett. 2006, 163, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Zunino, S.J.; Parelman, M.A.; Freytag, T.L.; Stephensen, C.B.; Kelley, D.S.; Mackey, B.E.; Woodhouse, L.R.; Bonnel, E.L. Effects of dietary strawberry powder on blood lipids and inflammatory markers in obese human subjects. Br. J. Nutr. 2012, 108, 900–909. [Google Scholar] [CrossRef] [PubMed]
- Meyers, K.J.; Watkins, C.B.; Pritts, M.P.; Liu, R.H. Antioxidant and antiproliferative activities of strawberries. J. Agric. Food Chem. 2003, 51, 6887–6892. [Google Scholar] [CrossRef] [PubMed]
- Aaby, K.; Skrede, G.; Wrolstad, R.E. Phenolic composition and antioxidant activities in flesh and achenes of strawberries (Fragaria ananassa). J. Agric. Food Chem. 2005, 53, 4032–4040. [Google Scholar] [CrossRef] [PubMed]
- Andres-Lacueva, C.; Shukitt-Hale, B.; Galli, R.L.; Jauregui, O.; Lamuela-Raventos, R.M.; Joseph, J.A. Anthocyanins in aged blueberry-fed rats are found centrally and may enhance memory. Nutr. Neurosci. 2005, 8, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Loren, D.J.; Seeram, N.P.; Schulman, R.N.; Holtzman, D.M. Maternal dietary supplementation with pomegranate juice is neuroprotective in an animal model of neonatal hypoxic-ischemic brain injury. Pediatr. Res. 2005, 57, 858–864. [Google Scholar] [CrossRef] [PubMed]
- Rechner, A.R.; Kroner, C. Anthocyanins and colonic metabolites of dietary polyphenols inhibit platelet function. Thromb. Res. 2005, 116, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Ding, M.; Feng, R.; Wang, S.Y.; Bowman, L.; Lu, Y.; Qian, Y.; Castranova, V.; Jiang, B.H.; Shi, X. Cyanidin 3-glucoside, a natural product derived from black berry, exhibits chemopreventive and chemotherapeutic activity. J. Biol. Chem. 2006, 281, 17359–17368. [Google Scholar] [CrossRef] [PubMed]
- Hartman, R.E.; Shah, A.; Fagan, A.M.; Hartman, R.E.; Shah, A.; Fagan, A.M.; Schwetye, K.E.; Parsadanian, M.; Schulman, R.N.; Finn, M.B.; et al. Pomegranate juice decreases amyloid load and improves behavior in a mouse model of Alzheimer’s disease. Neurobiol. Dis. 2006, 24, 506–515. [Google Scholar] [CrossRef] [PubMed]
- Hecht, S.S.; Huang, C.; Stoner, G.D.; Li, J.; Kenney, P.M.; Sturla, S.J.; Carmella, S.G. Identification of cyanidin glycosides as constituents of freezedried black raspberries which inhibit anti-benzo [a] pyrene-7,8-diol-9,10-epoxide induced NF-κB and AP-1 activity. Carcinogenesis 2006, 27, 1617–1626. [Google Scholar] [CrossRef] [PubMed]
- Olsson, M.E.; Andersson, C.S.; Oredsson, S.; Berglund, R.H.; Gustavsson, K.E. Antioxidant levels and inhibition of cancer cell proliferation in vitro by extracts from organically and conventionally cultivated strawberries. J. Agric. Food Chem. 2006, 54, 1248–1255. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Patel, J.D.; Mumper, R.J. Characterization of black-berry extract and its antiproliferative and anti-inflammatory properties. J. Med. Food 2007, 10, 258–265. [Google Scholar] [CrossRef] [PubMed]
- West, T.; Atzeva, M.; Holtzman, D.M. Pomegranate polyphenols and resveratrol protect the neonatal brain against hypoxic-ischemic injury. Dev. Neurosci. 2007, 29, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Henning, S.M.; Zhang, Y.; Rontoyanni, V.G.; Huang, J.; Lee, R.P.; Trang, A.; Nuernberger, G.; Heber, D. Variability in the antioxidant activity of dietary supplements from pomegranate, milk thistle, green tea, grape seed, Goji, and Acai: Effects of in vitro digestion. J. Agric. Food Chem. 2014, 62, 4313–4321. [Google Scholar] [CrossRef] [PubMed]
- Del Río, D.; Rodriguez-Mateos, A.; Spencer, J.P.; Tognolini, M.; Borges, G.; Crozier, A. Dietary (poly)phenolics in human health: Structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxid. Redox Signal. 2013, 18, 1818–1892. [Google Scholar]
- Kosińska-Cagnazzo, A.; Diering, S.; Prim, D.; Andlauer, W. Identification of bioaccessible and uptaken phenolic compounds from strawberry fruits in in vitro digestion/Caco-2 absorption model. Food Chem. 2015, 170, 288–294. [Google Scholar]
- Scalbert, A.; Williamson, G. Dietary intake and bioavailability of polyphenols. J. Nutr. 2000, 130, 2073S–2085S. [Google Scholar] [PubMed]
- Passamonti, S.; Vrhovsek, U.; Vanzo, A.; Mattivi, F. The stomach as a site for anthocyanins absorption from food. FEBS Lett. 2003, 544, 210–213. [Google Scholar] [CrossRef]
- Talavera, S.; Felgines, C.; Texier, O.; Besson, C.; Lamaison, J.L.; Rémésy, C. Anthocyanins are efficiently absorbed from the stomach in anesthetized rats. J. Nutr. 2003, 133, 4178–4182. [Google Scholar] [PubMed]
- Fang, J. Bioavailability of anthocyanins. Drug Metab. Rev. 2014, 46, 508–520. [Google Scholar] [CrossRef] [PubMed]
- Miyazawa, T.; Nakagawa, K.; Kudo, M.; Muraishi, K.; Someya, K. Direct intestinal absorption of red fruit anthocyanins, cyanidin-3-glucoside and cyanidin-3,5-diglucoside, into rats and humans. J. Agric. Food Chem. 1999, 47, 1083–1091. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, Y.; Chen, L.; Xin, X.; Yuan, Q. A study of controlled uptake and release of anthocyanins by oxidized starch microgels. J. Agric. Food Chem. 2013, 61, 5880–5887. [Google Scholar] [CrossRef] [PubMed]
- Hancock, J.F. Structural and Developmental Physiology. Strawberries; CABI Publishing: Wallingford, UK, 1999; pp. 131–148. [Google Scholar]
- Aaby, K.; Wrolstad, R.E.; Ekeberg, D.; Skrede, G. Polyphenol composition and antioxidant activity in strawberry purees; impact of achene level and storage. J. Agric. Food Chem. 2007, 55, 5156–5166. [Google Scholar] [CrossRef] [PubMed]
- Williner, M.R.; Pirovani, M.E.; Guemes, D.R. Ellagic acid content in strawberries of different cultivars and ripening stages. J. Sci. Food Agric. 2003, 83, 842–845. [Google Scholar] [CrossRef]
- Braadbaarta, F.; Wrightc, P.J.; van der Horsta, J.; Boona, J.J. A laboratory simulation of the carbonization of sunflower achenes and seeds. J. Anal. Appl. Pyrolysis 2007, 78, 316–327. [Google Scholar] [CrossRef]
- Contreras-Calderón, J.; Calderón-Jaimes, L.; Guerra-Hernández, E.; García-Villanova, B. Antioxidant capacity, phenolic content and vitamin C in pulp, peel and seed from 24 exotic fruits from Colombia. Food Res. Int. 2011, 44, 2047–2053. [Google Scholar]
- Vinha, A.F.; Alves, R.C.; Barreira, S.V.P.; Castro, A.; Costa, A.S.G.; Oliveira, M.B.P.P. Effect of peel and seed removal on the nutritional value and antioxidant activity of tomato (Lycopersicon esculentum L.) fruits. LTW-Food Sci. Technol. 2014, 55, 197–202. [Google Scholar] [CrossRef]
- Gil-Izquierdo, A.; Zafrilla, P.; Tomás-Barberán, F.A. An in vitro method to simulate phenolic compound release from the food matrix in the gastrointestinal tract. Eur. Food Res. Technol. 2002, 214, 155–159. [Google Scholar] [CrossRef]
- Ryan, L.; Prescott, S.L. Stability of the antioxidant capacity of twenty-five commercially available fruit juices subjected to an in vitro digestion. Int. J. Food Sci. Technol. 2010, 45, 1191–1197. [Google Scholar] [CrossRef]
- Navarro, G.; Navarro, S. Química Agrícola Química del Suelo Y de Nutrientes Esencial; Editorial Mundi-Prensa Libros: Madrid, Spain, 2013. [Google Scholar]
- Brouillard, R.; Figueiredo, P.; Elhabiri, M.; Dangles, O. Molecular interactions of phenolic compounds in relation to the colour of fruit and vegetables. In Phytochemistry of Fruit and Vegetables; Tomás-Barberán, F.A., Robins, R.J., Eds.; Clarendon Press: Oxford, UK, 1997; pp. 29–49. [Google Scholar]
- Bao, J.; Cai, Y.; Sun, M.; Wang, G.; Corke, H. Anthocyanins, flavonols, and free radical scavenging activity of chinese bayberry (Myrica rubra) extracts and their color properties and stability. J. Agric. Food Chem. 2005, 53, 2327–2332. [Google Scholar] [CrossRef] [PubMed]
- Clifford, M.N.; Scalbert, A. Ellagitannins nature, occurrence and dietary burden. J. Sci. Food Agric. 2004, 80, 1118–1125. [Google Scholar] [CrossRef]
- Murkovic, M.; Adam, U.; Pfannhauser, W. Analysis of anthocyane glycosides in human serum. Fresenius J. Anal. Chem. 2000, 366, 379–381. [Google Scholar] [CrossRef] [PubMed]
- Pérez Vicente, A.; Gil-Izquierdo, A.; García Viguera, C. In vitro gastrointestinal digestion study of pomegranate juice phenolic compounds, anthocyanins, and vitamin C. J. Agric. Food Chem. 2002, 50, 2308–2312. [Google Scholar]
- Vázquez-Flores, A.; Álvarez-Parrilla, E.; López-Díaz, J.A.; Wall-Medrano, A.; de la Rosa, L. Hydrolyzable and condensed tannins: chemistry, advantages and disadvantages of their intake. Tecnociencia 2012, 4, 84–93. [Google Scholar]
- Jiménez, A.; Selga, A.; Torres, J.L.; Julia, L. Reducing activity of polyphenols with stable radicals of the TTM series. Electron transfer versus H-abstraction reactions in flavan-3-OLS. Org. Lett. 2004, 6, 4583–4586. [Google Scholar]
- Kähkönen, M.P.; Heinämäki, J.; Ollilainen, V.; Heinonen, M. Berry anthocyanins: Isolation, identification and antioxidant activities. J. Sci. Food Agric. 2003, 83, 1403–1411. [Google Scholar]
- Espín, J.C.; Soler-Rivas, C.; Wichers, H.J. Characterization of the total free radical scavenger capacity of vegetable oils and oil fractions using 2,2-diphenyl-1-picrylhydrazyl radical. J. Agric. Food Chem. 2000, 48, 648–656. [Google Scholar]
- Ariza, M.T.; Soria, C.; Medina, J.J.; Martínez-Ferri, E. Fruit misshapen in strawberry cultivars (Fragaria x ananassa) is related to achenes functionality. Ann. Appl. Biol. 2011, 158, 130–138. [Google Scholar] [CrossRef]
- Ariza, M.T.; Martinez-Ferri, E.; Dominguez, P.; Medina, J.J.; Miranda, L.; Soria, C. Effect of harvest time on functional compounds and fruit antioxidant capacity in ten strawberry cultivars. J. Berry Res. 2015, 5, 71–80. [Google Scholar] [CrossRef]
- Slinkard, K.; Singleton, V.L. Total phenol analysis: Automation and comparison with manual methods. Am. J. Enol. Vitic. 1977, 28, 49–55. [Google Scholar]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Dewanto, V.; Wu, X.; Adom, K.K.; Liu, R.H. Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. J. Agric. Food Chem. 2002, 50, 3010–3014. [Google Scholar] [CrossRef] [PubMed]
- Giusti, M.M.; Wrolstad, R.E. Anthocyanins. Characterization and measurement with UV visible spectroscopy. In Current Protocols in Food Analytical Chemistry; Wrosltad, R.E., Ed.; Wiley: New York, NY, USA, 2001; pp. 1–13. [Google Scholar]
- Cerezo, A.B.; Cuevas, E.; Winterhalter, P.; García-Parrilla, M.C.; Troncoso, A.M. Isolation, identification, and antioxidant activity of anthocyanin compounds in “Camarosa” strawberry. Food Chem. 2010, 123, 574–582. [Google Scholar] [CrossRef]
- Deighton, N.; Brennan, R.; Finn, C.; Davies, H.V. Antioxidant properties of domesticated and wild Rubus species. J. Sci. Food Agric. 2000, 80, 1307–1313. [Google Scholar] [CrossRef]
- Kumaran, A.; Karunakaran, R.J. Activity-guided isolation and identification of free radical-scavenging components from an aqueous extract of Coleus aromaticus. Food Chem. 2007, 100, 356–361. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Schieber, A.; Keller, P.; Carle, R. Determination of phenolic acids and flavonoids of apple and pear by high-performance liquid chromatography. J. Chromatogr. A 2001, 910, 265–273. [Google Scholar] [CrossRef]
- Fredericks, C.H.; Fanning, K.J.; Gidley, M.J.; Netzel, G.; Zabaras, D.; Herrington, M.; Netzel, M. High-anthocyanin strawberries through cultivar selection. J. Sci. Food Agric. 2013, 93, 846–852. [Google Scholar] [CrossRef] [PubMed]
| TPC | TFC | AC | FRAP | DPPH | TEAC | |
|---|---|---|---|---|---|---|
| Flesh | 59 | 77 | 92 | 47 | 55 | 19 |
| Achene | 41 | 23 | 8 | 53 | 45 | 81 |
| Non-Digested | Gastric Fraction | Intestinal Fraction | Ee 1 | R (%) 2 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Flesh | Achene | Flesh | Achene | Flesh | Achene | Flesh | Achene | Flesh | Achene | |
| TPC | 37.68 ± 0.95 | 382.87 ± 4.69 * | 255.02 ± 4.40 | 600.34 ± 33.51 * | 41.17 ± 0.61 | 16.55 ± 0.39 * | 6.8 | 1.6 | 16.1 | 2.8 |
| TFC | 22.04 ± 0.49 | 93.92 ± 4.70 * | 135.32 ± 4.08 | 133.66 ± 9.13 ns | 14.40 ± 0.13 | 7.68 ± 1.60 ns | 6.1 | 1.4 | 10.6 | 5.7 |
| AC | 5.4 ± 0.78 | 6.97 ± 0.79 ns | 41.58 ± 5.37 | 17.59 ± 2.42* | 0.00 ± 0.00 | 0.00 ± 0.00 ns | 7.7 | 2.5 | 0.0 | 0.0 |
| FRAP | 149.40 ± 18.69 | 2474.22 ± 38.92 * | 1254.45 ± 46.66 | 4522.91 ± 223.18 * | 101.31 ± 1.87 | 75.25 ± 1.95 * | 8.4 | 1.8 | 8.1 | 1.7 |
| DPPH | 100.54 ± 4.49 | 1192.21 ± 62.09 * | 119.25 ± 14.30 | 3931.60 ± 771.70 * | 90.04 ± 4.58 | 32.85 ± 2.15 * | 1.2 | 3.3 | 75.5 | 0.8 |
| TEAC | 20.13 ± 7.64 | 1286.39 ± 110.39 * | 2174.79 ± 46.74 | 2100.68 ± 298.81 ns | 286.82 ± 2.79 | 86.57 ± 4.69 * | 108.0 | 1.6 | 13.2 | 4.1 |
| Non-Digested | Gastric Fraction | Intestinal Fraction | Ee 1 | R (%) 2 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Flesh | Achene | Flesh | Achene | Flesh | Achene | Flesh | Achene | Flesh | Achene | |
| Phenolic acids | ||||||||||
| Caffeic acid | 0.45 ± 0.04 | 0.40 ± 0.03 * | 0.28 ± 0.02 | 0.21 ± 0.02 ns | 0.16 ± 0.01 | 0.49 ± 0.04 * | 0.6 | 0.5 | 58.3 | 236.6 |
| Chlorogenic acid | 1.42 ± 0.12 | 1.22 ± 0.11 * | 1.25 ± 0.11 | 1.06 ± 0.09 ns | 0.57 ± 0.05 | 0.75 ± 0.07 ns | 0.9 | 0.9 | 45.5 | 70.6 |
| Ellagic acid | 1.26 ± 0.11 | 2.09 ± 0.18 * | 0.77 ± 0.07 | 0.71 ± 0.06 ns | 0.19 ± 0.02 | 0.35 ± 0.03 * | 0.6 | 0.3 | 24.4 | 48.6 |
| Anthocyanins | ||||||||||
| Cyanidin 3-glc | 0.65 ± 0.07 | 1.98 ± 0.11 * | 1.98 ± 0.11 | 2.57 ± 0.01 * | 0.00 ± 0.00 | 0.76 ± 0.11 * | 3.0 | 1.3 | 0.0 | 29.5 |
| Pelargonidin 3-glc | 4.10 ± 0.36 | 8.55 ± 0.07 * | 6.28 ± 0.11 | 13.55 ± 0.88 * | 0.90 ± 0.07 | 1.43 ± 0.22 ns | 1.5 | 1.6 | 14.3 | 10.5 |
| Pelargonidin 3-rut | 0.51 ± 0.04 | 0.32 ± 0.05 ns | 1.85 ± 0.33 | 4.26 ± 0.18 * | 0.44 ± 0.02 | 0.00 ± 0.00 * | 3.6 | 13.2 | 24.0 | 0.0 |
| DPPH | FRAP | TEAC | |
|---|---|---|---|
| TPC | 0.507 | 0.944 | 0.657 |
| 0.010 | 0.005 | 0.010 | |
| TFC | 0.146 | 0.641 | 0.900 |
| 0.020 | 0.005 | 0.010 | |
| AC | 0.002 | 0.080 | 0.545 |
| 0.246 | 0.060 | 0.010 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ariza, M.T.; Reboredo-Rodríguez, P.; Mazzoni, L.; Forbes-Hernández, T.Y.; Giampieri, F.; Afrin, S.; Gasparrini, M.; Soria, C.; Martínez-Ferri, E.; Battino, M.; et al. Strawberry Achenes Are an Important Source of Bioactive Compounds for Human Health. Int. J. Mol. Sci. 2016, 17, 1103. https://doi.org/10.3390/ijms17071103
Ariza MT, Reboredo-Rodríguez P, Mazzoni L, Forbes-Hernández TY, Giampieri F, Afrin S, Gasparrini M, Soria C, Martínez-Ferri E, Battino M, et al. Strawberry Achenes Are an Important Source of Bioactive Compounds for Human Health. International Journal of Molecular Sciences. 2016; 17(7):1103. https://doi.org/10.3390/ijms17071103
Chicago/Turabian StyleAriza, María Teresa, Patricia Reboredo-Rodríguez, Luca Mazzoni, Tamara Yuliett Forbes-Hernández, Francesca Giampieri, Sadia Afrin, Massimiliano Gasparrini, Carmen Soria, Elsa Martínez-Ferri, Maurizio Battino, and et al. 2016. "Strawberry Achenes Are an Important Source of Bioactive Compounds for Human Health" International Journal of Molecular Sciences 17, no. 7: 1103. https://doi.org/10.3390/ijms17071103
APA StyleAriza, M. T., Reboredo-Rodríguez, P., Mazzoni, L., Forbes-Hernández, T. Y., Giampieri, F., Afrin, S., Gasparrini, M., Soria, C., Martínez-Ferri, E., Battino, M., & Mezzetti, B. (2016). Strawberry Achenes Are an Important Source of Bioactive Compounds for Human Health. International Journal of Molecular Sciences, 17(7), 1103. https://doi.org/10.3390/ijms17071103















